Abstract
For appreciable time, the pathophysiology leading to ultimate melanocyte destruction remained uncertain. This is because skin-infiltrating T cells involved in melanocyte loss are few in number and are only observed in actively depigmenting skin; thus, such infiltrates are easily overlooked. Moreover, T cells were more difficult to distinguish before antibodies became readily available for immunohistology. Ample support exists for autoimmunity as a chief etiopathological factor in vitiligo: susceptible individuals exhibit polymorphisms in immune regulatory genes which promote autoimmunity in the cutaneous microenvironment; additionally, vitiligo has a well-established association with other autoimmune diseases. Among the possibly involved cell populations, Langerhans cells might contribute to depigmentation on-site (perhaps through continued melanocyte antigen presentation to cytotoxic T cells), thereby preventing viability of any melanocytes attempting to repopulate the skin. The HSP-native protein complexes can trigger a local immune response directed at the cells from which the native proteins originate. Upon melanocyte stress and subsequent HSP70i release, antigen-presenting cells will recruit an initial cohort of melanocyte-reactive T cells that produce IFN-γ upon antigen recognition. This would lead to CXCL10 production and further recruitment to the epidermis. The absence of Tregs in vitiligo skin is likewise best explained by differential chemokine expression in lesional skin, mainly involving CCL22.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
-
Progressive depigmentation in vitiligo relies on skin-infiltrating cytotoxic T cells specific for melanocyte self-antigens.
-
Melanocytes in perilesional skin are also sensitive to self-reactive antibodies.
-
Regulatory T cells (Tregs) are present in decreased numbers in vitiligo-affected skin.
-
Stress proteins including inducible heat shock protein 70 (HSP70i) link initial skin trauma to the adaptive immune responses that follow.
-
Interferon-gamma (IFN-γ) signaling through the JAK-STAT pathway drives vitiligo pathogenesis by recruiting cytotoxic T cells to the skin.
-
Stress protein upregulation, activation of antigen-presenting cells, recruitment of melanocyte-reactive T cells, and a paucity of regulatory T cells are intertwined phenomena that lie at the heart of vitiligo pathogenesis.
-
Promising immunotherapeutic strategies are being considered for vitiligo treatment including modified HSP70 delivery, chemokine receptor blockade, JAK-STAT inhibitor application, adoptive Treg transfer, and anti-cytokine therapies.
-
Vitiligo development is considered a positive prognostic factor in melanoma, as it serves as a marker of generation of robust anti-melanocyte tumor responses.
1 Establishment of Vitiligo as an Autoimmune Disease Entity
The disease course of vitiligo is variable, and it affects between 0.5% and 1% of the population [1]. The clinical finding of depigmented skin patches correlates with the finding of nearly complete absence of melanocytes histologically [2, 3]. This observation prompted studies that would explain disease etiology in terms of melanocyte destruction rather than suppression of pigmentation. For an appreciable time, the pathophysiology leading to ultimate melanocyte destruction remained uncertain. This is because skin-infiltrating T cells involved in melanocyte loss are few in number and are only observed in actively depigmenting skin; thus, such infiltrates are easily overlooked. Moreover, T cells were more difficult to distinguish before antibodies became readily available for immunohistology [4, 5], and, initially, most attention was directed toward possible humoral involvement in vitiligo [6, 7]. Observations including the transmission of disease through adoptive transfer of antibodies [8, 9], as well as reduced surface expression of factors such as decay-accelerating factor (DAF) that otherwise protects from complement-mediated destruction, also led to antibodies being considered as the culprits in melanocyte destruction [10]. For the morphology of cultured melanocytes required for such studies, see Fig. 28.1. However, as the target antigens identified by these studies are not necessarily expressed in the cell membrane, anti-melanocyte antibodies were ultimately considered an epiphenomenon of the disease. Since B cell activation and T cell activation are intimately related and mutually supportive [11,12,13], renewed attention to B cell and antibody involvement is to be expected.
