Abstract
The plant kingdom encompasses a plethora of beneficial compounds which are the main source of chemicals needed for different industries and medicinal purposes. Slow growth rate, low yields, and difficulties to grow many of the medicinal plants in commercial scale are motivating factors for those searching for an efficient and alternative method. Tissue culture-mediated production of metabolites offers the advantages that are economically competitive over extraction from whole plant systems. Despite several solutions that have been proposed to overcome the potential problems of in vitro-mediated production of plant bioactive compounds, the success appears to require integration of a variety of tools. The quality, yield, stability, and the processing steps of a specific metabolite are subjected to change through metabolite engineering. Suppression of unwanted metabolite or introduction of new pathway for de novo synthesis of a dedicated metabolite are the other advantages of genetic engineering. Manipulation of secondary metabolites is a complicated process due to the fact that there are competing pathways with potential intervention for the same substrate or intermediates with the pathway of interest. Metabolic engineering using a single structural gene can stimulate a contest in accessibility of cofactors and development of feedback-inhibition mechanisms by accumulation of end products. It seems that successful engineering of plant secondary metabolites using a single structural gene should coincide with the manipulation of regulatory proteins and or targeting of a specific metabolite to subcellular compartments or even to the culture medium to reduce the total cost. This chapter highlights the recent strategies and achievements in metabolite engineering of medicinal plants for secondary metabolite production in cell or tissue culture systems.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 Introduction
More than 50,000 of plant species all around the world are used for medicinal purposes (http://www.fao.org) and it is believed that one-third of the flora on earth have therapeutic properties (Maitra and Satya 2014). Each of these plants has the potential of producing 5000–25,000 different metabolites (Trethewey 2004). Plant secondary metabolites comprise a wide variety of organic compounds such as terpenes , phenolics , and alkaloids that show different biological activities. Their uses include pharmaceuticals (Atropa belladonna, Digitalis lanata, Taxus sp., Hypericum perforatum), agrochemicals (Calceolaria andina), colors (Lythospermum erithroryzum), flavors (Mentha spp., Camellia sinensis and Vanilla planifolia), fragrances (Lavandula spp.), biopesticides (Chrysanthemum cinenariaefolium), and food and food additives (Coffea arabiga, Vanilla tahitiensis and Piper spp.). Until now, the FDA has approved several plant-derived active metabolites such as taxol/paclitaxel (Taxus spp.), topotecan and irinotecan (Camptotheca acuminata), etoposide and teniposide (Podophyllum peltatum), and vinblastine and vincristine (Catharanthus roseus) as anticancer compounds. These chemicals are structurally too complex to be developed by semi- or total-chemical synthesis. So, the only feasible choice was extraction of these compounds from their natural sources (Gomez-Galera et al. 2007). Herbal remedy is increasing throughout the world. Around 25% of the new pharmaceutical drugs in the market are based on plant originated molecules (Raskin et al. 2002; Terryn and Van Montagu 2006). The 16.50 billion USD worth of worldwide plant-derived secondary metabolite industries in the 1990s extended to about 70 billion USD in 2010 (Chatterjee 2010). It is estimated that plant-derived medicines hit around 35.4 billion USD between 2013–2020 (Giri and Zaheer 2016) and that the global market of medicinal plants will reach to five trillion USD by the end of 2050 (Kalia 2005). The demand for medicinal herbs, even in developed countries, was fascinating. Since 2010, the yearly growth rate of phyto-pharmaceuticals in European and North American market were 15–20% and 25–30%, respectively (Chatterjee 2010).
Because of global warming, the calamitous environmental conditions worsen throughout the world. This situation resulted in more biotic and abiotic stresses and destruction of natural areas for most of the economically valuable plant species. Besides, over-harvesting of plants with economically valuable active compounds from natural resources is one of the main reasons for plant extinction. Populations of medicinal plants on their natural habitats are dwindling because of indiscriminate harvesting for medicinal uses and food, overgrazing, landslides and degradation, climate changes, and the other anthropogenic activities. Between 4000 and 10,000 species of medicinal plants are categorized as endangered (Canter et al. 2005) and unfortunately the number is increasing. For example, Panax ginseng has nearly disappeared in their natural growing area. It is reported that Dioscorea balcanica , Podophyllum hexandrum , and Pilocarpus jaborandis are now regarded as extinct species (Nosov 2012). Up to 80–90% of commercial supply of medicinal plants are collected from natural resources (Nosov 2012). Cultivation of most of medicinal plants is unprofitable. Only 10% of 1200–1300 conventional medicinal and aromatic plant species in Europe are cultivated on approximately 70,000 hectares (Nosov 2012). Slow growth rate, low yields, difficulties in growing many of these medicinal plants in commercial scale, and fluctuations of metabolite concentrations due to varietal, physiological, developmental stage, geographical and seasonal differences are examples of problems to produce secondary metabolites via field cultivation of medicinal plants (Rao and Ravishankar 2002; Gomez-Galera et al. 2007). Production of 1 kg of taxol, an anticancer drug, needs to collect around 10,000 kg of dry bark of Taxus sp. (Vidensek et al. 1990). These reasons and additional concerns regarding the instability of secondary metabolites related to species and environmental conditions are motivating factors for those searching for an efficient alternative method.
Plant cell and tissue culture introduced as new sources of plant originated chemicals (Rao and Ravishankar 2002). This approach has been attempted since the late 1950s to produce beneficial secondary metabolites. The following results stimulated new studies to develop efficient in vitro systems in many countries. But, many barriers and problems still exist and need to be overcome for commercialization of the products. Production of active metabolites using in vitro systems is influenced by the fact that most of the secondary metabolites are produced in low quantities. In addition, the slow growth rate of plant cells and tissues, and differences between the metabolomes of the original tissue in the whole plant with the tissue cultured cells and tissues are major considerations. These can lead to a high product price or unsuccessful production of the metabolites. Different techniques have been attempted to increase the efficiency of secondary metabolite production using in vitro methods, and further elucidation of metabolic pathways and dedicated molecular mechanisms is going to facilitate manipulation of plant cells as green factories.
There are several reports indicating elevated level of terpenoid indole alkaloids (TIAs) in tissue culture of genetically modified periwinkle cells, tissue, and plants. Because of the two important anti-tumor compounds, vinblastine and vincristine obtained from Madagascar Periwinkle (Catharanthus roseus (L.) ), it is one of the most extensively studied medicinal plants. But these bisindole alkaloids accumulated in small quantities (0.001%) in the plant leaves (O’Keefe et al. 1997). The researchers are seeking to increase these metabolite using tissue culture techniques as an alternative, alongside genetic manipulation for enhanced concentrations of vinblastine and vincristine. Overexpression of the strictosidine synthase (Str) gene in cell lines of periwinkle resulted in tenfold STR activity with higher level of strictosidine , ajmalicine , catharanthine , serpentine , and tabersonine than wild type (Canel et al. 1998). In another experiment, the cell suspension culture of transgenic cell lines in periwinkle overexpressing tryptophan decarboxylase (Tdc) gene accumulated more TIAs (serpentine, catharanthine, strictosidine) compared to wild type (Whitmer et al. 2002).
7.2 Secondary Metabolisms and Economically Active Metabolites of Interest
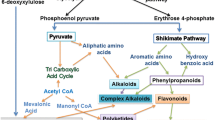
Schematic representation of the primary and secondary metabolisms in plants is demonstrated in Fig. 7.1. Plants are able to synthesize the primary complex molecules like carbohydrates using sunlight, CO2 and H2O in a process known as photosynthesis . Primary metabolism is the generator of chemical energy in the form of ATP for the ongoing cellular processes. Secondary metabolites are by-products of primary metabolism. The biochemist Albrecht Kossel was the first person who used the term “secondary metabolites” to describe the cellular chemical components that originated from “primary metabolites” (Talapatra and Talapatra 2015). These substances serve as defense compounds, repellants, and attractants in planta, and display several biological activities to be used by human (Table 7.1). Plant secondary metabolites have been classified structurally into several major groups. Also, these compounds can be categorized according to their precursors or the metabolic pathways. Bourgaud et al. (2001) subdivided these compounds into three groups termed terpenes and steroids, phenolics, and alkaloids (Bourgaud et al. 2001). Complexity of the secondary metabolites makes it difficult to place them under these three groups. In another classification, they have been divided into the following five groups: phenylpropanoids, alkaloids, polyketides, isoprenoids, and flavonoids (Verpoorte et al. 2000). Some of secondary metabolites are present in all plants, while the others are synthesized in specific species or tissues and sometimes in response to external stimuli.
The mevalonic acid (MVA) , shikimic acid , 1-deoxy-D-xylulose phosphate (DXP) , and polyketide pathways are examples of the well-known secondary metabolic pathways. The biosynthesis of terpenoids, steroids, and related natural molecules are related to MVA and DXP pathways, while shikimic acid pathway is correlated with the biosynthesis of various aromatic amino acids and structural carbon containing metabolites. Biosynthesis of secondary metabolites in higher plants take places in separate compartments under the influence of the conventional acetate-mevalonic acid and non-mevalonic acid pathways. The biosynthesis of sterols, sesquiterpenes, and ubiquinones is related to acetate-mevalonic acid pathway that operates mainly in the cytosol and mitochondria, while the non-mevalonic acid pathway takes place in the plastid, operates the biosynthesis of carotenoids, hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), and diterpenes (C20) (Singh and Sharma 2014). Biosynthesis of most of the chemicals is not dedicated to a single route. For example, C6 from polyketide pathway and C3–C6 from shikimic acid pathway come together to form flavonoids.
7.2.1 Terpenes and Steroids
Terpenes and steroids constitute the largest groups of secondary metabolites in the plant kingdom with cyclic structure and cyclopentane perhydrophenanthrene backbone, respectively. Terpenes are metabolites of interest because of their roles in plant defense system under biotic and abiotic stresses, and their beneficial biological activities in the medical and industrial sectors as scent, flavours, fragrances, spices, sweetener, anti-inflammatory, anti-parasitic, and anti-tumor. These active metabolites show diversity in the number of C5-units: hemiterpenes (C5), monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), tetraterpenes (C40), and polyterpenes (>C40) (Ashour et al. 2010; Alvarez 2014). The mevalonate and methylerythritol phosphate (MEP) pathways are two compartmentalized pathways involved in the first step of the terpenoids biosynthesis. Brassinosteroids, carotenoids, and saponins are groups of plant steroidal compounds. Ergosterol, stigmasterol, β-sitosterol, campesterol, and stigmasterol are examples of plant steroids with health-promoting properties as anti-atherosclerotic, anti-inflammatory, cholesterol lowering, and anti-oxidative activities (Alvarez 2014). The terpenes such as amarogentin (Swertia japonica), hernandulcin (Lippia dulcis), shikonin (Lithospermum erythrorhizon), digitoxin (Digitalis purpurea), ginsenosode (Panax ginseng), and astragaloside (Astragalus membranaceus) have received much attention for production using transformed root culture (Bajaj and Ishimaru 1999).
