Abstract
Pulmonary hypertension is a complex disease of the pulmonary vasculature, which in severe cases terminates in right heart failure. Complex remodeling of pulmonary arteries comprises the central issue of its pathology. This includes extensive proliferation, apoptotic resistance and inflammation. As such, the molecular and cellular features of pulmonary hypertension resemble hallmark characteristics of cancer cell behavior. The vascular remodeling derives from significant metabolic changes in resident cells, which we describe in detail. It affects not only cells of pulmonary artery wall, but also its immediate microenvironment involving cells of immune system (i.e., macrophages). Thus aberrant metabolism constitutes principle component of the cancer-like theory of pulmonary hypertension. The metabolic changes in pulmonary artery cells resemble the cancer associated Warburg effect, involving incomplete glucose oxidation through aerobic glycolysis with depressed mitochondrial catabolism enabling the fueling of anabolic reactions with amino acids, nucleotides and lipids to sustain proliferation. Macrophages also undergo overlapping but distinct metabolic reprogramming inducing specific activation or polarization states that enable their participation in the vascular remodeling process. Such metabolic synergy drives chronic inflammation further contributing to remodeling. Enhanced glycolytic flux together with suppressed mitochondrial bioenergetics promotes the accumulation of reducing equivalents, NAD(P)H. We discuss the enzymes and reactions involved. The reducing equivalents modulate the regulation of proteins using NAD(P)H as the transcriptional co-repressor C-terminal binding protein 1 cofactor and significantly impact redox status (through GSH, NAD(P)H oxidases, etc.), which together act to control the phenotype of the cells of pulmonary arteries. The altered mitochondrial metabolism changes its redox poise, which together with enhanced NAD(P)H oxidase activity and reduced enzymatic antioxidant activity promotes a pro-oxidative cellular status. Herein we discuss all described metabolic changes along with resultant alterations in redox status, which result in excessive proliferation, apoptotic resistance, and inflammation, further leading to pulmonary arterial wall remodeling and thus establishing pulmonary artery hypertension pathology.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Pulmonary hypertension
- Pulmonary arterial wall remodeling
- Immune system
- Aerobic glycolysis
- Mitochondrial catabolism
- NAD(P)H oxidase
1 Introduction
Pulmonary hypertension (PH) is a serious and often fatal pulmonary vascular condition that is becoming increasingly prevalent worldwide. PH occurs in a variety of clinical conditions and is associated with a broad spectrum of pathologic abnormalities in the pulmonary arteries of affected patients. All patients with PH exhibit a common set of clinical signs, such as exertional dyspnea, marked exercise limitation, and in severe cases, right heart failure and death. Because of the diverse causes and mechanisms contributing, PH has been classified into five categories related to common clinical parameters, potential etiologic mechanisms and pathological, pathophysiological, and therapeutic characteristics [1, 2]. PH in all categories is characterized by a complex panvasculopathy involving excessive proliferation and apoptosis resistance of multiple cell types, as well as inflammation and fibrosis throughout the vasculature. The characteristics of this remodeling have led many to hypothesize that the cellular and molecular features of PH resemble hallmark characteristics of cancer cell behavior [3,4,5,6]. In cancer, it is widely recognized that changes in metabolism in the cancer cell, as well as cells in surrounding stromal cells, including fibroblasts and macrophages, are essential for cancer cells to proliferate, migrate, and exhibit pro-inflammatory characteristics [7,8,9]. Because of this there have been intense efforts in oncology to define the molecular mechanisms that underlie the coordinated cross talk between cancer cells and cells in their immediate microenvironment. Indeed, while it is acknowledged that there is evidence to suggest that metabolic rewiring is orchestrated by the concerted action of oncogenes and tumor suppressor genes, it is now recognized that in some circumstances altered metabolism can play a primary role in oncogenesis. Indeed, aberrant metabolism, rather than simply an epigenetic phenomenon of oncogenic reprogramming, is now thought to play a key role in oncogenesis with the power to control both genetic and epigenetic events in cells [8, 10]. Certainly, questions arise as to whether this may be the case in PH as well.
Metabolic adaptations akin to aerobic glycolysis (“Warburg-like”), historically assigned to cancer cells, have also recently been reported in PH where hypoxia has significant regulatory role [3, 5, 11,12,13]. These changes have been described to occur in smooth muscle cells (SMCs), endothelial cells (ECs), and fibroblasts (Fibs) [11, 13,14,15,16]. Additional data in support of the importance of this metabolic adaptation are supported by 18-fluorodeoxyglucose (18FDG) PET imaging, which has demonstrated increased glucose uptake and metabolism in PH patients as well as in the monocrotaline rat model of PH [13, 17]. Furthermore, 18FDG uptake and gene-expression studies in pulmonary arterial fibroblasts, isolated from iPAH patients, tend support to the concept that a proliferative and inflammatory pulmonary vascular pathology contributes to the lung 18FDG PET signal [13]. This study also showed that 18FDG uptake occurs in perivascular mononuclear cells, which accumulate in the adventitial perivascular regions. In vivo studies in the monocrotaline model demonstrated a close correlation between lung18FDG uptake and pulmonary vascular remodeling. Importantly, enhancement of oxidative metabolism with dichloroacetate-mediated inhibition of the enzyme pyruvate dehydrogenase kinase attenuated PH and vascular remodeling in this model and also reduced expression of the glucose transporter (Glut1) typically upregulated in cells exhibiting high glycolysis. These findings correlated with reduced 18FDG PET signals , which were associated with decreased peripheral vascular muscularization and inflammatory cell accumulation. Collectively, these in vivo and ex vivo observations support a “metabolic hypothesis” for the pathogenesis of PH, whereby a rearrangement of the mitochondrial and cytosolic metabolism, known as the “Warburg effect,” might explain, at least partially, the molecular and functional abnormalities seen in PH cells, including excessive proliferation, apoptosis resistance, and inflammatory activation [12].
2 Metabolic Reprogramming in Cells of Pulmonary Artery Wall in PH Pathology
Excessive proliferation and apoptotic resistance of ECs, SMCs, and Fibs from the PH vessel wall phenocopies the Warburg-like metabolic reprogramming observed in highly proliferating cancer cells. Incomplete oxidation of glucose through aerobic glycolysis depresses catabolism through mitochondria and fuels anabolic reactions of amino acids, lipids and nucleosides to sustain proliferation. This is often induced by dysregulation of oncogenes, hypoxic signaling and aberrant responses in cancer cells [18]. The metabolic switch needs to cover the energy demands of highly proliferating cells , such as DNA replication and gene expression, macromolecule biosynthesis, ion gradients’ homeostasis and maintenance of cellular structure. Transcriptional upregulation and increased expression of glycolytic enzymes is observed in these cells and hypoxic signaling can play a pivotal role in these responses (see Fig. 1). Generalized or microenvironmental hypoxia can participate in the development of pulmonary hypertension (PH) through regulation of the oxygen sensitive subunit of hypoxia inducible factor (HIF) [19]. Consistent with a hallmark of metabolic reprogramming in proliferating cancer cells, cells from the hypertensive pulmonary vasculature express specific isoforms of glycolytic enzymes that promote faster fluxes through glycolysis, such as glucose transporter 1 (GLUT1), phosphofructokinase 3 (PFKB3) and pyruvate dehydrogenase kinase 1, and lactate dehydrogenase A (LDHA) [3, 12, 20]. Increased uptake of glucose not only provides a rate-limiting substrate to promote glycolysis, but also results in the upregulation of the pentose phosphate pathway. Hexokinase responsible for glucose phosphorylation, the initial step of glycolysis, was found to be able to suppress apoptosis by reversible binding to VDAC in mitochondrial membrane, thus preventing cytochrome c release [21]. Similar to highly proliferating cancer cells, activation of the oxidative branch of the pentose phosphate pathway in PH cells provides reducing equivalents (NADPH) to sustain NADPH-dependent anabolic reactions (e.g., de novo synthesis of fatty acids) and, at the same time, it generates ribose phosphate moieties for the de novo synthesis and salvage of nucleosides—the building blocks of RNA and DNA. It can inhibit full AMPK activation, which increases fatty acid and cholesterol synthesis while inhibiting acetyl-CoA carboxylase 1 and 2, otherwise participating in fatty acid oxidation (see Fig. 2). In addition to serving as a key cofactor for anabolic reactions, NADPH represents a key-reducing equivalent to preserve the redox poise. Indeed, NADPH is used as an antioxidant substrate to reduce oxidized glutathione or to promote pro-oxidant reactions through the activity of NADPH oxidase (NOX). Moreover, NADPH derived from the pentose phosphate pathway seems to be important during the initial phase of PH development for vasoconstriction of pulmonary arteries, as it forms an essential cofactor for nitric oxide (NO) production by NO synthase (NOS) [22]. In agreement with this, a deficiency of glucose-6-phosphate dehydrogenase in the African, Middle East, and Asian population was associated with enhanced endothelial oxidative stress, decreased NO bioavailability and increased risk for vascular disease such as PH [23]. Interestingly, glucose-6-phosphate dehydrogenase was also found to be involved under certain conditions in apoptosis [24]. Excess NADPH can then induce calcium/calmodulin-dependent kinase II to phosphorylate, thus inhibit pro-apoptotic caspase2 activation.