Dendritic morphology of recently plated melanocytes in culture. Cells were isolated from (C) neonatal foreskin or (V) a scalp biopsy from a vitiligo patient by overnight enzymatic treatment and plated in replete media. Note that vitiligo melanocytes exhibit multiple, branched dendrites (arrows) in contrast to the more bipolar morphology of healthy neonatal melanocytes. K keratinocytes
While many environmental factors can contribute to disease precipitation, the consensus reached by the Vitiligo European Task Force (VETF) is that autoimmunity contributes to all cases of vitiligo [14]. Interestingly, this includes segmental vitiligo [15], which was initially considered a developmental defect [16]. Vitiligo likely manifests itself when certain environmental factors affect individuals with a genetic predisposition for the condition. Interestingly, many of the proposed vitiligo susceptibility genes have immune modulatory functions, including interleukin-2 receptor alpha chain (IL2RA), ubiquitin-associated and SH3 domain-containing A protein (UBASH3A), and C1q and tumor necrosis factor-related protein (C1QTNF6) [17]. Human leukocyte antigens (HLA)-A2 [17, 18] and HLA-DR4 [17, 19] have also been linked with vitiligo development. Indeed, MHC involvement is a hallmark of autoimmunity [20]. In the following sections, we describe the interplay between the cutaneous microenvironment and immunologic factors that lead to ultimate melanocyte destruction in vitiligo.
2 Cellular Immunity in Vitiligo
Ample support exists for autoimmunity as a chief etiopathological factor in vitiligo. As discussed above, susceptible individuals exhibit polymorphisms in immune regulatory genes which promote autoimmunity in the cutaneous microenvironment. Additionally, vitiligo has a well-established association with other autoimmune diseases [6], the most common of which is Hashimoto’s thyroiditis [21]. In fact, this association is common enough to warrant checking antithyroid antibodies, including antithyroid peroxidase antibody and anti-thyroglobulin levels, in addition to baseline thyroid-stimulating hormone (TSH) levels in newly diagnosed vitiligo patients [22, 23]. Other associated autoimmune diseases include alopecia areata, diabetes mellitus type I, pernicious anemia, and Addison’s disease [21] (see Chap. 13). Some of the more pertinent findings supporting contributory autoimmune mechanisms in vitiligo involve the inflammatory infiltrates consistently identified in newly vitiligo-afflicted skin. Specifically, lymphocytic infiltrates exhibiting a decreased ratio of CD4+/CD8+ T cells have been identified in perilesional areas of patients with active depigmentation [24, 25]. The cytotoxic (CD8+) T cells present in perilesional areas are activated [26] and are located in close proximity to melanocytes and melanocyte fragments in the basal layer of the epidermis (Fig. 28.2). Notably, their presence correlates with melanocyte disappearance [25]. These cytotoxic T cell infiltrates are found in perilesional skin of vitiligo patients [24], suggesting a role in melanocyte destruction. Indeed, these skin-infiltrating T cells are reactive with melanocyte self-antigens [27, 28]. Circulating melanocyte antigen-specific T cells have been found in increased levels in vitiligo patients as well [29], but, given their location, the skin-homing T cells are of greater pathogenic significance. Further supporting the role of antigen-specific T cells in vitiligo pathogenesis is their epidermal tropism [30, 31].
T cells in vitiligo and melanoma. Sections of (a) depigmenting vitiligo skin and (b) a melanoma tumor metastasis were stained with antibodies to CD3 (blue) and CD8 (red). Note infiltrates consisting of primarily CD8+ T cells in vitiligo skin with some cytotoxic T cells approaching the basal layer of the epidermis. A larger proportion of the tumor-infiltrating T cells belongs to the CD4+ subset of helper and regulatory T cells (blue)
3 Antigen-Presenting Cells in Vitiligo
The development of cutaneous immune responses begins with antigen-presenting cells (APCs) residing in the epidermis and dermis. Indeed, these APCs ultimately promote T cell activation, which, in turn, begets both further T cell responses (cellular immunity), as well as B cell activation and antibody formation (humoral immunity). Numerous types of professional antigen-presenting cells reside in the skin, including macrophages, Langerhans cells (LC), and dermal dendritic cells [32]. Dendritic cells (DCs) play a particularly important role in cutaneous immune responses by patrolling for pathogens. Pathogenic signals such as microbial peptides interact with receptors on resident dendritic cells, resulting in DC maturation and antigen presentation. This maturation involves a marked increase in a number of MHC class II and costimulatory molecules on the DC cell surface [33] and allows DCs to process antigens and activate T cells efficiently [34]. Two important receptor families which commonly stimulate DC maturation are the toll-like receptors (TLRs) and tumor necrosis factor (TNF) receptors [35,36,37,38,39]. Dendritic cells then endocytose, process, and present antigenic peptides on the cell surface in the context of major histocompatibility complex (MHC) molecules [40]. After migrating to skin-draining lymph nodes, they interact with, activate, and recruit cytotoxic and helper T cells specific to the antigens presented [41, 42]. Thus, antigen-presenting cells govern the type of helper T cell response elicited. Through cross-presentation, melanocyte-specific antigens displayed via MHC class I molecules give rise to the cytotoxic T cell cohort known to mediate melanocyte loss in vitiligo. Interestingly, a viral trigger may exist in some cases of vitiligo [43].