7.2.2 Phenolics
The aromatic compounds like lignin, coumarins, isoflavonoids, stilbenes, proanthocyanidines, and condensed tannins are phenolic secondary metabolites involved in several aspects of plant growth and developments. As indicated in Fig. 7.1, most of phenolic compounds originated from phenylpropanoid pathway. Other alternative pathways also drive the biosynthesis of some phenolics. It is believed that the polyketide pathway mediates the synthesis of several phenolic compounds. Tetrahydrocannabinoids is catalyzed from terpenoids (Alvarez 2014). The flavonoids and stilbenes are the two main subgroups of phenolics. Most of the fruits and vegetables contain flavonoids. There are several substances categorized as flavonoids: anthocyanidins, flavanones, naringen, silymarin, hesperitin, pelargonidins, cyanidins, delphinidins, rutin, and kaempferol (Gibbs 2007; Alvarez 2014). These compounds are very beneficial as anticancer, natural anti-inflammatory, potent antioxidant, scavenger of free radicals, and plant UV-protectants. These compounds are subjected to degradation during storage and cooking and it is believed that only 9% of the people receive enough flavonoids through consuming fruits and vegetables (Gibbs 2007). Stilbenes are another small group of phenolics that participate in plant defense system and display multiple beneficial function as medicine. A number of plant families like Liliaceae , Fagaceae , Vitaceae , and Mirtaceae produce stilbenes (Giorcelli et al. 2004). The 3,5,4′-trihydoxystilbene, also known as resveratrol, is the most common stilbene and its trans isomer has beneficial effects on some of the serious human diseases (Bradamante et al. 2004; Halls and Yu 2008). It is desirable to manipulate for resveratrol in plants, because of its potential nutraceutical property and as a cosmetic ingredient or for enhancing plant resistance against diseases. Engineering for enhanced level of resveratrol in plant is simply achievable by ectopic expression of stilbene synthase (STS) . It has been demonstrated that introduction of STS gene can result in increased level of resveratrol in several plant species (Fischer et al. 1997; Liu et al. 2006; Nicoletti et al. 2007; Hanhineva et al. 2009). The Anthraquinone (Rubia tinctorum), Xanthone (Swertia japonica), Lignan (Linum flavum), Rosmarinic acid (Ocimum basilicum), Anthocyanin (Lobelia chinensis), and Rutin (Fagopyrum esculentum) are examples of phenolic compounds in medicinal plants that have been tested for production from transformed root culture (Bajaj and Ishimaru 1999).
7.2.3 Alkaloids
Alkaloids are nitrogen-containing compounds (tropane-, morphinan-, indole-, pyrrolidine-, piperidine-, pyrrolizidine-, quinoline-, isoquinoline-, aporphine-, imidazole-, diazocin-, purine-, steroidal-, amino-, and terpenoid indole alkaloids) capable to form complex with metal ions and salts with acids (Alvarez 2014). Biosynthesis of alkaloids is performed mainly in mevalonic acid (MVA)/deoxy-D-xylulose P (DXP) and shikimic acid pathways (Talapatra and Talapatra 2015). While the nitrogen atoms in alkaloids are provided from L-amino acids.
People of 1200–1400 B.C. took advantage of opium as medicine. The latex of Papaver somniferum or opium is a rich source of codeine and morphine. These compounds are alkaloids. Alkaloids that are biosynthesized in different organs provide protective advantages for plants. Ruminants keep off browsing the herbs containing alkaloids. Alkaloids can cause severe damage to liver. Pyrrolizidine alkaloids have been recognized as the main cause of the cirrhosis and failure of the liver. These compounds are hepatotoxic, mutagenic, and carcinogenic. Some of the Crotalaria spp. ( Leguminosae ) and Heliotropium spp. ( Boraginaceae ) contain pyrrolizidine alkaloids. The calabash-curare alkaloids have strong paralysis properties and was used as dart poisons for hunting. With more than 21,000 structures, alkaloids comprise the biggest group of secondary metabolites. The purine alkaloids are synthesized from small molecules such as amino acids L-aspartic acid, L-glutamine, and L-glycine. The tea (Camellia sinensis), coffee (Coffea spp.), cacao (Theobroma cacao), guarana (Ilex paraguariensis), and cola (Cola nitida) are the rich source of purine alkaloids (Springob and Kutchan 2009). Caffeine is one of the major purine alkaloids that we receive daily by drinking coffee. This drink originated from 1000 A.D. in Ethiopia. Caffeine is an analgesic and defensive agent against herbivores in plants (Hollingsworth et al. 2002) and as inhibitor of germination of coffee seedling (Friedmann and Waller 1985). The alkaloids (S)-hyoscyamine and (S)-scopolamine are tropane alkaloids occurring only in Solanaceae family. These alkaloids originated from the amino acids ornithine and/or arginine. The Solanaceae, Brassicaceae, Erythroxylaceae, Euphorbiaceae, Rhizophoraceae, and Proteaeceae families are well known for having tropane alkaloids. Some of the alkaloids with medicinal properties are given in Table 7.1. These compounds are of interest to human societies because of their prominent role in human health care. For example, atropine is an anti-cholinergic agent and has an antidote activity against intoxication. Besides, before surgery the patient will receive atropine to decrease salivation and respiratory secretion (Springob and Kutchan 2009). Hyoscyamine and scopolamine have been used as analgesic and sleeping poisons, aphrodisiacs and psychoactive drugs. Some of the plant species like Atropa belladonna, Datura species , Hyoscyamus niger, and Mandragora officinarum are recognized as lethal plants because of these toxic alkaloids.
7.3 Tissue Culture of Medicinal Plants
The levels of secondary metabolite found in plants are subjected to variation due to unfavorable environmental conditions. Production of active metabolites in some medicinal plants is usually a time-consuming process, which sometimes take around 20 years. In ginseng, the root growth rate in the plantation is around 1 gram/year, while the rate for tissue cultured roots was reported to be up to 2 gram dry weight/liter/day (Nosov 2012). Besides, the quantity and quality of the medicinal plant active metabolites are influenced by climate and growing conditions in the field or natural habitats. It has been shown that composition of ginsenoside in ginseng roots harvested from plantation is different from wild ginseng (Li and Mazza 1999). Tissue culture of some plants exhibits an advantage over field grown materials. For example, the rhizomes of the field grown mountain arnica lack the smell and taste which are present in tissue cultured plants (Ellenberger 1998). One alternative method to produce the economically bioactive compounds is the cell and hairy root culture system. Plant cell and tissue cultures hold great promise for controlled production of myriad of useful secondary metabolites on demand. Transgenic hairy root cultures have revolutionized the role of plant tissue culture in secondary metabolite production. They are unique in their genetic and biosynthetic stability, faster in growth, and more easily maintained. Using this methodology, a wide range of chemical compounds has been synthesized. Theoretically, in vitro grown plant cells and tissues have the potential to serve as a renewable source of economically valuable bioactive compounds. The following are some of the potential advantages of cell and tissue cultures compared to extraction of useful metabolites from field grown herbs:
-
Continuous production of active metabolites independent of the environmental variations
-
Production of uniform and pure metabolites by Good Manufacturing Practice (GMP) in a predictable system that guarantee a product free of agrochemicals throughout the cultivation process
-
The possibility for enrichment of target metabolites and an increase in biomass production
-
Easier and simple extraction procedures that can help to reduce aggressive solvents
-
Authoritative and reliable identity of particular species of medicinal plant
-
Simplicity to monitor genotype variability
-
Easy to handle the cell lines to produce new compounds
Feasibility of in vitro techniques for production of the pharmaceuticals falls in doubt by the failure of Charles Pfizer Co. first attempts in the 1950s. The first successful strive for production of substances through tissue culture technique was reported in 1956 (Chandra and Chandra 2011). Later on in 1978, additional progresses were made in other countries particularly in Germany and Japan which led to the use of tissue culture as a sustainable source for production of secondary metabolites. Despite the significant progress, plant cell culture is still a complex process with relatively long period compared to bacterial culture and it is sensitive to mechanical damage. All these factors are influencing production of bioactive metabolites in in vitro condition. The difficulty of in vitro techniques is not the only limiting factor for commercialization of products. Sometimes, the products from this technology failed because of regulatory reasons. This was the reason that curbed the production of sanguinarine and vanilla in the USA, despite the successful achievement of in vitro production of these compounds.
In addition to the bioactive secondary metabolites, production of foreign proteins such as antibodies, vaccines, enzymes, and therapeutics medicinal proteins have been achieved using plant tissue culture techniques. The plant systems provide a less expensive and often clean source for these groups of chemicals. This is a low contamination system with reduced risk of mammalian pathogens and viruses. But significant challenges related to plant-based expression systems still remain to be overcome.
Production of plant metabolites via tissue culture system has been used for a limited number of medicinal plants mainly due to insufficient production of metabolites and high culture cost to meet economic production. Nosov (2012) provided a table indicating some of the successful examples of metabolites produced from medicinal plants by in vitro techniques in commercial scale. For example, Mitsui Petrochemical Industries in Japan applied a submerged fermentation processes to produce the bioactive metabolites berberine, arbutin, ginseng, and shikonin. These chemicals are produced in bioreactors with 4000–20,000 L capacity. Ginsenosides has been produced from tissue culture of Panax ginseng in bioreactor. It was reported that 27% of in vitro-derived cell dry weight contained Ginsenosides compared with 4.5% in whole plants (Chandra and Chandra 2011). In Japan, the Mitsui Petrochemical Industries Group started to produce berberine in bioreactor. They obtained a high yielding line with more than 0.1 g/L/day berberine. Selection of high yielding cell lines of Coptis japonica resulted in accumulation of 7 g/L of the alkaloid berberine in suspension cell culture. Until now, several metabolites of different groups have been synthesized using in vitro culture of plant cells. Production of phenolic compounds (hydroxycinnamic acids, coumarins, lignans, stilbenes, flavonoids, tannins, isoflavonoids, anthraquinones, benzoquinones, naphthaquinones), alkaloids (acridine, quinolizidine, pyridine), isoprenoids (sesquiterpenes, mono-, di-, tri-, and tetraterpenes), and some other minor groups (betalains, polyacetylenes, thiophenes, alliin, pyrethrins, nonproteinogenic amino acids) have been reported (Nosov 2012).
Several factors are affecting production of plant-derived compounds using tissue culture systems. The cell line, medium, temperature, pH, light, inoculum, gas composition, mixing technique, elicitors, bioreactor type, and growing mode are the main elements determining the level of secondary metabolites and cell biomass. Tissue culture-mediated secondary metabolite production is affected by several limiting factors such as slow growth rate and doubling time (approx. 30–40 h), low specific production rates, instability of cell lines, shear sensitivity, high cost of bioreactors, and accumulation of the active metabolites in the cells. All these factors should be overcome by applying appropriate strategies.
The bioreactor culture system is the promising way of commercial production of secondary metabolites. The engineering of adventitious and hairy roots represents one of today’s fastest growing areas in pharmaceutical and nutraceutical economy of the world. Efficient nutrients supply to the cells, possibly combined with the application of mechanical stimulation to direct cellular activity as well as differentiation and function of adventitious and hairy roots in the bioreactor, are the significant steps towards development of high-tech products (Sivakumar 2006). Currently, metabolic engineering in hairy roots opened a new opportunity to improve the metabolic pathway of desired molecules in bioreactors. Most of the bioactive molecules, such as camptothecin, vinblastine, and ginsenosides, are of root origin. Harvesting roots is destructive for the plants and hence there has been increasing interest in developing in vitro cultures of adventitious and hairy roots from various medicinal plant species. The gap between discovery and commercialization can be bridged when biotechnologists and bioengineers join forces and integrate their research disciplines to develop bioreactor technology for the production of bioactive compounds (Sivakumar 2006). For example, constitutive expression of hyoscyamine 6β-hydroxylase from Hyoscyamus niger into hyoscyamine-rich Atropa belladonna plants using A. rhizogenes resulted in induction of hairy roots with enhanced levels of hydroxylase activity and up to fivefold higher concentrations of scopolamine than control (Hashimoto et al. 1993).