Anabolic metabolism of hypertensive cells from pulmonary artery wall. Increased glycolytic pathway, where numerous enzymes is upregulated by HIF1/2. Enhanced pentose phosphate pathway, leading to NADPH production. Altered mitochondrial metabolism with suppressed oxidative phosphorylation. Elevated one carbon metabolism. Metabolic changes in glycolysis, Krebs cycle, redox homeostasis, and amino acid metabolism (color coded) are documented by UHPLC-mass spectrometry metabolomics data (left) of calf control and pulmonary hypertensive fibroblasts from pulmonary arteries. Relative metabolite quantities were graphed through heat maps, upon Z score normalization of values determined across samples (blue, lowest values; red, highest values). Each sample is represented by three independent experiments depicted in columns. All metabolic changes are leading to increased NAD(P)H, which then participates in several signaling reactions
Mitochondrial metabolism of hypertensive cells from pulmonary artery wall. Pyruvate oxidation is inhibited. β-oxidation is induced, since there is low level of inhibitory metabolite malonyl-CoA. Glutaminolysis is induced leading among others to production of α ketoglutarate, which incorporated into TCA cycle can serve for catabolic reactions to fuel further respiratory chain through succinate production or can serve for anabolic reactions in reversed flow of TCA cycle called reductive carboxylation. It will then provide citrate for lipogenesis. Electron transport chain is retarded, mainly through complex I, leading to increased production of superoxide, which after dismutation to H2O2 participates in stabilization of HIF1 and further signaling reactions
Increased glucose oxidation also promotes the accumulation of reducing equivalents other than NADPH, such as NADH. Increased NADH/NAD+ ratios are promoted by increased fluxes through glycolysis, consistent with the law of mass action indicating that excess lactic fermentation under hypoxic conditions unbalances the NAD-cofactor poise towards the accumulation of reduced equivalents. Hypoxia also compromises physiological electron transfer through the electron transport chain, resulting in the incapacity to regenerate reducing equivalents (NADH, FADH2) in the mitochondria owing to the lack of oxygen as the final acceptor of electrons in the electron transport chain. Disruption of the normal electron flow in the electron transport chain does not impair mitochondrial Krebs cycle reactions upstream to succinate dehydrogenase (SDH) (as part of complex II of the electron transport chain), though it promotes metabolic bottlenecks in complex I, which is involved in the reactions catalyzing the conversion of malate to oxaloacetate [25, 26]. Isocitrate dehydrogenase (IDH) activity either in cytosol (IDH1) or in mitochondria (IDH2,3), which can generate NADH or NADPH, depending on the isoform of the enzyme catalyzing the reaction and redox equilibrium, has been reported to be increased in PH patients [14]. Further, increased NAD(P)H can significantly impact redox status and also the regulation of proteins using NAD(P)H as a cofactor. The transcriptional repressor C-terminal-binding protein1 (CtBP1) is such an example. Oligomerization of CtBP1 into active dimers is dependent on the presence of increased free NADH and NADH/NAD+ ratios [27]. CtBP1 interacts with gene specific transcriptional factors besides recruiting several epigenetic modifying enzymes on the target genes [27]. Recently, the coauthors of this chapter found its increased expression in fibroblasts generated from the pulmonary arteries of chronically hypoxic calves or patients with iPAH (termed PH-Fibs) that were characterized by an aerobic glycolytic state and exhibited increased free NADH, even under normoxic conditions and with enhanced proliferation and inflammation. Genetic or chemical suppression of CtBP1 led to downregulation of proliferation (and upregulation of the cyclin-dependent kinase inhibitor genes, P21 and P15), apoptosis (e.g., upregulation of NOXA and PERP) and upregulation of anti-inflammatory (HMOX1 ) markers [28]. Other studies showed that CtBP1 also participates in the maintenance of metabolic homeostasis through the inhibition of apoptosis. In this respect, it was found that the transcriptional repressor CtBP1 directly inhibits pro-apoptotic Bax or SIRT4, the repressor of glutaminolysis in mitochondria. The activation of glutaminolysis promotes the generation of alternative substrate for mitochondrial catabolism and also release of the acidification pressure of highly proliferative lactate-producing cells [29, 30]. These studies highlight the importance of metabolic regulation on gene expression during PH development and identify feed forward mechanisms at the crossroads of transcriptional regulation and metabolic reprogramming.
Another glycolytic intermediate, fructose-1,6-bisphosphate, was shown to inhibit mitochondrial respiratory rate in highly glycolytic cells, an observation that may contribute to explain the so-called Crabtree effect—i.e., the reliance on glycolysis rather than the tricarboxylic acid cycle (TCA) to generate high energy compounds (ATP) [31].
Analogous to cancer, metabolic reprogramming in PH is accompanied by post-transcriptional reprogramming triggering the expression of specific isoforms of key metabolic enzymes. For example, an isoform switch in pyruvate kinase M plays an important role in the glycolytic state of highly proliferative cells. PKM1 and 2 isoforms are alternative splice isoforms of pyruvate kinase M. While PKM1 is expressed in terminally differentiated tissues, PKM2 isoform is expressed mostly in proliferating cells and tissues with anabolic functions (cancer, embryonic cells) and cells with high intrinsic self-renewal properties. While PKM1 has high catalytic activity, PKM2 is allosterically regulated between a high and a low activity state. PKM2 exists in the catalytically distinct tetrameric and dimeric states [32] and is present usually in its less active dimer form in highly proliferative cells. This leads to inhibition of the last step of glycolysis and to accumulation of glycolytic intermediates including lactate for biosynthetic processes. The dissociation of PKM2 tetramer into dimers is a reversible process and determines the balance between anabolic and catabolic phases of cell metabolism. Besides direct activation of PKM2 transcription by HIF1, PKM2 also directly interacts with HIF1 and thus promotes transactivation of genes with HIF response elements in their promoters [33]. This regulatory mechanism contributes to the escalation of the efficiency of glycolytic fluxes. The PKM2 isoform is also subject to many regulatory post-translational modifications such as phosphorylation, which participates in the conversion of the tetrameric form into the dimeric one [34]. On the other hand, PKM2 is positively allosterically regulated by fructose-1,6 bisphosphate and serine, which is produced from glycolytic intermediate 3-phosphoglycerate [35]. Moreover, oxidation of cysteine 358 in PKM2 impairs enzymatic function and decreases its activity, leading to the accumulation of glucose-6-phosphate and increased glucose flux through pentose phosphate pathway [36], thus contributing to redox homeostasis. Such complex regulation of this protein isoform is crucial for highly proliferating cells as a variety of molecules further interact with PKM2. Specifically, dimeric PKM2 was shown to translocate into the nucleus, where it can act as an active protein kinase to phosphorylate specific nuclear proteins such as STAT3 and β-catenin [29, 37, 38]. PKM2 can interact directly with the HIF-1α subunit in the nucleus to promote transactivation of HIF-1α target genes by enhancing HIF-1 binding and recruitment of p300 [39]. PKM2 gene transcription is also activated by HIF-1, which creates a positive feedback loop that promotes HIF-1 transactivation and alters glucose metabolism [39]. The coauthors of this chapter recently found that PMK2/PKM1 ratio is increased in PH Fibs from calves and iPAH humans [40]. Moreover, this alternative splicing resulting in the increased PKM2/PKM1 ratio was found to be under direct control of polypyrimidine-tract binding protein 1 (PTBP1) and miR124 acting upstream. Genetic manipulations of miR124 (miR-124 mimic) or PTBP1 (siPTBP1) normalized the PKM splicing ratio. This induced mitochondrial activity and returned metabolism and proliferation toward that of normal control fibroblasts. Collectively this evidence supports the idea that PKM2 is a crucial enzyme controlling overall metabolic state of cells during the development of PH.
PKM catalyzes the final step of glycolysis , which results in the production of pyruvate. The destiny of pyruvate is another crucial point in highly proliferating cells. Under physiological conditions in most cells, pyruvate enters mitochondria where is further processed by pyruvate dehydrogenase (PDH) to acetyl-CoA fueling the TCA cycle and mitochondrial respiratory chain. However, under hypoxic conditions, the activity of mitochondrial respiratory chain is disrupted, as anticipated above, and cells require alternative pathways to regenerate reducing equivalents (NADH to NAD+) to keep glycolysis going. Reduction of pyruvate to lactate through lactic fermentation serves this purpose under hypoxic conditions. This process can be enzymatically regulated under hypoxic conditions in PH. Indeed, inactivating pyruvate oxidation in mitochondria by PDH phosphorylation in hypoxia is directly regulated by HIF1α. This transcription factor induces PDH kinase in proliferating cells, an adaptation that can become independent from oxygen availability in cancers undergoing constitutive HIF activation or PDH kinase mutations [41]. Recently, we have shown that this metabolic adaptation also occurs in PH Fibs from chronic hypoxia exposed calves and iPAH humans [16]. Suppressed glucose oxidation was reflected by decreased mitochondrial bioenergetics (see below).
Termination of the glycolytic pathway by production of lactate is important for the later stage of hypoxia in cancer cells. It was found that accumulated lactate can stabilize NDRG3 protein which binds to c-Raf and mediates hypoxic cell growth through c-Raf-ERK pathway in various types of cancer cells [42].
Recent interest in one-carbon metabolism has been generated by experiments showing its importance in rapidly proliferating cells. One-carbon metabolism contributes key substrates for de novo synthesis of purine bases, participates in redox homeostasis by regulating the synthesis of amino acid substrates for glutathione synthesis and directly contributes to the redox poise through NADPH-generating reactions [43,44,45,46,47,48,49]. Notably, glycolytic isoform switch in cancer modulates the activity of rate limiting enzymes of one-carbon metabolism, as documented by evidence indicating a role for serine as an allosteric modulator for PKM2 activity [49]. Further studies will be necessary to investigate the role of this pathway in PH development.
