Abstract
The growth hormone (GH)-IGF-1 axis, which regulates postnatal growth and metabolism, progressively declines after puberty. This decline causes alterations in body composition and thus physical frailty in aging animals. By contrast, attenuation of the GH-IGF-1 axis consistently increases lifespan in a range of animals. Studies using mutant animals reveal key molecules for longevity in cytoplasmic IGF-1 signaling, including mechanistic target of rapamycin (mTOR) and forkhead box, subgroup O (FoxO) transcription factors. Dietary calorie restriction, a robust experimental intervention to extend lifespan in animals, also inhibits the GH-IGF-1 axis. Studies in human dwarf cohorts report lower incidences of cancers and cardiovascular diseases, though there is no scientific evidence of extended lifespan in these people. Genome-wide studies in long-lived people indicate an association between longevity and minor alleles of genes that lead to a reduction in IGF-1 signaling. Evolutionary views suggest a trade-off relation between growth and longevity. Therefore, it is rational to conclude that the GH-IGF-1 axis is the central pathway that regulates lifespan and thus aging in animals.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Growth hormone (GH) secreted from the pituitary gland is essential for postnatal growth in mammals (Butler and Le Roith 2001). The anabolic effect of GH is mostly mediated by IGF-1 , although GH also exerts direct effects on peripheral tissues. GH receptor (GHR) is expressed not only in the liver but also in many peripheral organs. Three-quarters of the circulating IGF-1 is estimated to originate from the liver, with the remainder probably derived from other tissues including adipose tissues. IGF-1 receptor is also expressed in many tissues. GH and IGF-1 mostly work in concert to affect tissue growth and metabolism, but work independently in some physiological conditions.
Synthesis and pulsatile release of GH from the pituitary gland are governed mainly by the opposing actions of hypothalamic GH-releasing hormone (GHRH) and somatostatin (Butler and Le Roith 2001). Secretion is also modulated depending on nutritional conditions, e.g., long-term reduction in calorie intake or protein deficiency inhibits GH release and lowers plasma concentrations of IGF-1.
Twenty-four-hour secretion of GH, which reaches a peak around puberty, has begun to decrease in young adults (Savine and Sonksen 2000; Sattler 2013). In most adults, GH secretion has progressively declined by the age of 60 to levels indistinguishable from those of adult GH deficiency patients with organic lesions in the pituitary gland. GH deficiency with aging causes a decrease in lean muscle mass and an increase in fat mass. These alterations in body composition can cause physical frailty as well as an increased risk of cardiovascular diseases in elderly people. As a result, clinical trials of GH administration have been conducted in elderly people to improve such conditions (Rudman et al. 1990).
By contrast, loss-of- or reduction-of-function mutations of genes involved in GH signaling consistently extend lifespan in a range of vertebrates and invertebrates (Bartke 2008) Dietary calorie restriction (DR), a simple but robust experimental intervention to extend lifespan in animals, inhibits the GH-IGF-1 axis (Shimokawa 2015). Genome-wide human studies also report a potential relationship between longevity and reductions in IGF-1 (van Heemst et al. 2005). This chapter describes the antagonistic pleiotropic effects of GH in the aging process.
2 Decline of the GH-IGF-1 Axis and Replacement Therapy in Elderly People
Acquired GH deficiency (GHD) in adults due to structural defects in the pituitary gland causes alterations in body composition such as reduced skeletal muscle and increased total and trunk fat mass (Sattler 2013). Adults with GHD also show worsened cardiovascular disease risk factors including insulin resistance, increments of blood total and LDL cholesterol, and high blood pressure. GH replacement therapy improves some, but not all, of these factors (Sattler 2013). In elderly people, GH levels, and thus circulating IGF-1 concentrations, are as low as those in GHD adults. However, whether the alterations in body composition in elderly people are caused by the reduced GH-IGF-1 axis and thus whether GH replacement can improve the aging-related changes in body composition remained unclear. To address this question, Rudman et al. (1990) conducted clinical trials in which biosynthetic human GH (hGH) was administered for 6 or 12 months in healthy people aged 61–81 years. The doses of hGH were adjusted to elevate plasma IGF-1 levels according to the range shown in young people. The 6-month trial resulted in increases in lean muscle mass and decreases in adipose tissue mass with no significant changes in body weight or bone density. There were also no adverse effects such as edema or hypertension. This study supports the hypothesis that reduced bioavailability of GH results in an altered body composition causing physical frailty in elderly people. In contrast, a subsequent 12-month study involving administration of hGH to a larger number of subjects identified a high frequency of side effects such as carpal tunnel syndrome, gynecomastia, and hyperglycemia (Cohn et al. 1993). A systematic review based on 31 articles describing randomized, controlled trials in 18 unique study populations indicates that GH treatment in elderly overweight (mean BMI 28 kg/m2) people increased lean body mass and decreased fat mass and total cholesterol without significant changes in body weight (Liu et al. 2007). However, the people treated with GH were significantly more likely to experience soft tissue edema, arthralgias, carpal tunnel syndrome, and gynecomastia. Some subjects also showed impaired fasting glucose and diabetes. The review concludes that GH administration cannot be recommended as an anti-aging therapy in elderly people because it increases the rates of adverse events, even though it induces small beneficial changes in body composition (Liu et al. 2007).
3 Loss-of- or Reduction-of Function Mutations of Genes in the GH Signal Transduction Pathway Consistently Extend Lifespan in a Range of Experimental Animals
Despite the aging–related decline of the GH-IGF-1 axis in animals, a large body of evidence indicates an extension of lifespan by inhibition of the GH-IGF-1 axis in mice (Fig. 5.1) and invertebrates.