Langerhans cells are epidermal antigen-presenting cells that form the first line of defense against foreign antigens entering via the skin. They can stimulate CD4+ T cells [44] and are largely responsible for inducing contact hypersensitivity reactions [45]. Sensitization to contact allergen was not observed in Langerhans cell-deficient skin [46]. The role of Langerhans cells in vitiligo is not as clearly established. Interestingly, Langerhans cells reposition themselves, settling into the basal layer of the epidermis in melanocyte-depleted vitiliginous skin [47]. Furthermore, repigmentation in response to treatment can be associated with Langerhans cell depletion [48]. Thus, Langerhans cells might contribute to depigmentation on-site (perhaps through continued melanocyte antigen presentation to cytotoxic T cells), thereby preventing viability of any melanocytes attempting to repopulate the skin. This model could be likened to a delayed type hypersensitivity response, whereby the melanocyte self-antigens represent the “contact allergen.” Indeed, peptide-pulsed Langerhans cells can stimulate and expand CD8+ MART-I specific T cells [44]. However, as Langerhans cells are relatively inefficient at processing antigen [49, 50], their actual role in recruiting melanocyte-reactive T cells and initiating disease remains to be firmly established. Macrophages have been implicated in autoimmune disease development [51], and diabetes will not develop in macrophage-deficient mice [52]. Macrophages may likewise be pathogenic in vitiligo as they consistently infiltrate depigmenting perilesional vitiligo skin (Fig. 28.3), and their appearance correlates with melanocyte loss [24, 25]. However, the role of macrophages in vitiligo has not been clearly established [53]. Macrophages may phagocytize already apoptotic melanocytes or may actively contribute to melanocyte destruction. Once activated by interferon-gamma (IFN-γ) [54], macrophages secrete proinflammatory cytokines. Among these, TNF-α can contribute to melanocyte apoptosis [55]. Moreover, phagocytic macrophages [56] generate proinflammatory cytokines, such as IL-8, when engulfing apoptotic cells [57]. They may also serve as APCs to promote continued activation of autoreactive T cells. Taken together, there is ample cause for further studies into the pathogenic role of macrophages in vitiligo.
Abundance of CD36+ cells in perilesional vitiligo skin. Overexpression of the thrombospondin receptor CD36 has been associated with monocyte-to-macrophage differentiation and activation of the phagocytic process. CD36 can be expressed by other cells including thrombocytes, endothelial cells, and melanocytes, yet the morphology and location of the majority of cells observed in the dermis of this patient’s non-lesional and perilesional skin (detectable as a red AEC precipitate, red arrows) support the reported abundance of macrophages in depigmenting vitiligo skin (P) as compared to non-lesional skin (N)
4 Heat Shock Proteins in Immune Activation
Heat shock proteins (HSPs) play an integral role in the development of several autoimmune diseases, including vitiligo [58, 59]. Expression of HSPs is induced by stress [60, 61]. HSPs bind native proteins to prevent misfolding and cellular apoptosis [62]. In addition to facilitating cellular self-preservation, these HSP-native protein complexes can also trigger a local immune response directed at the cells from which the native proteins originate [63]. Once the HSP-protein complexes are released into the microenvironment, they bind receptors located on antigen-presenting cells [64, 65]. Surrounding APCs subsequently process the bound peptides and cross-present them to CD8+ T cells [66], eliciting immune responses toward the chaperoned antigens. Inducible heat shock protein 70 (HSP70i) can also be secreted from live cells under stress as a “chaperokine” [67]. HSP70 and other stress proteins can activate dendritic cells and subsequent T cell responses, leading to autoimmunity. HSP70 mediates development of autoimmune diabetes in mice [68] and likely plays a role in other autoimmune diseases as well. Overexpression of inducible HSP70 is sufficient to precipitate vitiligo disease [69], and vitiligo does not develop in mice that lack expression of HSP70i [70]. Importantly, the stress protein is overexpressed in vitiligo skin [71, 72]. These findings support a critical role for HSP70i in T cell-mediated melanocyte destruction.