7.4 Enhance Metabolic Output of Cellular Lines Using Conventional Approaches
Several methods have been used to increase metabolic output and levels of valuable molecules using in vitro culture of medicinal plants. The biomass growth rate and level of bioactive compounds are the two main determinants for economical production of secondary metabolites in in vitro condition. Different factors are impacting these parameters. Generally, the majority of cell lines in callus or suspension cultures do not fully differentiate, and yield low amounts of secondary metabolites. Strain improvement is often carried out which include selection of a mother plant with high contents of desired bioactive products for callus induction to obtain high-producing cell lines. Appropriate techniques must be adopted for selecting those cell lines with higher yield.
Various physicochemical aspects including media composition, plant growth regulators (PGRs), temperature, light, pH, and aeration influence the culture productivity of plant cells. The type and concentration of the carbon source have important effects on cell growth and yield of secondary metabolites. Available concentration of nitrogen was also found to affect the contents of proteinaceous or amino acid products in cell suspension cultures. General plant tissue culture medium including MS, LS, or B5 usually have both NO3− and NH4+ ions as sources of nitrogen. The ratio of the NH4+/NO3− and the total content of nitrogen are significantly affective for successful plant tissue culture systems. Phosphate level in the medium may have a major impact on the production of secondary metabolites in plant cell cultures. Higher concentrations of phosphate ion can enhance the cell growth with negative influence on secondary product accumulation. The effects of PGRs on secondary metabolite production are variable. Important biochemical changes are induced by some PGRs. The PGRs have remarkable consequences on physiological and biochemical processes and gene regulation as well as plant growth and development. Secondary metabolite production was strongly affected by the presence of 6-benzylaminopurine (BA), kinetin (Kin), or other cytokinines such as 4-chloro-2-diphenylurea in the medium (Karuppusamy 2009). Production of secondary metabolites in in vitro culture of Mentha piperita was monitored only by the addition of cytokinin, which resulted in about 40% increase in the total production of essential oils (Santoro et al. 2013). Gibberellic acid is also effective on plant cell cultures. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots (Abbasi et al. 2012). A considerable rise in the contents of phenols and flavonoids in culture of Stevia rebaudiana was proven in response to a combination of BA either with gibberellic acid (GA3) or indol-3-acetic acid (IAA) compared to singly applied of PGRs indicating synergistic effects of PGRs (Radić et al. 2016).
The biosynthetic pathway is also controlled by physical conditions of cell culture like temperature and pH. Each plant species may favor a specific temperature for optimum in vitro metabolic activity usually in the range of 17–25 ° C. In the case of Eleutherococcus senticosus , the low (12 and 18 °C) and high (30 °C) temperatures resulted in significant reduction in fresh weight, dry weight, total phenolics, flavonoids, and total eleutheroside accumulation, while low temperature increased eleutheroside E accumulation in somatic embryos (Abdullah et al. 2006). It has been shown that the pH value of Stevia rebaudiana’s nod culture is a determinant of leaf metabolite, polyphenolics levels, and their distribution between different tissues (Radić et al. 2016). The inoculum density is another determinant for plant cell growth and accumulation of secondary metabolites. The flavonoid accumulation in cell suspension cultures of Glycyrrhizin inflata was shown to be correlated with the inoculum density as well as sucrose and nitrogen concentrations (Yang et al. 2009).
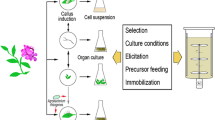
Elicitation is one of the most useful strategies for elevating the in vitro production of secondary metabolites. Elicitors can be categorized into biotic or abiotic factors. Endophytes are microbes that may stimulate host plant growth, improve nutrient supply, and protect host plants from biotic and abiotic stresses. There are several reports of endophyte-induced biosynthesis of secondary metabolites in host plants. Two fungal endophytes, Curvularia sp. CATDLF5 and Choanephora infundibulifera CATDLF6 isolated from the leaves of Catharanthus roseus were found to elevate vindoline content by 229–403% (Pandey et al. 2016). Abiotic elicitors have various effects on the production of secondary metabolites and can be divided into three types on the basis of physical (light, ultrasound, UV, thermal stress, salt stress, drought, and osmotic stress), chemical (heavy metals, nanoparticles mineral salts, amino acids, and gaseous toxins), and hormonal (salicylic acid and jasmonates). Elicitation effects of 1,2,4,5-tetraoxane and 2,5-diphenylthiophene in shoot cultures of two Nepeta species have been studied (Dmitrović et al. 2016). Addition of 150 mg/L of tryptophan resulted in elevated level of thymol to 390%, compared to mother plant (Abedaljasim et al. 2016). The other strategy such as permeabilization (Hussain et al. 2012), immobilization (Rao and Ravishankar 2002; Hussain et al. 2012), addition of precursors (Masoumian et al. 2011; Dheeranupattana and Natthiya 2013; Paek et al. 2014), biotransformation (Sabir et al. 2011; Banerjee et al. 2012), and hairy root and organ culture (Srivastava and Srivastava 2008; Hussain et al. 2012; Paek et al. 2014) have been studied extensively for production of different bioactive compounds from alkaloids, phenolics, and terpenoids.
7.5 Metabolome Differences Between Whole Plant and Tissue Cultured Materials
The properties of major metabolites found in the whole (organized) plants are generally similar to those in cell, tissue, and organ culture systems. The in vitro cultured cells often exhibit the same features of primary metabolic processes as those found in the whole plant cells. Differences that occur between the two systems can lead to altered secondary metabolite profiles. A cell suspension culture is submersed in a liquid medium containing the necessary nutrient factors. The different kinds of nutrients can enter the cells either through diffusion or by active uptake. Plant growth regulators (PGRs) which are incorporated exogenously to most plant cell cultures cause a special metabolic and cell differentiation changes in the in vitro cultured cells.
Although the plants have all the genetic information for the synthesis of PGRs, but in the whole plant not all the cells synthesize the PGRs. Growth and developmental processes are regulated by PGR gradient across the plant organs. The cell cultures mostly need exogenous PGR. A comparison study of the metabolome for primary metabolites extracted from the leaves of Arabidopsis thaliana and Arabidopsis’s T87 cell suspension culture indicated that some metabolites that were produced in the leaves were missing in the T87 cell culture and vice versa (Fukusakia et al. 2006). Primary metabolites are essential for the biosynthesis of the secondary; it is therefore expected that each variation in primary metabolites would be reflected in the secondary metabolite profiles. In fact, whole plant secondary metabolism exhibits much more variation compared to the plant organ or undifferentiated cultures. Production of secondary metabolities in cell suspension cultures have been widely published and it was proposed as a technology to overcome problems of variable product quantity and quality from whole plants due to the effects of different environmental factors, such as climate, diseases, and pests. Despite the differences, the in vitro systems using cell culture were successful in producing bioactive secondary metabolites using cell culture.
In the cell suspension culture of Anthriscus sylvestris , trace amounts of deoxypodophyllotoxin could be detected. Koulman et al. (2003) carried out feeding experiments using deoxy-podophyllotoxin, yatein, and anhydropodorhizol. Their results showed that only deoxypodophyllotoxin was converted into podophyllotixin, yielding significantly higher concentration than measured in whole plants. Another research indicated that the roots of A. sylvestris produced a diverse range of lignins, but cell suspension cultures have a hydroxylating activity specific for deoxypodophyllotoxin . Hence suspension culture is a useful bioconversion catalyst for the synthesis of podophyllotoxin from deoxypodophyllotoxin (Koulman et al. 2003). Induction of these properties in plant cells would result in an interesting new source for podophyllotoxin. The presence of total phenols, leucocyanidins and (−)-epicatechin was compared between the mature plants, seedling and callus cultures of Theobroma cacao by Jalal and Collin (1979). They reported some compounds were found in both callus and intact plants and some were dedicated to callus, only. For example, the callus was found to contain two new flavonoid glycosides, phenolic acid complexes, p-coumaric and caffeic acids (Jalal and Collin 1979). In Papaver bracteatum, 2–3 seasons is necessary to accumulate thebaine (Razdan 2003). Novel synthetic pathways can be recovered from deviant and mutant cell lines which can lead to the production of new compounds, not previously found in whole plants. In some examples the cultured cell produces novel secondary metabolites that are not found in the whole plant. The Lithospermum erythrorhizon cell culture synthesized the rosmarinic acid which has not been detected in intact plant (Fukui et al. 1984). This shows that a novel unexpected compound can sometimes be observed after modifications of a metabolic pathway. Breitenbach et al. (2014) investigated the carotenoids contents of engineered rice callus. Manipulation of carotenoid pathway resulted in formation of a novel carotenoid 4-keto-α-carotene in rice callus. In further assessment, they applied a bacterial ketolase gene in callus. The transgenic callus was able to produce astaxanthin, a non-plant carotenoid. The authors suggested that astaxanthin could have been produced from the combination of ketolase gene products and the native endogenous enzymes (Breitenbach et al. 2014).
Generally, cultured plant cells produce lower quantities and different features of secondary metabolites when compared with the whole plant. Also, the quantitative and qualitative profiles may change over the time. Inadequate production of secondary metabolites in cell culture systems is often caused by the lack of cell differentiation. Regardless of the disappointing statements, there are several examples indicating the potential of tissue culture over whole plant biosynthesis of some bioactive compounds. In the cell suspension culture of Lithospermum erythrorizon, 20% of the cell’s dry weight contained shikonin compared to 1.5% in the whole plant (Takahashi and Fujita 1991). The same results have been reported for a few compounds such as Ginsenoside (Panax ginseg), Anthraquinones (Morindaci trifolia, Galiumverum, Galiumaparine), Ajmalicine (Catharanthus roseus), Rosmarinic acid (Coleus blumeii), Ubiquinone-10 (Nicotiana tabacum) (Zhong 2001), Diosgenin (Dioscorea deltoids) (Rokem et al. 1984), Anthocyanin (Vitis sp., Euphorbia milli, Perilla frutescens), Benzyl isoquinolone alkaloids (Coptis japonica), Berberine (Thalictrum minor, Coptis japonica), Nicotine (Nicotiana tabacum), Bisoclaurine (Stephania cepharantha), and Tripdiolide (Triptreyqium wilfordii) (Ulbrich et al. 1985; Zhong 2001) in which the tissue cultured cells accumulated more metabolites compared to the whole plant.
7.6 Genetic Transformation and Manipulation of Metabolic Pathway
The in vitro techniques can serve as one of the alternative tools to produce valuable active metabolites, but the yield and stability of the product in in vitro condition are the main concern. Examples of successful production of economically valuable secondary metabolites are limited to 16 plant species for production of 13 bioactive compounds of interest (Nosov 2012). It seems that our failure to set up in vitro-mediated production of valuable metabolites in other medicinal plants could be partly due to the shortage or lack of our knowledge on the molecular mechanisms of different metabolic pathways. Manipulation of the metabolic pathway can be achieved after elucidation of the functional and regulatory proteins involved in the biosynthesis of the specific compound and even the related pathways. The quality, yield, stability, and the processing steps of a specific metabolite are subjected to change through metabolite engineering. Suppression of unwanted metabolite (e.g., toxic compounds) or introduction of a new pathway for de novo synthesis of dedicated metabolite (e.g., biofuel) are the other advantages of genetic engineering. The traditional limitations on in vitro culture-mediated production of plant secondary metabolites can be overcome using genetic engineering.