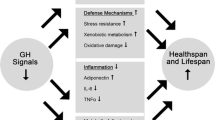
Regulation of the GH-IGF-1 axis. Deletion of genes encoding ligands or receptors (dark rectangles) in the axis consistently extends lifespan in mice. Overexpression of genes and thus proteins (shaded rectangles) also inhibits components of the GH-IGF-1 axis, leading to prolonged lifespan. IGF-1 signaling in the hypothalamus during the adult phase inhibits GHRH neurons (solid lines); during the early postnatal phase, IGF-1 promotes development of the GHRH-GH axis. There are no reports indicating an involvement of somatostatin (SIRF) or its specific receptors (SSTR) in the regulation of lifespan or aging in mammals
GH secretion is stimulated by GHRH and inhibited by somatostatin. The little mouse strain, in which the GHRH receptor gene is spontaneously mutated, lives longer than wild-type mice (Flurkey et al. 2001). Disruption of the Ghrh gene in mice also increases lifespan in both males and females (Sun et al. 2013). Little mice and Ghrh knockout (KO) mice both show dwarfism and obesity (an increase in fat pad/body weight), and have similar phenotypes.
Mutations of paired-like homeodomain factor 1 (Prop1) and POU domain, class 1, transcription factor 1 (Pou1f1 or Pit-1) genes cause a deficiency of GH, prolactin (PRL), and thyroid stimulating hormone (TSH), because these factors are required for development of anterior pituitary cells (Li et al. 1990; Sornson et al. 1996). Ames mice with a mutation of the Prop1 gene and Snell mice with a mutation of the Pit-1 gene display similar phenotypes including dwarfism and extended lifespan, compared with wild-type littermates (Brown-Borg et al. 1996; Flurkey et al. 2002). Although the potential effects of PRL and/or TSH deficiency on lifespan are not eliminated in these models, most researchers believe GH to be a key factor in the regulation of lifespan and aging , because overexpression of the GH gene in mice produces premature aging phenotypes and shortens lifespan (Steger et al. 1993). A recent study also indicates that twice-daily treatment with bovine GH in Ames mice aged 2–8 weeks reverses their long lifespan phenotype, indicating not only the importance of but also the critical stage of reduction in GH for longevity in mice (Panici et al. 2010). Moreover, there are no reports to indicate extension of lifespan by PRL or TSH deficiency in mice. The one exception to this is a study involving the chemical removal of TSH in rats, which reported an extension of lifespan compared with wild-type control rats (Ooka and Shinkai 1986).
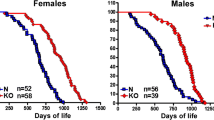
The significance of isolated GH deficiency on lifespan has been confirmed by gene disruption of GH receptor/binding protein (GHR/BP) in mice (Coschigano et al. 2000). Male and female Ghr −/− mice, in which plasma IGF-1 and IGFBP-3 are very low, outlived wild-type control mice.
Disruption of the IGF-1 receptor gene (Igf1r) is lethal at birth. Heterozygous mutants, Igf1r +/−, show a modest reduction in body weight (8% in males; 6% in females) compared with wild-type mice after weaning (Holzenberger et al. 2003). Lifespan is extended in female Igf1r +/− mice but not male Igf1r +/− mice. Female, but not male, Igf1r +/− mice also display resistance to oxidative stress induced by intraperitoneal injection of paraquat, compared with wild-type mice. A reduction in cytoplasmic IGF-1 signaling is demonstrated by a comparison of MEF cells derived from Igf1r +/− mice and those from wild-type mice. The original study was conducted on mice with the genetic background of 129/SvPas. In a subsequent study using C57BL/6J mice, Xu et al. (2014) confirmed the lifespan extension in female Igf1r +/− mice only; however, the life-prolonging effect was smaller in C57BL/6J females than in 129/SvPas females (11% increase in C57BL/6J Igf1r +/− mice versus 33% increase in 129/SvPas mice). This difference between mouse strains in the extent of lifespan extension by Igf1r +/− mutation could be due to differences in plasma IGF-1 levels and activation of IGF-1 signaling between the two strains. Plasma levels of IGF-1 were lower in C57BL/6J mice than in 129/SvPas mice (Xu et al. 2014). Similarly, indices of activation of IGF-1 signaling in tissues such as phosphorylated IGF-1R and IRS-1 co-immunoprecipitated with IGF-1R were lower in C57BL/6J mice than in 129 Sv/Pas mice. Even in control wild-type mice, the strain with lower plasma IGF-1 and thus less activated IGF-1 signaling lived longer than the strain with higher IGF-1 levels. Therefore, the life-prolonging effect of the Igf1r +/− mutation seems to be smaller in C57BL/6J mice. Xu et al. (2014) also reported that oxidative stress hyper-activates IGF-1 signaling in tissues. They speculated that hyper-activation of IGF-1 signaling injures tissues; however, Igf1r +/− mutation limits damage and promotes survival by blocking an acute overreaction of IGF-1. This is a possible mechanism underlying the life-extending effect of reduced IGF-1 signaling.
Heterozygous mice for brain-specific deletion of Igf1r (bIgf1r) also show modest dwarfism . The body weight of bIgf1r +/− mice at 90 days of age is approximately 90% of the control mice in both males and females (Kappeler et al. 2008). In these bIgf1r +/− mice, development of hypothalamic GHRH neurons and pituitary GH secreting cells is impaired. Subsequently, the total GH content in the pituitary gland remains low throughout development. Plasma IGF-1 does not show any pubertal increase whereas control mice display the normal surge. The survival rates of bIgf1r +/− mice are greater in the first half of life in both males and females compared with control mice; however, there is no such increase in survival (lifespan) in the last quarter of life (Kappeler et al. 2008). Thus, the life-extending effect of a brain-specific reduction in IGF-1R could be minor.
Three-quarters of circulating IGF-1 derives from the liver. Svensson et al. (2011) tested the effect of liver-specific IGF-1 inactivation (LI-Igf1 −/−) on lifespan . Using the LoxP and Mx-Cre system, the Igf1 gene was inactivated at 1 month of age in C57BL/6 mice. Serum IGF-1 is reduced by 20% in LI-Igf1 −/− mice compared with wild-type mice. LI-Igf1 −/− male and female mice display reduced body weight mostly due to a decrease in body fat, but not lean mass. The mutant mice show a compensatory increase in GH levels, which are 3.1-fold greater in LI-Igf1 −/− mice than in wild-type mice. This compensatory change in GH levels may cause the alterations in body composition in LI-Igf1 −/− mice. Energy expenditure is modestly increased in LI-Igf1 −/− mice. Finally, the mean lifespan is 10% greater in female mice but not male mice (Svensson et al. 2011).