3 Metabolic Remodeling in Macrophage Activation
Pulmonary arterial wall remodeling during PH development develops in concert with the innate immune system where macrophages play a pivotal role. Macrophages contribute to maintenance of tissue homeostasis [50, 51] through paracrine communication with tissue parenchymal cells [52], but also by sensing and responding to environmental cues and signals [53]. They are innate immune cells known to be important in the initiation and propagation of sterile inflammatory processes , like chronic inflammatory pathologies such as rheumatoid arthritis and remodeling pathologies such as liver fibrosis and vascular remodeling as it occurs in association with pulmonary hypertension [51, 54]. Indeed, macrophages have been recognized to promote tumor growth [55]. They are equipped with a sophisticated system to detect microenvironmental differences across various organs and conditions, and exhibit a high degree of functional versatility/plasticity in order to adequately contribute to homeostatic tissue maintenance, infectious and inflammatory processes and to tissue repair processes [51]. Continuous adjustments in the metabolic programming of macrophages have been suggested to be a key component in the regulation of their gene transcriptional profile and thus their functional phenotype within these processes [56]. There is now consensus that metabolic reprogramming consistent with a Warburg-like state is a prerequisite for inflammatory macrophages (here referred to as macrophages stimulated with lipopolysacharide (LPS) or with LPS in combination with interferon gamma (IFNg) to generate pro-inflammatory mediators [57, 58]. Metabolic reprogramming results at least in part from LPS mediated increases in HIF1 and expression of HIF1 target genes, encoding for enzymes of the glycolysis pathway [57,58,59]. LPS also promotes PKM2 expression, and IDH and SDH suppression [58]. Accumulated succinate derived from SDH suppression can generate ROS by reverse electron transfer on Complex I of respiratory chain, which has been suggested to be critical for activation of genes encoding for pro-inflammatory mediators [60]. LPS, especially in combination with IFNg, also upregulates expression of citrulline-arginine-succinate cycle enzymes, such as iNOS (inducible nitric oxide synthase), ASS (argininosuccinate synthetase), ASL (arginine succinate lyase), and Arginase1 [61]. As a result, inflammatory macrophages consume large amounts of glucose to generate lactate, accumulate citrate, succinate, and produce nitric oxide (NO).
Lactate and succinate have also been suggested to induce macrophage activation, thus providing a potential autocrine and paracrine feed forward loop [55]. Citrate, as a critical substrate for fatty acid synthesis and precursor for leukotrienes and prostaglandins, has been shown to be also important macrophage derived pro-inflammatory mediator production [62]. Succinate can positively regulate pro-inflammatory IL1b through HIF1α stabilization [57] as well as iNOS [63] and Arginase1 [54, 55]. Citrulline derived from iNOS metabolism of arginine can react with aspartate to regenerate arginine [58], which is a critical event under arginine limiting conditions [61]. In addition, this reaction generates fumarate, which is a critical step in replenishing the reprogrammed TCA cycle when SDH is reduced [58].
While these concepts help explain how inflammatory macrophages that are activated by LPS and IFNg use metabolic reprogramming to mount generation of pro-inflammatory mediators, much less is known about if and how metabolic programming underlies macrophage phenotypes in chronic inflammatory conditions that are not characterized by LPS, but instead involve a more complex microenvironmental makeup, e.g., cancer or rheumatoid arthritis, or vascular remodeling. In these conditions a metabolic synergy or symbiosis between macrophages and cancer cells and/or macrophages and fibroblasts might be a key mechanistic foundation for chronic inflammatory activation towards a pro-cancer or pro-remodeling phenotype. In such a metabolic symbiosis both cells, the macrophage and the tissue cell (for instance the fibroblast) undergo overlapping but also distinct metabolic reprogramming, such that both anabolic and catabolic pathways are engaged. As a result catabolic metabolites and anabolic substrates can be exchanged and shared between the two cells in order to sustain the respective metabolic reprogramming and thus functional phenotype. For example, cancer cell derived lactate has been shown to induce activation of HIF1 and expression of Arg1 in cancer associated macrophages, which in turn promotes generation of polyamines (anabolic pathway) through utilization of Arginine by Arginase1 [55]. In that study, polyamines were proposed to sustain cancer growth, since macrophages genetically deficient in Arginase1 were less capable of promoting cancer growth in vivo. Recent studies investigating the mechanism of vascular remodeling in PH have identified perivascular fibroblasts with a distinct pro-inflammatory phenotype characterized by generation of cytokines and chemokines [16, 54, 64]. Intriguingly, the activated fibroblast is capable of activating macrophage expression of Arginase1 at least in part through paracrine IL6 [54, 64]. As pointed out previously, these fibroblasts display metabolic reprogramming similar to that observed in inflammatory macrophages, including generation of lactate. Inhibition of glycolysis prevents inflammatory activation of these fibroblasts. Therefore, a metabolic symbiosis could potentially underlie the macrophage fibroblast crosstalk in vascular remodeling associated with PH in which fibroblast derived lactate (catabolism of glucose) and fibroblast derived IL6 (anabolic cytokine generation as a result of rewired TCA cycle and mitochondrial ROS production [16]) activate macrophage expression of HIF1 and Arginase1 [54]. Macrophage derived polyamines downstream of Arginase1 expression and macrophage derived IL1b (as a catabolic event mediated by HIF induced Il1 generation) could in turn activate fibroblasts and promote their proliferation.
4 Mitochondrial Alterations and Superoxide Production in Pulmonary Arterial Hypertension
Observations of incomplete glucose oxidation terminating in lactate production in PH cells lead to the discovery of altered mitochondrial bioenergetics, similar to cancer cells (see Fig. 2). These changes have been described for cells from all layers of the hypertensive pulmonary artery wall [15, 16]. The role of mitochondria in apoptosis resistance, redox and calcium homeostasis assign them a central position within the cell which can determine cell fate. Changes in fluxes in the TCA cycle provide intermediates for biosynthetic pathways in highly proliferative cells. Thus, mitochondrial respiration in PH cells is still operating and needs to be preserved [16]. To overcome suppressed pyruvate oxidation, mitochondria adjust the fluxes from carbohydrates (pyruvate) to other significant substrate sources, such as fatty acid oxidation or glutaminolysis (Fig. 2). Glycolysis followed by pyruvate oxidation is assumed to contribute to 50–70% of total ATP production in cancer cells with the remainder contributed by mitochondrial oxidation of glutamine and fatty acids [65,66,67].
The feedback mechanism in which phosphorylation of PDH, leading to its downregulation, is critical for the mitochondrial substrate switch to fatty acid oxidation was described originally by Randle [68]. Upregulation of the expression of genes involved in fatty acid oxidation as well as an increase in metabolic intermediates was found in lung tissue from PH patients [69]. The importance of fatty acid oxidation in PH development is evident from the experiment where mice lacking malonyl-coA decarboxylase (MDC ), which otherwise induces fatty acid oxidation at the expense of its synthesis, do not develop PH upon chronic hypoxic exposure [70]. Interestingly, MDC can be deacetylated by SIRT4 and thus inactivated [71]. This is consistent with our finding of increased CtBP1 levels in hypoxic PH-Fibs in that inhibition of SIRT4 by increased CtBP1 activity allows MDC to be active and drive fatty acid oxidation in mitochondria [28].
Another potential fuel for the TCA cycle is glutamine, the most abundant amino acid in blood. It is processed through glutaminolysis to α-ketoglutarate which can enter the TCA cycle. In highly proliferative cells, glutaminolysis plays a major anaplerotic role, since α-ketoglutarate production either fuels the TCA cycle or provides a carbon backbone for the biosynthesis of nonessential amino acids. We have observed elevation of glutamine and α-ketoglutarate in PH Fibs (see Fig. 1). Glutamine catabolism can thus provide a carbon source that supports further proliferation. In highly proliferative cells, it can also serve as the source of nitrogen for transamination reactions, which is necessary for GSH synthesis (serine and thus cysteine/glycine anabolism depends on glutamine for rate-limiting transamination reactions branching from glycolysis) and protect the cell from excessive acidification, by converting pyruvate into alanine, thus preventing accumulation of excess lactate in highly glycolytic cells. Under hypoxic conditions glutamine can also contribute to de novo lipogenesis by fueling reductive carboxylation reactions (reverse fluxes through the TCA cycle) [72] (See Fig. 2). Among others, α-ketoglutarate can give rise to 2-hydroxyglutarate, a metabolite that accumulates significantly in response to hypoxia in cells and plasma [73]. On the other hand, certain types of cancer cells (e.g., glioma) are characterized by IDH 1/2 mutations or a switch in the expression pattern of IDH isoforms in favor of NADPH-generating IDH1 [74]. Some of these mutations, together with excess glutaminolysis contribute to increased α-ketoglutarate–citrate ratio [75], which in turn promotes reductive carboxylation fluxes to sustain acetyl-CoA generation and anabolic reactions (e.g., de novo synthesis of fatty acids). We have found that the α-ketoglutarate–citrate ratio was significantly increased in PH Fibs [64]. On the other hand, 2-hydroxyglutarate has been described as oncometabolite and was found to elicit significant epigenetic changes that can contribute to cancer phenotype [76]. Citrate can be also processed through oxaloacetate or malate for amino acid synthesis required for building proteins.
Mitochondrial bioenergetics terminates with the production of ATP where the respiratory chain is the key part of this machinery. Mitochondrial respiratory capacity is defined by availability of substrate, i.e., reducing equivalents coming either from the cytosol (NADH) or mitochondrial TCA cycle (NADH and FADH) and integrity of electron transport supercomplexes. The optimal flow of electrons through respiratory supercomplexes then defines the maximal production of ATP by complex V. However, in rapidly proliferating cells pyruvate stays in the cytosol instead of fueling TCA cycle and possibly the respiratory chain (Fig. 1). In that case, glutaminolysis or fatty acid oxidation can significantly substitute for the fuel in the TCA cycle. This might be the case of PH-Fibs, which were found to have enhanced succinate levels in mitochondria [16]. Succinate can then, in addition to other TCA cycle intermediates like α-ketoglutarate or fumarate, regulate histone and DNA methylation [77]. Besides shaping epigenetic regulation of DNA expression, upregulation of succinate and fumarate can also stimulate HIF1 stabilization though inhibition of HIF-prolyl hydroxylase, thus enhancing the Warburg effect.
