Abstract
Craniopharyngioma tumors are histologically benign, but their management remains challenging because of their intimate relationship to vital neurovascular structures such as the optic apparatus, pituitary stalk, hypothalamus, and vessels of the circle of Willis. Surgery remains the mainstay of suprasellar craniopharyngioma management. Although total resection should be the goal, a subtotal resection with postoperative radiation is a reasonable option if the lesion is adherent to the hypothalamus or other eloquent midline structures. Open transcranial approaches are the standard of care for surgical intervention, which have stood the test of time. More recently, minimally invasive transcranial keyhole approaches and endoscopic endonasal approaches have been added to the armamentarium of skull base neurosurgeons. In 18.2, we discuss the options available for open transcranial approaches to craniopharyngioma tumors and consider their relative merits and limitations, patient selection criteria, perioperative care, complication avoidance, management principles, and surgical outcomes. No single operative technique can be considered as the “best approach” for all patients. Each patient is best served by the formulation of an individualized, tailored surgical plan that aims to realistically and safely achieve the expected management goals. The supraorbital “eyebrow” craniotomy is an ideal approach for those tumors that are anterior or superior to the optic apparatus, and we discuss this in 18.3. Meticulous attention to detail during the approach and tumor dissection as well as the closure will help achieve good tumor resection, minimize morbidity, and maintain cosmesis. The use of surgical tools such as the micro-Doppler probe and neuroendoscopy are helpful to visualize and preserve critical neurovascular structures. Attentive postoperative management is necessary for good long-term outcomes with close monitoring and treatment of endocrinopathies. The use of stereotactic radiation therapy and targeted molecular therapy should be utilized as necessary.
Recently, there has been increased application of the endoscopic endonasal approach for these tumors. In appropriately selected cases, the endoscopic endonasal approach offers excellent direct visualization of the undersurface of the optic chiasm and its vascular perforators, hypothalamus, and third ventricle, which contributes to safer microdissection and a more complete removal while minimizing potential complications. In 18.4, we review the technical nuances and surgical pearls for resection of suprasellar craniopharyngiomas using the endoscopic endonasal approach. We also discuss factors involved in approach selection and complication avoidance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Craniopharyngioma
- Open transcranial approaches
- Technical nuances
- Surgical outcomes
- Keyhole craniotomy
- Eyebrow craniotomy
- Supraorbital craniotomy
- Pituitary tumor
- Hypopituitarism
- Optic nerve
- Vision preservation
- Retrochiasmatic
- Endoscopic skull base surgery
- Endoscopic endonasal approach
- Microsurgical transcranial approach
18.1 Suprasellar Craniopharyngiomas: Editors’ Introduction
Craniopharyngiomas are histologically benign World Health Organization (WHO) grade I intracranial tumors that originate from remnants of Rathke’s pouch and can involve the sellar, parasellar, and suprasellar areas. Although craniopharyngiomas constitute less than 1% of all primary CNS tumors, they are the most common non-glial tumor in children [1]. The incidence of craniopharyngiomas is estimated at 0.13 per 100,000 person years, with a bimodal age distribution between 5–14 years and 65–74 years [2] and a higher prevalence in childhood. Two pathological types of craniopharyngiomas have been described. The adamantinomatous subtype is more common in children, frequently is cystic, and accounts for 5–10% of pediatric intracranial malignancies. The papillary subtype is more commonly solid with calcifications and occurs almost exclusively in adults.
Craniopharyngiomas are most commonly found in the suprasellar region but can occur along the entire length of the craniopharyngeal duct. Five basic growth patterns have been observed: infradiaphragmatic (sellar), subarachnoid extraventricular, and subpial intraventricular (third ventricle) based on the origin from infrasellar; infradiaphragmatic; transinfundibular; suprasellar; and subarachnoid subpial ventricular locations [3]. The extent of the tumor, coupled with the propensity for local invasion to surrounding critical neurovascular structures such as the hypothalamus, infundibulum, pituitary gland, optic chiasm, and carotid arteries, substantially increases the difficulty of achieving complete surgical resection. Thus, multimodal treatment including radiation and molecular-targeted agents may often be necessary for tumor control. In this chapter, authors will discuss both open and endoscopic approaches to the suprasellar region. In addition, each author will discuss the advantages and disadvantages of their approach to treat the case example below (Fig. 18.1a–c).
(a–c) CASE EXAMPLE: suprasellar craniopharyngioma. A 24-year-old lady presents with mild bilateral visual blurriness. Examination: OS, 20/20 (corrected); Humphrey VF, mild arcuate superior and inferior temporal defects. OD, 20/20 (corrected); Humphrey VF, mild arcuate superior and inferior temporal defects. Rest of neurological exam normal. Laboratory workup: normal endocrine function
18.2 Suprasellar Craniopharyngiomas: Open Transcranial Approaches
Introduction
Craniopharyngiomas are benign tumors that arise from remnants of Rathke’s pouch. Despite their histologically benign nature, their management remains challenging because of the tumor’s close proximity to vital neurovascular structures such as the optic apparatus, pituitary stalk, hypothalamus, and vessels of the circle of Willis. They are primarily located in the sellar-suprasellar region, but intraventricular and multicompartmental extensions into the anterior, middle, and posterior cranial fossae are also frequently seen.
The constraints imposed by nearby critical neurovascular structures in the suprasellar region have led to the development of several approaches, including transcranial and transsphenoidal [4,5,6]. Transcranial approaches are further subdivided into open and minimally invasive keyhole approaches, while transsphenoidal procedures can be either microscopic or endoscopic. This chapter will discuss the current available options for open transcranial approaches to craniopharyngioma tumors and consider their relative merits and limitations, patient selection criteria, perioperative care, complication avoidance, management principles, and surgical outcomes. We will discuss in detail the pros and cons of open surgical technique compared to endonasal technique and discuss the relative merits/demerits of each open technique over another using the illustrative case example.
Open Transcranial Approaches
There are four primary open transcranial approaches to craniopharyngioma tumors: the pterional/orbitozygomatic, the subfrontal/transbasal, the subtemporal approach alone or in combination with a transpetrosal approach, and the interhemispheric transcallosal/transcortical transventricular approaches. The use of extended skull base modifications such as the orbitozygomatic, transbasal, and transpetrous approaches helps to attain a much flatter and inferior-to-superior operative trajectory to more extensive skull base tumors while reducing the amount of brain retraction required. Each of the techniques has its own set of merits and demerits, and the appropriate surgical corridor must be chosen according to the individual tumor and patient characteristics. Many of these approaches require preoperative planning for adequate intraoperative brain relaxation. This goal can be achieved via intraoperative elevation of the head (reverse Trendelenburg position), mannitol or hypertonic saline bolus infusion, lumbar drain placement, ventricular tapping, and early release of cerebrospinal fluid through the sylvian and basal cisterns. One or more of these modalities can be used to achieve adequate brain relaxation depending upon the tumor morphology, patient symptomatology, and ventricular status. The principles of tumor dissection and craniopharyngioma removal include maintaining the arachnoid plane to reduce neurovascular damage, progressive tumor debulking, precise sharp microsurgical extracapsular dissection, and assiduous preservation of neurovascular structures.
Pterional/Orbitozygomatic Approach
The frontotemporal pterional approach, as advocated by Yasargil, uses the parachiasmal spaces (interoptic, opticocarotid, and carotid-oculomotor/carotid-tentorial corridors) and (less commonly) the triangle superior to the carotid bifurcation to access the suprasellar cistern. Performing an additional orbitozygomatic osteotomy can augment the operative access to the interpeduncular, parasellar, and posterior/superior third ventricular regions over and beyond the usual suprasellar access. Drilling of the anterior clinoid process along with optic nerve decompression either extradurally or intradurally helps to alleviate any mass effect on the optic nerve and increase the maneuverability of surgical instruments between the interoptic and opticocarotid corridors for more radical resection of tumor. Although the pterional approach is a versatile skull base approach that provides the shortest distance and most direct transcranial route to the suprasellar region and can be used for a wide spectrum of craniopharyngioma tumors, its best indication is in the resection of prechiasmatic tumors with a secondarily postfixed chiasm. Tumors with large retrochiasmatic and intraventricular extensions may be better managed with other approaches, although many such cases can also be managed by adding a lamina terminalis corridor through the standard pterional craniotomy for better surgical access. The primary disadvantages of this approach include limited access, poor visualization of the contralateral opticocarotid triangle, ipsilateral infrachiasmatic and hypothalamic surfaces and retrocarotid space, and the need for optic nerve/chiasm manipulation to gain access to the suprasellar and interpeduncular regions. Complications associated with this approach for craniopharyngioma include frontalis palsy if the frontalis facial nerve branches are injured with the craniotomy approach, visual deterioration due to manipulation of the optic apparatus, and vascular injury to the ipsilateral carotid or its branches. Occasionally, especially in children, fusiform dilatation of the carotid may develop after manipulation of the vessel during tumor dissection.
Subfrontal/Transbasal Approach
The subfrontal approach with bifrontal/unilateral craniotomy, as popularized by Tessier et al. and Derome et al., primarily relies on the interoptic, lamina terminalis, and opticocarotid corridors for suprasellar and third ventricular access. As opposed to the pterional/anterolateral approach, the primary advantage for this approach is its midline anterior surgical trajectory, which provides a direct and straight access to the prechiasmatic space and lamina terminalis corridor and offers visualization of the bilateral opticocarotid cisterns. It also provides excellent visualization of both walls of the third ventricle and hypothalamus through the translamina terminalis corridor; however, both the pterional and subfrontal approaches have an inherent limitation of accessing the retrochiasmatic space. If the lamina terminalis approach is chosen to approach tumors with third ventricular extension, a midline transbasal approach offers the optimal trajectory. The presence of a primary or secondary prefixed chiasm becomes a relative contraindication for subfrontal approach if the lamina terminalis approach is not added. The transbasal approach involves the addition of a bilateral orbitotomy and drilling of the crista galli to the bifrontal craniotomy so as to provide a much more inferior surgical trajectory to access craniopharyngiomas with significant third ventricular extension. If the prechiasmatic space is still narrow despite adequate transbasal exposure and the tumor extends inferiorly into the sella turcica, the working channel can be further expanded by drilling the anterior wall of the sella turcica and tuberculum and planum sphenoidale to allow tumor resection. This transsphenoidal transsellar variant of the transbasal approach is associated with higher incidence of cerebrospinal fluid (CSF) rhinorrhea and requires meticulous skull base reconstruction. These approaches can utilize both subfrontal and basal interhemispheric access routes to the suprasellar region. The disadvantage of the subfrontal route as compared with the basal interhemispheric approach is the risk of retraction injury to the bilateral frontal lobes and olfactory tracts; however, if the olfactory tracts are carefully and meticulously dissected from the frontal lobes, a wide operative exposure can be achieved safely, and lateral extensions of tumor can be handled. On the contrary, the basal interhemispheric route provides a relatively narrow operative corridor, requires interhemispheric brain retraction, and has difficulty accessing lateral extensions of the tumor, but it is associated with a lower incidence of olfactory tract injury and risk of CSF rhinorrhea. Complications pertinent to these approaches include anosmia/hyposmia, bifrontal contusions, venous infarctions, and CSF rhinorrhea if the frontal sinus is violated. Additional late complications (mucocele) can be associated with frontal sinus violation that is not addressed properly with the approach.