The mechanism underlying sexually dimorphic responses in lifespan and oxidative stress to reduced IGF-1 signaling remains to be elucidated. In addition, if GH levels are increased in Igf1r +/− mice and LI-Igf1 −/− mice from a negative feedback mechanism through the pituitary gland, this might have some adverse effects, particularly on male mice.
Circulating IGF-1 forms a ternary complex with one of the IGF binding proteins (IGFBPs) and acid-labile subunit (ALS) (Butler and Le Roith 2001). Of the six different IGFBPs, IGFBP3, produced by the liver under the regulation of GH, is the major carrier for IGF-1 in the blood. The remaining IGFBPs are expressed in a tissue-specific manner and regulate bioactivity in specific tissues and cells. Pregnancy-associated plasma protein A (PAPPA) is a metzincin superfamily metalloproteinase in the IGF system (Boldt and Conover 2007). PAPPA cleaves IGFBP4, leading to an increase in IGF-1 bioavailability and mitogenic effectiveness in vitro. Pappa-KO mice show dwarfism due to an increase in IGFBP4 expression that reduces the bioavailability of IGF-1 (Conover and Bale 2007). Plasma concentrations of GH and IGF-1 tend to show a 70% reduction in Pappa-KO mice compared with those of wild-type mice, though this is statistically insignificant. Male and female Pappa-KO mice also live longer than wild-type control mice (Conover et al. 2010).
In long-lived mouse models, in which circulating levels, bioavailability, or cytoplasmic signal transduction of IGF-1 is reduced, the extent of lifespan extension varies depending on the timing of gene disruption, gene dose, sex, and genetic background of mice.
In rats, the effect of inhibition of the GH-IGF-1 axis on lifespan and aging remains controversial. A modest suppression of the GH-IGF-1 axis by overexpression of an antisense of the Gh gene in Wistar rats results in dwarfism in adolescent rats heterozygous for the transgene (tg/−) versus wild-type rats (−/−) (Shimokawa et al. 2002). Compared with wild-type rats, dwarf (tg/−) rats show a 30% decrease in mean food intake and body weight with a 40% reduction in plasma IGF-1 (Shimokawa et al 2002). By contrast, dwarf rats originally derived from the Lewis strain do not live longer than control rats (Sonntag et al. 2005). However, GH administration after weaning for 10 weeks in dwarf rats from the Lewis strain extends lifespan compared with those without GH treatment and control normal-size rats (Sonntag et al. 2005). This rat model, in which GH is administered at a young age, is considered to represent adult onset GH deficiency, because the plasma IGF-1 concentration returns to the same level as that in control dwarf rats at 2 weeks after termination of GH replacement. This work indicates the importance of GH during adolescence, a contradictory finding to that from dwarf mice (Panici et al. 2010).
Spontaneous dwarf rats derived from the Sprague-Dawley (SD) strain are reported to live longer than control SD rats (Kuramoto et al. 2010). Unfortunately, this study does not contain a longevity group of control SD rats. Instead, the lifespan data from these dwarf rats are compared with multiple data sets from SD rats published from the other institutes.
In summary, the role of GH and IGF-1 in lifespan extension remains elusive in rats.
4 Lifespan Extension by Single Gene Mutations Related to the Reduction of the GH-IGF-1 Axis
Since the report on lifespan extension in Ames dwarf mice in 1996, a number of single genes have been reported to extend lifespan in mice if they are spontaneously mutated or genetically engineered (Shimokawa et al. 2008). Many of the genes are clustered in GH-IGF-1 signaling, as described above. Some of the gene mutations also seem to affect GH-IGF-1 signaling secondarily.
αMUPA transgenic mice, overexpressing the urokinase-type plasminogen activator gene (Plau, also known as uPA) in the brain, live longer than wild-type controls (Miskin and Masos 1997). Plau is a serine protease that activates plasminogen by proteolytic cleavage into plasmin. Transgenic Plau is also expressed in the hypothalamic paraventricular nuclei, which are involved in regulation of appetite and energy expenditure. αMUPA mice display reduced food intake and body weight, and plasma IGF-1 is reduced by 30% compared with wild-type mice. In αMUPA mice, body temperature is reduced and the incidence of spontaneously occurring tumors and carcinogen-induced neoplastic lesions is decreased. These phenotypes resemble those of DR mice and some phenotypes are also displayed in GH-IGF-1-reducing mice (Miskin et al. 2005). Therefore, brain-specific, particularly hypothalamic, overexpression of Plau may affect GHRH neurons and their downstream signaling.
Overexpression of the Fgf21 gene in mice results in an extension of lifespan (Zhang et al. 2012). Fgf21, a hormone secreted by the liver, increases during periods of fasting, and sensitizes insulin actions (Potthoff et al. 2012). Fgf21 blocks somatic growth by induction of GH resistance. Fgf21-overexpressing mice show a decrease in circulating IGF-1 despite elevated GH levels, as compared with wild-type mice. Correspondingly, Fgf21-overexpressing mice are smaller than wild-type mice (Zhang et al. 2012). These hormonal alterations in Fgf21 transgenic mice are similar to those of GHR/BP-KO mice (Coschigano et al. 2000). Gene expression analysis of Fgf21 transgenic and DR mice has confirmed an overlap of genes significantly regulated in the liver, suggesting that Fgf21 signaling is a part of DR (Zhang et al. 2012).
Sirtuin 6 (Sirt6) acts in the DNA double-strand break repair system via deacetylation of CtBP-interacting protein (CtIp) and ADP ribosylation of poly ADP-ribose polymerase 1 (PARP1) (Gertler and Cohen 2013). Deletion of the Sirt6 gene in cells induces genomic instability (Mostoslavsky et al. 2006). Sirt6-KO mice exhibit premature aging phenotypes similar to those of XPA/CA or XPA/TTD null mice (Mostoslavsky et al. 2006). Conversely, overexpression of Sirt6 extends the lifespan of male mice but not female mice (Kanfi et al. 2012). In Sirt6-overexpressing mice, plasma IGF-1 levels are lower than those in wild-type mice. In addition, IGFBP1 is increased in Sirt6-KO mice. Decreased levels of phosphorylated IGF-1R and Akt indicate attenuation of IGF-1 signaling in tissues. Molecular mechanisms underlying the inhibition of IGF-1 by Sirt6 overexpression remain elusive.
