Abstract
The endocrine system coordinates and regulates various physiological functions and has a profound influence on the aging process. Studies from multiple model organisms indicate that hormones that regulate growth and metabolism can also play central roles in aging and the incidence of age-related diseases. The growth hormone (GH) and insulin-like growth factor 1 (IGF-1) signaling pathways represent perhaps the most potent and best characterized pro-aging axis. In mammals, GH and IGF-1 activity also contributes to age-related diseases, including cancer and diabetes. Growth factors can affect disease progress, in part, by regulating cellular resistance to stress and by the inhibiting stem cell-dependent regeneration. Here, we discuss the GH-IGF-1 axis, its connections with the recently identified mitochondrial-derived endocrine factor, and their effect on aging and age-related diseases.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Growth hormone
- Insulin-like growth factor 1 (IGF-1)
- Caloric restriction
- Mitochondrial-derived peptides
- Humanin
- MOTS-c
Introduction
Aging is accompanied by a progressive dysfunction of the endocrine system that ultimately affects hormone levels. Based on studies from model organisms, hormones, particularly those involved in growth, can regulate the rate of aging and incidence of age-related diseases. The growth hormone (GH) and insulin-like growth factor 1 (IGF-1) (GH/IGF-1) axis includes a set of genes, which have well characterized pro-aging functions in various model organisms. In mice and possibly humans, high GH/IGF-1 axis activity also contributes to diseases, including cancer and diabetes. One possible connection between growth factors and diseases is their effect on both the sensitization to stress of a variety of cell types and their role in the inhibition of stem cell-dependent regeneration. Here, we discuss the GH/IGF-1 axis, its effect on aging and age-related diseases, and its connections with the recently identified mitochondrial-derived endocrine factors in the regulation of aging.
The Somatotropic Axis
The GH/IGF-1 Axis Overview
Growth hormone (GH), also called somatotropin, is synthesized and secreted by somatotropic cells in the anterior pituitary gland in a pulsatile manner. Release of GH in humans, which is strongly associated with sleep, exercise, and metabolism, begins to decline after age 20 (Bartke et al. 2013). Its secretion is largely regulated by the hypothalamic growth hormone-releasing hormone (GHRH) and somatostatin (SST). A key role of GH is to induce IGF-1 expression in the liver via specific receptors, which are also expressed in other cell types including adipocytes and muscle (156, 208). Hepatic production accounts for >80% of circulating IGF-1, which is known to be a major mediator of many GH actions. The sharp age-related fall in GH secretion and serum concentration is accompanied by the decline in circulating IGF-1 levels (Bartke et al. 2013; Ho et al. 1987; Iranmanesh et al. 1991; Zadik et al. 1985; Juul et al. 1994), which is affected by protein intake (Levine et al. 2014) (Fig. 1).
Circulating IGF-1 levels of subjects with different protein consumptions among 50–65 and >66 years of age. IGF-1 levels were measured in 2,253 participants of NHANES III, a nationally representative, cross-sectional study. Subjects were categorized by percent of calorie intake from protein into a (i) high-protein group (20% or more of calories from proteins), a (ii) moderate-protein group (10–19% of calories from proteins), or a (iii) low-protein group (less than 10% of calories from proteins). Data points represent the mean ± SEM. ∗p < 0.01 (Figure adapted from Levine et al. 2014)
GH exerts mitogenic and metabolic effects both directly and through the stimulation of IGF-1 and the inhibition of insulin actions. The GH/IGF-1 axis is a major regulator of fetal and postnatal growth (Baker et al. 1993; Cohen et al. 2010; Randhawa and Cohen 2005). Also, when nutrients are abundant, the GH-induced stimulation of IGF-1 and insulin is important for anabolic storage and growth of lean body mass (LBM), adipose tissue, and glycogen reserves (Kaplan and Cohen 2007). However, under nutrient-restricted conditions, such as fasting, GH acts as a lipolytic factor that largely targets adipose tissue, leading to an increased release of free fatty acids (FFAs) into the circulation (Vijayakumar et al. 2010; Gormsen et al. 2007; Moller et al. 2003).