Subtemporal/Transpetrosal Approach
Given the limitations of the anterior and anterolateral skull base approaches to access retrochiasmatic craniopharyngiomas, Hakuba et al. and Al-Mefty et al. pioneered the subtemporal transtentorial posterior transpetrosal (presigmoid translabyrinthine) approach for such lesions. This posterolateral approach provides a caudocranial operative corridor, as opposed to the craniocaudal trajectory provided by the anterior and anterolateral skull base approaches. The primary advantage is that it allows direct visualization of the infrachiasmatic surface, pituitary stalk, and hypothalamus, thereby allowing for dissection of the upper pole of the tumor from these vital neural structures. It also offers lower risk to anterior circulation perforators, which are more often encountered with anterior approaches; however, it has the limitation of manipulating the vital neurovascular contents of the ambient cistern (oculomotor nerve, trochlear nerve, second segment of posterior cerebral artery, and posterior communicating artery along with their perforating branches). In addition, this approach also carries the risk of temporal contusion due to prolonged retraction of the temporal lobe, seizures, speech disturbances, and vein of Labbé injury-associated venous infarct. With this approach, the posterior communicating artery may be in the corridor; some authors have described division of this artery to enhance the trajectory to the tumor.
Interhemispheric Transcallosal/Transcortical Transventricular Approach
Unlike the other approaches to craniopharyngiomas described earlier, the interhemispheric transcallosal and transcortical transventricular approaches do not employ skull base corridors but rather provide a vertical trajectory from a calvarial aspect. They are essentially reserved for primarily intraventricular lesions with large superior extensions along the foramen of Monro with or without accompanying obstructive hydrocephalus. It is vital to differentiate the primary intraventricular lesions from tumors invading the third ventricle floor secondarily from the suprasellar region, because the former would indicate a transcallosal/transcortical approach while the latter requires a skull base approach. The senior author pays particular attention to the pituitary stalk in this differentiation. Tumors that arise wholly within the third ventricle do not deviate from the pituitary stalk, which may be foreshortened by the mass from above, but is not deviated laterally. Suprasellar tumors will deviate from the pituitary stalk. Therefore, careful preoperative assessment of radiological imaging and appropriate surgical planning is mandatory to preserve the hypothalamus and third ventricular floor. The choice between the transcallosal and transcortical approaches is based primarily on the degree of ventricular dilation, venous drainage anatomy into the superior sagittal sinus, tumor growth pattern, and surgeon’s preference. The interhemispheric transcallosal approach offers the advantage of accessing the tumor from the midline and does not require enlargement of the ventricles, whereas the latter has the potential benefits of having lower risk of disconnection syndrome from corpus callosotomy and injury to pericallosal/callosomarginal arteries. The use of intraoperative neuronavigation can be handy for these approaches to better delineate the operative trajectory and accordingly the skin incision and craniotomy. Once the lateral ventricle is entered and the laterality is confirmed using the orientation of the choroid plexus, foramen of Monro, and thalamostriate vein, the third ventricle can be accessed using the transforaminal, interforniceal, suprachoroidal, or subchoroidal corridors. Care has to be taken to avoid iatrogenic forniceal damage and injury to the thalamostriate vein to prevent any memory deficits and postoperative limb weakness, respectively. There is a risk of retraction injury to the frontal lobe and iatrogenic injury to the superior sagittal sinus along with potential venous air embolism with the transcallosal approach. The primary disadvantages of these approaches include long operative distance, putting the hypothalamus and pituitary stalk at risk during tumor dissection, limited access to the anteroinferior aspect of the third ventricular and lateral sellar lesions, and postoperative tendency toward ventricular inflammation and obstructive hydrocephalus.
Surgical Outcomes
Gross Total Versus Subtotal Resection and Adjuvant Radiotherapy
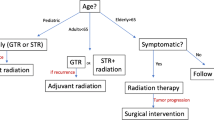
The conventional strategy for craniopharyngioma surgery aims at gross total resection (GTR) because the benign histopathology of these lesions potentially offers the lowest chance of tumor recurrence with complete removal. The factors governing the aggressive resectability of a craniopharyngioma include the presence of preoperative hypothalamic disturbance, evidence of hypothalamic involvement by the tumor on preoperative imaging or intraoperative dissection, intraoperative adherence of tumor to the floor of the third ventricle, vascular encasement, and presence of dense peripheral calcifications stuck to vital neurovascular structures. Radical resection is associated with increased morbidity associated with hypothalamic dysfunction, visual deterioration, and major vessel injury, which may also translate into increased mortality and reduced overall survival. Recent studies have validated that long-term outcomes (progression-free and overall survival) in patients with GTR are similar to those receiving partial resection with adjuvant radiation. Because of these potential sequelae of radical tumor resection, modern surgical strategy takes into account the functional outcome of the surgical resection and emphasizes subtotal resection of the lesion/maximally safe resection and using adjuvant modalities such as radiotherapy to control the residual tumor progression and maintain a good quality of life. It is important to understand that treatment strategy needs to be tailored according to individual case-to-case basis, realistically matching the patient’s expectations and optimal surgical outcome. For example, the risk of radiation-associated neurocognitive disturbance, necrosis, arteritis, and secondary malignancy is of paramount concern for pediatric patients.
Pituitary Stalk Preservation Versus Sacrifice
Craniopharyngiomas are often intimately related to the pituitary stalk because of its origin from remnants of Rathke’s pouch. Tumor adherence to the pituitary stalk often precludes radical resection without sectioning the pituitary stalk. There are two schools of thought about whether to preserve the pituitary stalk during craniopharyngioma resection. The first presumes that the pituitary stalk is essential to maintaining optimal posterior pituitary function, so near-total resection of tumor is the goal, and tumor adherent to the stalk is left behind. Subsequently, the residual tumor can be either monitored with close radiological surveillance or given up-front adjuvant radiotherapy. Various studies have demonstrated no correlation of stalk preservation with recurrence-free survival rates, validating this surgical strategy. Jung et al. [7] highlighted another important rationale for stalk preservation: its preservation also increases the likelihood of maintaining intact anterior pituitary function. Honegger et al. noted that the attempt to preserve the stalk is a time-consuming and sometimes demanding effort, but it is rewarded with improved endocrinological results. The school of thought regarding stalk resection argues that sacrificing the pituitary stalk during craniopharyngioma resection is a small price to pay for achieving GTR of a benign tumor. Adherents cite strong evidence from the literature supporting the fact that high rates of diabetes insipidus are seen with radical resection of craniopharyngioma, irrespective of the pituitary stalk integrity. Therefore, attempted stalk preservation should not preclude GTR. In these cases, care has to be taken to section the stalk as distal to the hypothalamus as possible without compromising the negative margins, so as to preserve as much antidiuretic hormone production as possible, despite permanent impairment of anterior pituitary function. It is also important to emphasize that preservation of the pituitary stalk does not imply preservation of pituitary function.
Transcranial Approach Outcomes
In a meta-analysis of surgical management of craniopharyngiomas in children, Elliot et al. [8] included 2955 patients operated via transcranial approaches. In these patients, the average GTR rate was 60.9%, and tumor recurrence rate after GTR was 17.6%. The operative mortality was 2.6% and iatrogenic neurological morbidity was 9.4%. Overall, the incidence of postoperative diabetes insipidus (DI), vision improvement, visual deterioration, obesity/hyperphagia, and overall survival were 69.1%, 47.7%, 13%, 32.2%, and 90.3%, respectively.
Transcranial Versus Endoscopic Endonasal Transsphenoidal Approaches
Many studies have attempted to compare the conventional and the newer minimally invasive techniques based on the extent of resection, recurrence rates, and complication profiles. The primary caveats to this comparison include heterogeneity of tumor characteristics (size, location, extent, and neurovascular adhesions), progression of clinical symptomatology, surgical experience, aggressiveness of surgical resection, sample size, and duration of follow-up to assess recurrence. There is increasing experience with using the endoscopic endonasal approach. It has some inherent advantages, largely with the trajectory of approach. It enables direct visualization of tumors growing in a superior direction, with extension directly into the third ventricle. A recent meta-analysis by Elliott et al. [8] compared the transcranial and transsphenoidal approaches for surgical management of craniopharyngiomas in children. A total of 2955 patients operated via transcranial and 373 patients operated via transsphenoidal routes were included. The authors concluded that directly comparing outcomes after the two approaches for pediatric craniopharyngiomas does not appear valid. Baseline differences in patients who underwent each approach create selection bias that may explain the improved rates of disease control and lower morbidity of transsphenoidal resection. It is also pertinent to understand that transsphenoidal approaches are being increasingly used primarily for smaller intrasellar tumors as compared with transcranial approaches, which are often used for much larger tumors with significant suprasellar and parasellar components, those with significant peripheral calcification, and those that engulf vascular structures. In addition, the data for such minimally invasive approaches are limited and have shorter follow-up duration as compared with traditional transcranial approaches.
Pearls |
|---|
Anterior (subfrontal) and anterolateral (pterional) skull base approaches are suitable for prechiasmatic craniopharyngioma tumors with limited intraventricular and retrochiasmatic extension. |
Augmentation of standard anterior/anterolateral skull base approaches with orbitozygomatic and transbasal extensions may facilitate removal of even larger tumors with significant intraventricular and retrochiasmatic extensions. |
Posterolateral (presigmoid translabyrinthine) approach provides a viable alternative for extensive retrochiasmatic lesions and residual tumors along posterior aspect of optic apparatus, which are otherwise not accessible by conventional anterior and anterolateral approaches. |
Interhemispheric transcallosal/transcortical transventricular approaches are primarily reserved for purely intraventricular craniopharyngiomas where the bulk of tumor is above the floor of third ventricle. |
Commentary on Case Presentation in Section 18.1
Case Presentation
A 24-year-old woman presents with mild bilateral visual blurriness, and the examination reveals OS, 20/20 (corrected); Humphrey VF, mild arcuate superior and inferior temporal defects, and OD, 20/20 (corrected); Humphrey VF, mild arcuate superior and inferior temporal defects. The rest of her neurological examination is within normal limits. The patient’s endocrine function is within normal limits. Preoperative gadolinium-enhanced MRI demonstrates a heterogeneously enhancing suprasellar region in close proximity to the pituitary stalk and floor of the third ventricle (see Fig. 18.1). The lesion is splaying and compressing the optic chiasm (left > right side). The primary radiological differential is suprasellar subchiasmatic craniopharyngioma. Ideally, further imaging in the form of computed tomography and coronal T2/FLAIR MR imaging should be evaluated preoperatively to assess the status of peripheral calcification and better delineate for the presence of any radiological hypothalamic involvement, respectively, which might have a bearing on our surgical strategy.