Mitochondria of PH Fibs are hyperpolarized, reflecting less efficient oxidative phosphorylation [16]. This can be due to several factors, primarily involving mitochondrial respiratory chain complexes , some of which (Complex I, III and IV) are pumping protons across the mitochondrial membrane, which at insufficient protons backflow via ATP synthase creates increased membrane potential. However, the involvement of fatty acid stimulated uncoupling proteins in attenuation of mitochondrial membrane potential is also well recognized. Resulting mild uncoupling has antioxidant effect. Studies using mitochondrial uncoupling protein 2 deficient (UCP2−/−) mice showed that vascular remodeling with development of pulmonary hypertension occurred primarily via increased ROS production, not by regulation of membrane potential itself [78]. Another study explained spontaneous PH development in UCP2−/− mice by decreased mitochondrial calcium levels [79]. Many mitochondrial enzymes are calcium dependent so loss of UCP2 could lead to decreased mitochondrial bioenergetics. Another reason for hyperpolarized mitochondria in cells from the hypertensive vessel wall was attributed to translocation of hexokinase II into mitochondria via a GCK3-β dependent mechanism. This was shown to inhibit the voltage dependent anion channel (VDAC) and thus sustain mitochondrial hyperpolarization [80].
Mitochondrial membrane potential together with substrate availability, local oxygen concentration and electron flow through respiratory chain determines the mitochondrial redox status. Moreover, mitochondria contain numerous redox active metals , i.e., Fe–S cluster, cytochromes and thus are a primary site for single-electron reactions in the cell. The primary reactive oxygen species (ROS) produced in mitochondria is superoxide, which is formed mainly within complexes I and III of the respiratory chain [81,82,83,84]. The respiratory complexes with multiple redox centers form electron transport chain, which normally facilitates transfer of electrons to their final acceptor, molecular oxygen. It is reduced by four electrons to water at complex IV. The premature single electron reduction of molecular oxygen gives rise to superoxide formation. This can happen when electron flow is either retarded (i.e., ATP synthase is inhibited, cytochrome c or coenzyme Q cycling is retarded) or there is substrate pressure (high load of NADH, FADH2) on respiratory complexes. Notably Complex I elevates superoxide formation at NADH≫NAD+ and at reverse electron transport after succinate accumulation [84]. Also alterations in the assembly of electron transport chain complexes can lead to stoichiometric mismatches, which can result in delay of electron flow on sites of the complexes mediating production of superoxide. It is important to realize that antioxidant redox couples (i.e., GSH/GSSG) are closely linked to the metabolic redox couples (NADH, FADH2) which serves also as the substrate of respiratory chain [82]. There are two sites within the complex I, where superoxide can be formed [85]. The first site is the flavin in the NADH-oxidizing site , producing superoxide at NADH››NAD+, i.e., high substrate pressure and the ubiquinone-reducing site, pumping superoxide at high protonmotive force or retarded proton pumping [86] or under condition of reverse electron pumping. Both sites produce superoxide into mitochondrial matrix. In complex III, superoxide is thought to arise from the quinol oxidizing site, producing superoxide about equally into mitochondrial intermembrane space and matrix. It was proposed that Complex I driven increased superoxide production is predominantly deleterious as they can react in high concentration with mitochondrial DNA or other matrix components vulnerable to oxidative damage, whereas Complex III driven superoxide may rather serve as a second messenger in cellular signaling. Their various but different targets were experimentally determined [87]. However, other sites of mitochondrial superoxide production were also suggested in context of various cell types. This involves complex II [88], glycerol phosphate dehydrogenase [89], pyruvate dehydrogenase [90], or α-ketoglutarate dehydrogenase [91]. How the individual ROS source contributes to increased ROS production during PH development remains to be elucidated.
Additional regulation of superoxide production might come directly from alterations in Complex I and III. For example, mutations in 9 out of 13 assembly factors of Complex I were proven as disease causing due to impairments in complex biogenesis [92]. There is evidence regarding direct downregulation of subunits of Complex I (NDUFA4L2) in murine embryonic fibroblasts [93] regulating further superoxide production. We have recently shown altered activity of Complex I in PH-Fibs from chronically hypoxic calves due to downregulation of the assembly subunit NDUFS4. This can cause complex I instability which leads to an increased disconnection of electron influx of the NADH dehydrogenase module from the complex I holo-complex. Subsequent mitochondrial hyperpolarization and increased superoxide production occurs [16]. Deficiency of NDUFS4 was shown previously to cause aberrant mitochondrial morphology and elevated ROS production in primary fibroblasts of NDUFS4 knockout mice, a model of Leigh syndrome [94]. Specific inhibition of Complex I, in this case NDUFV1 gene knockdown, reduces NAD+/NADH ratio, however, does not drastically inhibit oxidative phosphorylation. This was shown to significantly enhance metastatic activity and cell proliferation [95]. On the other hand, enhancement of Complex I was shown to increase tumor cell autophagy and to inhibit proliferation in vivo. Based on these data from cancer research, Complex I seem to be involved in efficiency of proliferation when glycolysis takes place .
Mitochondrial superoxide production was shown to be increased by hypoxia in pulmonary artery SMCs [96, 97]. However, another group suggested it was decreased [98]. This discrepancy might be explained by differences in the techniques used for ROS detection, the experimental PH model used, the level of PH development, and handling of the cells in vitro. We have confirmed accumulation of mitochondrial superoxide in PH-Fibs from chronically hypoxic calves grown and studied under normoxic conditions [16]. Mitochondrial superoxide production from Complex III has unique role in hypoxia as produced superoxide was shown to be crucial for stabilization of HIF1α [99]. Superoxide burst inhibits HIF-prolyl hydroxylases, which under normoxic conditions hydroxylate oxygen sensitive HIF1α thus priming it for proteolytic degradation. That this superoxide production is crucial for response of pulmonary artery SMCs in hypoxia and further PH development was confirmed by Sabharwal et al., who targeted peroxiredoxin5 into mitochondrial intermembrane space of these cells [100]. ROS decay by peroxiredoxin5 then suppressed ROS production from mitochondria and the acute activation of cytosolic calcium, which regulates vasoconstriction, the initial step in PH development.
Mitochondrial ROS production can be regulated by SIRT3 protein, the predominant mitochondrial deacetylase. This enzyme requires NAD+ for its activity. Thus changes in nutrient status can be directed through this protein to mitochondrial redox metabolism. The observed increased ratio of NADH/NAD+ in PH-Fibs [28] together with increased glycolysis will repress SIRT3 activity. This was confirmed in human and rat PH cells. Moreover, the human iPAH was associated with loss of function polymorphism of SIRT3 protein [101]. The targets of SIRT3, such as IDH2, SDH A, Lon protease, or NDUFA9 subunit of Complex I remain acetylated and inactivated [102]. Inactivated SDH A can then lead to accumulation of succinate. In case of the complexes of electron transport chain, the acetylation can negatively regulate assembly or subunits interaction. This might negatively impact superoxide production. SIRT3 was also shown to regulate PDHA1, one of the components of PDH complex [103]. The decreased activity of SIRT3 in PH cells might keep PDHA1 acetylated, which will attenuate its activity and induce its phosphorylation, thus directing pyruvate into lactate production. Moreover, SIRT3 knockout mice were shown to develop spontaneous PH [101]. SMCs from pulmonary arteries derived from SIRT3 knockout mice showed enhanced stabilization of HIF1 and STAT3 phosphorylation, increased phosphorylation of PDH and thus suppressed glucose oxidation in favor of glycolysis. Thus, the metabolic regulations observed in SIRT3 knockout mice contribute to PH development .
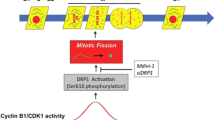
Efficiency of mitochondrial ATP synthesis was previously associated with mitochondrial morphology. Low respiratory mitochondrial bioenergetics was associated with condensed state of cristae structure (i.e., cristae expansion and matrix condensation) in situ and mitochondrial fission. Such mitochondrial ultrastructure was found to accompany mitochondria linked diseases such as diabetes and selected neurological diseases [104]. On the other hand, orthodox cristae state (i.e., matrix expansion and thin cristae) in situ and fused mitochondrial morphology corresponds with higher respiratory capacity. We have recently shown that under chronic hypoxia (72 h), metabolism of hepatocellular cancer cells (HepG2) establishes Warburg effect and downregulated mitochondrial bioenergetics is accompanied by condensed mitochondrial cristae structure [16]. A metabolic link is obvious as proteins participating in mitochondrial fusion and fission are often GTPases (Opa1, Mfn1 and 2, and Drp1) posttranslationally modified and moreover, often sensitive to mitochondrial membrane potential (Opa1). Also redox homeostasis will be important regulator [105]. Indeed, there are several reports showing that mitochondria of SMCs or Fibs from humans or experimental models with PH are fragmented with corresponding downregulation of pro-fusion proteins (Mfn2 and Opa1) and upregulated pro-fission proteins (Drp1 and Fis1) [16, 106]. Moreover, Drp1 was shown to be phosphorylated thus activated by cyclin B CDK1 in PH mitochondria of PH cells, while Mfn2 was suggested to be downregulated through transcriptional coactivator PGC1 or platelet-derived growth factor and endothelin-1 [106]. How mitochondrial dynamics in concert with mitochondrial bioenergetics participate in PH development remains to be elucidated. However, mitochondrial fusion/fission can modify rates of cell proliferation and apoptotic resistance. Thus, increased fission of mitochondria in PH cells might augment proliferation and inhibit apoptosis .