Stress exposures that precipitate and worsen vitiligo include trauma to the affected area (koebnerization) [73], psychological stress [74], UV irradiation (oxidative stress) [75], and exposure to bleaching phenols [76]. HSP70 is upregulated in melanocytes after exposure to bleaching phenols [77], which have a well-established role in precipitating and worsening depigmentation [76, 78]. Other heat shock proteins are less likely to induce vitiligo as they are released only after cell death [79]. HSP70i is unique in that it is secreted by viable cells under stress, including melanocytes [80,81,82], which are ultimately later killed as the result of HSP70i-mediated cellular immune activation.
5 T Cell Subset Imbalance in Vitiligo
Infiltrates with decreased CD4+/CD8+ T cell ratios are found in actively depigmenting vitiligo patient skin [24, 25, 83]. T cells infiltrating vitiligo skin were shown to express the cutaneous lymphocyte antigen (CLA), a skin-homing receptor [25]. Circulating T cells in vitiligo patients are reactive with several melanocyte-specific antigens, including gp-100, tyrosinase, and Melan-A/MART-1 [83, 85]. Moreover, a T cell receptor reactive with the gp100 antigen has been characterized and cloned from patient skin [86]. T cells derived from vitiligo patient skin secrete predominantly type I cytokines in response to melanocytes, again indicative of cytotoxic T cell involvement [28].
In addition to increased numbers of skin-homing effector T cells [83], decreased skin homing of their inhibitory counterparts, the regulatory T cells (Tregs), is observed [87]. Others have reported a decreased abundance of Tregs in the circulation [88] or mentioned that Treg function may be reduced among circulating Tregs [89], but such deficiencies are expected to accompany more systemic autoimmune consequences. Decreased numbers or impaired function of Tregs have been established in systemic autoimmune disease [90]. This deficiency can well explain why tolerance to self-antigens is inadequately maintained [91]. The T cell imbalance in vitiligo skin thus creates the perfect environment for depigmentation. Destruction of melanocytes in the skin not only results in clinical depigmentation but also leads to a large number of resident memory T cells capable of recognizing melanocytic self-antigens [92, 93]. These self-reactive T cells have been found to persist in the skin long after melanocyte destruction [93]. This might well explain disease relapse in patients following periods of repigmentation.
6 T Cell Trafficking
In order for vitiligo to develop, melanocyte-specific cytotoxic T cells must migrate toward the epidermis in response to chemoattractant cytokines. The presence of IFN-γ is crucial for active depigmentation to ensue [94]; it induces the expression of the Th1 chemokine, CXCL10 [95], which is produced by numerous cell types, including keratinocytes, macrophages, fibroblasts, neutrophils [95, 96], and, importantly, activated T cells [97, 98]. Upon melanocyte stress and subsequent HSP70i release, antigen-presenting cells will recruit an initial cohort of melanocyte-reactive T cells that produce IFN-γ upon antigen recognition. This would lead to CXCL10 production and further T cell recruitment to the epidermis. Indeed, neutralization of CXCL10 prevented depigmentation and promoted repigmentation in mice [99]. Aside from T cells, CXCL10 can recruit monocytes/macrophages and natural killer (NK) cells [96]. Yet, the primary contribution of CXCL10 to vitiligo development lies in skin recruitment of self-reactive T cells expressing its receptor CXCR3, as found in vitiligo patient skin [99].
The absence of Tregs in vitiligo skin is likewise best explained by differential chemokine expression in lesional skin. Cells expressing the Treg chemoattractant CCL22 are present in markedly decreased numbers in vitiligo as compared to control skin [82]. CCL22 is secreted by macrophages and dendritic cells [100]. As the CCL22 receptor, CCR4, is expressed in similar amounts by circulating Tregs from patients and controls, the decreased amounts of CCL22 may be primarily responsible for the significant underrepresentation of Tregs in vitiligo skin.