Three long-lived mouse models also support the significance of inhibition of the GH-IGF-1 axis for longevity.
5 Genes Downstream of IGF-1 Signaling that Lead to Lifespan Extension
IGF-1 binds to specific tyrosine kinase receptors and activates downstream signaling molecules such as insulin receptor substrate (IRS) proteins, PI3K, and then Akt (O’Neill 2013). Akt phosphorylates multiple substrates such as mechanistic target of rapamycin (mTOR, activated), glycogen synthesis kinase 3 beta (GSK3β, inactivated), and FoxO transcription factors (inactivated). A number of genes encoding these cytoplasmic molecules are also reported to regulate lifespan in mice.
Whole body or brain-specific deletion of the Irs2 gene is reported to extend lifespan in mice (Taguchi et al. 2007). Brain-specific Irs2-KO mice, either homozygous or heterozygous, show hyperinsulinemia and mild glucose intolerance. However, the reduced IRS2 prevents aging-related decreases in FoxO1 and SOD2. Taguchi et al. (2007) speculated that attenuation of IRS2 signaling in the brain shielded against the harmful effects of hyperinsulinemia. However, controversial findings are reported by another group in Irs2 −/− mice (Selman et al. 2008). In their experiment, Irs2 −/− mice exhibited diabetic phenotypes and died earlier than wild-type mice. By contrast, it is reported that deletion of the Irs1 gene extends lifespan in female mice.
Akt1 +/− mice are also reported to outlive wild-type mice with 8% increase of lifespan in males and 14% increase in females (Nojima et al. 2013). In Akt1 +/− mice, the mTOR pathway, which regulates ribosomal biogenesis, protein synthesis, and mitochondrial activity, is attenuated. Glucose tolerance and insulin sensitivity are comparable between Akt1 +/− and wild-type mice, although Akt1 +/− mice display lower body weight and decreased body fat content compared with wild-type mice. The total protein and phosphorylated form of FoxO3 do not differ between Akt1 +/− and wild-type mice (Nojima et al. 2013).
The mTOR pathway exerts main effector proteins such as eukaryotic translation initiation factor 4E (eIF-4E)-binding protein 1 (4EBP1) and 70-kDa ribosomal S6 kinase (S6K) (O’Neill 2013). When activated, the mTOR pathway phosphorylates 4EBP1 and S6K and promotes protein translation and synthesis. Deletion of the S6K peptide 1 (Rps6kb1) in mice leads to extension of lifespan , though the effect is significant in females but not in males (Selman et al. 2009). Rps6kb −/− mice show a dwarf phenotype without reductions in plasma IGF-1 or pituitary GH. Motor functions, bone volume, and glucose tolerance are reported to be improved in Rps6kb −/− mice. The genetic model is a counterpart of a non-genetic, rapamycin (an mTOR kinase inhibitor) model for longevity (Harrison et al. 2009).
FoxO transcription factors are mammalian orthologs of DAF-16 in C. elegans (Greer and Brunet 2005). DAF-16 is needed for extension of lifespan following reductions in DAF-2 (mammalian IGF-1 receptor) and AGE-1 (a subunit of PI3K) signaling (Kenyon et al. 1993); that is, insulin-like signaling in C. elegans inhibits DAF-16 in nourished conditions. Target genes of FoxOs regulate cell cycle, DNA repair, stress resistance, apoptosis, autophagy, and metabolism in response to cellular and genotoxic stress (Greer and Brunet 2005). Negative energy balance is also a stimulator of FoxOs. In mice, under standard ad libitum feeding conditions, single deletion of the Foxo1, Foxo3, or Foxo4 gene resulted in minor alterations in the incidence of neoplasms and in lifespan. Only triple knockout of the Foxo1, Foxo3, and Foxo4 genes caused the cancer-prone phenotype and shortened lifespan, suggesting functional redundancy of FoxOs in the regulation of cancer and lifespan (Paik et al. 2007). However, under 30% DR conditions, in which wild-type mice live longer and are protected against neoplastic processes, FoxO1 and FoxO3 play differential roles in the life-extending and antineoplastic effects. In Foxo1 +/− mice, the antineoplastic effect of DR is diminished, though lifespan is extended to the same extent as that of wild-type mice (Yamaza et al. 2010). In contrast, Foxo3 +/− mice show a significant reduction in cancer incidence by DR in a lifespan study but little extension of lifespan (Shimokawa et al. 2015). These studies clearly demonstrate distinct roles for Foxo1 and Foxo3 in mammals. Isoform-specific functions of DAF-16 are also reported in C. elegans. A meta-analysis of direct FoxO targets across tissues and organisms using data of mammals, C. elegans, and Drosophila revealed evolutionarily conserved targets, enriched in growth factor signaling, metabolism, stress resistance and proteostasis (Webb et al. 2016). This study also identified candidate cofactors at conserved FoxO targets, e.g., CREB and ETS family factors.
A series of lifespan studies using mutant mice indicate a central role of the GH-IGF-1 axis and subsequent cytoplasmic signaling that inhibits mTOR and activates FoxO3 for longevity in mammals. DR inhibits GH secretion and lowers plasma concentrations of IGF-1. Therefore, it is probable that DR exerts its effects in part via the GH-IGF-1 axis and subsequent cytoplasmic signaling.
6 Evidence in Humans Indicating a Relation Between Longevity and Inhibition of the GH-IGF-1 Axis
Mouse and invertebrate models consistently indicate that reduction or deficiency of GH-IGF-1 signaling leads to longer lifespans compared with those of wild-type controls. This raises the question of whether similar mutations result in the extension of overall and healthy lifespans in humans.