GH/IGF-1 Axis and Aging
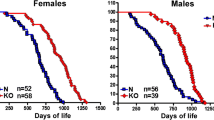
The GH/IGF-1 axis is strongly implicated in the regulation of aging and age-related diseases. Mice with congenital GH deficiency experience a dwarf phenotype and significantly extended lifespan and healthspan. Ames, Snell, and little mice carry mutations (prop-1, pit-1, Ghrhr, respectively) that cause GH deficiency, leading to dwarfism and extended lifespan (>50%, >40%, and >20%, respectively) (Brown-Borg et al. 1996; Flurkey et al. 2001). GH receptor/binding protein (GHR/BP) knockout mice (GHRKO), which were initially developed to model the human Laron syndrome, exhibit a 90% reduction in circulating IGF-1 levels and dwarfism and an approximately 50% increase in lifespan (Zhou et al. 1997; Coschigano et al. 2000). On the contrary, GH-overexpressing transgenic mice can exhibit a 50% decrease in mean lifespan (Bartke 2003) and experience kidney dysfunction, increased age-dependent liver alterations, and neoplasms (Wolf et al. 1993). IGF-1 is potentially a major mediator of the effects of GH on aging, and inhibiting its signaling can result in lifespan extension. Notably, a common characteristic of the GH-deficient and GHRKO mice is the significant decrease of circulating IGF-1 levels. The heterozygous deletion of IGF-1 receptor (IGF-1R) extends lifespan in female, but not male, mice (Holzenberger et al. 2003). In contrast, transgenic mice expressing an antagonistic analog of GH, which has a very modest reduction in serum IGF-1 and eventually reach normal body size, are not long-lived (Coschigano et al. 2003). However, mice with liver-specific IGF-1 gene deletion (LID) or inactivation at 1-month postnatal (LI-IGF-1 −/−), which lead to ~75% reduction in circulating IGF-1 and a compensatory increase in GH levels, are slightly smaller and only live longer (16%) if they are female (Sjogren et al. 1999; Yakar et al. 1999; Svensson et al. 2011). Because both GH- and GHR-deficient mice live over 40% longer and considering that they affect both IGF-1 and insulin, as well as many other pathways, it is likely that IGF-1 signaling is only an effector of the pro-aging role of GH. In humans, GHR deficiency also causes severe IGF-1 deficiency and has been shown to protect against cancer (Guevara-Aguirre et al. 2011; Steuerman et al. 2011) and diabetes (Guevara-Aguirre et al. 2011, 2015), but data on lifespan is yet to be collected.
At the cellular level, IGF-1 acts through its specific receptor (IGF-1R) and, with less affinity, to the structurally related insulin receptors (IRs) in various tissues (Slaaby 2015). Insulin receptor substrates 1 and 2 (IRS-1 and IRS-2) transduce signals from the IGF-1R and the IR that activate several signaling cascades. The global deletion of Irs1 (Irs1 −/−) caused a 17% increase in lifespan in female mice (Selman et al. 2008a, 2011), and the global deletion of Irs2 (Irs2 −/−) significantly shortened lifespan, largely due to diabetes (Selman et al. 2008a). In contrast, mice carrying a heterozygous mutant Irs2 (Irs2 +/−) have been reported to experience an increase in mean lifespan of 17% by one group (Taguchi et al. 2007), but not by another group due to unresolved differences (Selman et al. 2008b). The insulin/IGF-1 signaling (IIS) pathway also involves a highly conserved nutrient-sensing network, which includes PI3K/AKT, the target of rapamycin (TOR) kinase and its downstream S6 kinase (S6K), all implicated as pro-aging factors (Fontana et al. 2010). Recently, IGF-1 signaling has also been connected to another pro-aging enzyme implicated in aging in both yeast and mice: the protein kinase A (PKA) (Fabrizio et al. 2001; Cheng et al. 2014).
