Abstract
The present investigation is carried out for the production of biofuel (biodiesel) from animal fat with an active and promising nano-catalyst (Cs/Al/Fe3O4) via transesterification process. The catalyst was prepared by chemical precipitation and impregnation method through which nano size was controlled. Characterization was done by using SEM, XRD, TGA and BET to identify morphology, chemical composition, thermal stability and surface area respectively. The conversion of animal fat to fatty acid methyl ester was influenced by reaction conditions such as molar ratio of methanol to oil, catalyst concentration, reaction time and temperature. The optimum results were obtained at 60 min reaction time at 60 °C for 3 wt% nano-catalyst and 12:1 methanol to oil molar ratio to achieve a maximum yield of 97 wt%. The processed biodiesel was characterized as per ASTM methods and compare with ASTM standards.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
15.1 Introduction
The dependence on fossil fuel resources has been waning due to the concern about the environment and decrease in petroleum reserve [1]. Therefore, researches have been focused on finding an alternative source for the growth of mankind. Biodiesel (Fatty acid methyl ester) will be the best option to replace petro diesel. Use of biodiesel comparatively reduces the unburned hydrocarbon, carbon monoxide and particulate matter. They are biodegradable, non toxic and renewable being derived from organic materials such as animal fats, edible and non edible oils. It is produced through transesterification reaction using homogeneous and heterogeneous catalyst with methanol. The use of conventional catalyst has more problems in purification process, and recycling and hence economically undesirable [2]. Implementation of nano catalysts can reduce the time for purification and recycling process and also their high catalytic surface increases biodiesel conversion. This catalyst will eliminate the problems related to conventional catalysts. Recent study on nano form of catalyst showed high activity, less reaction time, better catalytic activity and stability for the biodiesel production. Beef tallow is one of the cheap raw materials used for the production of biodiesel. In all the developing as well as the under developing countries, huge amount of animal fat is thrown out as waste. Only partial amount of animal fat is utilized by the soap manufacturing industries. Beef tallow contains lesser free fatty acid when compared to other edible and non edible oils due to which the acid value range lies between 2–10 mg KOHg−1 of oil. Therefore, in order to reduce the production cost of biodiesel combination of waste beef tallow with nano based catalyst was identified.
The nano catalyst (CS/Al/Fe3O4) was prepared from CS, Al and Fe3O4 via impregnation method. The physical appearance, thermal properties, shape and structure were characterized using scanning electron microscopy (SEM), Thermo-gravimetric analyzer (TGA) and X-ray diffractometer (XRD) respectively. The optimum condition for transesterification and the various parameters that affects biodiesel production were investigated using nano catalyst.
15.2 Experimental Materials and Methods
15.2.1 Preparation of (CS/Al/Fe3O4) Nano-catalyst
The catalyst Cs/Al/Fe3O4 was prepared by impregnation method. Three moles of NaOH were added to 1 L of deionized water and dissolved. Sodium metaaluminate solution was produced by the addition of aluminum sheet into it. Then, a 2 M of CsNO3 aqueous solution was mixed with sodium metaaluminate solution and this mixture was taken in a round bottomed flask fitted with a condenser heating around 60 °C. The mixture was kept under rigorous stirring at 60 °C to obtain pH to 12. The obtained precipitate was then dried in the oven at 120 °C for 12 h to produce the catalyst precursor [3]. Then the precursor was thermally treated at 650 °C for 6 h to give CsO. The magnetic property of the catalyst was identified via co precipitation method while adding Fe3O4 nanoparticles into the mixed solution of NaOH, Al and CsOH under vigorous stirring at 70 °C for 6 h and the wet powder was dried under vacuum for 10 h. In order to achieve the magnetic composite solid catalyst the dried powder was calcined at 700 °C for 6 h.
15.2.2 Catalyst Characterization
The morphology of the nano-catalyst was identified using SEM (Hitachi S 4300). TGA for the catalyst was done to find the thermal degradation at a higher temperature using TA Instrument, TGA Q50. The powder form of catalyst was analyzed using XRD to determine the specific structure in TF-XRD, Rint-2500 diffractometer [4].
15.2.3 Feedstock
Waste beef tallow was collected from the slaughter house at Coimbatore, India. It was melted at 80 °C and filtered to remove the suspended particles and residue. The melted tallow was further centrifuged and decanted to make the oil clean for the next step. The chemicals required for the experiments were purchased from Merk, Mumbai, India.
15.2.4 Transesterification
The experiments were carried out in a 250 mL three-neck flat bottom round flask equipped with a reflux condenser to condense the methanol vapor. The reactants were mixed thoroughly by using a magnetic stirrer at 500 rpm. The whole setup was placed in a heating oil bath to maintain desirable reaction temperature. The mixture of methanol with catalyst was prepared separately and charged into the reactor containing 50 g tallow. Then it was heated up to a desired temperature. The process parameters like methanol to oil molar ratio, catalyst quantity, reaction temperature and reaction time were varied from 3:1 to 15:1, 1 to 6 wt%, 30 to 65 °C and 10 to 70 min respectively. When the reaction was completed, the sample was poured into a separating funnel kept without any disturbance. Due to gravity two layers were formed, the upper layer containing biodiesel and the lower layer containing glycerol [5]. The biodiesel yield was determined gravimetrically after removing methanol.