Besides our understanding on plant metabolome, availability of in vitro regeneration methods and genetic manipulation systems are prerequisites to engineer metabolic pathways of secondary products in plants. Several plant transformation methods have been grouped into two main categories: (a) direct gene delivery such as biolistics, protoplast fusion, microinjection, and electroporation; and (b) indirect transformation system using natural genetic engineers mainly A. tumefaciens and A. rhizogenes (Zarate and Verpoorte 2007). Alongside limitations and attractive applications provided from all these methods, transformation procedure using natural genetic engineers is most preferred due to the simplicity, inexpensive handling, ability for transformation of long DNA fragments, low frequency of transgene rearrangement, preferential integration of DNA segments into transcriptionally active sites, low rate of transgene silencing, and integration of several single copy of transgene. These factors make the Agrobacterium as attractive engineers (Kumar et al. 2013).
Extensive attempts have been made to manipulate for production of secondary metabolites using tissue culture several years ago (Bajaj and Ishimaru 1999). Production of nicotine from transgenic roots of Nicotiana rustica expressing ornithine decarboxylase gene was reported by Hamill et al. (1990). Overexpression of hyoscyamine 6β-hydroxylase (H6H) from Hyoscyamus niger in hairy roots of Atropa belladonna resulted in an enhanced level of scopolamine (Hashimoto et al. 1993). The transgenic hairy roots showed fivefold concentration of this tropane alkaloid in genetically engineered root cultures. In another attempt, Kang et al. (2011) reported elevated level of hyoscyamine and scopolamine in Scopolia parviflora root culture. Co-expression of two key enzymes putrescine N-methyl transferase (PMT) and H6H in hairy roots not only increased the level of tropane alkaloids, but also showed a potential role in root development (Kang et al. 2011). Tanshinones produced in Salvia miltiorrhiza are health-promoting diterpenoids with many important applications. According to Shi et al. (2016) two genes including geranylgeranyl diphosphate synthase (SmGGPPS) and 1-deoxy-d-xylulose-5-phosphate synthase (SmDXSII) were introduced into hairy roots of S. miltiorrhiza. Overexpression of SmGGPPS and SmDXSII in hairy roots produced higher content of tanshinone than control and single-gene transformed lines. Tanshinone production in the double-gene transformed line GDII10 reached 12.93 mg/g dry weight, which is the highest tanshinone content that has been achieved through genetic engineering. Furthermore, transgenic hairy root lines showed higher antioxidant and antitumor activities than control lines. Also, contents of chlorophylls, carotenoids, indole acetic acid, and gibberellins were significantly increased in the transgenic Arabidopsis thaliana plants (Shi et al. 2016).
As mentioned earlier, plant natural products are divided into three main categories known as terpenes and steroids, phenolics, and alkaloids. By far, with around 40,000 compounds, isoprenoids represent the largest secondary metabolites in plants. These valuable terpenoids are produced in low quantities in specialized cells such as glandular trichomes and laticifers. The squalene epoxide and several prenyl diphosphates, including isopentenyl diphosphate (IDP), dimethyl allyl diphosphate (DMADP), geranyl diphosphate (GDP), farnesyl diphosphate (FDP), and geranylgeranyl diphosphate (GGDP), are the precursors that catalyze the production of isoprenoids through two different pathways located in the cytosol or plastids. The two main precursors, IDP and DMADP together originated from mevalonic acid (MVA) pathway, in the cytosol and from pyruvate and a non-mevalonate or methylerythritol phosphate (MEP) pathways in the plastids. The process consists of a dephosphorylation step and is often followed by cyclization (Staniek et al. 2013). The resulted isoprenoid structures are subjected to hydroxylation process catalyzed by specialized enzymes termed cytochrome P450. These enzymes have been recognized as candidate gene for enhanced production of triterpenoides using genetic engineering (Fukushima et al. 2011). The triterpene glycyrrhizin is a natural sweetener derived from the rhizomes of licorice (Glycyrrhiza spp. ) plants. Due to overharvesting from its natural resources, licorice is under extinction and has received considerable attention for production through in vitro techniques. Several enzymes mediate the biosynthesis of glycyrrhizin from 2,3-oxidosqualene, a common precursor of triterpenes and phytosterols (Abe et al. 1993). It has been shown that Cytochrome P450s are critical enzymes in the biosynthesis of terpenoids and other natural products in plants. Seki et al. (2008) reported that CYP88D6 (a cytochrome P450 mono-oxygenase) catalyzes the biosynthesis of 11-oxo-β-amyrin, an intermediate between β-amyrin and glycyrrhizin. This group also isolated a second P450 (CYP72A154) responsible for C-30 oxidation of 11-oxo-β-amyrin in the glycyrrhizin pathway to produce glycyrrhetinic acid (Seki et al. 2011). However, tissue cultures attempts for production of glycyrrhizin in licorice cell culture were unsuccessful (Hayashi et al. 1988, 1990; Ayabe et al. 1990). Seki et al. (2011) showed that β-amyrin synthase (bAS), CYP88D6 and CYP72A154 were expressed in both stolons and roots of licorice with no transcript detection in the leaves. These enzymes play critical function on the biosynthesis of glycyrrhizin in the roots and stolons. Although the first attempts on establishments of hairy root culture of licorice failed to produce glycyrrhizin, the ongoing researches on metabolite engineering of hairy roots were promising. Toivonen and Rosenqvist (1995) were not able to report glycyrrhizin production on transformed hairy roots (Toivonen and Rosenqvist 1995). They applied A. rhizogenesis to induce hairy roots on explants without engineering for any enzymes responsible for glycyrrhizin biosynthetic pathway. Instead, Ri-mediated transformation of G. uralensis using A. rhizogenesis harboring a cassette with constitutive expression of Squalene Synthase Gene (GuSQS1) resulted in enhanced level of glycyrrhizin (Lu et al. 2008). One of the transgenic lines was able to produce 2.5 mg/g dry weight glycyrrhizin which was 2.6-fold higher than control. Despite the success, the resulted quantity was not high enough to meet the prerequisites of an economically valuable amount of glycyrrhizin using hairy root culture. By recent gene discovery efforts and characterization, it is now expected that engineering for a single gene in biosynthetic pathway of glycyrrhizin should not be enough to yield a high-producing line (Seki et al. 2008, 2011). As mentioned above, Cytochrome P450s have critical functions in different steps of converting 2,3-oxidosqualene to glycyrrhizin.
The recent findings using functional genomics revealed several Cytochrome P450s. This family consists of specialized enzymes responsible for the biosynthesis of terpenoids. Taxol or paclitaxel is another example from diterpenes with high anticancer activity. Extraction of taxol from its natural source faces serious limitations. At least 2–4 mature trees must be cut for extraction of adequate amount of the drug for treatment of a single patient (Schoendorf et al. 2001; Jennewein et al. 2001). The tissue culture has been introduced as an alternative for production of taxol. Elucidation of the taxol biosynthetic pathway and the other rate-limiting steps lead to the development of high yielding recombinant plant or microbial cells. The taxadiene synthase was the first cloned enzyme that mediates for cyclization of the isoprenoid precursor geranylgeranyl diphosphate (GGDP) to taxa-4(5), 11(12)-diene (Wildung and Croteau 1996). The following reactions are mainly catalyzed by Cytochrome P450 enzymes (Walker and Croteau 2001). Although several attempts have been made for ectopic expression of the taxol related biosynthesis genes in microbial host like E. coli (Walker and Croteau 2000a, b), but the main conventional source of this drug in the market originated from plant cell culture technologies optimized by the companies such as ESCA genetics (United States), Phyton Catalytic (United States/Germany), Nippon Oil (Japan), and Samyang Genex Co. (Korea) (Nosov 2012). Among the enzymes involved in the biosynthesis of terpenoid, cytochrome P450 hydroxylases are the big challenge for functionalization in microbial systems (Khosla and Keasling 2003). They are decorators of the carbon skeleton in terpenoid . Until now, about 14 cytochrome P450s genes were reported to be involved in the biosynthesis of taxol (Morant et al. 2003). Difficulties of engineering for plant terpenoid biosynthetic pathways related genes in microbial cells motivated the use of the native plant species for production of some economically valuable bioactive compounds. Several genes have been cloned and identified with potential role in taxol biosynthesis such as Geranylgeranyl diphosphate synthase (Hefner et al. 1998), Taxadiene synthase (Wildung and Croteau 1996), Taxane 5α-hydroxylase (Jennewein et al. 2004), Taxa-4(20), 11(12)-dien-5α-ol-O-acetyltransferase (Walker and Croteau 2000a), Taxane 10β-hydroxylase (Schoendorf et al. 2001), Taxane 13α-hydroxylase (Jennewein et al. 2001), 10-Deacetylbaccatin III-10-O-acetyltransferase (Walker and Croteau 2000a), Taxane 2α-O-benzoyltransferase (Walker and Croteau 2000b), Phenylalanine aminomutase (Walker et al. 2004), Baccatin III:3-amino-3-phenylpropanoyltransferase (Walker et al. 2002), and 3′-N-debenzoyl-2′-deoxytaxol N-benzoyltransferase (Walker et al. 2002).
Hypericum perforatum or St John’s wort is another interesting candidate with potentials for in vitro-mediated production of economically valuable medicinal compounds through genetic engineering of metabolic pathways. H. perforatum is one of the ancient medicinal herbs with two thousand years old history in remedy of human being (Zobayed et al. 2005). This plant contains several valuable chemical groups such as phenolics, flavonoids, naphthodianthrones, and phloroglucinols with approved bioactivity (Barnes et al. 2001). It has been reported that the hypericins content in the field grown plants varies and the difference can be up to 50-fold depending on the season (Southwell and Bourke 2001). This fluctuation in metabolites is a critical issue when the active metabolite is recorded in different phytopharmaceutical preparations. It can result in up to 13- to 17-fold differences in hypericin and pseudohypericin amounts, respectively (Greeson et al. 2001). So, the cell and tissue cultures of this plant have been attempted for stable and large-scale production of pharmaceutically important compounds. However, the in vitro system was not successful due to the low performance and unreliable yield of both bioactive compounds and the biomass.
The hypericins and hyperforins are the two main bioactive compounds of H. perforatum (Barnes et al. 2001) belonging to naphthodianthrones and phloroglucinols, respectively (Schröder 1997). It has been shown that type III polyketide synthases (PKSs) are the key enzymes in the biosynthesis of these compounds (Klingauf et al. 2005; Karioti and Bilia 2010). The PKSs multi-gene family mediates the biosynthetic reactions for most of the plant secondary metabolites as well as naphthodianthrones, phloroglucinols, xanthones, and flavonoids. In higher plants the PKSs are categorized in three groups: chalcone synthase (CHS-type), stilbene synthase (STS-type), and coumaroyl triacetic acid synthase (CTAS-type). The PKSs responsible for the biosynthesis of hypericins and hyperforins is the CHS-type (Flores-Sanchez and Verpoorte 2009).
Biosynthesis of hypericin is related to the metabolism of anthranoid. The type III PKS-mediated cyclization process is performed using one molecule of Acetyl-CoA and seven molecules of malonyle-CoA. The resulted octaketide participates in the following cyclization and decarboxylation process to produce emodin anthrone as an intermediate (Kirakosyan et al. 2004; Zobayed et al. 2005). This compound serves as a substrate for the biosynthesis of hypericin. In the following, another key enzyme Hypericum perforatum phenolic oxidative coupling protein (Hyp-1) catalyzes the ongoing reactions to produce hypericin (Lin-Fang et al. 2014). It is believed that Hyp-1 is not a limiting enzyme. In contrast to PKSs with differential expression limited to flowers and leaves (He et al. 2012), maximum expression of Hyp-1 was reported in all tissues as well as the roots, the non-hypericin producing organ in H. perforatum (Kosuth et al. 2011). So, it is axiomatic that hypericin should not be detectable in the roots. Despite the contradictory reports (Cui et al. 2010), this finding is consistent with Gaid et al. (2016) who indicated that hypericin was not detectable in hairy root culture of H. perforatum.