In yeast, which does not express an IGF-1-like gene, deleting the orthologs of genes functioning in mammalian GH/IGF-1 signaling causes a major extension of longevity (Fabrizio et al. 2001). Mutations in RAS (RAS2) and/or TOR-S6K (TOR-SCH9) increase lifespan by more than 200% while elevating stress resistance against oxidants, genotoxins, and heat shock (Longo and Finch 2003). Similarly, in C. elegans, mutations in the human homologs of insulin/IGF-1 receptor (daf-2) and PI3K (age-1) extend lifespan to 200% and show increased resistance to thermal and oxidative stress (Kleemann and Murphy 2009). In D. melanogaster, mutations in the insulin receptor substrate (Chico) lead to a 150% lifespan extension (Giannakou and Partridge 2007). In humans, a functional mutation in the IGF-1R, which confers partial IGF-1 resistance, was more prevalent in centenarians, as compared to controls without familial longevity (Suh et al. 2008). Further, in exceptionally long-lived individuals (nonagenarians), low levels of circulating IGF-1 were correlated with their prolonged survival in cancer-free females, but not males, and in both sexes with a history of cancer (Milman et al. 2014). Another study showed that the offspring of centenarians had relatively lower circulating IGF-1 bioactivity compared to offspring-matched controls, which was inversely related to insulin sensitivity (Vitale et al. 2012).
Dietary Restriction (DR) GH/IGF-1 Signaling and Aging
Caloric restriction (CR) , which if it includes a restriction of protein intake reduces also both insulin and IGF-1 levels, is the most effective and reproducible intervention known to decelerate the rate of aging and increase healthspan in model organisms ranging from yeast to worms, flies, rodents, and nonhuman primates (Fontana et al. 2010). In 1934, Crowell and McCay reported that white rats fed with a calorie-restricted diet with sufficient nutrients from the time of weaning resulted in lifespans nearly doubling (McCay et al. 1989). A 30+ year longitudinal adult-onset CR study in rhesus monkeys performed at the Wisconsin National Primate Research Center (WNPRC) shows that CR (30%) delays disease onset and mortality, with a 50% decrease in cancer incidence (Colman et al. 2009, 2014). However, a comparable 20+ year study performed at the National Institute on Aging (NIA) does not show extended lifespan in CR monkeys, although this dietary intervention reduces the incidence of diabetes and cancer and thus improves healthspan (Mattison et al. 2012). The disparities between the WNPRC and NIA studies were largely attributed to differences in diet composition and the genetic origin of the monkeys. However, there are multiple trade-offs resulting from a severe and chronic CR, which include impaired wound healing (Hunt et al. 2012) and immune responses (Kristan 2008). Also, because proteins and amino acids are the major regulators of GH/IGF-1 axis activity, it is not clear whether CR without protein restriction can actually be effective in lifespan and healthspan. In both mice and humans, a high-protein intake combined with CR reversed its beneficial effects (Brandhorst et al. 2013; Fontana et al. 2008).
The long-lived GH/IGF-1 mutant mice and wild-type mice on CR share several phenotypes, including reduced levels of circulating IGF-1 and enhanced insulin sensitivity (Lee and Longo 2011; Masternak et al. 2009; Bonkowski et al. 2009; Dominici et al. 2002). They are also both protected against several age-related pathologies and conditions, such as cancer and insulin resistance (Omodei and Fontana 2011; Ikeno et al. 2013). The anticancer effect of CR on spontaneous and induced tumors can be mediated, at least in part, by the reduction in IGF-1 levels and an increase in corticosteroid levels (Longo and Fontana 2010). GHRKO mice do not benefit from further lifespan extension and improved insulin sensitivity by CR, suggesting overlapping mechanisms (Bonkowski et al. 2009; Arum et al. 2009), whereas the GH-deficient Ames mice showed further increase in lifespan by CR (Bartke et al. 2001). These studies suggest that the GH/IGF-1 axis and CR affect aging in overlapping, but not identical, mechanisms, and additional components, such as diet and metabolic signals, should be carefully considered.