5 Activation of NADPH Oxidases in Pulmonary Arterial Hypertension
The activity of mitochondrial produced superoxide is often restricted to close proximity of its origin. Although, it can be dismutated into more diffusible hydrogen peroxide (H2O2), it is suggested to have signaling properties rather than pro-oxidative features outside the mitochondria. In contrast both pro-oxidative status and signaling function in cells is provided by NADPH oxidases (NOXs) (Figs. 1 and 2). Vascular NOXs are multi-subunit complexes bound to plasma membranes. Based on real-time PCR, NOX1, NOX2, and NOX4 isoforms were found to be expressed in ECs (predominantly NOX4), SMCs (predominantly NOX1,4) and Fibs (predominantly NOX4) in pulmonary arteries [107]. Expression of NOX5 was found to be present only in pulmonary artery ECs and SMCs [108]. It is important to mention, that mRNA expression profiles do not correspond to protein expression and enzyme activity. Yet, because currently available antibodies are highly nonspecific, it is a widely used method for localization of quantification. NOXs associate in membranes with signaling domains, such as calveolae (NOX1, NOX2, and NOX4) or lipid rafts (NOX2 and NOX4). They catalyze the transfer of electrons from NADPH to molecular oxygen and thus generating ROS . Vascular NOXs produce superoxide, except for NOX4 isoform, which primarily produces H2O2. The stability of ROS produced by NOXs makes them likely to act as signaling molecules. Activation of NOX1,2 requires interaction with additional proteins. NOX5 is activated directly through calcium binding. The NOX2 catalytic subunit needs to be associated with p22phox membrane regulatory subunit and p47phox, p40phos, Rac2, and p67phox cytosolic regulatory subunits. Activation of this enzymatic isoform thus requires both assembly and phosphorylation steps. Similarly, NOX1 forms a complex with p22phox. NOX5 is lacking p22phox regulatory subunit for its function. NOX4 activity is different from other NOXs, as it is the only constitutively expressed NOX enzyme. It does not require p22phox regulatory subunit, but the polymerase delta-interacting protein (Poldip2) and the p47phox-related adaptor protein Tks5 have been reported to modestly enhance its activity [109, 110]. Thus its activity is regulated mainly by its expression and availability of reducing equivalents. Moreover, this isoform is upregulated under hypoxic conditions, as the promoter of this protein isoform has a HIF binding site [111]. Together with enhanced glycolysis and pentose phosphate pathway and suppressed mitochondrial respiration in PH arterial cells (being NADH and NADPH producers), NOX4 activity is significantly elevated [16, 112]. As such, it can regulate cellular function through growth factors (TGF-β and PPAR-γ), cytokines, HIF, vasoactive agents (endothelin-1) or G protein-coupled receptor agonists allowing it to regulate enzymes and ion channels with proliferative, inflammatory, and apoptotic properties. Indeed, increased NOX4 activity was sufficient to enha,nce fibroblast migration and proliferation [112, 113]. Similarly, it has been shown to be critical for HIF2 transcriptional activity in renal carcinoma [114]. Another target was described to be Ca2+ permeable cation channel TRPC, through which 60% of Ca2+ enters the pulmonary artery SMC. It can thus initiate vasoconstriction, later remodeling of pulmonary artery and also endothelial barrier dysfunction [115]. Its importance highlight the fact that it was found to be the only isoform present in all layers of pulmonary artery which is increased in various models of PH [112]. Thus, NOX4 was suggested to play a key role in chronic hypoxia-induced pulmonary vascular remodeling, which can be suppressed using new NOX1/4 inhibitor (GKT137831) [116]. Unfortunately, many previous studies using NOXs inhibitors such as diphenyliodonium (DPI) or apocynin cannot be interpreted as being solely due to NOX activity involvement because, for example, DPI was found to be a general flavoprotein inhibitor and apocynin was described to behave like an antioxidant in the cell. However, new generation inhibitors are also not specific for individual protein isoforms. Therefore, the results of these studies often reflect manipulation of redox status within whole cell, i.e., mitochondrial and other sources.
The subcellular localization of NOX4 in vascular cells is more complex than other NOX isoforms. In lung ECs, expression of native NOX4 is predominantly localized to the nucleus as compared to endoplasmic reticulum, mitochondria or plasma membrane [117]. NOX4 has a cis-acting ARE sequence, which is regulated by Nrf2 under stress conditions (i.e., hyperoxia), and, thus, the catalyzed H2O2 signal is part of a cellular adaptation response to oxidative stress. For that, nuclear localization of NOX4 is important and its expression can be regulated. The Km of NOX4 for oxygen has been described to be unusually high. This together with non-inducible production of H2O2 could mean that it responds directly and acutely to oxygen tension with output of the signal molecule (H2O2), rather than responding to external signals via intermediate signaling mechanisms such as phosphorylation of regulatory subunits or changes in cellular calcium levels. Thus NOX4 signaling may participate in both acute mechanisms and slower ones involving induction of NOX4 expression [118]. As mentioned previously, enhanced glycolytic metabolism of PH arterial cells is similar in certain ways to cancer metabolism. Cancer cells with compromised mitochondrial function due to p53 loss showed increased NOX4 activity and enhanced sensitivity to NOX4 inhibition. Activation of NOX4 in that case was suggested to provide additional NAD+ to support the highly active glycolysis [119] in addition to regulation of several signaling pathways (JAK-STAT, protein kinase C, MAPK, AKT, and inflammatory mediators via the NF-κB pathways etc.). TGF-β was described to be involved in pathophysiological vascular remodeling in PH and interestingly, to increase NOX4 protein levels [120]. Protein kinases C, angiotensin II, and cyclic adenosine monophosphate are other stimuli that modulate NOX4 expression.
Another isoform found in PH cells, NOX2 , was originally found to be highly expressed in phagocytes. In mice, NOX2 deletion reverses hypoxia induced PH [121]. However, how NOX2 contributes to PH is not fully understood. It might regulate inflammation as a part of vessel remodeling.
Thus, NOXs are important sources of ROS production in PH pathology; however, the activity of individual isoforms present, their regulation and signaling properties need to be better determined in relation to PH development [16].
6 Interplay Between Mitochondria and NADPH Oxidases
The nonspecific nature of in situ ROS detection methods combined with a disregard of ROS compartmentalization within a cell has led to still unresolved controversies regarding the contribution of individual ROS sources to cell signaling. However, it is suggested that substantial interplay between different sources exists. For example, mitochondrial ROS can be amplified by cytoplasmic NOX and vice versa, leading to feed-forward process that augments the pro-oxidative status required for pathological signaling in PH. There is evidence regarding mitochondrial redox involvement in NOX activity [122, 123]. One report by Rathore et al. [122] showed that inhibition of mitochondrial ROS production either with rotenone (Complex I) and myxothiazol (Complex III) or by mitochondrial Gpx1 overexpression prevented activation of NOX in pulmonary artery SMCs under hypoxic conditions. This points out a requirement of mitochondrial ROS for HIF stabilization, which can then further activate NOXs in pulmonary arteries through a mitochondrial ROS-PKCε-NOX4 axis. Wedgwood et al. described another example where attenuation of stretch-induced NOX4 expression was found to be a consequence of mitochondrial complex III inhibition in fetal pulmonary artery SMCs [123]. Moreover, Complex III lies upstream of NF-ҡB and NOX4 and is sensitive to ROS. Enhanced H2O2 production by NOX4 activity then leads to activation of genes necessary for cell cycle progression and thus induction of vascular remodeling.
On the other hand, changes in NOX activity also have an impact on mitochondrial redox status. For example, PKC dependent activation of NOXs by angiotensin is an early response seen in endothelial cells. This induces mitochondrial superoxide production, which becomes deleterious for mitochondrial function. It opens the mitochondrial KATP channel, causes matrix alkalization, swelling, mild mitochondrial uncoupling and further ROS production [124]. Another report describes inhibition of mitochondrial Complex I (NDUFA9 subunit) by increased NOX4 activity in HUVEC cells [125]. NOX was suggested to affect the synthesis and/or stability of Complex I subunits encoded in the mitochondria.
Based on the evidence above it is obvious that mitochondrial ROS interact with NOXs and collectively induce a pro-oxidative redox status in cells which further participates in signaling during PH development. Adesina et al. performed elegant experiments to establish this relationship [126]. They overexpressed mitochondrial superoxide dismutase (SOD2) in mice, which converts superoxide to H2O2 in mitochondria. This lead to enhanced mtH2O2 production, which induced NOX activity (NOX2 and NOX4 isoforms), leading to pulmonary vascular cell proliferation. On the other hand, mice with increased expression of mitochondria-targeted catalase, which decompose mitochondrial H2O2, suppressed NOX expression and attenuated ECs proliferation (i.e., downregulated cyclinD1 and PCNA) in hypoxia induced PH [126]. This suggests that mitochondrial redox balance might be suitable target for pharmacological treatment of PH .
7 Other ROS Sources and Antioxidant Balance
Most intracellular ROS are derived from the superoxide radical. Mitochondrial superoxide production is not the only cellular source. It was shown that the exposure of pulmonary artery endothelial cells to hypoxic conditions results in increased activity of xanthine oxidase, which then generates a surplus of superoxide [127]. Enhanced xanthine oxidase activity was also reported in the arteries of PH patients [128]. ROS produced by xanthine oxidase upregulates Erg1 via ERK1/2 and increases its phosphorylation thus enhancing proliferation and reducing apoptosis [129]. However, superoxide can be converted to non-radical H2O2 by superoxide dismutases (SOD), present in mitochondria as well as the cytosol. H2O2 has a capacity to cross the membranes and its stability is much higher. This allows H2O2 to encounter susceptible residues on target molecules and display selectivity. Such signaling includes reversible oxidation of cysteines . This can cause allosteric changes of proteins to modify their activity or can affect binding with partners and thus alter signaling cascades. Interestingly, pulmonary hypertensive SMCs show decreased mitochondrial manganese superoxide dismutase (MnSOD), due to hypermethylation of the SOD2 gene [130]. Similarly, the disruption of extracellular SOD (EC-SOD) either globally or in specific cells of pulmonary arteries, results in worsening of experimental PH [131,132,133]. Decreased activity of EC-SOD in chronically hypoxic calves was shown to lead to upregulation of Erk1 via ERK1/2 [129]. The decreased enzymatic antioxidant protection in PH thus enables excessive production of ROS which can proceed to oxidative stress, the phenotype accompanying PH pathology. Several studies confirmed enhanced lipid peroxidation in human PH samples, i.e., increased F2 isoprostane and plasma malondialdehyde [134, 135]. On the other hand, nonenzymatic antioxidants, such as thioredoxin, were found to increase catalytic activity in many cancer cells. Moreover, the level of its activity was associated with aggressive tumor growth and poorer prognosis. Enhanced thioredoxin activity was shown to significantly increase HIF1α under both normoxic and hypoxic conditions [136]. This was confirmed to be valid in SMCs of chronic hypoxia-induced PH in mice, which is interesting with respect to downregulated enzymatic antioxidant protection. Increased activity of thioredoxin1 was reported to modulate HIF1 activation and subsequent phosphatidylinositol 3 kinase (PI3K)–seronine/threonine kinase (Akt) activation inducing proliferation [137]. Collectively, these data show that redox maintenance in PH is complex and compartmentalized. Thus, we need to consider not only the levels and type of ROS produced, but also state of specific antioxidant defense. ROS has naturally compartmentalized signaling properties; however, while exceeding antioxidant capacity they can establish rather pathological oxidative stress. Thus, cellular redox balance is important feature of cell proliferation in PH development.