Hyperforin is the other important bioactive compound in H. perforatum. It has been shown that expression of HpPKS1 and HpPKS2 are related to the biosynthesis of hyperforin and hypericin, respectively (Karppinen and Hohtola 2008). Karppinen and Hohtola (2008) showed that the maximum transcript level of HpPKS1 is in the flowers, while the HpPKS2 demonstrated high expression level in the flower buds and leaves. The minimum expression was detected in the roots, for both of these genes. The HpPKS1 mediates the biosynthesis of hyperforin by cyclization of one molecule of isobutyryl-CoA with three molecules of malonyl-CoA to form phlorisobutyrophenone. Then phlorisobutyrophenone is converted to dimethylallyl-phlorisobutyrophenone in a reaction catalyzed by dimethylallyltranstransferase (MAT) and dimethylallyl diphosphate (DMAPP). De Novo sequencing of transcriptome indicated that MAT is expressed in almost all tissues of H. perforatum (He et al. 2012). These findings corroborate that inadequate production of hyperforin and hypericin in hairy root culture of H. perforatum should be partly due to the lack of expression of HpPKS1 and HpPKS2.
As mentioned earlier, metabolite engineering has been introduced as a promising tool to overcome low performance, instability, and unreliable yield of secondary metabolites using plant cell and tissue cultures. But, implementation of this method for manipulation of metabolic pathways in medicinal plants encounters significant difficulties related to the efficiency of transformation and accurate assessment of the consequences on the metabolic pathways following genetic engineering at the cellular and subcellular level. Manipulation of H. perforatum for enhanced production of bioactive metabolites has so far been hampered due to the lack of an efficient transformation system. Until now, there are few reports on establishment of transgenic events in H. perforatum (Di Guardo et al. 2003; Vinterhalter et al. 2006; Franklin et al. 2007; Bertoli et al. 2008; Santarem et al. 2008, 2010; Tusevski et al. 2013, 2014). Agrobacterium-mediated transformation of H. perforatum was shown to be affected by factors such as early oxidative burst and enhanced level of antimicrobial factors which kills ~99% of agrobacteria after 12 h of cocultivation (Pitzschke 2013). Besides, the low level accumulation of foreign proteins and even plant originated secondary metabolites, and silencing and instability of the transgene in engineered lines, are the problems that need to be overcome in metabolic pathway engineering of medicinal plants.
7.7 Regulatory Proteins Involved in Secondary Metabolism
Manipulation of secondary metabolites is influenced by the fact that there are competing pathways with potential intervention effects as they compete for the same substrate or intermediates with the pathway of interest. Metabolic engineering using a single structural gene can stimulate a contest in accessibility of cofactors and development of feedback-inhibition mechanisms by accumulation of end products. The turnover number (k cat values) of specialized enzymes is significantly lower than their related corresponding enzymes of core metabolism (Bar-Even and Tawfik 2013). Incidentally, the other rate-limiting enzymatic steps and compartmentation related issues of pathways are the problems that need to be overcome in plant metabolite engineering. A general believe is that most of the metabolic enzymes in plant are promiscuous (Bar-Even and Tawfik 2013). These enzymes can often function on different substrates. It seems that successful engineering for plant secondary metabolites using single structural genes should coincide with the manipulation of regulatory proteins located upstream of the metabolic pathways. According to Sato et al. (2001), the suggestion was “fortification of multiple steps by over-expression of multiple biosynthetic genes, manipulation of regulatory genes that control expression of multiple pathway enzymes, or both.”
The enzyme activity and specificity of the substrates are not the only factors affecting biosynthesis of secondary metabolites in plants. Availability of cofactors, negative feedback, intracellular chemical flux, source of chemicals, and pH are among the parameters influencing secondary metabolism. Although modification of a single gene helps in identifying the limiting steps involved in the biosynthesis of certain metabolites, but it does not result in an enhanced level of secondary metabolites. As the biosynthetic pathways are usually complicated, application of regulatory genes seems more helpful than a single gene. General regulation of the biosynthetic pathway is more trustable to increase production of bioactive compounds (Verpoorte et al. 2000). Manipulation for master transcription factors can lead to the regulation of the entire pathway or critical steps in the biosynthesis of dedicated metabolites. The early reports on feasibility of metabolite engineering using regulatory genes have been published by several researchers including Lloyd et al. (1992) and Martin (1996) for enhanced production of anthocyanins in maize and Arabidopsis. Anthocyanin is the end products of the flavonoid biosynthetic pathway. It has been shown that expression of the structural genes involved in the biosynthesis of these pigments is directly regulated by a combination of two regulatory proteins with homology to c-MYB and bHLH transcription factors (Mol et al. 1998). Ectopic expression of R (a MYB protein) and C1 (a bHLH protein) resulted in accumulation of anthocyanins in in vitro cultured maize cells (Bruce et al. 2000). The biosynthesis of flavonoid as well as terpenoid indole alkaloids (TIA) correlated with ethylene- and jasmonate-mediated transcriptional machinery in plants (Zhou et al. 2010; Zhu et al. 2015).
Hormone-elicited transcriptional machinery plays a major role on secondary metabolism in plants. Plant hormones are considered as conserved elicitors of expression for a wide range of transcription factors. Ethylene serves as a signal to trigger specific biological responses. Accumulation of secondary metabolites in tomato fruit is partly correlated with ethylene-mediated transcriptional machinery. The signals are perceived by a multi-gene family of membrane-localized ethylene receptors (ETRs) (Wang et al. 2002) that negatively regulate ethylene responses through Constitutive Triple Response1 (CTR1) , a putative MAP-kinase kinase kinase (MAPKKK) (Kieber et al. 1993; Huang et al. 2003). So far, six members of ETRs (SlETR1, SlETR2, SlETR3 or SlNR, SlETR4, SlETR5, and SlETR6) with ethylene binding ability (Zhang et al. 2010) and four CTRs (SlCTR1, SlCTR2, SlCTR3, and SlCTR4) with differential expression have been isolated from tomato (Lin et al. 2008). There are several positive regulators (EIN2, EIN3, EIN5, EIN6, and EIL1) downstream of the CTRs. Existence or absence of ethylene is a determining factor that mediates the ongoing regulatory events. The ethylene downstream responsive signals will be inactivated in the absence of ethylene. This process is mediated by binding of ETRs to CTR1 and their interaction with EIN2. While presence of ethylene inactivates the ETRs followed by deactivation of CTR1, resulting in the EIN2 functioning as a positive regulator of the ethylene pathway (Klee 2004; Joo et al. 2007; Li and Guo 2007) through EIN3 and other Ethylene Insensitive-Like Proteins (EILs) transcription factors (Roman et al. 1995). These transcription factors can bind to the primary ethylene response element (PERE) on the promoter of ethylene responsive factors (ERFs) (Solano et al. 1998). Altered level of the genes with GCC-box and DRE/CRT containing promoters are the consequences of ETRs expression (Joo et al. 2007; Li and Guo 2007). This process indicates the importance of precise choices of regulatory proteins to manipulate for secondary metabolism in plants. Here, selection of AP2/ERF related proteins could be helpful.
Jasmonate is another master regulator of transcriptional machinery of secondary metabolism. This metabolite is an effective elicitors originated from oxidation of unsaturated fatty acids in plant cells. Jasmonate is directly correlated with stress in plants. Stress can result in subcellular damage followed by impairs electron transport system. These events lead to enhanced level of reactive oxygen species (ROS) (Price et al. 1989). Treatment of the adventitious root culture with higher levels of sucrose increased the total phenolics in H. perforatum (Tian and Russell 1999). These stress-induced metabolic processes can be partly dictated by jasmonate-elicited transcription machinery. It is speculated that the resulted osmotic stress of sucrose treatment on adventitious roots was followed by oxidative stress and other subsequent events, including lipid peroxidation and accumulation of secondary metabolite. Expression of Octadecanoid-Responsive Catharanthus AP2/ERF-domain (ORCAs) is induced by methyl jasmonate (MeJA). These transcription factors regulate the biosynthesis of several TIAs (Vom Endt et al. 2002). There is a direct target site (G-box) for MYC2, a basic helix-loop-helix (bHLH) factor in ORCA3 gene in Arabidopsis, N. tabaccum, and C. roseus (Geyter et al. 2012). MYC2 is a jasmonate-elicited transcription factor that regulates the secondary metabolism. Our knowledge on jasmonate-mediated secondary metabolism in Arabidopsis indicates that although an enhanced level of jasmonate can promote JA-responsive gene expression by MYC2 transcription factors, but overexpression of these transcription factors does not theoretically means that expression of JA-responsive gene will be elevated. In this regards, several repressor and co-repressor proteins have to be released off from their binding to the transactivation domain of MYC protein. JA ZIM domain (JAZ) is a repressor protein that directly binds to MYC. The other protein is a Skp-Cullin-F-box-type E3 ubiquitin ligase complex (SCF + COI1(CORONATIVE INSENSITIVE 1)). Existence of JA mediates degradation of this complex from JAZ protein through 26S proteasome. This release can promote expression of JA-responsive gene by MYC protein (Geyter et al. 2012).
Transcriptional reprogramming of secondary metabolism in plants is triggered by JA through recruitment of transcription factors from several families. Geyter et al. (2012) have published a review that summarizes the jasmonate-elicited regulatory proteins involved in metabolism of plant bioactive compound. Several examples of regulatory genes from AP2/ERF, bHLH, R2R3-MYB, WRKY, NAC, DOF, HD-ZIP, and TFIIIA zinc finger were shown to be recruited by JA signaling with regulatory role to navigate the metabolism in plants.
7.8 Transporters and Secretion of Secondary Metabolites to Culture Medium or Subcellular Compartments
Production of valuable bioactive compounds using cell culture systems or transgenic plants overexpressing biosynthetic enzyme genes have been reported (Yazaki 2004: Rischer et al. 2013) with limited successes. Some secondary metabolites, especially alkaloids (e.g., berberine and benzylisoquinoline ), are toxic to prokaryotic and eukaryotic cells. One of the mechanisms in plants to escape from such cytotoxicity is excretion of those chemicals from the cytosol to the apoplast or the other subcellular compartments such as vacuole (Shitan 2016). Transporter proteins provide such opportunities. Tissue culture-mediated production of metabolites offers the advantages that made it economically competitive for the extraction of chemicals from the whole plant systems. The total cost of secondary metabolite purification can be reduced if the desired compound is released into the liquid culture medium. Recovery of chemicals from plant biomass requires a series of complicated operations that increases the total cost of production greater than secreted one. According to Shitan (2016) the secondary metabolites transporter proteins can be roughly classified into 4 families: the ABC (ATP-binding cassette) transporter, nitrate-peptide transporter (NRT) , multidrug and toxic compound extrusion (MATE) , and purine permease (PUP) families. Some examples of current understanding of these transporters are described as follows. CrTPT2 is a plasma membrane-localized G-type ABC transporter responsible for the efflux of catharanthine to the leaf surface, where it plays a role in protection of plants against herbivores (Yu and luca 2013). In Nicotiana tabacum , nicotine is biosynthesized in roots and translocated to aerial parts via the xylem (Shoji and Hashimoto 2013). The MATE transporters jasmonate-inducible alkaloid transporter 1 (JAT1) , JAT2, and MATE1 show nicotine transport activities, probably acting as nicotine/proton antiporters and localize to the vacuolar membrane. JAT1 and JAT2 likely transport nicotine into leaf vacuoles (Morita et al. 2009); MATE1 and MATE2 likely transport nicotine into root vacuoles (Shoji et al. 2009). Biochemical analysis of VvABCC1 of grapevine ( Vitis vinifera ) proved that this C-type ABC transporter mediates cotransport of glucosylated anthocyanidin, malvidin 3-O-glucoside with glutathione (GSH) in an ATP-dependent manner (Francisco et al. 2013). As transporters for terpenoids, a G-type ABC transporters have been reported so far. Nicotiana plumbaginifolia pleiotropic drug resistance1 (NpPDR1/NpABC1) was first isolated as a plasma membrane-localized protein induced by treatment with sclareolide, a close analog of the endogenous antifungal diterpene compound sclareol. NpPDR1 is preferentially expressed throughout the root, in the leaf glandular trichomes, and in the flower petals and is proposed to transport sclareol (Jasinski et al. 2001).