Although CR can extend lifespan and/or healthspan, it is undoubtedly a very challenging dietary regimen with potential malnutrition. Thus, a CR-mimicking diet that delivers the beneficial effects without severe abstinence from food would be an exciting field of investigation. A recent report showed that a fasting-mimicking diet (FMD), designed based on its ability to reduce IGF-1, increases IGFBP-1, reduces glucose, and increases ketone bodies comparably to water-only fasting, while maximizing nourishment, and minimizing adverse effects, showed significant increase in median lifespan (25.5 vs. 28.3 months) in mice (Brandhorst et al. 2015). A pilot randomized clinical trial in which subjects consumed an FMD for five consecutive days every month for 3 months and returned to normal diet in between FMDs showed a significant reduction of circulating IGF-1 (~24%) and had beneficial effects on fasting glucose, the inflammatory marker CRP, and abdominal fat (Brandhorst et al. 2015). Notably, the FMD reduced body weight without loss of lean mass relative to total weight (Brandhorst et al. 2015).
Interventions Targeting the GH/IGF-1 Axis
Interventions to inhibit the GH/IGF-1 axis to increase lifespan and healthspan are promising but should be approached with caution. As mentioned above, this axis is integrated with other endocrine systems, such as insulin, and, thus, targeted interventions may lead to diabetic symptoms. For instance, somatostatin analogs, which lower GH production and reduce circulating IGF-1 levels, also suppress insulin secretion. A more promising approach may be to target the GHR using compounds similar to pegvisomant, a dominant negative GH mimetic that is FDA approved for the treatment of acromegaly. Pegvisomant reduces serum IGF-1 levels by up to 90% (20 mg/day for 12 weeks) (Trainer et al. 2000) and also acts as an insulin sensitizer that counters the diabetogenic action of GH and thus improves glucose metabolism (Thankamony et al. 2014; Higham et al. 2009).
GH/IGF-1 Axis and Mitochondrial-Derived Endocrine Factors
Mitochondrial dysfunction is strongly implicated in aging, but the mechanistic details are poorly understood. Mitochondria are increasingly being appreciated as signaling organelles, especially in light of recent studies that clearly indicate the importance of a balanced mitochondrial-to-nuclear communication in coordinating homeostasis. Mitochondria have retained a semiautonomous genetic system, complete with its own independent genome known to encode for 13 proteins that are all part of the electron transport chain. The recently discovered two peptides (MDPs), as described below, expand the mitochondrial genetic repertoire and provide a novel category of hormones that may influence aging and age-related diseases. Notably, MDPs may represent a novel mitochondrial component of the GH/IGF-1 signaling pathway that regulates longevity.