Discussion
Based on the clinical, endocrinological, and radiological information, we infer that the lesion is presumably a moderate-sized suprasellar subchiasmatic craniopharyngioma (Yasargil type C, Puget grade 0, Kassam infundibular type). The lesion does not seem to have any sellar extension as evident by the normal-sized sella turcica. Therefore, endonasal endoscopic TS approach seems inappropriate for the surgical access to this tumor, as it will require pituitary transposition, putting anterior pituitary function at risk. Also, because the sella is not dilated, the surgical corridor for tumor access via a transsphenoidal approach will be quite narrow. A lesser concern is the higher CSF leak associated with the transsphenoidal approach, which may translate into poor patient outcome. The chiasm appears to be just anterosuperior to the lesion and seems to be slightly prefixed, due to the bulk of tumor pushing and splaying it, but there is a reasonable interoptic corridor to this moderate-sized tumor for it to be accessed through both anterior and anterolateral skull base approaches. Because the lesion is located slightly eccentrically toward the left side, performing a unilateral (right-sided) pterional approach would be a reasonable first-line strategy. It will provide us with the benefit of both transsylvian and unilateral subfrontal working corridors for safe access to tumor. In addition to the use of the interoptic corridor primarily, the opticocarotid corridor may also be instrumental for complete resection of tumor safely. We would access this tumor through the right side and not the left side because the oblique field of view from the right side would help us to clearly define the tumor located at the undersurface of left optic apparatus. The vision is affected to the same extent bilaterally so the side of surgical access is not governed by vision in this patient. Had the vision been affected more on the left side, using a left pterional approach would have given us the option of performing ipsilateral optic canal deroofing for better maneuverability of surgical instruments around the optic apparatus and safe resection of the tumor.
Bifrontal craniotomy using anterior subfrontal/interhemispheric approach is another reasonable option and can also be performed in this case depending upon surgeon’s preference; however, retraction of the bilateral frontal lobes has a potential for damage to the bilateral olfactory tracts. Had there been significant midline intraventricular extension, this approach would have been the first choice, with a lamina terminalis approach. A posterolateral skull base approach via subtemporal transtentorial posterior transpetrosal (presigmoid translabyrinthine) approach would be our second-line strategy for resection of any tumor left behind from first surgery. Because this approach provides a caudocranial access route and excellent view of the retrochiasmatic region, it can provide the advantage of removal of any residual lesion under direct vision, ensuring safe resection and optimal outcome. This approach has more morbidity than a frontotemporal approach. Lastly, because there does not seem to be any apparent tumor within the third ventricle, interhemispheric transcallosal and transcortical transventricular approaches are not approaches of choice for this case. Another important aspect of this case is the radicality of resection and sacrifice of infundibulum if deemed necessary for radical resection of craniopharyngioma. Considering the normal endocrinological status of the patient and infundibular type of the tumor, the pros and cons of stalk preservation must be discussed in detail with the patient before proceeding with our final surgical planning. Intraoperative adherence of tumor tissue to the infundibulum will also govern our resection strategy and needs to be tailored according to patient’s expectations.
Acknowledgments We thank Kristin Kraus, MSc, our medical editor, for her contribution to manuscript editing.
18.3 Suprasellar Craniopharyngiomas: “Eyebrow” Supraorbital Craniotomy Approach
Introduction
Craniopharyngiomas have been approached surgically via a variety of surgical corridors. Parameters evaluated for surgical approach selection include tumor location relative to adjacent neurovascular structures, prior treatments, patient’s neurological and endocrinological status, and surgeon’s experience with each approach. The supraorbital transciliary (eyebrow) or transpalpebral (eyelid) craniotomy is a versatile approach to the parasellar region and is a preferred transcranial approach utilized at our institution for a minority of select craniopharyngiomas.
Patient Selection
The primary factor in surgical approach selection for craniopharyngiomas is the anatomical relationship with the optic chiasm. Most de novo craniopharyngiomas are retrochiasmal in location with their long axis along the sinonasal-sellar-hypothalamic corridor and are best approached via an endonasal endoscopic route along the undersurface of the chiasm. For such retrochiasmal craniopharyngiomas, most anterior transcranial approaches will require significant manipulation of the optic chiasm, nerves, and tracts, thereby increasing the risks of vision loss and vascular injury particularly to the superior hypophyseal arteries (Fig. 18.2), as well as limiting the ability to achieve a safe gross total resection. However, a minority of craniopharyngiomas are suprachiasmatic, either extending directly above the chiasm, anterior to the chasm, or lateral to the chiasm, while some are completely intraventricular and may thus be more safely approached via a transcranial route.
Other important considerations in determining the surgical approach include tumor consistency, prior surgery or radiation, vision, and endocrine function. For example, in many cystic recurrent craniopharyngiomas that were originally approached from the endonasal route, a transcranial route may provide excellent safe access for cyst drainage without the potential additional morbidity of reopening a prior skull base reconstruction and nasal-septal flap via the endonasal route. Thus, the supraorbital approach is ideal for supraoptic and preoptic tumors, as the optic apparatus acts as a barrier to this region from an endonasal or transpetrous approach and is particularly appropriate for recurrent cystic craniopharyngiomas with accessible cystic components in which the main goal is cyst drainage and fenestration [9].
Index Case
Commentary on Case Presentation in Section 18.1
Case Presentation
A 24-year-old lady presents with mild bilateral visual blurriness. Examination: OS, 20/20 (corrected), Humphrey VF, mild arcuate superior and inferior temporal defects; and OD, 20/20 (corrected), Humphrey VF, mild arcuate superior and inferior temporal defects. The rest of neurological exam is normal. Laboratory work-up revealed: normal endocrine function (see Fig. 18.1).
Discussion
The MRI of the index craniopharyngioma patient demonstrates a suprasellar, supraglandular tumor. This lesion is heterogeneous in nature and is consistent with a craniopharyngioma. Though there are numerous surgical approaches to this tumor for resection, specific aspects of this lesion make it ideal for the supraorbital eyebrow approach.
-
The primary determinant to differentiate between endonasal and supraorbital approaches is the location of the optic chiasm. Retrochiasmal tumors are better accessed from an endonasal approach as the surgical trajectory is ideal, and the optic chiasm would be obstructing the supraorbital surgical corridor. However, this tumor is subchiasmal in location, providing an adequate window beneath the optic chiasm for tumor resection (Fig. 18.3). In many cases, the optic chiasm is not well visualized, particularly in the sagittal plane. However, the anterior communicating artery (AComm) is a good surrogate marker of the optic chiasm as this relationship is not distorted unless the pathology is within the optic apparatus itself (e.g., optic nerve glioma). Additionally, this tumor is not in the third ventricle, hence a trans-lamina terminalis approach would not be feasible here.
-
A secondary determinant is the relation of the tumor and the tuberculum sella. Most craniopharyngiomas reside in both the sellar and suprasellar regions (though typically supradiaphragmatic). A high-riding tuberculum sella will result in a blind spot of line of sight in the sellar fossa. This lack of visualization can compromise the dissection of the tumor off the superior hypophyseal arteries and the carotid arteries. However, this can be overcome in some patients by the use of neuroendoscopy and bimanual microsurgical dissection. This tumor is situated just below the plane of the planum sphenoidale/tuberculum sella, allowing for adequate visualization and instrument access with direct line of sight with the microscope alone.
-
Relative features that can benefit the supraorbital approach but are not critical include the relationship with the infundibulum as well as the dorsum sella. This tumor is predominantly anterior to the infundibulum (type I), though it does appear to be wrapping around it as well [10]. Hence, there is the possibility to preserve pituitary structure, which may need to be incised via an endonasal approach. Conversely, the technique of incising the pituitary gland has been shown to be safe and does not cause increased pituitary dysfunction [11].
This tumor sits atop the dorsum sella with a subtle extension beyond it. The supraorbital approach offers the ability to directly view this region. For tumors with more significant retrodorsal extension, the use of neuroendoscopy can help visualize the relationship of the tumor and the mesencephalic/pontine structures. Nevertheless, the endonasal approach can access this region via gland manipulation/translocation and drilling of the dorsum sella.
-
Potential additional features that are not explicit in the imaging provided include the patient’s pituitary hormonal function, visual function, frontal sinus anatomy, age, and comorbidities. Often, patients with craniopharyngiomas have some level of hypopituitarism (although diabetes insipidus is not a common preoperative finding). In the setting of panhypopituitarism, the infundibulum may need to be sacrificed to help facilitate dissection. This is avoided when possible in the attempt to preserve pituitary function. A contemporary series noted 20% of patients had improved postoperative pituitary function after tumor resection [12].
-
A typical presentation with large suprasellar craniopharyngiomas is bitemporal hemianopsia. If vision loss is significant, an approach that would minimize optic apparatus manipulation (such as the endonasal approach) may be beneficial. Nevertheless, the small case series of the supraorbital approach demonstrate vision preservation in the majority of patients.
-
Often, the craniotomy exposed with the supraorbital eyebrow approach is lateral to the frontal sinus. However, some patients have enlarged and over-pneumatized frontal sinuses. This can be a conduit of postoperative cerebrospinal fluid (CSF) rhinorrhea. This can be prevented with fat graft occlusion of the sinus. However, a very large frontal sinus may be a relative contraindication to this approach if alternative approaches can provide comparable tumor resection.
-
Ultimately, patient age and comorbidities can contribute to the approach selection. The approach and closure of the supraorbital eyebrow craniotomy takes about 30–45 min in our experience. However, the endonasal approach can add additional time (often two- to threefold longer) under anesthesia, particularly with the elevation of a nasoseptal flap, securing the reverse flap and gasket seal buttress closure. Additionally, patients with obesity, sleep apnea, or chronic obstructive pulmonary disease (COPD) may have a higher risk of CSF rhinorrhea with the endonasal approach [13,14,15].
Surgical Technique
When deciding which approach to utilize for a craniopharyngioma in the setting of recurrent or postradiation tumors, a thorough understanding of the vasculature is necessary. Hence, a preoperative CT angiogram (CTA) is helpful to study the relationship of the carotid artery and its branches with the tumor. This can be fused with the preoperative MRI and used for operative neuronavigation. The frontal sinuses are assessed, and, if sinus entry is anticipated, the patient is advised that an abdominal fat graft harvest would be necessary to augment the surgical closure.
The side of the approach is determined by the anatomy of the tumor and its relation with the optic apparatus. A “blind spot” of the supraorbital approach is the region inferior to the ipsilateral optic nerve (Fig. 18.4). Hence, the side with the least volume of tumor beneath the ipsilateral optic nerve is chosen for the approach. If this is not a consideration, then the side with the smaller frontal sinus is chosen. Typical medical and cardiac assessments are conducted to ensure that the patient is optimized for general anesthesia.
The working area of the supraorbital craniotomy is denoted with the blue shade. Relative “blind spots” are denoted with orange shades and include the anterior medial middle fossa, olfactory grooves, sellar fossa, and inferior aspect of the ipsilateral optic nerve (Courtesy of D.F. Kelly Neurological, Inc.)
Adjuncts used in the operating suite are planned prospectively. These include neuronavigation, neuromonitoring, micro-Doppler probe, and surgical endoscopes. Neuromonitoring typically includes somatosensory evoked potentials (SSEPs) to detect early vascular ischemia. In cases with cavernous sinus or brainstem involvement, monitoring of cranial nerves III and VI is also performed with direct intraoperative nerve stimulation and EMG. The micro-Doppler probe is particularly helpful in identifying and dissecting the involved branches of the carotid artery. The endoscopes are used as adjuncts to the microscope and are helpful for identification and resection of tumor that is located in the blind spots of this approach.