A 22-year study of an Ecuadorian cohort with GHR gene mutations and IGF-1 deficiency, i.e., Laron syndrome, revealed that subjects showed a low incidence of cancer and diabetes compared with control relatives (Guevara-Aguirre et al. 2011). Subjects with Laron syndrome were obese, with lower serum insulin and increased insulin sensitivity. Subjects in the cohort displayed high mortality from common diseases of childhood. There was no evidence of extended lifespan in these subjects, although a small fraction (10%) of the cohort lived longer than 80 years (Guevara-Aguirre et al. 2011). A study of an Israeli cohort of Laron syndrome also indicates that these subjects have a lower incidence of cancers, despite the high prevalence of obesity (Shevah and Laron 2007).
Subjects in an Itabaianinha cohort with dwarfism from a mutation of the gene encoding the GHRH receptor also show a reduction in lean mass and an increase in percentage of fat mass (Oliveira et al. 2010). Some of the risk factors for cardiovascular diseases are also increased. However, these people do not display insulin resistance or evidence of premature atherosclerosis. A study from this cohort reports that serum adiponectin is increased without any changes in serum leptin (Oliveira et al. 2010). Insulin levels are also lower compared with control subjects. These hormonal changes may delay progression of atherosclerosis.
Congenital deficiency of the GH-IGF-1 axis in humans may induce protective effects against cancers, insulin resistance and atherosclerosis. Some studies describe a greater fraction of the extant population in older ages; however, there is no scientific evidence for extension of lifespan .
In a cohort of Ashkenazi Jewish centenarians , their female offspring show a smaller stature with high serum IGF-1 concentrations, when compared with offspring-matched controls (Suh et al. 2008). Male offspring of centenarians do not show these traits. Sequence analysis of the IGF-1 and IGF-1R genes of female centenarians with a short stature revealed a number of variants of the IGF-1R gene but not the IGF-1 gene. Immortalized lymphocytes established from the female centenarians carrying mutations of IGF1R show significant reductions in IGF1R levels compared with lymphocytes from female centenarians without gene mutations. IGF-1 signaling is also attenuated in lymphocytes from the IGF1R mutation carriers of female centenarians, compared with those from female centenarians with no mutations. The study found overrepresentation of those mutations in the IGF1R gene among female centenarians relative to controls. These findings suggest correlation between longevity and reduced IGF-1 signaling in females. These traits (reduction-of-function mutations of the IGF-1R gene, high concentrations of serum IGF-1—a compensatory increase in IGF-1, and extended lifespan) in female centenarians are similar to those in Igf1r +/− female mice (Holzenberger et al. 2003).
Human genetic studies have suggested an association between polymorphisms in IGF-1 signaling genes and longevity (Willcox et al. 2008; Ziv and Hu 2011; Di Bona et al. 2014). In an Italian cohort (Bonafe et al. 2003), long-lived people (aged 86–109 years) had lower BMI, fasting glucose, plasma insulin, insulin resistance (HOMA) and plasma free IGF-1 levels than young people (aged 17–85 years). The study indicates that IGF1R and PIK3CB (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit beta) polymorphisms affect IGF-1 plasma levels. Carriers of the A allele at the IGF1R locus show lower plasma free IGF-1 compared with the GG genotype. Carriers of the T allele at the PIK3CB locus also display lower plasma IGF-1 than the CC genotype. Finally, carriers of the A allele of IGF1R were overrepresented among long-lived people compared with young people. These polymorphisms in the IGF1R gene contrast with those in the IGF1R gene mutations reported in the cohort of Ashkenazi Jewish centenarians regarding the levels of plasma IGF-1 (Suh et al. 2008). However, both studies conclude that the attenuation in IGF-1 signaling could result in longer life in humans.
In a genetic analysis of the population-based Leiden 85-plus study (subjects were 85 years old and over), variant allele carriers of the GH1 single nucleotide polymorphism (SNP) were 2 cm shorter in body height and showed reduced mortality, compared with wild-type allele carriers (van Heemst et al. 2005). Other selected polymorphisms that lead to reduced insulin/IGF-1 activity such as the IGF-1 CA repeat also showed a trend of lower mortality but did not reach statistical significance. These findings were noted only in females.
A genome-wide meta-analysis of up to 30,844 adults of European ancestry from 21 studies confirms that the known longevity-associated FOXO3 variant rs2153960 is a genome-wide significant SNP for lowering IGF-1 concentrations (Teumer et al. 2016). The study also reports the IGF-1 decreasing allele of SNP rs934073, an eQTL of ASXL2, to be associated with higher adiposity and higher likelihood of survival beyond 90 years.
A study on regulation of GH secretion in offspring of long-lived families indicates that 24-h total GH secretion is lower and that the secretion is more tightly feedback- or feedforward-controlled in these offspring compared with control subjects (van der Spoel et al. 2016). However, no significant differences were observed in circulating levels of IGF-1 and IGFBP3 between offspring and controls.
In conclusion, human genome studies have identified alleles for reduced GH and/or IGF-1 signaling, some of which are overrepresented in long-lived people. These findings suggest common mechanisms for regulation of aging and longevity among animals including humans.
7 Evolutionary Perspective on Regulation of Aging and Lifespan
Within a species, individuals with lower plasma concentrations of IGF-1 are smaller in body size but live longer than those with relatively higher levels of IGF-1 and thus bigger body size. The correlation between body size and plasma IGF-1 concentration is significant in female mice but not male mice (Miller et al. 2002).
A study on genetically-diverse inbred mouse strains at the Jackson Aging Center showed a negative correlation between median lifespan and plasma IGF-1 levels, particularly in long-lived strains (Yuan et al. 2009). A subsequent study revealed that in female mice, IGF-1 levels at 7 weeks of age significantly correlated with the age of vaginal patency, i.e., sexual maturation (Yuan et al. 2015) and IGF-1 levels at 52 and 76 weeks of age negatively correlated with longevity. Thus, female mice with lower IGF-1 levels delay sexual maturation but outlive mice with higher IGF-1 levels. Using the QTL method and combining human GWAS results, proprotein convertase subtilisin/kexin type 2 (Pcsk2) was found to regulate female sexual maturation and IGF-1 , and thus Pcsk2 may play a role in regulating aging and longevity in mammals (Yuan et al. 2015).