Humanin
Humanin was the first short open reading frame (sORFs) to be identified in the mtDNA, suggesting the existence of a larger mitochondrial genetic repertoire (Lee et al. 2013; Hashimoto et al. 2001a; Guo et al. 2003). Humanin is a conserved polypeptide (Guo et al. 2003) encoded as a 75 bp polycistronic sORF within the 16S rRNA, discovered in 2001 from an unbiased functional screen using a cDNA library created from the surviving brain fraction of an Alzheimer’s disease (AD) patient (Hashimoto et al. 2001a, b). Its expression is age dependent (Bachar et al. 2010; Muzumdar et al. 2009) and found in various tissues and also in circulation in rodents and humans (Hashimoto et al. 2001a). Humanin is secreted from cells and found in circulation (Hashimoto et al. 2001a; Muzumdar et al. 2010) and is proposed to act through two different types of receptors (Ying et al. 2004; Hashimoto et al. 2009). Humanin binds to IGF-binding protein 3 (IGFBP-3), which regulates IGF-1 bioactivity, with high affinity and specificity (Ikonen et al. 2003). Notably, circulating humanin levels are (i) negatively correlated with circulating IGF-1 levels and (ii) positively correlated with longevity (Lee et al. 2014). The long-lived Ames mice and Laron syndrome patients, mentioned above, had elevated circulating humanin levels, whereas short-lived GH-transgenic mice had reduced levels (Lee et al. 2014). Furthermore, GH or IGF-1 treatment reduced circulating humanin levels in both mice and human subjects (Lee et al. 2014). This suggests a potential coordinated mitochondrial element to the GH/IGF-1 regulation of aging. At the cellular level, humanin acts as an antiapoptotic factor that binds to and inhibits the activity of the proapoptotic protein BAX (Guo et al. 2003). Its cytoprotective effects were first tested and confirmed in vitro against mutant forms of amyloid precursor protein (APP) and presenilins 1 and 2 (PS1/2), which cause familial AD, in neurons (Hashimoto et al. 2001a). A wide range of in vitro and in vivo studies, which cause variable levels of oxidative stress, now indicate that humanin protects against various types of stress or disease states, including AD, cancer, and type 1 diabetes (Lee et al. 2013; Yen et al. 2013).
MOTS-c
MOTS-c (mitochondrial ORF of the 12S rRNA-c) is a recently identified MDP. As its name implies, it is encoded as a polycistronic 51 bp gene within the 12S rRNA of the mtDNA, yielding a 16-amino acid peptide (Lee et al. 2015; Zarse and Ristow 2015). It is detected in various tissues and in circulation in an age-dependent manner (Lee et al. 2015). Its primary target organ appears to be the skeletal muscle, and its cellular actions inhibit the folate cycle and its tethered de novo purine biosynthesis, causing a significant accumulation of AICAR and consequent AMPK activation; MOTS-c shares strikingly similar effects with the pro-longevity diabetes drug metformin (Martin-Montalvo et al. 2013; Hall 2015), which has also shown to target the folate/purine cycle (Cabreiro et al. 2013; Corominas‐Faja et al. 2012). The intracellular effect of MOTS-c on glucose metabolism is mediated by AMPK and SIRT1 (Lee et al. 2015), two major proteins that work in concert with the GH/IGF-1 pathway to regulate aging (Lopez-Otin et al. 2013). This suggests that MOTS-c, similar to Humanin, may be a mitochondrial signal that is integrated with the GH/IGF-1 axis. In mice, MOTS-c regulates glucose homeostasis and significantly improves insulin sensitivity in aged and high-fat diet-fed mice, largely by targeting skeletal muscle (Lee et al. 2015). Notably, the mitochondrial m.1382A>C polymorphism, which is found specifically in the D4b2 haplogroup that is associated with exceptional longevity in the Japanese population (Alexe et al. 2007), causes a Lys14Gln variation that may have functional alterations (Fuku et al. 2015). Further studies are required to test if MOTS-c, and its analogs, can positively extend healthspan and/or lifespan in model organisms.
Conclusion
The GH/IGF-1 axis and its conserved downstream IIS pathways have been central to aging research and have provided much mechanistic insight into the regulation of lifespan and healthspan. Recent discoveries of additional age-dependent humoral factors, such as mitokines and MDPs, further expand the mechanistic breadth of the aging process. As mentioned above, the GH/IGF-1 axis seems to have overlapping, but not identical, mechanisms with CR on longevity, suggesting additional metabolism-related factors to be involved. Connecting these novel endocrine factors with DR and the GH/IGF-1 axis may unveil a more integrated multicomponent regulatory system of aging and also provide insight into the coordination of differential aging rates of various tissue types. Further, these endocrine factors can be used as biomarkers for the development of interventions that extend healthy lifespan.