Ample dialogue and interaction with the anesthesia team is necessary for quality surgical outcomes. Given the limited superficial exposure afforded by the eyebrow supraorbital approach, brain relaxation is of paramount importance. The patient is positioned in the typical position of a pterional craniotomy, with the malar eminence at the highest position and the patient’s back angled to 20–30°. The head is rotated slightly (10–15°) for parasellar lesions. A modest dose of mannitol is given (25 g for a typical adult), and the patient is mildly hyperventilated with a goal arterial pCO2 of 30 mmHg. High-dose dexamethasone is often administered – even in the absence of hypopituitarism – which helps to mitigate cerebral edema. Lumbar drainage/CSF diversion is not routinely utilized. If neuromonitoring is employed with cranial nerve stimulation, then muscle relaxants are avoided, and total intravenous anesthesia (TIVA) is employed. TIVA also allows for decreased postoperative nausea/vomiting and a potentially shorter postoperative hospital course. The abdomen is prepped in all patients, even if sinus entry appears unlikely on imaging.
The incision is planned through the center of the eyebrow, starting just medial to the superior orbital notch (or foramen) and extending along the orbital rim about 1 cm inferior to the superior orbital line. If the eyebrow is thin or absent laterally, the incision is continued along the orbital rim. The eyebrow is never shaved, as this results in significant cosmetic deficit with inadequate and delayed hair growth. When the incision is made, the angle of the scalpel blade is situated parallel to the direction of the hair follicles, aiming to minimize transection of the eyebrow hairs, which helps afford improved cosmesis.
The orbicularis oculi is incised sharply and the pericranium is exposed. Then, the supraorbital nerve is carefully dissected at the region of the supraorbital notch. This nerve is typically deep to the orbicularis oculi and often has small local branches that arise from the main nerve (Fig. 18.5a). Efforts are made to preserve each branch, though some branches are very low along the orbital rim and are sacrificed with little long-term clinical significance. A subgaleal pocket is then created to the extent of the planned craniotomy. The pericranium and the temporalis muscle fascia are then incised down to the frontal bone as a single layer and extended to the origin of the zygoma. This layer is elevated and secured with a stay suture. The dissection should avoid entry into the orbit, as this could result in postoperative periorbital ecchymosis. If the orbital rim is to be removed, then the dissection should be carried further, and the periorbital ecchymosis should be reflected off the orbital roof. Fishhooks are used to retract the skin and temporalis muscle superiorly. These are periodically readjusted throughout the operation to prevent laceration or necrosis of the skin edges.
(a–d) Intraoperative photographs of supraorbital eyebrow craniotomy. (a) Pericranial dissection is complete with preservation of the supraorbital nerve (arrowheads). (b) Craniotomy is completed with burr hole beneath superior temporal line and orbital roof flattened with drill. (c) Bone flap with titanium plate locations. (d) Positioning of bone flap with superior and medial edges flush with the skull. The gap is left beneath the eyebrow to preserve cosmesis
The craniotomy itself has three components: a single burr hole, turning a flap, and drilling the inner table. The burr hole is made with a small burr drill bit (e.g., “matchstick”) beneath the superior temporal line. This location allows for placement of a burr hole cover without major cosmetic deficits. The dura is carefully dissected, and the orbital roof is palpated. The craniotomy is performed with a craniotome while protecting the supraorbital nerve (the medial limit). The goal is to remain flush with the orbital roof; hence, this cut is made first, and the drill follows the orbital roof. It is key to ensure a symmetric, ovoid craniotomy, avoiding narrowing at either side. This will prevent a “pinching” effect of the keyhole craniotomy, limiting the use of two-tined instruments such as microforceps or bipolars. Minimal dimensions are 1.5 cm (anterior/posterior) and 2.5 cm (lateral). The inner table is then drilled with the “matchstick” drill bit, and the orbital roof is smoothed (Fig. 18.5b). If there is entry into the frontal sinuses, it is either sealed with bone wax (for small or pinpoint breaches) or betadine-soaked sponge for larger defects, to be more definitively addressed during the closure. Though we are not advocates of removing the orbital rim, some authors do this for certain pathologies [16]. There is, as expected, a larger working area and increased degrees of freedom [17]. If the tumor has significant superior extension, requiring a very superior working angle, this may be of some help, though we find the use of angled endoscopes to work just as well.
The dural opening is performed with the intent to achieve ultimately a watertight closure. The initial step is to expose either the optico-carotid or carotid-oculomotor cistern for CSF egress. For craniopharyngioma, this is quite feasible and obviates the need for lumbar drain CSF diversion. It is rare for craniopharyngioma to extend into the optic canal, but if this is noted intraoperatively, the optic canal roof is drilled, the falciform ligament is divided, and the tumor is removed from this location.
The ipsilateral Sylvian fissure is split, and the anterior cerebral artery is dissected, identifying the anterior communicating artery, the contralateral A1 and both A2 arteries. The tumor is dissected from the dorsal optic nerve and chiasm. Tumor extending into the third ventricle via the lamina terminalis is carefully resected while trying to avoid injuring the hypothalamus. Tumor is dissected off the anterior cerebral artery complex, preserving the perforator arterial branches. Most of the unnamed perforators in this region supply the hypothalamus, and a stroke in this region can result in debilitating hypothalamic dysfunction such as hypothalamic obesity, adipsic diabetes insipidus, and cognitive dysfunction.
If there is tumor extending below the plane of the optic apparatus (a relative contraindication to this approach), the interoptic cistern is dissected under direct visualization utilizing angled endoscopes and instruments. The endoscope of choice is the 30°, 4 mm rigid endoscope with HD camera. This offers a low-profile system while preserving high resolution and magnification. We prefer using both the standard and reverse light-post endoscopes to minimize instrument collision while visualizing regions such as the sellar fossa, perimesencephalic region, and infraoptic region. The endoscope is held by an experienced surgeon and brought into the field with the instruments to minimize inadvertent injury with blind movements. Static or pneumatic endoscope holders are not often utilized.
It is imperative that the superior hypophyseal arteries are visualized and preserved, as these are typically involved by the capsule of craniopharyngiomas. For tumors that are mostly cystic, the cyst is drained, and the capsule is resected where feasible. Often, the cyst wall is difficult to dissect off the brain pia mater and must be left as residual, to be treated with adjuvant therapies.
After hemostasis is achieved and the entire cavity is inspected for all resectable tumor, the vasculature is treated with papaverine if there is concern of the potential for vasospasm. Subsequently, the dura is closed primarily in a watertight fashion if possible. This is most relevant if the lamina terminalis was entered for tumor resection, resulting in a high-flow CSF communication.
If there was a significant breach of the frontal sinus, the mucosa is stripped back, and the cavity is obliterated with a fat graft and collagen sponge. The bone flap is plated with a low-profile titanium burr hole cover (lateral) and a short two-hole bar (medial) (Fig. 18.5c). The gap of the bony defect is situated inferiorly – beneath the eyebrow (Fig. 18.5d). In patients with thin eyebrows, bone cement is used to augment the cosmesis of the bone flap. The pericranium is then reapproximated, and the orbicularis/dermal layer is closed, followed by a subcuticular stitch for the skin (typically 4–0 or 5–0 unbraided dissolvable suture). During the closure, pressure is held on the skin to prevent a hematoma from accumulating. The wound is then covered with a nonstick gauze and gently wrapped with an elastic headwrap. This should be tight enough to prevent a hematoma but loose enough to avoid scalp necrosis or pressure urticaria.
Postoperative Care
Much of the success of any skull base surgery relies on appropriate and meticulous postoperative management. Similar to most skull base craniotomies, the patient is admitted to the intensive care unit for close neurovascular monitoring. Tight blood pressure parameters are applied, keeping the patient normotensive and avoiding blood pressure spikes. The patient’s vision, cranial nerve, and cognitive exam are routinely assessed and compared to the preoperative and immediate postoperative baselines. The head-of-bed is maintained at about 30°.
A postoperative CT head without contrast is performed immediately after the patient is discharged from the recovery unit. This assesses the tumor cavity for any potential hemorrhagic complication. An assessment of the degree of pneumocephalus is performed. If there is excessive pneumocephalus, the patient is treated with 100% oxygen for 24 h to help decrease postoperative hypotensive headaches. This CT can be compared with future scans, and if the pneumocephalus has not resolved or has worsened, this could reflect a possible cerebrospinal fluid leak.
A postoperative “pituitary protocol” MRI is performed on either the first or second postoperative day. The primary purpose is to assess for the extent of tumor resection. In the unlikely situation of a large residual tumor, the patient may require an early reoperation. Certain cystic craniopharyngioma can develop early cyst recurrence. Hence, the immediate postoperative MRI is helpful to compare to interval postoperative scans at 1–2 months which can also help for stereotactic radiotherapy planning.
A deviation from our standard cranial postoperative protocol is the endocrine assessment and treatment, which mirrors our endonasal postoperative protocol. As stated, these patients typically are prescribed dexamethasone or stress-dose hydrocortisone perioperatively. These medications are tapered down, and if the pituitary gland could be preserved, they may be discontinued to assess the adrenal axis with adequate morning serum cortisol levels. As many of these patients are at high risk for diabetes insipidus (DI), close monitoring of serum sodium and urine output would prompt administration of desmopressin (ddAVP). These patients are comanaged with our pituitary endocrinologists. In follow-up, the remaining pituitary axes are assessed including the thyrotrope, gonadotrope, and somatotrope axes. If the pituitary stalk was sacrificed, the patient is typically discharged with low-dose levothyroxine replacement to be adjusted at the 6-week follow-up visit. Whether or not the patient has developed diabetes insipidus, a delayed postoperative serum sodium level is performed at 5–7 days after surgery. If the gland is preserved, this would help diagnose a delayed syndrome of inappropriate antidiuretic hormone (SIADH) or DI. If the patient was discharged on ddAVP, the serum sodium helps assess for proper dosage.
Long-Term Follow-Up
Following the initial postoperative visit, the patient is seen at about 2 months after surgery. At this time, a repeat “pituitary protocol” MRI is performed, and the patient’s neurological symptoms and signs are assessed. If the patient had vision loss preoperatively, a formal visual field test is performed, primarily to serve as a baseline for future examinations. Given the high incidence of tumor recurrence, even in the setting of gross total resection (GTR), fractionated stereotactic radiation therapy is administered either prophylactically or at the earliest sign of tumor recurrence/progression [18, 19]. There are sufficient data to suggest that patients have superior outcomes with combinatorial therapy compared to surgical resection alone [19, 20].
Long-term endocrinological treatment is of paramount importance. In the setting of pituitary stalk preservation, there is possibility of hormonal recovery, and periodic assessments of the adrenal and thyroid axes in particular may be helpful. In these patients, radiation therapy can also result in hypopituitarism over time [21, 22].
Complication Avoidance
Approach-specific complications have overlap with more extensive craniotomies such as the pterional and orbitozygomatic approaches. These include cosmetic/wound healing issues, CSF rhinorrhea prevention, supraorbital nerve injury, frontotemporal nerve injury, stroke/vascular injury, optic nerve injury, and hypothalamic injury.