Accumulation of desirable metabolite(s) can be problematic and limiting factors that have to be overcomed by appropriate strategy. So, targeting of a specific metabolite to vacuole, other subcellular compartments or even to the culture medium following constitutive expression of specialized enzyme(s) or regulatory proteins in a transgenic medicinal plant is often essential for modulation of a particular pathway.
7.9 Conclusions and Future Prospects
Global warming is going to change the life on earth. Our planet is getting warmer and this phenomenon is threatening our resources. How can we provide food and the other necessaries for ~9 billion people in the year 2050? Besides food, most of the chemicals used in medicine and industries are of plant origin. It is axiomatic that most of the plant diversity and suitable lands will be lost because of climate changes. With this condition, invention and developments of new tools are promising. The bioreactor targeted production of plant secondary metabolites is a modern approach that has to be further developed using new strategies as an alternative for whole plant harvested in nature. We have witnessed a continuous intensive effort of several scientists from different laboratories on this field. The emergence of OMICS as state-of-the-art tools in biotechnology is giving indications of future successes in precise manipulation of metabolic pathway(s) in medicinal plant. These will lead to discovery of key nucleotide targets for modification using powerful genome editing clean technologies such as CRISPR/Cas9 system.
References
Abbasi BH, Stiles AR, Saxena PK, Liu CZ (2012) Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl Biochem Biotechnol 168(7):2057–2066
Abdullah MS, Ali MB, Yu KW, Hahn EJ, Paek KY (2006) Effect of temperature on secondary metabolites production and antioxidant enzyme activities in Eleutherococcus senticosus somatic embryos. Plant Cell Tiss Org Cult 85(2):219–228
Abe I, Rohmer M, Prestwich GD (1993) Enzymatic cyclization of squalene and oxidosqualene to sterols and triterpenes. Chem Rev 93:2189–2206
Abedaljasim MJ, Al-Jibouri AAS, Abass Ali AJ, Majeed DM (2016) Improvement of phenols production by amino acids in callus cultures of Verbascum thapsus L. Am J Plant Sci 7:84–91
Alvarez MA (2014) Plant biotechnology for health: from secondary metabolites to molecular farming. Spring, New York. doi:10.1007/978-3-319-05771-2
Anand P, Bley K (2011) Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth 107(4):490–502
Ashour M, Wink M, Gershenzon J (2010) Biochemistry of terpenoids: monoterpenes, sesquiterpenes and diterpenes. In: Wink M (ed) Annual plant reviews: biochemistry of plant secondary metabolism, vol 40, 2nd edn. Wiley, New York
Ayabe S, Takano H, Fujita T, Furuya T, Hirota H, Takahashi T (1990) Triterpenoid biosynthesis in tissue cultures of Glycyrrhiza glabra var. glandulifera. Plant Cell Rep 9:181–184
Bajaj YPS, Ishimaru K (1999) Genetic transformation of medicinal plants. In: YPS B (ed) Transgenic medicinal plants, Biotechnology in Agriculture and Forestry, vol 45. Springer, New Delhi, pp 1–29
Banerjee S, Singh S, Rahman LU (2012) Biotransformation studies using hairy root cultures–a review. Biotechnol Adv 30:461–468
Bar-Even A, Tawfik DS (2013) Engineering specialized metabolic pathways—is there room for enzyme improvements? Curr Opin Biotechnol 24:310–319
Barnes J, Anderson LA, Phillipson JD (2001) St. John’s wort (Hypericum perforatum L.): a review of its chemistry, pharmacology, and clinical properties. J Pharm Pharmacol 53:583–600
Bertoli A, Giovannini A, Ruffoni B, Guardo AD, Spinelli G, Mazzetti M et al (2008) Bioactive constituent production in St John’s wort in vitro hairy roots Regenerated plant lines. J Agric Food Chem 56:5078–5082. doi:10.1021/jf0729107
Bourgaud F, Gravot A, Milesi S, Gonntier E (2001) Production of plant secondary metabolites: a historical perspective. Plant Sci 161:839–851
Bradamante S, Barenghi L, Villa A (2004) Cardiovascular protective effects of resveratrol. Cardiovasc Drug Rev 22(3):169–188
Breitenbach JP, Bai C, Rivera SM, Anela RC, Capell T, Christou P, Zhu C, Sandmann G (2014) A novel carotenoid, 4-keto-α-carotene, as an unexpected by-product during genetic engineering of carotenogenesis in rice callus. Phytochemistry 98:85–91
Bruce W, Folkerts O, Garnaat C, Crasta O, Roth B, Bowen B (2000) Expression profiling of the maize flavonoid pathway genes controlled by estradiol-inducible transcription factors CRC and P. Plant Cell 12:65–80
Burns J, Yokota T, Ashihara H, Lean ME, Crozier A (2002) Plant foods and herbal sources of resveratrol. J Agric Food Chem 50:3337–3340
Calderón-Montaño J, Burgos-Morón E, Lopez-Lazaro M (2013) The cardiac glycosides digitoxin, digoxin and ouabain induce a potent inhibition of glycolysis in lung cancer cells. WebmedCentral CANCER 4:WMC004323
Canel C, Lopes-Cardoso MI, Whitmer S, van der Fits L, Pasquali G, van der Heijden R, Hoge JHC, Verpoorte R (1998) Effects of over-expression of strictosidine synthase and tryptophan decarboxylase on alkaloid production by cell cultures of Catharanthus roseus. Planta 205:414–419
Canter PH, Thomas H, Ernst E (2005) Bringing medicinal plants into cultivation: opportunities and challenges for biotechnology. Trends Biotechnol 23(4):180–185
Chandra S, Chandra R (2011) Engineering secondary metabolite production in hairy roots. Phytochem Rev 10:371–395
Chatterjee SK (2010) Status of medicinal plants in global perspective: biodiversity destruction and conservation. In: Proceedings of botanicals in integrated health care convention 2010, 26–28 December 2010. The Agriculture Horticultural Society of India, Kolkata, India, pp 32–36
Counet C, Callemien D, Collins S (2006) Chocolate and cocoa: new sources of trans-resveratrol and trans-ieced. Food Chem 98:649–657
Croteau R, Ketchum RE, Long RM, Kaspera R, Wildung MR (2006) Taxol biosynthesis and molecular genetics. Phytochem Rev 5:75–97
Cui XH, Chakrabarty D, Lee EJ, Paek KY (2010) Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor [J]. Bioresour Technol 101(12):4708–4716
Dheeranupattana S, Natthiya C (2013) Effects of sodium acetate and sucrose on in vitro alkaloid production from Stemona Sp. culture. Asian J Plant Sci 12(2):92–96
Di Guardo A, Cellarova E, Koperdáková J, Pistelli L, Ruffoni B, Allavena A et al (2003) Hairy root induction and plant regeneration in Hypericum perforatum L. J Genet Breed 57:269–278
Dmitrović S, Skorić M, Boljević J, Aničić N, Božić D, Mišić D, Filipović V, Opsenica D (2016) Elicitation effects of a synthetic 1,2,4,5-tetraoxane and a 2,5-diphenylthiophene in shoot cultures of two Nepeta species. J Serb Chem Soc 81(9):999–1012
Ellenberger A (1998) Assuming responsibility for a protected plant: Weleda’s endeavour to secure the Firm’s supply of Arnica Montana: first international symposium on the conservation of medicinal plants in trade in Europe. TRAFFIC Europe, Kew, pp 127–130
Erdelsky K (1978) In: Thorpe T (ed) Fourth international congress plant tissue and cell culture. Canada, University of Calgary
Fischer R, Budde I, Hain R (1997) Stilbene synthase gene expression causes changes in flower colour and male sterility in tobacco. Plant J 11(3):489–498
Flores-Sanchez IJ, Verpoorte R (2009) Plant polyketide synthases: a fascinating group of enzymes. Plant Physiol Biochem 47:167–174
Francisco RM, Regalado A, Ageorges A et al (2013) ABCC1, an ATP binding cassette protein from grape berry, transports anthocyanidin 3-O-glucosides. Plant Cell 25:1840–1854
Franklin G, Oliveira M, Dias ACP (2007) Production of transgenic Hypericum perforatum plants via particle bombardment-mediated transformation of novel organogenic cell suspension cultures. Plant Sci 172:1193–1203. doi:10.1016/j.plantsci.2007.02.017
Friedmann J, Waller GR (1985) Caffeine hazards and their prevention in germinating seeds of coffee (Coffea arabica L.) J Chem Ecol 9:1099–1106
Fukui H, Yamazaki K, Tabata M (1984) Two phenolic acids from Lithospermum erythrorhizon cell suspension cultures. Phytochemistry 23(10):2398–2399
Fukusakia E, Jumtee K, Bamba T, Yamaji T, Kobayashi A (2006) Metabolic fingerprinting and profiling of Arabidopsis thaliana leaf and its cultured cells T87 by GC/MS. Z Naturforsch C 61(3–4):267–272
Fukushima EO, Seki H, Ohyama K, Ono E, Umemoto N, Mizutani M, Saito K, Muranaka T (2011) CYP716A subfamily members are multifunctional oxidases in Triterpenoid biosynthesis. Plant Cell Physiol 52(12):2050–2061
Gaid MP, Haas BT, Scholl S, Beerhues L (2016) Hyperforin production in Hypericum perforatum root cultures. J Biotechnol 222:47–55
Geyter ND, Gholami A, Goormachtig S, Goossens A (2012) Transcriptional machineries in jasmonate-elicited plant secondary metabolism. Trends Plant Sci 17(6):349–359
Gibbs T (2007) Phytonutrients - the natural drugs of the future. In: Zhu YZ, Tan BKH, Bay BH, Liu CH (eds) Natural products: essential resources for human survival. World Scientific Publishing Co. Pte. Ltd, Hackensack, pp 1–26
Giorcelli AF, Sparvoli MF, Tava A, Balestrazzi A, Vrhovsek U, Calligari P, Bollini R, Confalonieri M (2004) Expression of the stilbene synthase (StSy) gene from grapevine in transgenic white poplar results in high accumulation of the antioxidant resveratrol glucosides. Transgenic Res 13:203–214
Giri CC, Zaheer M (2016) Chemical elicitors versus secondary metabolite production in vitro using plant cell, tissue and organ cultures: recent trends and a sky eye view appraisal. Plant Cell Tissue Org J 126(1):1–18
Gomez-Galera S, Pelacho AM, Gene A, Capell T, Christou P (2007) The genetic manipulation of medicinal and aromatic plants. Plant Cell Rep 26:1689–1715. doi:10.1007/s00299-007-0384-x
Greeson JM, Sanford B, Monti DA (2001) St. John’s wort (Hypericum perforatum): a review of the current pharmacological, toxicological and clinical literature. Psychopharmacology (Berl) 153:402–414
Halls C, Yu O (2008) Potential for metabolic engineering of resveratrol biosynthesis. Trends Biotechnol 26:77–81
Hamill JD, Robins RJ, Parr AJ, Evans DM, Furze JM, Rhodes MJC (1990) Over-expressing a yeast ornithine decarboxylase gene in transgenic roots of Nicotiana rustica can lead to enhanced nicotine accumulation. Plant Mol Biol 15:27–38