References
Alexe G, et al. Enrichment of longevity phenotype in mtDNA haplogroups D4b2b, D4a, and D5 in the Japanese population. Hum Genet. 2007;121(3–4):347–56.
Arum O, Bonkowski MS, Rocha JS, Bartke A. The growth hormone receptor gene-disrupted mouse fails to respond to an intermittent fasting diet. Aging Cell. 2009;8(6):756–60.
Bachar AR, et al. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res. 2010;88(2):360–6.
Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75(1):73–82.
Bartke A. Can growth hormone (GH) accelerate aging? Evidence from GH-transgenic mice. Neuroendocrinology. 2003;78(4):210–6.
Bartke A, et al. Extending the lifespan of long-lived mice. Nature. 2001;414(6862):412.
Bartke A, Sun LY, Longo V. Somatotropic signaling: trade-offs between growth, reproductive development, and longevity. Physiol Rev. 2013;93(2):571–98.
Bonkowski MS, et al. Disruption of growth hormone receptor prevents calorie restriction from improving insulin action and longevity. PLoS One. 2009;4(2):e4567.
Brandhorst S, Wei M, Hwang S, Morgan TE, Longo VD. Short-term calorie and protein restriction provide partial protection from chemotoxicity but do not delay glioma progression. Exp Gerontol. 2013;48(10):1120–8.
Brandhorst S, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22(1):86–99.
Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384(6604):33.
Cabreiro F, et al. Metformin retards aging in C. elegans by altering microbial folate and methionine metabolism. Cell. 2013;153(1):228–39.
Cheng CW, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14(6):810–23.
Cohen P, et al. Variable degree of growth hormone (GH) and insulin-like growth factor (IGF) sensitivity in children with idiopathic short stature compared with GH-deficient patients: evidence from an IGF-based dosing study of short children. J Clin Endocrinol Metab. 2010;95(5):2089–98.
Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–4.
Colman RJ, et al. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557.
Corominas-Faja B, et al. Metabolomic fingerprint reveals that metformin impairs one-carbon metabolism in a manner similar to the antifolate class of chemotherapy drugs. Aging. 2012;4(7):480–98.
Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141(7):2608–13.
Coschigano KT, et al. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144(9):3799–810.
Dominici FP, Hauck S, Argentino DP, Bartke A, Turyn D. Increased insulin sensitivity and upregulation of insulin receptor, insulin receptor substrate (IRS)-1 and IRS-2 in liver of Ames dwarf mice. J Endocrinol. 2002;173(1):81–94.
Fabrizio P, Pozza F, Pletcher SD, Gendron CM, Longo VD. Regulation of longevity and stress resistance by Sch9 in yeast. Science. 2001;292(5515):288–90.
Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci U S A. 2001;98(12):6736–41.
Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging Cell. 2008;7(5):681–7.
Fontana L, Partridge L, Longo VD. Extending healthy life span – from yeast to humans. Science. 2010;328(5976):321–6.
Fuku N, et al. The mitochondrial-derived peptide MOTS-c: a player in exceptional longevity? Aging Cell. 2015;14:921.
Giannakou ME, Partridge L. Role of insulin-like signalling in Drosophila lifespan. Trends Biochem Sci. 2007;32(4):180–8.
Gormsen LC, et al. Dose-response effects of free fatty acids on glucose and lipid metabolism during somatostatin blockade of growth hormone and insulin in humans. J Clin Endocrinol Metab. 2007;92(5):1834–42.
Guevara-Aguirre J, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3(70):70ra13.
Guevara-Aguirre J, et al. GH receptor deficiency in Ecuadorian adults is associated with obesity and enhanced insulin sensitivity. J Clin Endocrinol Metab. 2015;100(7):2589–96.
Guo B, et al. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423(6938):456–61.
Hall SS. A trial for the ages. Science. 2015;349(6254):1274–8.
Hashimoto Y, et al. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Abeta. Proc Natl Acad Sci U S A. 2001a;98(11):6336–41.