The supraorbital approach has the potential for very visible cosmetic deficits. Hence, much effort is taken to prevent wound issues. This includes a precision craniotomy that is re-plated and approximated to the superior edge of the defect to position the gap beneath the eyebrow. Bone cement is used to fill in the gap if the patient has a thin or absent eyebrow. Meticulous hemostasis helps prevent a postoperative hematoma that typically can result in periorbital ecchymosis. During the operation, the “fishhooks” are frequently repositioned to prevent laceration of the skin edges.
CSF rhinorrhea can occur if the frontal sinus is breached. A watertight closure is always attempted but sometimes not possible. Hence, abdominal fat grafting is helpful to seal this structure. A multilayer collagen sponge reinforcement is helpful as well.
The supraorbital nerve dissection is necessary to minimize the incidence of long-term postoperative supraorbital anesthesia. Once the nerve course is traced, the main branches are preserved. The “fishhooks” are positioned to avoid direct traction along this nerve. During closure, the medial plate is often positioned beneath this nerve. This requires gentle retraction during screw placement to prevent damage to the nerve. Similar efforts are made during pericranial flap closure. Overall, transient supraorbital hypesthesia occurs for about 1–2 months in many patients, but is permanent in about 3.4–7.5% [23, 24].
The frontotemporal branch of the facial nerve is not visualized during the operation. However, the majority of patients will develop an immediate postoperative frontalis paresis. Many of these will resolve within 3 months following surgery. Permanent paresis occurs in about 2% of patients [23]. The nerve has variable trajectories along the orbital rim, and its location correlates with postoperative paresis [25]. Frequent repositioning of the “fishhook” retractors during the operation will minimize the tension on this nerve and may prevent permanent injury.
A concern with a smaller exposure compared to pterional or orbitozygomatic approaches is vascular control and the management of vascular injury. A key adjunct to performing safe tumor resection is the micro-Doppler probe [26, 27]. Particularly in the setting of recurrent and/or radiated tumors, the carotid artery and its branches can be difficult to identify or dissect off the tumor. Hence, frequent use of Doppler ultrasound is helpful to avoid vascular injury.
Vessels that are most vulnerable to inadvertent injury are the superior hypophyseal arteries. These vessels are not only hidden by the optic chiasm but often adherent to the capsule of the craniopharyngioma. Other branches that can be involved include the anterior choroidal, posterior communicating, and recurrent Heubner arteries. Hence, blind dissection or excessive traction of the craniopharyngioma should be avoided.
Preserving the optic apparatus and vision function is dependent on vascular preservation (superior hypophyseal and anterior choroidal arteries) as well as careful optic sheath dissection. Often, recurrent/radiated tumors can be adherent to the optic chiasm. Hence, a small residual tumor is allowed to prevent optic nerve injury. Over the past few decades, the trend in craniopharyngioma surgery has shifted from attempting gross total resection to achieving maximal safe resection. A large series of craniopharyngioma patients cared for in the 1980s demonstrates a 90% gross total resection rate, but with 15% worsened vision and 17% mortality [28]. This is in stark contrast to a contemporary single-center, high-volume study that reported 38% gross total resection and 34% near-total resection with no new vision loss or increased mortality [12]. In the era of stereotactic radiation (IMRT) and targeted molecular therapy, permissive tumor residual is acceptable, even in the setting of recurrent tumor.
The hypothalamus borders the floor and the inferior half of the third ventricle lateral walls. Within this thin structure exists the numerous hypothalamic nuclei regulating pituitary hormone release as well as homeostatic functions. Hypothalamic injury can present with fatigue, memory loss, behavioral changes, adipsic diabetes insipidus, and obesity. These can be quite challenging to treat and very debilitating to the patient. Hence, measures to prevent hypothalamic injury are important for maintaining quality of life. Tumor that is densely adherent to the hypothalamus is debulked with deliberate residual left on the ependymal surface [29].
Surgical Outcomes
The supraorbital eyebrow craniotomy approach has been successfully used for a variety of pathologies. These include benign and malignant brain tumors as well as intracranial aneurysms, cavernous malformations, and other nonneoplastic lesions [24, 30,31,32,33]. Parasellar lesions are ideal for this approach, given its anatomic exposure previously described. Numerous surgical series have demonstrated the versatility of this approach with comparable outcomes to traditional frontal approaches such as the pterional, orbitofrontal, and orbitozygomatic craniotomies.
There are limited published series utilizing the supraorbital craniotomy for craniopharyngioma, primarily due to the infrequence of anterochiasmal tumors. Reisch et al. reported 39 of 1125 (3.5%) supraorbital “eyebrow” craniotomies over a 10-year period were for craniopharyngiomas [24]. Seventy-four percent of these patients achieved gross total resection, though only 36% were recurrent tumors. Fatemi et al. reported a more contemporary series comparing endonasal and supraorbital approaches for craniopharyngiomas [34]. Only four patients of 22 underwent the supraorbital approach, and 50% were recurrent tumors compared to 33% of the endonasal cohort. Conversely, only 50% gross or near-total resection was accomplished, compared to 67% with the endonasal approach [34]. In a follow-up article, McLaughlin et al. reported four supraorbital operations for recurrent tumors in patients that had previously been treated via craniotomy [9]. Overall outcomes were good, though one patient did experience a CSF leak, which required reoperation.
Pearls |
|---|
The supraorbital eyebrow craniotomy is ideal for the craniopharyngioma that is anterior or superior to the optic apparatus. |
Meticulous attention to detail during exposure, nerve dissection, and closure is necessary to achieve optimal cosmesis. |
Preservation of vascular and hypothalamic anatomy is essential to prevent postoperative morbidity. |
Permissive near-total resection is acceptable with adjunct stereotactic radiation therapy and targeted molecular therapy. |
18.4 Suprasellar Craniopharyngiomas: Endoscopic Endonasal Approach
Introduction
The endoscopic endonasal approach (EEA) has evolved significantly in the last decade. With more accurate neuronavigation, improved endoscope optics, and the evolution of skull base reconstruction materials and techniques, endoscopic endonasal surgery is safer and more effective and has become the preferred approach for a variety of skull base lesions. Traditional transcranial approaches to craniopharyngiomas often require some degree of brain retraction (and potential cerebral edema) and lack complete direct visualization of critical structures in the retrochiasmatic region. Traditional speculum-based microsurgical transsphenoidal approaches provide a direct endonasal route but with limited field of view and surgical freedom. On the other hand, the extended EEA via the transplanum transtuberculum corridor provides direct midline exposure to intrasellar/subdiaphragmatic, supradiaphragmatic, and retrochiasmatic craniopharyngiomas that extend up to the third ventricle without any brain retraction [35]. The extended EEA offers unmatched visualization of the undersurface of optic nerves and chiasm, pituitary stalk, third ventricle, perforators, and hypothalamus. Craniopharyngiomas that are retrochiasmatic in location should be strongly considered for resection via the EEA.
Patient Selection
When choosing a surgical approach for craniopharyngiomas, the optimal choice should be the shortest and most direct route that will provide maximal exposure and visualization of the tumor’s interface with surrounding critical structures. The anatomic location of the tumor and its degree of extension are of paramount importance, particularly its relation to the optic chiasm, the pituitary gland and stalk, the hypothalamus, the carotid artery, the anterior cerebral artery complex, as well as the sella and diaphragm. Retrochiasmatic lesions are particularly well suited for an extended EEA via the transplanum transtuberculum corridor rather than a transcranial route in order to avoid manipulation of the optic nerves and chiasm. It is important to note that tumor extension into the third ventricle can be removed via an EEA, as long as there is communication with suprasellar space [36]. However, pure intraventricular craniopharyngiomas situated within the third or lateral ventricles may be better accessed with a transcranial transventricular approach via a transcortical or transcallosal route [35]. A combined approach of both open and endoscopic techniques may be necessary for extensive lesions that involve multiple anatomic compartments. In cases of pure intrasellar craniopharyngiomas, a transsellar approach is favorable. However, the majority of suprasellar supradiaphragmatic craniopharyngiomas require an extended EEA via the transplanum transtuberculum corridor to gain optimal exposure. Tumors with significant lateral extension (>1 cm lateral to the carotids) may not be amenable to EEA, as are tumors with significant superior extension into the interhemispheric fissure [36]. Another limitation of the EEA is the inability to perform direct vascular repair or bypass in the case of arterial vessel injury.
The age of the patient and medical history must be taken into account when choosing surgical approach. For example, in an older patient with many medical comorbidities, a more conservative approach with a goal mainly to decompress neural structures may be most appropriate. In addition, surgeon’s preference, experience, and skill level are also important considerations. The EEA is associated with a significant learning curve, and the surgeon’s comfort level performing this approach should be considered. It is also crucial to have a collaborative experience with an otolaryngologist specializing in rhinology and endoscopic skull base surgery, which provides a multidisciplinary team approach to the patient. Some factors that make EEA less favorable include a hypoplastic sphenoid sinus, significant lateral extension of the tumor into the Sylvian fissure, significant superior extension into the interhemispheric fissure, a narrow intercarotid artery distance, and a narrow infrachiasmatic window [36].
Preoperative Evaluation
Preoperatively, in addition to conducting a thorough neurologic exam, it is crucial to obtain neuro-ophthalmologic evaluation to assess visual fields and acuity. It is also routine to have a neuroendocrine evaluation, which includes measurement of pituitary hormone levels and a body mass index measurement, as hypothalamic involvement can affect appetite and weight. Evaluation with an otolaryngologist should be obtained for surgical planning. Recent neuroimaging studies are imperative as well. CT scan shows the bony anatomy of the nasal sinuses and skull base that will be encountered during the approach and reveals calcifications and cystic components of the tumor [36]. MRI demonstrates tumor extension and can also differentiate solid and cystic components, position of the chiasm, and relationship of the tumor to neighboring vascular structures, the pituitary stalk, and the third ventricle [36].
Surgical Technique: Endoscopic Endonasal Transplanum
Transtuberculum Approach
Patient Positioning
The patient is placed under general anesthesia with the endotracheal tube secured to the left side of the patient. We generally place a lumbar drain at the time of surgery to minimize the risk of postoperative CSF leakage. The patient is positioned supine on the operating table with the head in a three-point Mayfield head holder. The bed is arranged to keep the head slightly elevated above the heart to promote venous return. The head is slightly rotated to the right to facilitate easier access for the operating surgeons standing on the right side of the patient. The head is also slightly extended to improve access to anterior skull base. Frameless stereotactic image guidance is used for intraoperative navigation and for anatomic localization. It also helps guide the extent of anterior bone removal from the planum sphenoidale based on the sagittal trajectory to the lesion [37]. The nose and nostrils are prepared with Betadine, and the nasal cavity is packed with Afrin-soaked pledgets. The abdomen and thigh are also prepared for harvest of autologous fat and/or fascia lata for dural repair and reconstruction. Intravenous antibiotics and 10 mg of dexamethasone are administered prior to incision. Mannitol and antiepileptics are usually not used because there is no brain retraction or manipulation during the EEA.