A comparative study of 36 mammalian species demonstrates a negative correlation between plasma IGF-1 and body mass, i.e., larger mammals have lower IGF-1 concentrations. Although there is no significant correlation between plasma IGF-1 levels and maximum lifespans among these species, it is tempting to speculate on the presence of a trade-off between growth rate and longevity. Larger animals with lower IGF-1 levels grow more slowly but live longer than animals with higher IGF-1 levels, who instead grow quickly to sexual maturation and have smaller body size. This trend is particularly notable in females.
8 Conclusion
The evolutionary view of aging predicts the presence of pleiotropic genes that regulate growth or sexual maturation and lifespan . Multiple genes or genetic loci are involved in the regulation of circulating GH and IGF-1 levels, and thus lifespan or aging is under complex genetic control. In considering extension of healthy lifespan in humans, we should avoid environments, including eating habits, which elevate circulating IGF-1. Experimental studies have identified key molecules, mTOR and FoxO3, downstream of IGF-1 signaling. The prospect of optimizing mTOR and FoxO3 activities in humans to not only increase lifespan but also reduce age-related disorders represents a fascinating avenue of clinical investigation.
References
Bartke A (2008) Growth hormone and aging: a challenging controversy. Clin Interv Aging 3:659–665
Boldt HB, Conover CA (2007) Pregnancy-associated plasma protein-A (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Horm IGF Res 17:10–18. doi:10.1016/j.ghir.2006.11.003
Bonafè M, Barbieri M, Marchegiani F, Olivieri F, Ragno E, Giampieri C, Mugianesi E, Centurelli M, Franceschi C, Paolisso G (2003) Polymorphic variants of insulin-like growth factor I (IGF-I) receptor and phosphoinositide 3-kinase genes affect IGF-I plasma levels and human longevity: cues for an evolutionarily conserved mechanism of life span control. J Clin Endocrinol Metab 88:3299–3304. doi:10.1210/jc.2002-021810
Brown-Borg HM, Borg KE, Meliska CJ, Bartke A (1996) Dwarf mice and the ageing process. Nature 384:33. doi:10.1038/384033a0
Butler AA, Le Roith D (2001) Control of growth by the somatropic axis: growth hormone and the insulin-like growth factors have related and independent roles. Annu Rev Physiol 63:141–164. doi:10.1146/annurev.physiol.63.1.141
Cohn L, Feller AG, Draper MW, Rudman IW, Rudman D (1993) Carpal tunnel syndrome and gynaecomastia during growth hormone treatment of elderly men with low circulating IGF-I concentrations. Clin Endocrinol (Oxf) 39:417–425
Conover CA, Bale LK (2007) Loss of pregnancy-associated plasma protein A extends lifespan in mice. Aging Cell 6:727–729. doi:10.1111/j.1474-9726.2007.00328.x
Conover CA, Bale LK, Mader JR, Mason MA, Keenan KP, Marler RJ (2010) Longevity and age-related pathology of mice deficient in pregnancy-associated plasma protein-A. J Gerontol A Biol Sci Med Sci 65:590–599. doi:10.1093/gerona/glq032
Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ (2000) Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology 141:2608–2613
Di Bona D, Accardi G, Virruso C, Candore G, Caruso C (2014) Association between genetic variations in the insulin/insulin-like growth factor (Igf-1) signaling pathway and longevity: a systematic review and meta-analysis. Curr Vasc Pharmacol 12:674–681
Flurkey K, Papaconstantinou J, Miller RA, Harrison DE (2001) Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA 98:6736–6741. doi:10.1073/pnas.111158898
Flurkey K, Papaconstantinou J, Harrison DE (2002) The Snell dwarf mutation Pit1(dw) can increase life span in mice. Mech Ageing Dev 123:121–130
Gertler AA, Cohen HY (2013) SIRT6, a protein with many faces. Biogerontology 14:629–639. doi:10.1007/s10522-013-9478-8
Greer EL, Brunet A (2005) FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene 24:7410–7425. doi:10.1038/sj.onc.1209086
Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, Wei M, Madia F, Cheng C-W, Hwang D, Martin-Montalvo A, Saavedra J, Ingles S, de Cabo R, Cohen P, Longo VD (2011) Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med 3:70ra13–70ra13. doi:10.1126/scitranslmed.3001845
Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pathor M, Javors MA, Fernandez E, Miller RA (2009) Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460:392–395. doi:10.10387/nature08221
Holzenberger M, Dupont J, Ducos B, Leneuve P, Géloën A, Even PC, Cervera P, Le Bouc Y (2003) IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature 421:182–187. doi:10.1038/nature01298
Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY (2012) The sirtuin SIRT6 regulates lifespan in male mice. Nature. doi:10.1038/nature10815
Kappeler L, De Magalhaes Filho C, Dupont J, Leneuve P, Cervera P, Périn L, Loudes C, Blaise A, Klein R, Epelbaum J, Le Bouc Y, Holzenberger M (2008) Brain IGF-1 receptors control mammalian growth and lifespan through a neuroendocrine mechanism. PLoS Biol 6:e254. doi:10.1371/journal.pbio.0060254
Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R (1993) A C. elegans mutant that lives twice as long as wild type. Nature 366:461–464. doi:10.1038/366461a0