Hashimoto Y, et al. Mechanisms of neuroprotection by a novel rescue factor humanin from Swedish mutant amyloid precursor protein. Biochem Biophys Res Commun. 2001b;283(2):460–8.
Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor alpha/WSX-1/gp130. Mol Biol Cell. 2009;20(12):2864–73.
Higham CE, Rowles S, Russell-Jones D, Umpleby AM, Trainer PJ. Pegvisomant improves insulin sensitivity and reduces overnight free fatty acid concentrations in patients with acromegaly. J Clin Endocrinol Metab. 2009;94(7):2459–63.
Ho KY, et al. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987;64(1):51–8.
Holzenberger M, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421(6919):182–7.
Hunt ND, et al. Effect of calorie restriction and refeeding on skin wound healing in the rat. Age (Dordr). 2012;34(6):1453–8.
Ikeno Y, et al. Do Ames dwarf and calorie-restricted mice share common effects on age-related pathology? Pathobiol Aging Age Relat Dis. 2013;3:20833.
Ikonen M, et al. Interaction between the Alzheimer’s survival peptide humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci U S A. 2003;100(22):13042–7.
Iranmanesh A, Lizarralde G, Veldhuis JD. Age and relative adiposity are specific negative determinants of the frequency and amplitude of growth hormone (GH) secretory bursts and the half-life of endogenous GH in healthy men. J Clin Endocrinol Metab. 1991;73(5):1081–8.
Juul A, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab. 1994;78(3):744–52.
Kaplan SA, Cohen P. The somatomedin hypothesis 2007: 50 years later. J Clin Endocrinol Metab. 2007;92(12):4529–35.
Kleemann GA, Murphy CT. The endocrine regulation of aging in Caenorhabditis elegans. Mol Cell Endocrinol. 2009;299(1):51–7.
Kristan DM. Calorie restriction and susceptibility to intact pathogens. Age (Dordr). 2008;30(2–3):147–56.
Lee C, Longo VD. Fasting vs dietary restriction in cellular protection and cancer treatment: from model organisms to patients. Oncogene. 2011;30(30):3305–16.
Lee C, Yen K, Cohen P. Humanin: a harbinger of mitochondrial-derived peptides? Trends Endocrinol Metab: TEM. 2013;24:222.
Lee C, et al. IGF-I regulates the age-dependent signaling peptide humanin. Aging Cell. 2014;13(5):958–61.
Lee C, et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015;21(3):443–54.
Levine ME, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19(3):407–17.
Longo VD, Finch CE. Evolutionary medicine: from dwarf model systems to healthy centenarians? Science. 2003;299(5611):1342–6.
Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci. 2010;31(2):89–98.
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–217.
Martin-Montalvo A, et al. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192.
Masternak MM, Panici JA, Bonkowski MS, Hughes LF, Bartke A. Insulin sensitivity as a key mediator of growth hormone actions on longevity. J Gerontol A Biol Sci Med Sci. 2009;64(5):516–21.
Mattison JA, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489(7415):318–21.
McCay CM, Crowell MF, Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989;5(3):155–71; discussion 172.
Milman S, et al. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13(4):769–71.
Moller N, Gjedsted J, Gormsen L, Fuglsang J, Djurhuus C. Effects of growth hormone on lipid metabolism in humans. Growth Horm IGF Res. 2003;13(Suppl A):S18–21.
Muzumdar RH, et al. Humanin: a novel central regulator of peripheral insulin action. PLoS One. 2009;4(7):e6334.
Muzumdar RH, et al. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol. 2010;30(10):1940–8.
Omodei D, Fontana L. Calorie restriction and prevention of age-associated chronic disease. FEBS Lett. 2011;585(11):1537–42.
Randhawa R, Cohen P. The role of the insulin-like growth factor system in prenatal growth. Mol Genet Metab. 2005;86(1–2):84–90.