Endonasal Sphenoid Sinus Exposure
In our center, we use a two-surgeon, three- to four-hand binostril technique with a neurosurgeon and otolaryngologist. The initial endonasal exposure to the sphenoid sinus is performed primarily by the otolaryngologist using a high-definition 30°-angled endoscope (Karl Storz, Tuttlingen, Germany). We prefer the 30°-angled endoscope because of the viewing capabilities around corners with simple rotation of the scope. The tail and anterosuperior attachment of the middle turbinates, as well as the nasal septum, are infiltrated with 1% lidocaine with epinephrine (1:100,000 dilution). Both middle and inferior turbinates are mobilized laterally. In some cases, the right middle turbinate can be removed to allow for more room for multiple instruments in the right nostril, if needed. The sphenoid ostium is identified bilaterally about 1–1.5 cm superior to the choanal arch and medial to the superior turbinate. A wide sphenoidotomy and posterior ethmoidectomy are performed with a microdebrider and Kerrison rongeurs. The same maneuvers are performed in the left nostril with an additional posterior septectomy of about 1.5–2 cm in order to create a unified working corridor to the anterior skull base. The posterior septectomy allows triangulation of surgical instruments through both nostrils so that bimanual dissection can be performed. It is important to recognize the presence of an Onodi cell (posterior ethmoid cell that is positioned superolateral to the sphenoid sinus), because the optic nerve and carotid artery may often course through the lateral aspect of that cell.
At this point, a vascularized, pedicled, nasoseptal flap is harvested from the nasal septum and rotated posteroinferiorly into the nasopharynx until later use at the time of reconstruction. Care must be taken to protect the vascular pedicle arising from the posterior septal branch of the sphenopalatine artery from inadvertent injury. At this juncture, the neurosurgeon and otolaryngologist work simultaneously using a binostril technique. The otolaryngologist provides guidance and optimal visualization with the 30° endoscope in the right nostril in the 6 o’clock position looking superiorly. The neurosurgeon uses bimanual surgical technique, with a suction device placed in the 12 o’clock position in the right nostril and the working instrument (drill, dissector, scissors, bipolar device, or tissue aspirator) in the left nostril.
Transplanum Transtuberculum Bony Opening
During the bone drilling, we prefer to use a double-barrel suction-irrigator in the right nostril. The self-irrigating system keeps the surgical field clear of bone dust and also cools the drill tip to protect underlying structures from heat injury. Irrigation is also provided from the self-irrigating high-speed drill and the irrigating endoscope sheath. The sphenoidotomy opening is maximally widened, removing all sphenoid septations and bony ridges that may hinder instrument maneuverability and surgical freedom. It is important to ensure that the line of sight to the transplanum transtuberculum region is unobstructed. A high-speed diamond drill with copious irrigation is used to remove bone over the sella turcica, planum sphenoidale, and tuberculum sellae. It is also important to identify the medial and lateral opticocarotid recesses on both sides. The medial opticocarotid recess is an indentation in bone that is formed at the medial junction of the parasellar carotid canal and the optic canal. This recess represents the lateral aspects of the tuberculum sellae as viewed from the endonasal perspective [10]. The lateral opticocarotid recess represents the optic strut from the endonasal perspective. Once the tuberculum strut and both medial opticocarotid recesses are thinned down to eggshell thickness, an up-angled 5-0 curette is used to remove the remaining remnant of tuberculum strut and medial opticocarotid recesses. By removing the medial opticocarotid recesses, the medial aspect of the optic canals are unroofed, which facilitates exposure of the optic nerves and paraclinoid carotid arteries in the opticocarotid cistern [10]. It is important to avoid using a Kerrison rongeur in the region of the optic canal since this can cause potential injury to the optic nerve.
Next, we prefer to open the dura in a transdiaphragmatic fashion, at the level of the planum and sella. An arachnoid knife or number 11 blade is used to make a cruciate incision over the sellar dura, and a second horizontal incision is made in the dura of the planum sphenoidale above the intercavernous sinus. The superior intercavernous sinus is coagulated with an endoscopic bipolar and divided sharply with scissors. This incision is continued along the diaphragma sella to expose the suprasellar cistern.
We typically use a 30° endoscope which gives the surgeon additional angled views. The endoscope is placed at the 6 o’clock position, with the suction at the 12 o’clock position in the right nostril when using the 30° endoscope to look up into suprasellar cistern or retrochiasmatic space. The neurosurgeon is therefore working “above” the endoscope while maintaining optimal surgical exposure. When using a 0° endoscope, we prefer to do the opposite and place the endoscope at the 12 o’clock and the suction at the 6 o’clock position.
Intradural Tumor Dissection and Removal
The arachnoid is dissected to expose the underlying tumor in the retrochiasmatic space. We recommend using an extra-arachnoidal dissection technique, in other words, dissecting in the plane between tumor capsule and the tumor arachnoid, instead of between tumor arachnoid and cisternal arachnoid. This allows mobilization of the arachnoid layers toward the critical neurovascular structures to provide a buffer of protection. Both carotid arteries are visualized underneath the optic nerves as they exit the distal dural ring. It is important to identify the superior hypophyseal arteries that arise from the carotid arteries and to preserve branches that supply the undersurface of the optic apparatus to avoid postoperative vision deficits. There is typically an arachnoid layer investing these perforators, and by working between the tumor capsule and the arachnoid layer, the perforators can be safely mobilized and preserved during tumor removal.
Initial decompression of the cystic contents is performed to allow collapse of the tumor capsule. This facilitates descent of the superior extent of the tumor and allows for subsequent extracapsular microdissection. Dissection of the tumor capsule from the undersurface of the optic chiasm and hypothalamus is achieved using conventional bimanual microsurgical techniques. The cyst wall is placed under gentle countertraction, while the suction is used as a dissector to sweep the capsule off of the neural structures. In some cases, arachnoid adhesions are lysed with sharp dissection using microscissors. By using bimanual microsurgical dissection techniques, the tumor capsule is identified in relation to the optic chiasm, optic nerves, and pituitary stalk. In retrochiasmatic craniopharyngiomas, the tumor is located underneath and posterior to the optic chiasm. It can be adherent to the undersurface of the optic apparatus and hypothalamus, with extension superiorly into the third ventricle and posteriorly into the interpeduncular fossa and retrosellar space. The anterior communicating artery complex is located superior to the optic chiasm and is therefore protected from the plane of dissection.
For solid craniopharyngiomas, the tumor is internally debulked with a side-cutting tumor aspirator device (NICO Myriad®, Indianapolis, IN, USA) or ultrasonic aspirator. Initial debulking of the solid component and/or aspiration of cystic fluid allows for decompression of the tumor capsule. Once the tumor is adequately debulked and decompressed, extracapsular dissection of the tumor capsule away from optic chiasm and hypothalamus is performed with careful bimanual microdissection. Care is taken not to prematurely amputate the tumor capsule, as it provides a surgical “handle” to provide countertraction for extracapsular dissection. After meticulous microdissection, the most superior extent of the tumor should descend into the retrochiasmatic space.
In most cases, the membrane of Liliequist is intact and can act as a plane of dissection to peel tumor safely from the basilar artery, posterior cerebral arteries, and P1 perforating vessels. To visualize this region, the 30° endoscope is pointed inferiorly and placed in the 12 o’clock position in the right nostril. The inferior aspect of the tumor is elevated from the top of the pituitary gland to identify the base of the pituitary stalk. We attempt to preserve the pituitary stalk, especially if the tumor can be readily dissected off of the stalk. However, if a gross total resection is possible and there is tumor invading or expanding the stalk (type II transinfundibular), we prefer to do a low stalk transection just above the pituitary gland to facilitate gross total removal and place the patient on postoperative hormone replacement therapy. We agree with the opinion of Dr. Oldfield that this strategy may be better to prevent tumor recurrence rather than leaving tumor on an anatomically intact stalk that may not retain normal pituitary function [38].
The tumor is generally most adherent at the level of the hypothalamus where meticulous and careful microdissection is performed. Once the tumor is free from all areas of adherence, the tumor is carefully delivered through the nose. Premature pulling of the tumor without complete dissection can potentially result in catastrophic nerve or vessel injury. If the floor of the third ventricle is open, a 30° and 70° endoscope can be used to look inside the walls to inspect for residual tumor.
Gross total resection (GTR) should be the goal of craniopharyngioma surgery if safely possible. However, in some cases, GTR may not be feasible due to the intimate nature of some of these lesions to critical neurovascular structures. Therefore, it may be necessary to leave some residual tumor that is densely adherent to important nerves or vessels yet maximize the extent of safe resection. In the event of subtotal or near-total resection, radiation therapy is an appropriate adjuvant treatment and has been shown to decrease recurrence rates [39].
Closure and Skull Base Reconstruction
Closure and reconstruction are of utmost importance to prevent a postoperative CSF leak. Although various techniques exist, we prefer a multilayered closure with autologous fascia lata and a vascularized pedicled nasoseptal flap for transplanum defects. An initial piece of Gelfoam is placed underneath the dural opening as an inlay to slow the pulsations of CSF out the dural defect. Next, an autologous fascia lata graft is placed over the dural opening and held in place with a monolayer of Surgicel® (Ethicon, Somerville, NJ, USA). This step is repeated with a second layer of fascia lata to reinforce the initial layer. Another monolayer of Surgicel is placed over the bone defect to hold the fascia graft in place. Finally, the vascularized pedicled nasoseptal flap is then rotated superiorly to cover the dural closure and bony skull base defect. Care is taken to ensure that the edges of the nasoseptal flap are in contact with the bone. The bony surface must be devoid of any sinus mucosa as this will increase risk of flap dehiscence and possibly delayed formation of mucoceles. Cottonoids are used to apply gentle pressure on the flap to ensure a good seal against the skull base without any trapped air pockets. After removing the cottonoids, another monolayer of Surgicel is placed over the edges of the flap against the surrounding bone to prevent flap migration. The flap is then bolstered with several layers of gentamicin-soaked Gelfoam pledgets followed by a Merocel® (Medtronic, Dublin, Ireland) expandable nasal tampon, positioned in the sphenoid sinus posterior to the nasal septum. The packing expands as it is hydrated with gentamicin irrigation. The lumbar drain is opened temporarily during extubation to allow preferential drainage of CSF through the lumbar catheter rather than through the repaired defect and to prevent increases in intracranial pressure [35].
Potential Complications
One of the most feared complications is direct injury to a major vascular structure. This can be devastating and requires prompt hemostasis via coagulation or clip ligation. A carotid injury may require packing with crushed autologous muscle followed by nasal packing to tamponade the bleeding. An emergent angiogram is then performed to rule out a pseudoaneurysm and to perform potential intervention. Postoperative vision worsening secondary to manipulation of the optic nerves and/or chiasm or vascular compromise is also possible. Anterior pituitary insufficiency and diabetes insipidus may also occur due to pituitary stalk manipulation or intentional stalk transection. The most frequent postoperative complications are discussed below.
Postoperative Care
Care is taken to monitor for signs of intracranial hypotension and CSF rhinorrhea postoperatively. The lumbar drain is kept open at 5–10 ml of drainage per hour for about 72 h after surgery. If a persistent CSF leak is suspected, reexploration and revision of the skull base repair in the operating room may be necessary. An MRI of the pituitary region with and without contrast is performed routinely on postoperative day 1 or 2 to evaluate extent of resection. These patients are also closely monitored for development of diabetes insipidus, which is managed with vasopressin. If the pituitary stalk is intentionally sacrificed, the patient is immediately placed on hormone replacement therapy with hydrocortisone, levothyroxine, and vasopressin. Patients are also preemptively kept on high-dose dexamethasone (10 mg every 6 h) with elevated systolic blood pressures to minimize optic nerve swelling and to optimize chiasmal perfusion, respectively. This is weaned off as tolerated over the course of 7 days. Formal visual field and acuity are assessed by the neuro-ophthalmology team. It is recommended to avoid nasal positive pressure ventilation out of concern for development of pneumocephalus. Patients are maintained on broad-spectrum antibiotics until the Merocel packing is removed by the otolaryngologist in the office on postoperative day 10–12.