Kuramoto K, Tahara S, Sasaki T, Matsumoto S, Kaneko T, Kondo H, Yanabe M, Takagi S, Shinkai T (2010) Spontaneous dwarf rat: a novel model for aging research. Geriatr Gerontol Int 10:94–101. doi:10.1111/j.1447-0594.2009.00559.x
Li S, Crenshaw EB, Rawson EJ, Simmons DM, Swanson LW, Rosenfeld MG (1990) Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature 347:528–533. doi:10.1038/347528a0
Liu H, Bravata DM, Olkin I, Nayak S, Roberts B, Garber AM, Hoffman AR (2007) Systematic review: the safety and efficacy of growth hormone in the healthy elderly. Ann Intern Med 146:104–115
Miller RA, Harper JM, Galecki A, Burke DT (2002) Big mice die young: early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell 1:22–29
Miskin R, Masos T (1997) Transgenic mice overexpressing urokinase-type plasminogen activator in the brain exhibit reduced food consumption, body weight and size, and increased longevity. J Gerontol A Biol Sci Med Sci 52:B24–B118
Miskin R, Tirosh O, Pardo M, Zusman I, Schwartz B, Yahav S, Dubnov G, Kohen R (2005) AlphaMUPA mice: a transgenic model for longevity induced by caloric restriction. Mech Ageing Dev 126:255–261. doi:10.1016/j.mad.2004.08.018
Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, Mills KD, Patel P, Hsu JT, Hong AL, Ford E, Cheng H-L, Kennedy C, Nunez N, Bronson R, Frendewey D, Auerbach W, Valenzuela D, Karow M, Hottiger MO, Hursting S, Barrett JC, Guarente L, Mulligan R, Demple B, Yancopoulos GD, Alt FW (2006) Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell 124:315–329. doi:10.1016/j.cell.2005.11.044
Nojima A, Yamashita M, Yoshida Y, Shimizu I, Ichimiya H, Kamimura N, Kobayashi Y, Ohta S, Ishii N, Minamino T (2013) Haploinsufficiency of akt1 prolongs the lifespan of mice. PLoS ONE 8:e69178. doi:10.1371/journal.pone.0069178
O’Neill C (2013) PI3-kinase/Akt/mTOR signaling: impaired on/off switches in aging, cognitive decline and Alzheimer’s disease. Exp Gerontol 48:647–653. doi:10.1016/j.exger.2013.02.025
Oliveira CRP, Salvatori R, Meneguz-Moreno RA, Aguiar-Oliveira MH, Pereira RMC, Valença EHA, Araujo VP, Farias NT, Silveira DCR, Vieira JGH, Barreto-Filho JAS (2010) Adipokine profile and urinary albumin excretion in isolated growth hormone deficiency. J Clin Endocrinol Metab 95:693–698. doi:10.1210/jc.2009-1919
Ooka H, Shinkai T (1986) Effects of chronic hyperthyroidism on the lifespan of the rat. Mech Ageing Dev 33:275–282
Paik J-H, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, Jiang S, Gilliland DG, Chin L, Wong WH, Castrillon DH, DePinho RA (2007) FoxOs are lineage-restricted redundant tumor suppressors and regulate endothelial cell homeostasis. Cell 128:309–323. doi:10.1016/j.cell.2006.12.029
Panici JA, Harper JM, Miller RA, Bartke A, Spong A, Masternak MM (2010) Early life growth hormone treatment shortens longevity and decreases cellular stress resistance in long-lived mutant mice. FASEB J 24:5073–5079. doi:10.1096/fj.10-163253
Potthoff MJ, Kliewer SA, Mangelsdorf DJ (2012) Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev 26:312–324. doi:10.1101/gad.184788.111
Rudman D, Feller AG, Nagraj HS, Gergans GA, Lalitha PY, Goldberg AF, Schlenker RA, Cohn L, Rudman IW, Mattson DE (1990) Effects of human growth hormone in men over 60 years old. N Engl J Med 323:1–6. doi:10.1056/NEJM199007053230101
Sattler FR (2013) Growth hormone in the aging male. Best Pract Res Clin Endocrinol Metab 27:541–555. doi:10.1016/j.beem.2013.05.003
Savine R, Sönksen P (2000) Growth hormone–hormone replacement for the somatopause? Horm Res 53(Suppl 3):37–41
Selman C, Lingard S, Choudhury AI, Batterham RL, Claret M, Clements M, Ramadani F, Okkenhaug K, Schuster E, Blanc E, Piper MD, Al-Qassab H, Speakman JR, Carmignac D, Robinson ICA, Thornton JM, Gems D, Partridge L, Withers DJ (2008) Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J 22:807–818. doi:10.1096/fj.07-9261com
Selman C, Tullet JMA, Wieser D, Irvine E, Lingard SJ, Choudhury AI, Claret M, Al-Qassab H, Carmignac D, Ramadani F, Woods A, Robinson ICA, Schuster E, Batterham RL, Kozma SC, Thomas G, Carling D, Okkenhaug K, Thornton JM, Partridge L, Gems D, Withers DJ (2009) Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326:140–144. doi:10.1126/science.1177221
Shevah O, Laron Z (2007) Patients with congenital deficiency of IGF-I seem protected from the development of malignancies: a preliminary report. Growth Horm IGF Res 17:54–57. doi:10.1016/j.ghir.2006.10.007
Shimokawa I, Higami Y, Utsuyama M, Tuchiya T, Komatsu T, Chiba T, Yamaza H (2002) Life span extension by reduction in growth hormone-insulin-like growth factor-1 axis in a transgenic rat model. Am J Pathol 160:2259–2265. doi:10.1016/S0002-9440(10)61173-X
Shimokawa I, Chiba T, Yamaza H, Komatsu T (2008) Longevity genes: insights from calorie restriction and genetic longevity models. Mol Cells 26:427–435
Shimokawa I (2015) Hormonal influence and modulation in aging (Chapter 4). In: Yu BP (ed) Nutrition. Aging Interventions, Exercise and Epigenetics, pp 69–83
Shimokawa I, Komatsu T, Hayashi N, Kim SE, Kawata T, Park S, Hayashi H, Yamaza H, Chiba T, Mori R (2015) The life-extending effect of dietary restriction requires Foxo3 in mice. Aging Cell n/a–n/a. doi:10.1111/acel.12340