15.2.5 Characterization of Biodiesel
The physical and chemical properties of biodiesel were identified as per ASTM test methods to determine their qualities. The tests were repeated three times to determine its repeatability. The results obtained were compared with ASTM D 6751 standard for biodiesel.
15.3 Result and Discussion
15.3.1 Characterization of Catalyst
The SEM results show that the catalyst have large pores and eventually distributed granules provide very less dense particles. TGA thermogram indicates that a weight loss of 1.1 wt% takes place between 220–420 °C due to the removal of bounded moisture. The weight remains constant even with a further increase in temperature up to 800 °C. This reveals that catalyst is stable even at higher temperature. The powder XRD identifies that the Cs/Al/Fe3O4 forms a specific structure which has been clearly specified in the peaks.
15.3.2 Effect of Methanol to Oil Molar Ratio
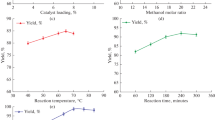
The yield of biodiesel increases with increase in methanol to oil ratio from 3:1 to 15:1. The addition of excess methanol will shift the equilibrium to right and brings better transesterification process yield. Optimum ratio was identified at 12:1 by consuming 3 wt% catalyst around 50 °C with the reaction being carried out for 50 min giving a conversion of 93 wt% of yield as shown in Fig. 15.1.
15.3.3 Effect of Catalyst Loading on Yield
Biodiesel yield was investigated for different catalyst quantity from 1 to 4 wt%. The maximum yield obtained was 93 wt% at 3 wt% where the reaction took place at 50 °C for 50 min and 12:1 methanol to oil molar ratio. Further increase in the amount of catalyst (4 wt%) resulted in no change in the yield as shown in Fig. 15.2.
15.3.4 Effect of Reaction Temperature
Figure 15.3, shows yield of biodiesel on varying the reaction temperature from 30 to 65 °C (approx. boiling point of methanol) with 3 wt% catalyst loading, 12:1 methanol to oil ratio and reaction time of 50 min. The yield increases with increase in temperature up to 60 °C. Thereafter, it remains constant at 96 wt%. Therefore, the optimum temperature for the biodiesel was at 60 °C.
15.3.5 Effect of Reaction Time
The variation of reaction time is also a parameter of conversion. Figure 15.4, shows that the conversion increases with the reaction time from 10–70 min. The reaction reaches the equilibrium at 60 min after which the yield of biodiesel remains constant. Therefore, 60 min reaction time is identified as an optimum for maximum yield of 97 wt% of biodiesel.
15.4 Characterization of Biodiesel
Table 15.1, shows the physical and chemical properties of the produced biodiesel. The fuel properties obtained were more compatible with the engines and in accordance with ASTM D 6751 standard.
15.5 Conclusion
The use of nano catalyst (Cs/Al/Fe3O4) took 60 min for the complete transesterification of tallow oil at 12:1 methanol to oil molar ratio, 3 wt% of catalyst at 60 °C thereby better result for synthesis of biodiesel. The catalyst confirms that it is effective in converting beef tallow into biodiesel. At optimum reaction condition the maximum yield of biodiesel was 97 wt%. The produced biodiesel was analyzed as per ASTM test methods and compared with ASTM D6751 standards. The results showed that they are in line with the standards and hence can be used in internal combustion engine without any modification.
References
J.W. Guo, T.S. Zhao, J. Prabhuram, R. Chen, C.W. Wong, Preparation and characterization of a PtRu/C nanocatalyst for direct methanol fuel cells. Electrochimi. Acta 51, 754–763 (2005)
M. Feyzi, A. Hassankhani, H.R. Rafiee, Preparation and characterization of Cs/Al/Fe3O4 Nano catalysts for biodiesel production. Energy Conv. Manag. 71, 62–68 (2013)
M. Kaur, A. Ali, Lithium ion impregated calcium oxide as nano catalyst for the biodiese production from karanja and jatropha ois. Renew. Energy 36, 2866–2871 (2011)
R. Madhuvilakku, S. Piraman, Biodiesel synthesis by TiO2-ZnO mixed oxide nano catalyst catayzed palm oil transesterification process. Bioresour. Technol. 150, 55–59 (2013)
F. Qiu, Y. Li, D. Yang, X. Li, P. Sun, Heterogeneous solid base nano catalyst: preparation, characterization and application in biodiesel production. Bioresour. Technol. 102, 4150–4156 (2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this paper
Cite this paper
Vijaya Kumar, B., Ramesh, K., Sivakumar, P. (2018). Production of Biofuel from Animal Fat Using Nano-catalyst via Single Step Transesterification Process. In: Anand, G., Pandey, J., Rana, S. (eds) Nanotechnology for Energy and Water . ICNEW 2017. Springer Proceedings in Energy. Springer, Cham. https://doi.org/10.1007/978-3-319-63085-4_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-63085-4_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-63084-7
Online ISBN: 978-3-319-63085-4
eBook Packages: EnergyEnergy (R0)