7 Cytokines in Vitiligo Skin
Activated perilesional vitiligo T cells secrete cytokines and chemokines, which either propagate immune mechanisms or contribute directly or indirectly to melanocyte destruction. Vitiligo development is critically dependent on IFN-γ in mouse models of vitiligo [94, 101]. Similarly, T cells derived from vitiligo patient skin secrete IFN-γ in response to melanocytes, again indicative of a type 1 cytokine response and cytotoxic T cell involvement [28]. IFN-γ-responsive effector chemokines, including CXCL9, CCL5, and especially CXCL10, are reportedly elevated in the vitiligo skin [102] and can serve to facilitate T cell trafficking toward resident melanocytes. IFN-γ also increases T cell expression of CXCR3, activates macrophages, and increases ICAM expression on endothelial cells [54, 103,104,105]. Each of these factors can facilitate melanocyte destruction. Importantly, IFN-γ also inhibits Treg generation through STAT1-signaling [106]. However, in mice knockout for IFN-γ, spontaneous depigmentation was restored when Tregs were depleted from the circulation [107], supporting the concept that IFN-γ involvement is not the sole pathogenic cytokine in vitiligo progression.
TNF-α contributes to vitiligo pathogenesis by supporting cytotoxic T cell development [108] and enhancing IFN-γ secretion [109]. TNF-α also inhibits melanocyte proliferation in vivo [110] and promotes melanocyte apoptosis in vitro [111]. However, its presence is not required for disease development, as vitiligo development ensued in TNF-α knockout mice [107]. Cytotoxic T cell-mediated apoptosis occurs through either the perforin-granzyme pathway [112] or the Fas/Fas ligand pathways [112,113,114]. Because melanocytes proved resistant to Fas ligand-mediated cell death [115], and most perilesional T cells in vitiligo skin are perforin and granzyme-B immunoreactive [25], this has implicated perforin-granzyme-mediated cell death in vitiligo. However, mice knockout for perforin can still develop vitiligo [100, 107], as can granzyme knockout mice [115]. This implies that neither mechanism is exclusively responsible for depigmentation.
IL-17 is a cytokine with controversial involvement in vitiligo pathogenesis. IL-17 is produced by T cells and natural killer cells [116] and is increased both in vitiligo patient serum and in the lesional skin [117]. Furthermore, IL-17A mRNA and IL-17A+ T cells were present in increased quantities in perilesional compared to non-lesional vitiligo skin [118]. In mice, adoptive transfer of Th17 cells induced vitiligo, supporting a possible role for their cytokines, including IL-17, in vitiligo pathogenesis. However, a role for IFN-γ cannot be ruled out [119]. Thus, focusing on the blockade of production or action of the latter cytokine may be of greater utility.
8 Additional Players in Cellular Immunity
Importantly, melanocytes from vitiligo patients are increasingly sensitive to cellular stress, as evidenced by dilation of the endoplasmic reticulum [120] and increased levels of oxidative byproducts [121,122,123]. When melanocytes release stress signals into the microenvironment (reactive oxygen species and HSP70i), this activates pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs) [124]. In addition to induction of adaptive immune responses via dendritic cell activation, there is evidence to support that HSP70 also stimulates local innate immune responses. Indeed, HSP70+ exosomes were found to stimulate activation and migration of natural killer (NK) cells in vitro [125]. This likely translates to human disease, as patients with vitiligo were found to have increased amounts of natural killer cells in both lesional and non-lesional skin compared to their non-vitiligo counterparts [126]. This likely renders vitiligo patients capable of generating robust innate immune responses. Interestingly, NKs taken from lesional skin of vitiligo patients expressed high levels of granzyme B [126], the serine protease that can mediate melanocyte destruction. Inflammatory dendritic cells are also increased in vitiliginous skin [59, 72] and are induced by HSP70i in vitro [127]. Gene transcription evaluation in Smyth chickens that develop spontaneous vitiligo revealed increased transcription of innate immune response genes in response to oxidative stress [128], further supporting the role of innate immune responses in vitiligo.
The role of humoral immunity in vitiligo pathogenesis may be less contributory than cellular immune responses, but it likely fuels disease progression. Vitiligo patients have elevated anti-melanocyte antibody titers [29, 129]. Such antibodies could mediate cytotoxicity toward melanocytes [130]. Moreover, B cells may be involved in propagating T cell activation [11,12,13] to drive depigmentation. A noteworthy observation to discriminate between causative and bystander roles for autoantibodies is that of rarely reported congenital vitiligo [131, 132], where depigmentation was postulated to result from transplacental transfer of melanocyte-specific IgG antibodies in utero.