Selman C, et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J: Off Publ Fed Am Soc Exp Biol. 2008a;22(3):807–18.
Selman C, Lingard S, Gems D, Partridge L, Withers DJ. Comment on “Brain IRS2 signaling coordinates life span and nutrient homeostasis”. Science. 2008b;320(5879):1012; author reply 1012.
Selman C, Partridge L, Withers DJ. Replication of extended lifespan phenotype in mice with deletion of insulin receptor substrate 1. PLoS One. 2011;6(1):e16144.
Sjogren K, et al. Liver-derived insulin-like growth factor I (IGF-I) is the principal source of IGF-I in blood but is not required for postnatal body growth in mice. Proc Natl Acad Sci U S A. 1999;96(12):7088–92.
Slaaby R. Specific insulin/IGF1 hybrid receptor activation assay reveals IGF1 as a more potent ligand than insulin. Sci Rep. 2015;5:7911.
Steuerman R, Shevah O, Laron Z. Congenital IGF1 deficiency tends to confer protection against post-natal development of malignancies. Eur J Endocrinol/Eur Fed Endocr Soc. 2011;164(4):485–9.
Suh Y, et al. Functionally significant insulin-like growth factor I receptor mutations in centenarians. Proc Natl Acad Sci U S A. 2008;105(9):3438–42.
Svensson J, et al. Liver-derived IGF-I regulates mean life span in mice. PLoS One. 2011;6(7):e22640.
Taguchi A, Wartschow LM, White MF. Brain IRS2 signaling coordinates life span and nutrient homeostasis. Science. 2007;317(5836):369–72.
Thankamony A, et al. Short-term administration of pegvisomant improves hepatic insulin sensitivity and reduces soleus muscle intramyocellular lipid content in young adults with type 1 diabetes. J Clin Endocrinol Metab. 2014;99(2):639–47.
Trainer PJ, et al. Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med. 2000;342(16):1171–7.
Vijayakumar A, Novosyadlyy R, Wu Y, Yakar S, LeRoith D. Biological effects of growth hormone on carbohydrate and lipid metabolism. Growth Horm IGF Res. 2010;20(1):1–7.
Vitale G, et al. Low circulating IGF-I bioactivity is associated with human longevity: findings in centenarians’ offspring. Aging. 2012;4(9):580–9.
Wolf E, et al. Effects of long-term elevated serum levels of growth hormone on life expectancy of mice: lessons from transgenic animal models. Mech Ageing Dev. 1993;68(1–3):71–87.
Yakar S, et al. Normal growth and development in the absence of hepatic insulin-like growth factor I. Proc Natl Acad Sci U S A. 1999;96(13):7324–9.
Yen K, Lee C, Mehta H, Cohen P. The emerging role of the mitochondrial-derived peptide humanin in stress resistance. J Mol Endocrinol. 2013;50(1):R11–9.
Ying G, et al. Humanin, a newly identified neuroprotective factor, uses the G protein-coupled formylpeptide receptor-like-1 as a functional receptor. J Immunol. 2004;172(11):7078–85.
Zadik Z, Chalew SA, McCarter Jr RJ, Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985;60(3):513–6.
Zarse K, Ristow M. A mitochondrially encoded hormone ameliorates obesity and insulin resistance. Cell Metab. 2015;21(3):355–6.
Zhou Y, et al. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci U S A. 1997;94(24):13215–20.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this entry
Cite this entry
Lee, C.D., Longo, V.D. (2018). Growth Hormones and Aging. In: Belfiore, A., LeRoith, D. (eds) Principles of Endocrinology and Hormone Action. Endocrinology. Springer, Cham. https://doi.org/10.1007/978-3-319-44675-2_27
Download citation
DOI: https://doi.org/10.1007/978-3-319-44675-2_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-44674-5
Online ISBN: 978-3-319-44675-2
eBook Packages: MedicineReference Module Medicine