Hanhineva K, Kokko H, Siljanen H, Rogachev I, Aharoni A, Kärenlampi SO (2009) Stilbene synthase gene transfer caused alterations in the phenylpropanoid metabolism of transgenic strawberry (Fragaria x ananassa). J Exp Bot 60(7):2093–2106
Hashimoto T, Yun DJ, Yamada Y (1993) Production of tropane alkaloids in genetically engineered root cultures. Phytochemistry 32:713–718
Hayashi H, Fukui H, Tabata M (1988) Examination of triterpenoids produced by callus and cell suspension cultures of Glycyrrhiza glabra. Plant Cell Rep 7:508–511
Hayashi H, Fukui H, Tabata M (1990) Biotransformation of 18-β-Glycyrrhetinic acid by cell suspension cultures of Glycyrrhiza glabra. Phytochemistry 29:2149–2152
He M, Wang Y, Hua W, Zhang Y, Wang Z (2012) De novo sequencing of Hypericum perforatum transcriptome to identify potential genes involved in the biosynthesis of active metabolites. PLoS One 7(7):e42081
Hefner J, Ketchum REB, Croteau R (1998) Cloning and functional expression of a cDNA encoding geranylgeranyl diphosphate synthase from Taxus canadensis and assessment of the role of this prenyltransferase in cells induced for Taxol production. Arch Biochem Biophys 360:62–74
Hollingsworth RG, Armstrong JW, Campbell E (2002) Caffeine as a repellent for slugs and snails. Nature 417:915–916
Huang Y, Li H, Hutchison CE, Laskey J, Kieber JJ (2003) Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J 33:221–233
Hussain MS, Fareed S, Ansari S, Rahman MA, Ahmad IZ, Saeed M (2012) Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci 4(1):10–20
Ishima N, Katayama O (1976) Sensory evaluation of stevioside as a sweetener. Rep Natl Food Res Inst 31:80–85
Jalal MAF, Collin HA (1979) Secondary metabolism in tissue cultures of Theobroma cacao. New Phytol 83(2):343–349
Jasinski M, Stukkens Y, Degand H et al (2001) A plant plasma membrane ATP binding cassette-type transporter is involved in antifungal terpenoid secretion. Plant Cell 13:1095–1107
Jennewein S, Rithner C, Williams R, Croteau R (2001) Taxol biosynthesis: taxane 13a-hydroxylase is a cytochrome P450-dependent monooxygenase. Proc Natl Acad Sci U S A 98:13595–13600
Jennewein S, Wildung MR, Chau M, Walker K, Croteau R (2004) Random sequencing of an induced Taxus cell cDNA library for identification of clones involved in Taxol biosynthesis. Proc Natl Acad Sci U S A 101:9149–9154
Joo S, Kim WT, Louis S (2007) A gaseous plant hormone ethylene: the signalling pathway. J Plant Biol 50:109–116
Kalia AN (2005) Worldwide trade scenario of medicinal and aromatic plants. In: Tyagi CS, Verma PK, Hooda JS, Yadav OP, Goyal RK (eds) Course compendium winter school on advances in medicinal, aromatic and under-utilized plants research, 29 September–19 October 2005 at CCSHAU. Hisar, Haryana, India, pp 271–273
Kang YM, Park DJ, Min JY, Song HJ, Jeong MJ, Kim YD, Kang SM, Karigar CS, Choi MS (2011) Enhanced production of tropane alkaloids in transgenic Scopolia parviflora hairy root cultures over-expressing putrescine N-methyl transferase (PMT) and hyoscyamine-6β-hydroxylase (H6H). In Vitro Cell Dev Biol Plant 47:516–524
Karioti A, Bilia AR (2010) Hypericins as potential leads for new therapeutics. Int J Mol Sci 11:562–594
Karppinen K, Hohtola A (2008) Molecular cloning and tissue-specific expression of two cDNAs encoding polyketide synthases from Hypericum perforatum. J Plant Physiol 165:1079–1086
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res Vol 3(13):1222–1239
Khosla C, Keasling JD (2003) Metabolic engineering for drug discovery and development. Nat. Rev. Drug Des Discov 2:1019–1025
Kieber JJ, Rothenberg M, Roman G, Feldman KA, Ecker JR (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the Raf family of protein kinases. Cell 72:427–441
Kirakosyan A, Sirvent TM, Gibson DM (2004) The production of hypericins and hyperforin by in vitro cultures of St. John's Wort (Hypericum perforatum) [J]. Biotechnol Appl Biochem 39(1):71–81
Kitagawa I (2002) Licorice root. A natural sweetener and an important ingredient in Chinese medicine. Pure Appl Chem 74(7):1189–1198
Klee HJ (2004) Ethylene signal transduction. Moving beyond arabidopsis. Plant Physiol 135:660–667
Klingauf P, Beuerle T, Mellenthin A, El-Moghazy SA, Boubakir Z et al (2005) Biosynthesis of the hyperforin skeleton in Hypericum calycinum cell cultures. Phytochemistry 66:139–145
Kosuth J, Smelcerovic A, Borsch T, Zuehlke S, Karppinen K, Spiteller M et al (2011) The hyp-1 gene is not a limiting factor for hypericin biosynthesis in the genus hypericum. Funct Plant Biol 38:35–43. doi:10.1071/FP10144
Koulman A, Kubbinga ME, Batterman S, Woerdenbag HJ, Pras N, Woolley JG, Quax WJ (2003) A phytochemical study of Lignans in whole plants and cell suspension cultures of Anthriscus sylvestris. Planta 69(8):733–738
Kumar N, Gulati A, Bhattacharya A (2013) L-glutamine and L-glutamic acid facilitate successful agrobacterium infection of recalcitrant tea cultivars. Appl Biochem Biotechnol 170:1649–1664. doi:10.1007/s12010-013-0286-z
Kurz WG, Chatson KB, Constabel F et al (1981) Alakloid production in Catharanthus roseus cell cultures. Planta Med 42:22–31
Li H, Guo H (2007) Molecular basis of the ethylene signaling and response pathway in Arabidopsis. J Plant Growth Regul 26:106–117
Li TSC, Mazza G (1999) Hortscience 34:85–87
Lin Z, Alexander L, Hackett R, Grierson D (2008) LeCTR2, a CTR1-like protein kinase from tomato, plays a role in ethylene signaling, development and defense. Plant J 54:1083–1093
Lin-Fang H, Zeng-Hui W, Shi-Lin C (2014) Hypericin: chemical synthesis and biosynthesis. Chin J Nat Med 12(2):0081–0088
Liu S, Hu Y, Wang X, Zhong J, Lin Z (2006) High content of resveratrol in lettuce transformed with a stilbene synthase gene of Parthenocissus henryana. J Agric Food Chem 54(21):8082–8085
Lloyd AM, Walbot V, Davis RW (1992) Arabidopsis and nicotiana anthocyanin production activated by maize regulators R and C1. Science 258:1773–1775
Lu HY, Liu JM, Zhang HC, Yin T, Gao SL (2008) Ri-mediated transformation of Glycyrrhiza uralensis with a squalene synthase gene (GuSQS1) for production of glycyrrhizin. Plant Mol Biol Rep 26:1–11
Maitra S, Satya P (2014) Value added products from medicinal plants. In: Sharangi AB, Datta S (eds) Value addition of horticultural crops: recent trends and future directions. Springer, India, pp 113–124
Martin C (1996) Transcription factors and the manipulation of plant traits. Curr Opin Chem Biol 7:130–138
Masoumian M, Arbakariya A, Syahida A, Maziah M (2011) Effect of precursors on flavonoid production by Hydrocotyle bonariensis callus tissues. Afr J Biotechnol 10(32):6021–6029
Mol J, Grotewold E, Koes R (1998) How genes paint flowers and seeds. Trends Plant Sci 3:212–217
Morant M, Bak S, Moller BL, Werck-Reichhart D (2003) Plant cytochromes P450: tools for pharmacology, plant protection and phytoremediation. Curr Opin Biotechnol 14:151–162
Morita M, Shitan N, Sawada K et al (2009) Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc Natl Acad Sci U S A 106:2447–2452
Muller JM, Schlittler E, Bein HJ (1952) Reserpin, der sedative Wirkstoffaus Rauwofia serpentina Benth. Experientia 8:338
Nicoletti I, De Rossi A, Giovinazzo G, Corradini D (2007) Identification and quantification of stilbenes in fruits of transgenic tomato plants (Lycopersicon esculentum Mill.) by reversed phase HPLC with photodiode Array and mass spectrometry detection. J Agric Food Chem 55(9):3304–3311
Nosov AM (2012) Application of cell Technologies for production of plant derived bioactive substances of plant origin. Appl Biochem Microbiol 48(7):609–624
O’Keefe BR, Mahady GB, Gills JJ, Beecher CWW (1997) Stable vindoline production in transformed cell cultures of Catharanthus roseus. J Nat Prod 60:261–264
Paek KY, Murthy HN, Zhong JJ (2014) Production of biomass and bioactive compounds using bioreactor technology. Springer, Dordrecht. doi:10.1007/978-94-017-9223-3
Pandey SS, Singh S, Vivek Babu CS, Shanker K, Srivastava NK, Shukla AK, Kalra A (2016) Fungal endophytes of Catharanthus roseus enhance vindoline content by modulating structural and regulatory genes related to terpenoid indole alkaloid biosynthesis. Sci Rep 25(6):26583
Pitzschke A (2013) Agrobacterium infection and plant defense—transformation success hangs by a thread. Front Plant Sci 4(519):1–12. doi:10.3389/fpls.2013.00519
Plowman T, Rivier L (1983) Cocaine and cinnamoylcocaine content of Erythroxylum species. Ann Bot 51:641–659
Price AH, Atherton NM, Hendry GAF (1989) Plants under drought stress generate activated oxygen. Free Radic Res Commun 8:61–66
Radić S, Vujčić V, Glogoški M, Radić-Stojković M (2016) Influence of pH and plant growth regulators on secondary metabolite production and antioxidant activity of Stevia rebaudiana (Bert). Period Biol 118(1):9–19
Rajput H (2013) Effects of Atropa belladonna as an anti-cholinergic. Nat Prod Chem Res 1:1. 1000104
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N, O'Neal JM, Cornwell T, Pastor I, Fridlender B (2002) Plants and human health in the twenty-first century. Trends Biotechnol 20:522–531
Razdan MK (2003) Introduction to plant tissue culture. Science Publishers. p 375. ISBN, 1578082374, 9781578082377
Rischer H, Hakkinen ST, Ritala A et al (2013) Plant cells as pharmaceutical factories. Curr Pharm Des 19:5640–5660
Rokem JS, Schwarzberg J, Goldberg I (1984) Autoclaved fungal mycelia increase diosgenin production in cell suspension cultures of Dioscorea deltoidea. Plant Cell Rep 3:159–160
Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR (1995) Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139:1393–1409
Sabir F, Sangwan RS, Singh J, Misra LN, Pathak N, Sangwan NS (2011) Biotransformation of withanolides by cell suspension cultures of Withania somnifera (Dunal.) Plant Biotechnol Rep 5:127–134
Santarem ER, Zamban DC, Felix LM, Astarita LV (2008) Secondary metabolism of Hypericum perforatum induced by agrobacterium rhizogenes. In Vitro Cell Dev Biol Anim 44:S52
Santarem E, Silva T, Freitas K, Sartor T, Astarita L (2010) Agrobacterium rhizogenes and salicylic acid trigger defense responses in Hypericum perforatum shoots. In vitro cell Dev. Biol Anim 46:S159–S160