Sonntag WE, Carter CS, Ikeno Y, Ekenstedt K, Carlson CS, Loeser RF, Chakrabarty S, Lee S, Bennett C, Ingram R, Moore T, Ramsey M (2005) Adult-onset growth hormone and insulin-like growth factor I deficiency reduces neoplastic disease, modifies age-related pathology, and increases life span. Endocrinology 146:2920–2932. doi:10.1210/en.2005-0058
Sornson MW, Wu W, Dasen JS, Flynn SE, Norman DJ, O’Connell SM, Gukovsky I, Carrière C, Ryan AK, Miller AP, Zuo L, Gleiberman AS, Andersen B, Beamer WG, Rosenfeld MG (1996) Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature 384:327–333. doi:10.1038/384327a0
Steger RW, Bartke A, Cecim M (1993) Premature ageing in transgenic mice expressing different growth hormone genes. J Reprod Fertil Suppl 46:61–75
Suh Y, Atzmon G, Cho M-O, Hwang D, Liu B, Leahy DJ, Barzilai N, Cohen P (2008) Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci USA 105:3438–3442. doi:10.1073/pnas.0705467105
Sun LY, Spong A, Swindell WR, Fang Y, Hill C, Huber JA, Boehm JD, Westbrook R, Salvatori R, Bartke A (2013) Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. eLife 2:e01098. doi:10.7554/eLife.01098
Svensson J, Sjögren K, Fäldt J, Andersson N, Isaksson O, Jansson J-O, Ohlsson C (2011) Liver-derived IGF-I regulates mean life span in mice. PLoS ONE 6:e22640. doi:10.1371/journal.pone.0022640
Taguchi A, Wartschow LM, White MF (2007) Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science 317:369–372. doi:10.1126/science.1142179
Teumer A, Qi Q, Nethander M, Aschard H, Bandinelli S, Beekman M, Berndt SI, Bidlingmaier M, Broer L, CHARGE Longevity Working Group, Cappola A, Ceda GP, Chanock S, Chen M-H, Chen TC, Chen Y-DI, Chung J, Del Greco Miglianico F, Eriksson J, Ferrucci L, Friedrich N, Gnewuch C, Goodarzi MO, Grarup N, Guo T, Hammer E, Hayes RB, Hicks AA, Hofman A, Houwing-Duistermaat JJ, Hu F, Hunter DJ, Husemoen LL, Isaacs A, Jacobs KB, Janssen JAMJL, Jansson J-O, Jehmlich N, Johnson S, Juul A, Karlsson M, Kilpeläinen TO, Kovacs P, Kraft P, Li C, Linneberg A, Liu Y, Loos RJF, Body Composition Genetics Consortium, Lorentzon M, Lu Y, Maggio M, Magi R, Meigs J, Mellström D, Nauck M, Newman AB, Pollak MN, Pramstaller PP, Prokopenko I, Psaty BM, Reincke M, Rimm EB, Rotter JI, Saint Pierre A, Schurmann C, Seshadri S, Sjögren K, Slagboom PE, Strickler HD, Stumvoll M, Suh Y, Sun Q, Zhang C, Svensson J, Tanaka T, Tare A, Tönjes A, Uh H-W, van Duijn CM, van Heemst D, Vandenput L, Vasan RS, Völker U, Willems SM, Ohlsson C, Wallaschofski H, Kaplan RC (2016) Genomewide meta-analysis identifies loci associated with IGF-I and IGFBP-3 levels with impact on age-related traits. Aging Cell 15:811–824. doi:10.1111/acel.12490
van der Spoel E, Jansen SW, Akintola AA, Ballieux BE, Cobbaert CM, Slagboom PE, Blauw GJ, Westendorp RGJ, Pijl H, Roelfsema F, van Heemst D (2016) Growth hormone secretion is diminished and tightly controlled in humans enriched for familial longevity. Aging Cell 15:1126–1131. doi:10.1111/acel.12519
van Heemst D, Beekman M, Mooijaart SP, Heijmans BT, Brandt BW, Zwaan BJ, Slagboom PE, Westendorp RGJ (2005) Reduced insulin/IGF-1 signalling and human longevity. Aging Cell 4:79–85. doi:10.1111/j.1474-9728.2005.00148.x
Webb AE, Kundaje A, Brunet A (2016) Characterization of the direct targets of FOXO transcription factors throughout evolution. Aging Cell 15:673–685. doi:10.1111/acel.12479
Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD (2008) FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA 105:13987–13992. doi:10.1073/pnas.0801030105
Xu J, Gontier G, Chaker Z, Lacube P, Dupont J, Holzenberger M (2014) Longevity effect of IGF-1R(±) mutation depends on genetic background-specific receptor activation. Aging Cell 13:19–28. doi:10.1111/acel.12145
Yamaza H, Komatsu T, Wakita S, Kijogi C, Park S, Hayashi H, Chiba T, Mori R, Furuyama T, Mori N, Shimokawa I (2010) FoxO1 is involved in the antineoplastic effect of calorie restriction. Aging Cell 9:372–382. doi:10.1111/j.1474-9726.2010.00563.x
Yuan R, Tsaih S-W, Petkova SB, Marin de Evsikova C, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, Rosen CJ, Sundberg JP, Harrison DE, Churchill GA, Paigen B (2009) Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 8:277–287. doi:10.1111/j.1474-9726.2009.00478.x
Yuan R, Gatti DM, Krier R, Malay E, Schultz D, Peters LL, Churchill GA, Harrison DE, Paigen B (2015) Genetic regulation of female sexual maturation and longevity through circulating IGF1. J Gerontol A Biol Sci Med Sci 70:817–826. doi:10.1093/gerona/glu114
Zhang Y, Xie Y, Berglund ED, Coate KC, He TT, Katafuchi T, Xiao G, Potthoff MJ, Wei W, Wan Y, Yu RT, Evans RM, Kliewer SA, Mangelsdorf DJ (2012) The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1:e00065. doi:10.7554/eLife.00065
Ziv E, Hu D (2011) Genetic variation in insulin/IGF-1 signaling pathways and longevity. Ageing Res Rev 10:201–204. doi:10.1016/j.arr.2010.09.002
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Shimokawa, I. (2017). Growth Hormone and IGF-1 Axis in Aging and Longevity. In: Rattan, S., Sharma, R. (eds) Hormones in Ageing and Longevity. Healthy Ageing and Longevity, vol 6. Springer, Cham. https://doi.org/10.1007/978-3-319-63001-4_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-63001-4_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-63000-7
Online ISBN: 978-3-319-63001-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)