In the pulmonary circulation the production of nitric oxide (NO) by ECs is crucial for normal function, as it allows vessel vasodilation. It also regulates vascular SMC proliferation and migration [138]. There is general consensus that bioavailability of NO is decreased and NO signaling is impaired during PH development. However, conflicting results exist concerning endothelial nitric oxide synthase (eNOS) expression and activity. Diminished NO signaling can be explained by eNOS uncoupling, which is a dysfunctional state of eNOS enzyme forming superoxide rather than NO [139, 140]. NO works almost exclusively as a signaling molecule, because it becomes injurious only after reacting with superoxide to form peroxynitrite anion. This can happen in PH when superoxide levels are increased. Peroxynitrite can then induce loss of function of protein kinase G and lead to the development of PH [141]. Peroxynitrite can also activate ERK [142], p38 MAP kinase [113], and protein kinase C [143].
Thus it is increasingly evident that redox balance is diverted toward pro-oxidative status during the development of PH. However, we are still missing detailed knowledge regarding the interplay and importance of individual ROS/NO sources in PH.
8 Summary
Pulmonary vascular remodeling in PH results from a synergy of changes in metabolism and redox balance in resident and recruited cells within the microenvironment of the artery wall. It involves endothelial cells, smooth muscle cells, fibroblasts, cells of immune system such as macrophages and the sympathetic nervous system. A prominent characteristic of the pulmonary artery remodeling is enhanced proliferation and apoptosis resistance of each of the resident cells comprising the vessel wall. Rapid or uncontrolled proliferation requires metabolic changes in cells in order to provide sufficient quantities of building blocks for protein synthesis and cell proliferation . Thus, it is not surprising that cells of hypertensive pulmonary artery wall shift toward glycolysis while suppressing catabolic reactions of mitochondrial metabolism. This provides cells with anabolic intermediates required for synthesis of amino acids, lipids, and nucleotides. This altered metabolism resembles the Warburg-like effect described for rapidly proliferating cancer cells. The cells increase glucose uptake , increase expression of enzymes of glycolytic pathways, and enhance the pentose phosphate pathway and one carbon metabolism, and thus the glycolytic pathway becomes a crucial source of energy production and metabolic intermediates. Mitochondrial bioenergetics is largely suppressed; fluxes of TCA cycle intermediates become altered to maintain mitochondrial membrane potential, to change redox signaling and to support production of metabolites for growth. Adjustment of mitochondrial energy metabolism gives rise to generation of ROS, being the initial provider of redox signaling, which is further amplified in cytosol by upregulation of NOX activity. We believe that the increased oxidative status supports the proliferation and together with activated cells of immune system participates in remodeling of pulmonary artery wall in PH. Chronic inflammation accompanying development of PH was described to require macrophage activation, whose first step includes metabolic changes. Thus, such metabolic and redox synergy might be key factors in pro-remodeling phenotype of cells that ultimately leads to severe PH and right ventricular failure.
References
Pugliese, S. C., et al. (2015). The role of inflammation in hypoxic pulmonary hypertension: From cellular mechanisms to clinical phenotypes. American Journal of Physiology. Lung Cellular and Molecular Physiology, 308(3), L229–L252.
Simonneau, G., et al. (2013). Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology, 62(25 Suppl), D34–D41.
Cottrill, K. A., & Chan, S. Y. (2013). Metabolic dysfunction in pulmonary hypertension: The expanding relevance of the Warburg effect. European Journal of Clinical Investigation, 43(8), 855–865.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: The next generation. Cell, 144(5), 646–674.
Tuder, R. M., et al. (2013). Relevant issues in the pathology and pathobiology of pulmonary hypertension. Journal of the American College of Cardiology, 62(25 Suppl), D4–12.
Tuder, R. M., Davis, L. A., & Graham, B. B. (2012). Targeting energetic metabolism: A new frontier in the pathogenesis and treatment of pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine, 185(3), 260–266.
Doherty, J. R., & Cleveland, J. L. (2013). Targeting lactate metabolism for cancer therapeutics. The Journal of Clinical Investigation, 123(9), 3685–3692.
Martinez-Outschoorn, U. E., Lisanti, M. P., & Sotgia, F. (2014). Catabolic cancer-associated fibroblasts transfer energy and biomass to anabolic cancer cells, fueling tumor growth. Seminars in Cancer Biology, 25, 47–60.
Wellen, K. E., & Thompson, C. B. (2012). A two-way street: Reciprocal regulation of metabolism and signalling. Nature Reviews. Molecular Cell Biology, 13(4), 270–276.
Hirschey, M. D., et al. (2015). Dysregulated metabolism contributes to oncogenesis. Seminars in Cancer Biology, 35(Suppl), S129–S150.
Fijalkowska, I., et al. (2010). Hypoxia inducible-factor1alpha regulates the metabolic shift of pulmonary hypertensive endothelial cells. The American Journal of Pathology, 176(3), 1130–1138.
Paulin, R., & Michelakis, E. D. (2014). The metabolic theory of pulmonary arterial hypertension. Circulation Research, 115(1), 148–164.
Zhao, L., et al. (2013). Heterogeneity in lung (18)FDG uptake in pulmonary arterial hypertension: Potential of dynamic (18)FDG positron emission tomography with kinetic analysis as a bridging biomarker for pulmonary vascular remodeling targeted treatments. Circulation, 128(11), 1214–1224.
Fessel, J. P., et al. (2012). Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulmonary Circulation, 2(2), 201–213.
Freund-Michel, V., et al. (2014). Mitochondria: Roles in pulmonary hypertension. The International Journal of Biochemistry & Cell Biology, 55, 93–97.
Plecita-Hlavata, L., et al. (2016). Constitutive reprogramming of fibroblast mitochondrial metabolism in pulmonary hypertension. American Journal of Respiratory Cell and Molecular Biology, 55(1), 47–57.
Marsboom, G., et al. (2012). Lung (1)(8)F-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine, 185(6), 670–679.
Smolkova, K., et al. (2011). Waves of gene regulation suppress and then restore oxidative phosphorylation in cancer cells. The International Journal of Biochemistry & Cell Biology, 43(7), 950–968.
Shimoda, L. A., & Laurie, S. S. (2014). HIF and pulmonary vascular responses to hypoxia. Journal of Applied Physiology, 116(7), 867–874.
Semenza, G. L. (2014). Hypoxia-inducible factor 1 and cardiovascular disease. Annual Review of Physiology, 76, 39–56.
Pedersen, P. L. (2008). Voltage dependent anion channels (VDACs): A brief introduction with a focus on the outer mitochondrial compartment’s roles together with hexokinase-2 in the “Warburg effect” in cancer. Journal of Bioenergetics and Biomembranes, 40(3), 123–126.
Leopold, J. A., et al. (2001). Glucose-6-phosphate dehydrogenase deficiency promotes endothelial oxidant stress and decreases endothelial nitric oxide bioavailability. The FASEB Journal, 15(10), 1771–1773.
Forgione, M. A., & Loscalzo, J. (2000). Oxidant stress as a critical determinant of endothelial function. Drug News & Perspectives, 13(9), 523–529.
Nutt, L. K. (2012). The Xenopus oocyte: A model for studying the metabolic regulation of cancer cell death. Seminars in Cell & Developmental Biology, 23(4), 412–418.
Pell, V. R., et al. (2016). Succinate metabolism: A new therapeutic target for myocardial reperfusion injury. Cardiovascular Research, 111(2), 134–141.
Tretter, L., Patocs, A., & Chinopoulos, C. (2016). Succinate, an intermediate in metabolism, signal transduction, ROS, hypoxia, and tumorigenesis. Biochimica et Biophysica Acta, 1857(8), 1086–1101.
Byun, J. S., & Gardner, K. (2013). C-terminal binding protein: A molecular link between metabolic imbalance and epigenetic regulation in breast cancer. International Journal of Cell Biology, 2013, 647975.
Li, M., et al. (2016). Metabolic reprogramming regulates the proliferative and inflammatory phenotype of adventitial fibroblasts in pulmonary hypertension through the transcriptional co-repressor C-terminal binding protein-1. Circulation, 134(15), 1105–1121.
Kim, J. H., & Youn, H. D. (2009). C-terminal binding protein maintains mitochondrial activities. Cell Death and Differentiation, 16(4), 584–592.
Wang, L., et al. (2015). CtBP maintains cancer cell growth and metabolic homeostasis via regulating SIRT4. Cell Death & Disease, 6, e1620.
Diaz-Ruiz, R., et al. (2008). Mitochondrial oxidative phosphorylation is regulated by fructose 1,6-bisphosphate. A possible role in Crabtree effect induction? The Journal of Biological Chemistry, 283(40), 26948–26955.
Dong, G., et al. (2016). PKM2 and cancer: The function of PKM2 beyond glycolysis. Oncology Letters, 11(3), 1980–1986.
Luo, W., et al. (2011). Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell, 145(5), 732–744.