Surgical Outcomes
Tumor Resection
It has been reported that the EEA achieves greater rates of gross total resection (GTR) than open approaches. In a recent meta-analysis by Komotar et al., EEA had greater rates of GTR (66.9% vs. 48.3%) and decreased rates of tumor recurrence than transcranial approaches [40]. There was also improved postoperative visual outcomes (57%) but a higher rate of postoperative CSF leak (18.6%) with EEA compared to open approaches [40]. However, reported rates of postoperative CSF leak have declined significantly in recent years with the development of more sophisticated reconstructive techniques, particularly when using the nasoseptal flap. With our technique described above using a multilayered reconstruction technique, our CSF leak rate has been 3.2% [41]. Other groups have reported postoperative CSF leak rates after EEA under 5% as well when using the nasoseptal flap [42].
Visual and Endocrinological Outcomes
Improved visual outcomes after EEA compared to open approaches are routinely reported, likely due to less manipulation of the optic apparatus [12, 40]. Komotar et al. reported improved or stable visual outcomes in 66% of EEA patients [40]. Recent reports of permanent diabetes insipidus after EEA range from 27% to 48% and of panhypopituitarism ranging from 38% to 47% [12, 40, 42, 43].
Pearls |
|---|
The extended EEA via the transplanum transtuberculum corridor provides direct midline exposure for retrochiasmatic craniopharyngiomas with excellent visualization of the infrachiasmatic and hypothalamic region. |
The EEA is limited for craniopharyngiomas that extend laterally into the Sylvian fissure or superiorly into the interhemispheric fissure. |
Multilayered dural defect closure with a nasoseptal flap is essential to minimizing risk of CSF leak. |
Postoperative complications include diabetes insipidus, CSF leak, vision deficits, panhypopituitarism, and vascular injury. |
Case Illustration
This 56-year-old female presented with progressive headaches, confusion, memory loss, and bitemporal visual field loss. MRI demonstrated a solid, retrochiasmatic craniopharyngioma compressing the optic chiasm with an associated giant right frontal cyst causing significant mass effect (Fig. 18.6a–f). Various surgical approaches were considered including a transbasal subfrontal approach, right orbitozygomatic approach, EEA, and a combined microscopic/endoscopic endonasal approach. After careful deliberation, it was felt that the optic chiasm would be best decompressed with an EEA with decompression of the frontal cyst. If the cyst wall could not be completely removed endonasally, a second-stage transcranial procedure was anticipated if the cyst recurred.
(a–c) Preoperative MRI of solid retrochiasmatic craniopharyngioma with associated giant right frontal lobe cyst. Removal of the solid retrochiasmatic component was performed with wide fenestration of frontal lobe cyst into suprasellar cistern. (d–f) Postoperative MRI at 3 months follow-up shows regression and collapse of frontal lobe cyst. Optic chiasm is well decompressed, and the patient had normal pituitary function with stalk preservation (a–f: Copyright retained by Dr. James K. Liu. Used with permission)
At surgery, the solid, retrochiasmatic component of the tumor was readily dissected off of the optic chiasm and hypothalamus (Fig. 18.7a–f). The pituitary stalk was identified at the base of the tumor and was preserved anatomically. Remnants of microscopic calcifications were left adherent to the top of the optic chiasm and the anterior communicating artery complex. The right frontal lobe cyst was decompressed, but the cyst wall was very adherent to the frontal lobe and anterior communicating artery complex and could not be safely removed. The solid component of the tumor was completely removed with excellent decompression of the optic chiasm and preservation of the pituitary stalk. Here, a decision was made to preserve the stalk because it was anatomically intact, and a complete tumor resection was not achievable.
(a–f) Intraoperative photographs of EEA resection of solid retrochiasmatic craniopharyngioma with associated giant right frontal lobe cyst. (a, b) Extracapsular dissection of tumor (T) off of the left internal carotid artery (ICA), left posterior communicating artery (Pco), left optic nerve (ON), and optic chiasm (OC). (c) Right frontal cyst (FC) is widely fenestrated into suprasellar cistern. The tumor (T) is very adherent to the optic chiasm. (d) Elevation of the tumor from the top of the pituitary gland (PG) reveals the pituitary stalk (PS) which is able to be preserved. The basilar artery (BA) complex and left oculomotor nerve are visualized. (e, f) Final view after near-total resection. The optic chiasm is well decompressed, and there is adherent microscopic tumor to the both A1 vessels. The frontal lobe cyst (FC) was also very adherent to the brain and the A1 vessels (a–d: Copyright retained by Dr. James K. Liu. Used with permission)
Postoperatively, the patient had restoration of normal vision, preservation of normal pituitary function without requiring hormone replacement therapy, and no CSF leakage. MRI performed at 3 months follow-up showed no evidence of solid tumor recurrence and regression of the right frontal lobe cyst (Fig. 18.6a–f). It appeared that the frontal lobe cyst was well fenestrated into the suprasellar cistern. The patient was referred for radiation therapy to maintain tumor control. Further imaging follow-up is warranted to determine long-term tumor control. This case illustrates the limitations of complete tumor removal via an EEA when superiorly extending cyst walls are adherent to critical structures. However, we felt it was a reasonable first surgical option since it allowed complete removal of the solid component in the retrochiasmatic space with excellent visual and endocrine outcomes.
Commentary on Case Presentation in Section 18.1
Case Presentation
A 24-year-old lady presents with mild bilateral visual blurriness. Examination: OS, 20/20 (corrected); Humphrey VF, mild arcuate superior and inferior temporal defects; OD, 20/20 (corrected); Humphrey VF, mild arcuate superior and inferior temporal defects. The rest of neurological exam is normal. Laboratory workup: normal endocrine function (see Fig. 18.1).
Discussion
In the case provided by the editors, the patient has a midline suprasellar supradiaphragmatic craniopharyngioma that is primarily retrochiasmatic in location. The lesion is situated below the optic chiasm and does not have any third ventricular or lateral extension. It appears that the majority of the tumor is pre-infundibular and a smaller component is post-infundibular. Nevertheless, one must be prepared for a tumor that is invading or encasing the pituitary stalk. With that in mind, the patient is also a young female of childbearing age which factors into the goals as well as the limits of aggressive surgical resection. In this particular case, the authors would favor an endoscopic endonasal transplanum transtuberculum approach, which provides midline access to the tumor with excellent visualization of the retrochiasmatic region. The challenge here is primarily in preservation of the pituitary stalk and pituitary gland function due to the patient’s potential desire for having children. A gross total resection should be attempted if safely possible. However, if there is any adherence or invasion of tumor into the pituitary stalk, one should leave adherent remnants to the stalk if the patient wishes for normal gland function and the ability to have children. However, if the tumor is transinfundibular in nature, it may not be possible to preserve the stalk in order to remove the tumor, and stalk sectioning may be considered if gross total resection can be performed.
18.5 Suprasellar Craniopharyngiomas: Editors’ Commentary
Although histologically benign, craniopharyngiomas represent some of the more daunting intracranial tumors due to their propensity for dense adherence to critical neurovascular structures. Management of these tumors typically is aimed at surgical gross-total resection (GTR) in order to prevent recurrence. Attempts at GTR, however, can be accompanied by significant morbidity from endocrinopathies, hypothalamic dysfunction, or neurovascular injury. Therefore, more recently, the emphasis in the treatment of craniopharyngiomas has shifted from the need to obtain a GTR to a paradigm that focuses on maximal safe resection followed by radiotherapy as necessary for long-term tumor control.
Surgical treatment of craniopharyngiomas is aimed at pathologic diagnosis, relief of mass effect, restoration of any visual compromise, preservation of endocrinologic function, and prolonged oncologic control. This can be accomplished through multiple surgical approaches, which can be grouped into either transcranial or endonasal techniques. The transcranial approaches can be further subdivided into “minimally invasive” supraorbital craniotomies, traditional cranial base approaches, and interhemispheric transcallosal or transcortical transventricular approaches. Each of these has its advantages and disadvantages. The choice of surgical technique is dependent on a number of factors, including the tumor’s radiographic characteristics, the patient’s demographics and presenting symptoms, and the surgical team’s experience. Often, the primary factor in surgical approach selection is the anatomical relationship of the tumor with the optic chiasm.
Regarding the traditional open cranial base approaches, these present familiar anatomy to the neurosurgeon, and their use over many decades has resulted in ample experience with these techniques. They generally allow for better vascular control and management of any potential large vessel injury compared to endonasal or supraorbital trajectories. Also, with the exception of those approaches that add extensive skull base osteotomies, postoperative cerebrospinal fluid leaks are less common.
In general, anterolateral approaches permit access to any lateral tumor extension beyond the internal carotid arteries and into the sylvian fissures. Subfrontal transbasal craniotomies allow resection of more midline tumors, and opening the lamina terminalis permits removal of tumor located within the third ventricle. These anterior and anterolateral corridors are best indicated for prechiasmatic tumors with a secondarily post-fixed chiasm. Posterolateral approaches through subtemporal transtentorial and posterior transpetrosal craniotomies, however, provide a method for accessing the retro- and infra-chiasmatic space, but traversing significant neurovascular structures and cranial nerves along this surgical corridor may limit its use. These traditional “open” cranial base procedures have some additional disadvantages, namely: potential difficulty in visualizing the interface between the tumor and the hypothalamus with more rostral tumor expansion due to the craniocaudal surgical trajectory, potential release of caustic cyst tumor fluid into CSF spaces due to wider CSF arachnoidal openings, brain retraction that can be minimized by potentially cosmetically disfiguring skull base osteotomies, and the need to work around or mobilize critical neurovascular structures to access the tumor.
To circumvent some of these limitations, the endonasal corridor and supraorbital craniotomies have been utilized with increasing frequency. The eyebrow or eyelid approach can be more cosmetic than the incisions used for open craniotomies, while providing a very low subfrontal approach. With the addition of an orbital osteotomy or the use of angled endoscopes, the degree of brain retraction can be further minimized. Frontal sinus management can be a large concern with the supraorbital craniotomy, and any wound healing issues can be particularly disfiguring. The surgical freedom may be limited, and it is often difficult to adequately visualize the retrochiasmatic space. The endonasal endoscopic technique, conversely, does provide access posterior to the chiasm as well as into the third ventricle. As a direct route along the long axis of the tumor, the endonasal approach affords excellent retrochiasmatic visualization, optimal cosmesis, and perhaps the best method to identify vascular perforators to the chiasm and infundibulum. With no brain retraction or manipulation of the optic apparatus, excellent visual outcomes can be achieved, and a more clear distinction can often be made of the tumor-hypothalamic interface. Despite these advantages, there is a higher CSF leak rate, the potential for sino-nasal complications, limited access to extension of tumor lateral to the internal carotid artery, and possibly a greater risk of postoperative endocrinopathies.