Santoro MV, Nievas F, Zygadlo J, Giordano W, Banchio E (2013) Effects of Growth Regulators on Biomass and the Production of Secondary Metabolites in Peppermint (Mentha piperita) Micropropagated in Vitro. Am J Plant Sci 04:49–55
Santos AP, Moreno PRH (2013) Alkaloids derived from histidine: imidazole (Pilocarpine, pilosine). In: Ramawat KG, Merillon J-M (eds) Handbook of natural products. Chapter 6. Springer. Disponível Em: http://www.springerreference.com/docs/html/chapterdbid/363118.html
Sato F, Hashimoto T, Hachiya A, Tamura KI, Choi KB, Morishige T, Fujimoto H, Yamada Y (2001) Metabolic engineering of plant alkaloid biosynthesis. Proc Natl Acad Sci U S A 98:367–372
Schiel Om Berlin J (1987) Large scale fermentation and alkaloid production of cell suspension cultures of Catharanthus roseus. Plant Cell Tiss Org Cult 8:153–161
Schoendorf A, Rithner CD, Williams RM, Croteau R (2001) Molecular cloning of a cytochrome P450 taxane 10β-hydroxylase cDNA from Taxus and functional expression in yeast. Proc Natl Acad Sci U S A 98:1501–1506
Schröder J (1997) A family of plant-specific polyketide synthases facts and predictions. Trends Plant Sci 2:373–378
Seki H, Ohyama K, Sawai S, Mizutani M, Ohnishi T, Sudo H, Akashi T, Aoki T, Saito K, Muranaka T (2008) Licorice beta-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc Natl Acad Sci U S A 105(37):14204–14209
Seki H, Sawai S, Ohyama K, Mizutani M, Ohnishi T, Sudo H, Fukushima EO, Akashi T, Aoki T, Saito K, Muranaka T (2011) Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. Plant Cell 23(11):4112–4123
Shi M, Luo X, Li L, Huang S, Zhang T, Wang H, Kai G (2016) Enhanced DiterpeneTanshinone accumulation and bioactivity of transgenic Salvia miltiorrhiza hairy roots by pathway engineering. J Agric Food Chem 64(12):2523–2530
Shimomura K, Sudo H, Saga H, Kamada H (1991) Shikonin production and secretion by hairy root cultures of Lithospermum erythrorhizon. Plant Cell Rep 10:282–285
Shitan N (2016) Secondary metabolites in plants: transport and self-tolerance mechanisms. Biosci Biotechnol Biochem 80(7):1283–1293
Shoji T, Hashimoto T (2013) Smoking out the masters: transcriptional regulators for nicotine biosynthesis in tobacco. Plant Biotech 30:217–224
Shoji T, Inai K, Yazaki Y et al (2009) Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots. Plant Physiol 149:708–718
Singh B, Sharma RA (2014) Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 5(2):129–151. doi:10.1007/s13205-014-0220-2
Sivakumar G (2006) Bioreactor technology: a novel industrial tool for high-techproduction of bioactive molecules and biopharmaceuticals from plant roots. Biotechnol J 1:1419–1427
Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes Dev 12:3703–3714
Southwell IA, Bourke CA (2001) Seasonal variation in hypericin content of Hypericum perforatum L. (St. John’s wort). Phytochemistry 56:437–441
Springob K, Kutchan TM (2009) Introduction to the different classes of natural products. In: Osbourn AE, Lanzotti V (eds) Plant-derived natural products. Synthesis, function, and application. Springer, New York. doi:10.1007/978-0-387-85498-4
Srivastava S, Srivastava AK (2008) Hairy root culture for mass-production of high-value secondary metabolites. Crit Rev Biotechnol 27(1):29–43
Staniek A, Bouwmeester H, Fraser PD, Kayser O, Martens S, Tissier A, van-der Krol S, Wessjohann L, Warzecha H (2013) Natural products – modifying metabolite pathways in plants. Biotechnol J 8:1159–1171
Takahashi D, Fujita Y (1991) In: Plant cell culture in Japan. Komamine A, Misawa M, Dicosmo F (eds). Cosmetic Materials. 72–78
Talapatra SK, Talapatra B (2015) Chemistry of plant natural products: stereochemistry, conformation, synthesis, biology, and medicine. Springer, Heidelberg. doi:10.1007/978-3-642-45410-3
Terryn N, Van Montagu M, Inzé D, Goosens A (2006) Functional genomic approaches to study and engineer secondary metabolites in plant cell cultures. In: Bogers LJ, Craker LE, Jange D (eds) Medicinal and aromatic plants. Springer, Netherlands, pp 291–300
Tian HQ, Russell SD (1999) Culture-induced changes in osmolality of tobacco cell suspensions using four exogenous sugars. Plant Cell Tiss Org Cult 55:9–13
Toivonen L, Rosenqvist H (1995) Establishment and growth characteristics of glycyrrhiza glabra Hairy root cultures. Plant Cell Tiss Org Cult 41:249–258
Tominack RL, Spyker DA (1987) Capsicum and capsaicin–a review: case report of the use of hot peppers in child abuse. J Toxicol Clin Toxicol 25(7):591–601
Trethewey RN (2004) Metabolite pro fi ling as an aid to metabolic engineering in plants. Curr Opin Plant Biol 7:196–201
Tusevski O, Stanoeva JP, Stefova M, Kungulovski D, Pancevska NA, Sekulovski N et al (2013) Hairy roots of Hypericum perforatum L.: a promising system for xanthone production. Cent Eur J Biol 8:1010–1022. doi:10.2478/s11535-013-0224-7
Tusevski O, Petreska Stanoeva J, Stefova M, Pavokovic D, Gadzovska Simic S (2014) Identification and quantification of phenolic compounds in Hypericum perforatum L. transgenic shoots. Acta Physiol Plant 36:2555–2569. doi:10.1007/s11738-014-1627-4
Ulbrich B, Weisner W, Arens H (1985) In: Neumann KH, Reinhard E (eds) Primary and secondary metabolism of plant cell cultures. Springer, Berlin, pp 293–303
Verpoorte R, van der Heijden R, Memelink J (2000) Engineering the plant cell factory for secondary metabolite production. Transgenic Res 9:323–343
Vidensek N, Lim P, Campbell A, Carlson C (1990) Taxol content in bark, wood, root, leaf, twig, and seedling from several taxus species. J Nat Prod 53:1609–1610
Vinterhalter B, Ninkovic S, Cingel A, Vinterhalter D (2006) Shoot and root culture of Hypericum perforatum L. transformed with Agrobacterium rhizogenes A4M70GUS. Biol Plant 50:767–770. doi:10.1007/s10535-006-0127-9
Vom Endt D, Kijne JW, Memelink J (2002) Transcription factors controlling plant secondary metabolism: what regulates the regulators? Phytochemistry 61(2):107–114
Walker K, Croteau R (2000a) Molecular cloning of a 10-deacetylbaccatin III-10-O-acetyl transferase cDNA from Taxus and functional expression in Escherichia coli. Proc Natl Acad Sci U S A 97(2):583–587
Walker K, Croteau R (2000b) Taxol biosynthesis: molecular cloning of a benzoyl- CoA:taxane 2alpha-O-benzoyltransferase cDNA from Taxus and functional expression in Escherichia coli. Proc Natl Acad Sci U S A 97(25):13591–13596
Walker K, Croteau R (2001) Taxol biosynthetic genes. Phytochemistry 58(1):1–7
Walker K, Fujisaki S, Long R, Croteau R (2002) Molecular cloning and heterologous expression of the C-13 phenylpropanoid side chain-CoA acyltransferase that functions in Taxol biosynthesis. Proc Natl Acad Sci U S A 99:12715–12720
Walker K, Klettke K, Akiyama T, Croteau R (2004) Cloning, heterologous expression, and characterization of a phenylalanine aminomutase involved in taxol biosynthesis. J Biol Chem 279:53947–53954
Wang KLC, Li H, Ecker JR (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14:131–152
Weathers PJ, Arsenault PR, Covello PS, McMickle A, Teoh KH, Reed DW (2011) Artemisinin production in Artemisia annua: studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochem Rev 10(2):173–183
Wentworth JM, Agnostini M, Love J, Schwabe JW, Chatterjee VKK (2000) St John’s wort, a herbal antidepressant, activates the steroid X receptor. J Endocrinol 166:R11
Whitmer S, van der Heijden R, Verpoorte R (2002) Effect of precursor feeding on alkaloid accumulation by a tryptophan decarboxylase over-expressing transgenic cell line T22 of Catharanthus roseus. J Biotechnol 96:193–203
Wildung MR, Croteau R (1996) A cDNA clone for taxadiene synthase, the diterpene cyclase that catalyzes the committed step of taxol biosynthesis. J Biol Chem 271(16):9201–9204
Wink M, Roberts MW (1998) Alkaloids: biochemistry, ecology, and medicinal applications. Plenum Press, New York
Yamada Y, Hashimoto T (1982) Production of tropane alkaloids in cultured cells of hyoscyamus niger. Plant Cell Rep 1:101e103
Yang Y, He F, Yu L, Ji J, Wang Y (2009) Flavonoid accumulation in cell suspension cultures of Glycyrrhiza inflata Batal under optimizing conditions. Z Naturforsch C 64:68–72
Yazaki K (2004) Natural products and metabolites. In: Christou P, Klee H (eds) Handbook of plant biotechnology, vol 2. Wiley, Chichester, pp 811–857
Yu F, Luca DV (2013) ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus. Proc Natl Acad Sci U S A 110:15830–15835
Zarate R, Verpoorte R (2007) Strategies for the genetic modification of the medicinal plant Catharanthus roseus (L.) G. Don. Phytochem Rev 6:475–491
Zhang S, Li N, Gao F, Yang A, Zhang J (2010) Overexpression of TsCBF1 gene confers improved drought tolerance in transgenic maize. Mol Breeding 26:455–465
Zhong JJ (2001) Biochemical Engineering of the Production of Plant-Specific Secondary Metabolites by Cell Suspension Cultures. In: Scheper T (ed) Advances in biochemical engineering, Biotechnology, vol 72. Springer, Heidelberg, pp 1–26
Zhou ML, Hou HL, Zhu XM, Shao JR, Wu YM, Tang YX (2010) Molecular regulation of terpenoid indole alkaloids pathway in the medicinal plant, Catharanthus roseus. J Med Plants Res 4(25):2760–2772
Zhu J, Wang M, Wen W, Yu R (2015) Biosynthesis and regulation of terpenoid indole alkaloids in Catharanthus roseus. Pharmacogn Rev 9(17):24–28
Zobayed SMA, Afreen F, Kozai T (2005) Temperature stress can alter the photosynthetic efficiency and secondary metabolite concentrations in St John’s wort. Plant Physiol Biochem 43:977–984
Zobel AM, Wang J, March RE, Brown SA (1991) Identification of eight coumarins occuring with psoralen, xanthoxin, and bergapten on leaf surfaces. J Chem Ecol 17(9):1859–1870
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Ebrahimi, M., Mokhtari, A. (2017). Engineering of Secondary Metabolites in Tissue and Cell Culture of Medicinal Plants: An Alternative to Produce Beneficial Compounds Using Bioreactor Technologies. In: Abdullah, S., Chai-Ling, H., Wagstaff, C. (eds) Crop Improvement. Springer, Cham. https://doi.org/10.1007/978-3-319-65079-1_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-65079-1_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-65078-4
Online ISBN: 978-3-319-65079-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)