Christofk, H. R., et al. (2008). Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature, 452(7184), 181–186.
Ashizawa, K., et al. (1991). In vivo regulation of monomer-tetramer conversion of pyruvate kinase subtype M2 by glucose is mediated via fructose 1,6-bisphosphate. The Journal of Biological Chemistry, 266(25), 16842–16846.
Anastasiou, D., et al. (2011). Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science, 334(6060), 1278–1283.
Gao, X., et al. (2012). Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Molecular Cell, 45(5), 598–609.
Tamada, M., Suematsu, M., & Saya, H. (2012). Pyruvate kinase M2: Multiple faces for conferring benefits on cancer cells. Clinical Cancer Research, 18(20), 5554–5561.
Luo, W., & Semenza, G. L. (2011). Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells. Oncotarget, 2(7), 551–556.
Zhang, H., Wang, D., Li, M., Plecitá, L., D’alessandro, A., Riddle, S., McKeon, B. A., Flockton, A., Frid, M., Ježek, P., El Kasmi, K., & Stenmark, K. (2016). Using genetics, epigenetics and small molecules to reverse metabolic reprogramming in adventitia fibroblasts for pulmonary hypertension therapy. American Journal of Respiratory and Critical Care Medicine, 193, A3895. American thoracic society international conference, San Francisco.
Hitosugi, T., et al. (2011). Tyrosine phosphorylation of mitochondrial pyruvate dehydrogenase kinase 1 is important for cancer metabolism. Molecular Cell, 44(6), 864–877.
Lee, D. C., et al. (2015). A lactate-induced response to hypoxia. Cell, 161(3), 595–609.
Parker, S. J., & Metallo, C. M. (2016). Chasing one-carbon units to understand the role of serine in epigenetics. Molecular Cell, 61(2), 185–186.
Maddocks, O. D., et al. (2016). Serine metabolism supports the methionine cycle and DNA/RNA methylation through de novo ATP synthesis in cancer cells. Molecular Cell, 61(2), 210–221.
Deschoemaeker, S., et al. (2015). PHD1 regulates p53-mediated colorectal cancer chemoresistance. EMBO Molecular Medicine, 7(10), 1350–1365.
Labuschagne, C. F., et al. (2014). Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells. Cell Reports, 7(4), 1248–1258.
Tavana, O., & Gu, W. (2013). The Hunger Games: p53 regulates metabolism upon serine starvation. Cell Metabolism, 17(2), 159–161.
Maddocks, O. D., et al. (2013). Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature, 493(7433), 542–546.
Chaneton, B., et al. (2012). Serine is a natural ligand and allosteric activator of pyruvate kinase M2. Nature, 491(7424), 458–462.
Kotas, M. E., & Medzhitov, R. (2015). Homeostasis, inflammation, and disease susceptibility. Cell, 160(5), 816–827.
Okabe, Y., & Medzhitov, R. (2016). Tissue biology perspective on macrophages. Nature Immunology, 17(1), 9–17.
Okabe, Y., & Medzhitov, R. (2014). Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell, 157(4), 832–844.
Chovatiya, R., & Medzhitov, R. (2014). Stress, inflammation, and defense of homeostasis. Molecular Cell, 54(2), 281–288.
El Kasmi, K. C., et al. (2014). Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. Journal of Immunology, 193(2), 597–609.
Colegio, O. R., et al. (2014). Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature, 513(7519), 559–563.
O’Neill, L. A., & Pearce, E. J. (2016). Immunometabolism governs dendritic cell and macrophage function. The Journal of Experimental Medicine, 213(1), 15–23.
Tannahill, G. M., et al. (2013). Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature, 496(7444), 238–242.
Jha, A. K., et al. (2015). Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity, 42(3), 419–430.
Palsson-McDermott, E. M., et al. (2015). Pyruvate kinase M2 regulates Hif-1alpha activity and IL-1beta induction and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metabolism, 21(1), 65–80.
Kelly, B., et al. (2015). Metformin inhibits the production of reactive oxygen species from NADH:Ubiquinone oxidoreductase to limit induction of interleukin-1beta (IL-1beta) and boosts interleukin-10 (IL-10) in lipopolysaccharide (LPS)-activated macrophages. The Journal of Biological Chemistry, 290(33), 20348–20359.
Qualls, J. E., et al. (2012). Sustained generation of nitric oxide and control of mycobacterial infection requires argininosuccinate synthase 1. Cell Host & Microbe, 12(3), 313–323.
O’Neill, L. A. (2011). A critical role for citrate metabolism in LPS signalling. The Biochemical Journal, 438(3), e5–e6.
Imtiyaz, H. Z., & Simon, M. C. (2010). Hypoxia-inducible factors as essential regulators of inflammation. Current Topics in Microbiology and Immunology, 345, 105–120.
Li, M., et al. (2011). Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. Journal of Immunology, 187(5), 2711–2722.
DeBerardinis, R. J., et al. (2008). The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metabolism, 7(1), 11–20.
Mathupala, S. P., Ko, Y. H., & Pedersen, P. L. (2010). The pivotal roles of mitochondria in cancer: Warburg and beyond and encouraging prospects for effective therapies. Biochimica et Biophysica Acta, 1797(6–7), 1225–1230.
Vander Heiden, M. G., Cantley, L. C., & Thompson, C. B. (2009). Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science, 324(5930), 1029–1033.
Randle, P. J., et al. (1963). The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet, 1(7285), 785–789.
Zhao, Y., et al. (2014). Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS One, 9(2), e88727.
Sutendra, G., et al. (2010). Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Science Translational Medicine, 2(44), 44ra58.
Laurent, G., et al. (2013). SIRT4 represses peroxisome proliferator-activated receptor alpha activity to suppress hepatic fat oxidation. Molecular and Cellular Biology, 33(22), 4552–4561.
Metallo, C. M., et al. (2012). Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature, 481(7381), 380–384.
Intlekofer, A. M., et al. (2015). Hypoxia induces production of L-2-hydroxyglutarate. Cell Metabolism, 22(2), 304–311.
Dang, L., et al. (2009). Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature, 462(7274), 739–744.
Fendt, S. M., et al. (2013). Reductive glutamine metabolism is a function of the alpha-ketoglutarate to citrate ratio in cells. Nature Communications, 4, 2236.
Shim, E. H., et al. (2014). L-2-Hydroxyglutarate: An epigenetic modifier and putative oncometabolite in renal cancer. Cancer Discovery, 4(11), 1290–1298.
Salminen, A., et al. (2014). Krebs cycle dysfunction shapes epigenetic landscape of chromatin: Novel insights into mitochondrial regulation of aging process. Cellular Signalling, 26(7), 1598–1603.
Pak, O., et al. (2013). Mitochondrial hyperpolarization in pulmonary vascular remodeling. Mitochondrial uncoupling protein deficiency as disease model. American Journal of Respiratory Cell and Molecular Biology, 49(3), 358–367.
Dromparis, P., et al. (2013). Uncoupling protein 2 deficiency mimics the effects of hypoxia and endoplasmic reticulum stress on mitochondria and triggers pseudohypoxic pulmonary vascular remodeling and pulmonary hypertension. Circulation Research, 113(2), 126–136.
Dromparis, P., Sutendra, G., & Michelakis, E. D. (2010). The role of mitochondria in pulmonary vascular remodeling. Journal of Molecular Medicine, 88(10), 1003–1010.
Cortassa, S., O’Rourke, B., & Aon, M. A. (2014). Redox-optimized ROS balance and the relationship between mitochondrial respiration and ROS. Biochimica et Biophysica Acta, 1837(2), 287–295.
Aon, M. A., Cortassa, S., & O'Rourke, B. (2010). Redox-optimized ROS balance: A unifying hypothesis. Biochimica et Biophysica Acta, 1797(6–7), 865–877.
Brand, M. D. (2010). The sites and topology of mitochondrial superoxide production. Experimental Gerontology, 45(7–8), 466–472.
Chouchani, E. T., et al. (2014). Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature, 515(7527), 431–435.
Treberg, J. R., Quinlan, C. L., & Brand, M. D. (2011). Evidence for two sites of superoxide production by mitochondrial NADH-ubiquinone oxidoreductase (complex I). The Journal of Biological Chemistry, 286(31), 27103–27110.
Dlaskova, A., Hlavata, L., & Jezek, P. (2008). Oxidative stress caused by blocking of mitochondrial complex I H(+) pumping as a link in aging/disease vicious cycle. The International Journal of Biochemistry & Cell Biology, 40(9), 1792–1805.
Bleier, L., et al. (2015). Generator-specific targets of mitochondrial reactive oxygen species. Free Radical Biology & Medicine, 78, 1–10.
Quinlan, C. L., et al. (2012). Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. The Journal of Biological Chemistry, 287(32), 27255–27264.
Orr, A. L., et al. (2012). A refined analysis of superoxide production by mitochondrial sn-glycerol 3-phosphate dehydrogenase. The Journal of Biological Chemistry, 287(51), 42921–42935.
Starkov, A. A., et al. (2004). Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. The Journal of Neuroscience, 24(36), 7779–7788.
Tretter, L., & Adam-Vizi, V. (2004). Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. The Journal of Neuroscience, 24(36), 7771–7778.
Sanchez-Caballero, L., Guerrero-Castillo, S., & Nijtmans, L. (2016). Unraveling the complexity of mitochondrial complex I assembly: A dynamic process. Biochimica et Biophysica Acta, 1857(7), 980–990.
Tello, D., et al. (2011). Induction of the mitochondrial NDUFA4L2 protein by HIF-1alpha decreases oxygen consumption by inhibiting Complex I activity. Cell Metabolism, 14(6), 768–779.
Valsecchi, F., et al. (2013). Primary fibroblasts of NDUFS4(-/-) mice display increased ROS levels and aberrant mitochondrial morphology. Mitochondrion, 13(5), 436–443.