The index case (Fig. 18.1) demonstrates a multiloculated, partially cystic craniopharyngioma with intratumoral calcifications that is largely located in the midline suprasellar cistern. Although the lesion is retrochiasmatic in location and the sella is minimally expanded, there is a fairly sizeable space between the pituitary gland and the optic chiasm for the approach. Additionally, the intervals between the neurovascular structures in the basal cisterns are likely expanded, allowing for tumor resection through the corresponding triangles, and there is minimal rostral extension. These factors make this particular lesion amenable to removal through both transcranial and endonasal approaches, as detailed in the preceding sections. There is not significant intraventricular extension, and therefore only the interhemispheric transcallosal and transventricular approaches would not be considered appropriate for this lesion.
There are some additional characteristics of this particular case to be considered in deciding the optimal surgical approach. The patient’s gender and age are critical to note as an emphasis should be placed not only on achieving a maximal resection but also in maintaining normal endocrinologic function in a female of child-bearing age. In such a patient, preservation of the pituitary gland architecture as well as the pituitary infundibulum should be attempted, if possible. Additionally, this patient has only minimal visual compromise on presentation. Therefore, it is especially critical that maximal efforts are made to not sacrifice any vascular perforators to the optic nerves and chiasm and to limit manipulation of these structures in order to ensure an optimal visual outcome.
This illustrative case is fairly representative of most de novo craniopharyngiomas in that it is located largely within the retrochiasmatic suprasellar cistern. As the long axis of the tumor is midline along the sino-nasal-sellar-hypothalamic corridor, we feel that it is best approached via an endoscopic endonasal transplanum transtuberculum approach. Although in experienced hands the anterior transcranial approaches could achieve excellent results, these may require manipulation of the optic chiasm, nerves, and tracts, thereby increasing the risks of vision loss and vascular injury. A multilayered closure of the dural and cranial base opening with a nasoseptal flap is essential to minimize the risk of a postoperative CSF leak.
References (Key References Bolded)
Hoffman HJ. Surgical management of craniopharyngioma. Pediatr Neurosurg. 1994;21(Suppl 1):44–9.
Bunin GR, et al. The descriptive epidemiology of craniopharyngioma. Neurosurg Focus. 1997;3(6):e1.
Pan J, et al. Growth patterns of craniopharyngiomas: clinical analysis of 226 patients. J Neurosurg Pediatr. 2016;17(4):418–33.
Alli S, Isik S, Rutka JT. Microsurgical removal of craniopharyngioma: endoscopic and transcranial techniques for complication avoidance. J Neuro-Oncol. 2016 Nov;130(2):299–307.
Komotar RJ, Roguski M, Bruce JN. Surgical management of craniopharyngiomas. J Neurooncol. 2009 May;92(3):283–96.
Zada G, Laws ER. Surgical management of craniopharyngiomas in the pediatric population. Horm Res Paediatr. 2010;74(1):62–6.
Jung TY, Jung S, Choi JE, et al. Adult craniopharyngiomas: surgical results with a special focus on endocrinological outcomes and recurrence according to pituitary stalk preservation. J Neurosurg. 2009 Sep;111(3):572–7.
Elliott RE, Jane JA, Jr., Wisoff JH. Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery. 2011 Sep;69(3):630–43. Discussion 43.
McLaughlin N, Ditzel Filho L, Shahlaie K, Solari D, Kassam A, Kelly D. The supraorbital approach for recurrent or residual suprasellar tumors. Minim Invasive Neurosurg. 2011;54(04):155–161.
Kassam A, Gardner P, Snyderman C, Carrau R, Mintz A, Prevedello D. Expanded endonasal approach, a fully endoscopic transnasal approach for the resection of midline suprasellar craniopharyngiomas: a new classification based on the infundibulum. J Neurosurg. 2008;108(4):715.
Barkhoudarian G, Cutler AR, Yost S, Lobo B, Eisenberg A, Kelly DF. Impact of selective pituitary gland incision or resection on hormonal function after adenoma or cyst resection. Pituitary. 2015;18(6):868–75.
Koutourousiou M, Gardner P, Fernandez-Miranda J, Tyler-Kabara E, Wang E, Snyderman C. Endoscopic endonasal surgery for craniopharyngiomas: surgical outcome in 64 patients. J Neurosurg. 2013;119(5):1194.
Dlouhy B, Madhavan K, Clinger J, et al. Elevated body mass index and risk of postoperative CSF leak following transsphenoidal surgery. J Neurosurg. 2012;116(6):1311.
Ivan M, Iorgulescu J, El-Sayed I, et al. Risk factors for postoperative cerebrospinal fluid leak and meningitis after expanded endoscopic endonasal surgery. J Clin Neurosci. 2015;22(1):48.
LeVay A, Kveton J. Relationship between obesity, obstructive sleep apnea, and spontaneous cerebrospinal fluid otorrhea. Laryngoscope. 2008;118(2):275.
Dare A, Landi M, Lopes D, Grand W. Eyebrow incision for combined orbital osteotomy and supraorbital minicraniotomy: application to aneurysms of the anterior circulation. J Neurosurg. 2001;95(4):714.
Figueiredo E, Deshmukh V, Nakaji P, et al. An anatomical evaluation of the mini-supraorbital approach and comparison with standard craniotomies. Neurosurgery. 2006;59(4 Suppl 2):ONS212.
Kobayashi T, Kida Y, Mori Y, Hasegawa T. Long-term results of gamma knife surgery for the treatment of craniopharyngioma in 98 consecutive cases. J Neurosurg. 2005;103(6):482–8.
Stripp D, Maity A, Janss A, et al. Surgery with or without radiation therapy in the management of craniopharyngiomas in children and young adults. Int J Radiat Oncol Biol Phys. 2004;58(3):714.
Komotar R, Starke R, Raper D, Anand V, Schwartz T. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World Neurosurg. 2012;77(2):329.
Sheehan J, Niranjan A, Sheehan J, et al. Stereotactic radiosurgery for pituitary adenomas: an intermediate review of its safety, efficacy, and role in the neurosurgical treatment armamentarium. J Neurosurg. 2005;102(4):678.
Gopalan R, Dassoulas K, Rainey J, Sherman J, Sheehan J. Evaluation of the role of Gamma Knife surgery in the treatment of craniopharyngiomas. Neurosurg Focus. 2008;24(5):E5.
Reisch R, Marcus H, Hugelshofer M, Koechlin N, Stadie A, Kockro R. Patients’ cosmetic satisfaction, pain, and functional outcomes after supraorbital craniotomy through an eyebrow incision. J Neurosurg. 2014;121(3):730.
Reisch R, Perneczky A. Ten-year experience with the supraorbital subfrontal approach through an eyebrow skin incision. Neurosurgery. 2005;57(4 Suppl):242.
Park J, Jung T, Kang D, Lee S. Preoperative percutaneous mapping of the frontal branch of the facial nerve to assess the risk of frontalis muscle palsy after a supraorbital keyhole approach. J Neurosurg. 2013;118(5):1114.
Dusick J, Esposito F, Malkasian D, Kelly D. Avoidance of carotid artery injuries in transsphenoidal surgery with the Doppler probe and micro-hook blades. Neurosurgery. 2007;60(4 Suppl 2):322.
Oskouian R, Kelly D, Laws E Jr. Vascular injury and transsphenoidal surgery. Front Horm Res. 2006;34:256.
Yaşargil M, Curcic M, Kis M, Siegenthaler G, Teddy P, Roth P. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J Neurosurg. 1990;73(1):3.
Vinchon M, Weill J, Delestret I, Dhellemmes P. Craniopharyngioma and hypothalamic obesity in children. Child Nerv Syst. 2009;25(3):347.
Wilson D, Duong H, Teo C, Kelly D. The supraorbital endoscopic approach for tumors. World Neurosurg. 2014;82(6 Suppl):S72.
Ditzel FL, McLaughlin N, Bresson D, Solari D, Kassam A, Kelly D. Supraorbital eyebrow craniotomy for removal of intraaxial frontal brain tumors: a technical note. World Neurosurg. 2014;81(2):348.
Paladino J, Mrak G, Miklić P, Jednacak H, Mihaljević D. The keyhole concept in aneurysm surgery – a comparative study: keyhole versus standard craniotomy. Minim Invasive Neurosurg. 2005;48(5):251.
Cohen A, Perneczky A, Rodziewicz G, Gingold S. Endoscope-assisted craniotomy: approach to the rostral brain stem. Neurosurgery. 1995;36(6):1128.
Fatemi N, Dusick J, de Paiva NM, Malkasian D, Kelly D. Endonasal versus supraorbital keyhole removal of craniopharyngiomas and tuberculum sellae meningiomas. Neurosurgery. 2009;64(5 Suppl 2):269.
Liu JK, Sevak IA, Carmel PW, Eloy JA. Microscopic versus endoscopic approaches for craniopharyngiomas: choosing the optimal surgical corridor for maximizing extent of resection and complication avoidance using a personalized, tailored approach. Neurosurg Focus. 2016 Dec;41(6):E5.
Baldauf J, Hosemann W, Schroeder HW. Endoscopic endonasal approach for craniopharyngiomas. Neurosurg Clin N Am. 2015;26:363–75.
Liu JK, Christiano LD, Patel SK, Eloy JA. Surgical nuances for removal of retrochiasmatic craniopharyngioma via the endoscopic endonasal extended transsphenoidal transplanum transtuberculum approach. Neurosurg Focus. 2011 Apr;30(4):E14.
Oldfield EH. Transnasal endoscopic surgery for craniopharyngiomas. Neurosurg Focus. 2010 Apr;28(4):E8a. Discussion E8b.
Dhandapani S, et al. Endonasal endoscopic reoperation for residual or recurrent craniopharyngiomas. J Neurosurg. 2017;126:418–430.
Komotar RJ, Starke RM, Raper DM, Anand VK, Schwartz TH. Endoscopic endonasal compared with microscopic transsphenoidal and open transcranial resection of craniopharyngiomas. World Neurosurg. 2012;77:329–41.
Liu JK, Schmidt RF, Choudhry OJ, Shukla PA, Eloy JA. Surgical nuances for nasoseptal flap reconstruction of cranial base defects with high-flow cerebrospinal fluid leaks after endoscopic skull base surgery. Neurosurg Focus. 2012;32:E7.
Zacharia BE, Amine M, Anand V, Schwartz TH. Endoscopic endonasal management of craniopharyngioma. Otolaryngol Clin North Am. 2016;49:201–12.
Cavallo LM, et al. The endoscopic endonasal approach for the management of craniopharyngiomas: a series of 103 patients. J Neurosurg. 2014;121:100–13.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Couldwell, W.T., Kelly, D.F., Liu, J.K. (2019). 18 Suprasellar Craniopharyngiomas. In: Evans, J., Kenning, T., Farrell, C., Kshettry, V. (eds) Endoscopic and Keyhole Cranial Base Surgery . Springer, Cham. https://doi.org/10.1007/978-3-319-64379-3_18
Download citation
DOI: https://doi.org/10.1007/978-3-319-64379-3_18
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-64378-6
Online ISBN: 978-3-319-64379-3
eBook Packages: MedicineMedicine (R0)