Santidrian, A. F., et al. (2014). Nicotinamide phosphoribosyltransferase can affect metastatic activity and cell adhesive functions by regulating integrins in breast cancer. DNA Repair (Amst), 23, 79–87.
Rathore, R., et al. (2006). Mitochondrial ROS-PKC epsilon signaling axis is uniquely involved in hypoxic increase in [Ca2+]i in pulmonary artery smooth muscle cells. Biochemical and Biophysical Research Communications, 351(3), 784–790.
Waypa, G. B., et al. (2006). Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circulation Research, 99(9), 970–978.
Archer, S. L., et al. (1993). A redox-based O2 sensor in rat pulmonary vasculature. Circulation Research, 73(6), 1100–1112.
Chandel, N. S., et al. (1998). Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proceedings of the National Academy of Sciences of the United States of America, 95(20), 11715–11720.
Sabharwal, S. S., et al. (2013). Peroxiredoxin-5 targeted to the mitochondrial intermembrane space attenuates hypoxia-induced reactive oxygen species signalling. The Biochemical Journal, 456(3), 337–346.
Paulin, R., et al. (2014). Sirtuin 3 deficiency is associated with inhibited mitochondrial function and pulmonary arterial hypertension in rodents and humans. Cell Metabolism, 20(5), 827–839.
Kincaid, B., & Bossy-Wetzel, E. (2013). Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Frontiers in Aging Neuroscience, 5, 48.
Ozden, O., et al. (2014). SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radical Biology & Medicine, 76, 163–172.
Pagano, G., et al. (2014). Oxidative stress and mitochondrial dysfunction across broad-ranging pathologies: Toward mitochondria-targeted clinical strategies. Oxidative Medicine and Cellular Longevity, 2014, 541230.
Willems, P. H., et al. (2015). Redox homeostasis and mitochondrial dynamics. Cell Metabolism, 22(2), 207–218.
Ryan, J., et al. (2015). Mitochondrial dynamics in pulmonary arterial hypertension. Journal of Molecular Medicine, 93(3), 229–242.
Konior, A., et al. (2014). NADPH oxidases in vascular pathology. Antioxidants & Redox Signaling, 20(17), 2794–2814.
Pandey, D., et al. (2012). Expression and functional significance of NADPH oxidase 5 (Nox5) and its splice variants in human blood vessels. American Journal of Physiology. Heart and Circulatory Physiology, 302(10), H1919–H1928.
Lyle, A. N., et al. (2009). Poldip2, a novel regulator of Nox4 and cytoskeletal integrity in vascular smooth muscle cells. Circulation Research, 105(3), 249–259.
Sedeek, M., et al. (2009). Molecular mechanisms of hypertension: Role of Nox family NADPH oxidases. Current Opinion in Nephrology and Hypertension, 18(2), 122–127.
Diebold, I., et al. (2010). The NADPH oxidase subunit NOX4 is a new target gene of the hypoxia-inducible factor-1. Molecular Biology of the Cell, 21(12), 2087–2096.
Barman, S. A., et al. (2014). NADPH oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arteriosclerosis, Thrombosis, and Vascular Biology, 34(8), 1704–1715.
Li, S., et al. (2008). NOX4 regulates ROS levels under normoxic and hypoxic conditions, triggers proliferation, and inhibits apoptosis in pulmonary artery adventitial fibroblasts. Antioxidants & Redox Signaling, 10(10), 1687–1698.
Maranchie, J. K., & Zhan, Y. (2005). Nox4 is critical for hypoxia-inducible factor 2-alpha transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Research, 65(20), 9190–9193.
Malczyk, M., et al. (2016). NADPH oxidases-do they play a role in TRPC regulation under hypoxia? Pflügers Archiv, 468(1), 23–41.
Green, D. E., et al. (2012). The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. American Journal of Respiratory Cell and Molecular Biology, 47(5), 718–726.
Kuroda, J., et al. (2005). The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes to Cells, 10(12), 1139–1151.
Nisimoto, Y., et al. (2014). Nox4: A hydrogen peroxide-generating oxygen sensor. Biochemistry, 53(31), 5111–5120.
Lu, W., et al. (2012). Novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biology, 10(5), e1001326.
Ismail, S., et al. (2009). NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: The role of autocrine production of transforming growth factor-{beta}1 and insulin-like growth factor binding protein-3. American Journal of Physiology. Lung Cellular and Molecular Physiology, 296(3), L489–L499.
Liu, J. Q., et al. (2006). Hypoxic pulmonary hypertension: Role of superoxide and NADPH oxidase (gp91phox). American Journal of Physiology. Lung Cellular and Molecular Physiology, 290(1), L2–10.
Rathore, R., et al. (2008). Hypoxia activates NADPH oxidase to increase [ROS]i and [Ca2+]i through the mitochondrial ROS-PKC epsilon signaling axis in pulmonary artery smooth muscle cells. Free Radical Biology & Medicine, 45(9), 1223–1231.
Wedgwood, S., et al. (2013). Increased p22(phox)/Nox4 expression is involved in remodeling through hydrogen peroxide signaling in experimental persistent pulmonary hypertension of the newborn. Antioxidants & Redox Signaling, 18(14), 1765–1776.
Costa, A. D., et al. (2006). The direct physiological effects of mitoK(ATP) opening on heart mitochondria. American Journal of Physiology. Heart and Circulatory Physiology, 290(1), H406–H415.
Koziel, R., et al. (2013). Mitochondrial respiratory chain complex I is inactivated by NADPH oxidase Nox4. The Biochemical Journal, 452(2), 231–239.
Adesina, S. E., et al. (2015). Targeting mitochondrial reactive oxygen species to modulate hypoxia-induced pulmonary hypertension. Free Radical Biology & Medicine, 87, 36–47.
Terada, L. S., et al. (1992). Hypoxia injures endothelial cells by increasing endogenous xanthine oxidase activity. Proceedings of the National Academy of Sciences of the United States of America, 89(8), 3362–3366.
Spiekermann, S., Schenk, K., & Hoeper, M. M. (2009). Increased xanthine oxidase activity in idiopathic pulmonary arterial hypertension. The European Respiratory Journal, 34(1), 276.
Hartney, T., et al. (2011). Xanthine oxidase-derived ROS upregulate Egr-1 via ERK1/2 in PA smooth muscle cells; model to test impact of extracellular ROS in chronic hypoxia. PLoS One, 6(11), e27531.
Archer, S. L., et al. (2010). Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: A basis for excessive cell proliferation and a new therapeutic target. Circulation, 121(24), 2661–2671.
Chow, K., et al. (2013). Dysfunctional resident lung mesenchymal stem cells contribute to pulmonary microvascular remodeling. Pulmonary Circulation, 3(1), 31–49.
Kamezaki, F., et al. (2008). Gene transfer of extracellular superoxide dismutase ameliorates pulmonary hypertension in rats. American Journal of Respiratory and Critical Care Medicine, 177(2), 219–226.
Xu, D., et al. (2011). Exacerbated pulmonary arterial hypertension and right ventricular hypertrophy in animals with loss of function of extracellular superoxide dismutase. Hypertension, 58(2), 303–309.
Cracowski, J. L., et al. (2001). Increased lipid peroxidation in patients with pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine, 164(6), 1038–1042.
Irodova, N. L., et al. (2002). Oxidative stress in patients with primary pulmonary hypertension. Bulletin of Experimental Biology and Medicine, 133(6), 580–582.
Welsh, S. J., et al. (2002). The redox protein thioredoxin-1 (Trx-1) increases hypoxia-inducible factor 1alpha protein expression: Trx-1 overexpression results in increased vascular endothelial growth factor production and enhanced tumor angiogenesis. Cancer Research, 62(17), 5089–5095.
Chen, B., et al. (2013). Thioredoxin-1 mediates hypoxia-induced pulmonary artery smooth muscle cell proliferation. American Journal of Physiology. Lung Cellular and Molecular Physiology, 305(5), L389–L395.
Zuckerbraun, B. S., et al. (2007). Nitric oxide-induced inhibition of smooth muscle cell proliferation involves S-nitrosation and inactivation of RhoA. American Journal of Physiology. Cell Physiology, 292(2), C824–C831.
Beckman, J. S., & Koppenol, W. H. (1996). Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and ugly. The American Journal of Physiology, 271(5 Pt 1), C1424–C1437.
Tabima, D. M., Frizzell, S., & Gladwin, M. T. (2012). Reactive oxygen and nitrogen species in pulmonary hypertension. Free Radical Biology & Medicine, 52(9), 1970–1986.
Zhao, Y. Y., et al. (2009). Persistent eNOS activation secondary to caveolin-1 deficiency induces pulmonary hypertension in mice and humans through PKG nitration. The Journal of Clinical Investigation, 119(7), 2009–2018.
Jernigan, N. L., Walker, B. R., & Resta, T. C. (2004). Endothelium-derived reactive oxygen species and endothelin-1 attenuate NO-dependent pulmonary vasodilation following chronic hypoxia. American Journal of Physiology. Lung Cellular and Molecular Physiology, 287(4), L801–L808.
Agbani, E. O., et al. (2011). Peroxynitrite stimulates pulmonary artery endothelial and smooth muscle cell proliferation: Involvement of ERK and PKC. Pulmonary Pharmacology & Therapeutics, 24(1), 100–109.
Acknowledgments
This work has been supported by KONTAKT grant (LH 15071) from Czech Ministry of Education to LPH and by NIH Program Project Grant HL014985, NIH Axis Grant HL114887, and Department of Defense Grant PR140997 to KRS, KEK, ML, and HZ.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Plecitá-Hlavatá, L. et al. (2017). Metabolic Reprogramming and Redox Signaling in Pulmonary Hypertension. In: Wang, YX. (eds) Pulmonary Vasculature Redox Signaling in Health and Disease. Advances in Experimental Medicine and Biology, vol 967. Springer, Cham. https://doi.org/10.1007/978-3-319-63245-2_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-63245-2_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-63244-5
Online ISBN: 978-3-319-63245-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)