9 Opportunities for Immunotherapy
-
Phototherapy
Ultraviolet radiation (UVR) has immunosuppressive properties and is utilized to treat many skin conditions including psoriasis, atopic dermatitis, cutaneous T cell lymphoma, and vitiligo [133]. Immunosuppression is mediated by keratinocyte secretion of Th2 cytokines, primarily IL-10 and IL4 [134]. Specifically, IL-10 appears to decrease the ability of APCs to activate Th1 cells [134], inhibits development of delayed type hypersensitivity responses [135], and decreases T cell production of type I cytokines, including IL-12, IL-8, and interferon-γ [136]. Importantly, UVB was shown to inhibit phosphorylation of STAT-1, inhibiting IFN-γ signaling [137]. Narrowband UVB (nb-UVB) also markedly increases lesional and perilesional abundance of the Treg transcription factor FoxP3 [138], implying that nb-UVB increases the number of Tregs in the cutaneous environment. This likely contributes to the observed treatment success of nb-UVB in the clinic [139]. The immunosuppressive properties of UVR are evidenced by blunted T cell immune-mediated responses as well as decreased immune surveillance of UV-induced skin cancers following exposure [140,141,142,143,144].
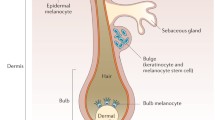
In addition to its immunosuppressive effects, UVR has direct effects on melanocyte function. Specifically, UVR increases melanosome accumulation and transfer to keratinocytes [145]; treatment with UVR also achieves clinical repigmentation. Narrowband UVB resulted in increased keratinocyte release of bFGF and ET-1, which induce melanocyte proliferation; nb-UVB also resulted in increased melanocyte expression of p125FAK and MMP-2, which enhance melanocyte migration [146]. Therefore, UVR may induce melanocyte stem cell proliferation, differentiation, and migration from their reservoir site upon exposure. The source of repigmenting melanocytes is presumably the hair follicle, which would clinically explain the appearance of perifollicular repigmentation as the first sign of treatment response in UVR-treated vitiligo patients. Inactive (Dopa-negative, non-melanin-producing) melanocyte stem cells are preserved in the outer root sheath of the hair follicles of vitiligo skin [147] and do not yet express the target molecules recognized by pathogenic T cells. When clinical repigmentation was noted, the first signs microscopically were an increased number of melanocytes in the outer root sheath of the hair follicle, migrating toward the epidermis and undergoing maturation [147]. UVA and nb-UVB (311 nm) and excimer laser (308 nm) have all been employed and have each met with varying degrees of success in treating vitiligo patients [148, 149]. While phototherapy is an efficacious and well-tolerated treatment option for vitiligo [139], it carries some limitations including limited accessibility to patients and its blunted efficacy in more resilient body sites. Therefore, development of additional immunotherapies is prudent. The principles of UV-based and other immunotherapeutic strategies are depicted in Fig. 28.4.
-
Modified HSP70
To be successful, treatment should both halt ongoing melanocyte destruction and stimulate repigmentation. HSP70 lends way to melanocyte destruction by activating dendritic cells, which subsequently leads to T cell recruitment and an anti-melanocyte immune response. HSP70iQ435A is a variant of inducible HSP70i of potential therapeutic benefit in vitiligo treatment [71] (=Sci. Transl. Med. 2013). Vitiligo-prone mice treated with DNA encoding HSP70iQ435A exhibited markedly reduced depigmentation [69]. This treatment is currently being applied to Sinclair swine with human-like skin and vitiligo lesions that develop in response to regressing melanomas [148]. In this application, the gene gun has been replaced by needle-less injectors, which are more translatable to use in the clinic and which are less traumatic than the gene gun delivery method [149]. The latter is important in vitiligo given its tendency to koebnerize. Such studies as well as further safety testing will be have to be completed before a clinical trial can be considered. By preventing dendritic cell activation, autoimmunity is prevented, and repigmentation can occur in the absence of infiltrating T cells.
-
Inhibiting T cell recruitment
Effector T cells engage the chemokine receptor, CXCR3, and its ligand, CXCL10, to migrate to the skin [99, 101, 152]. Recently, serum CXCL10 levels have been found to correlate with progressive disease, whereas levels decreased following successful treatment [153]. It follows that one proposed treatment strategy includes blocking the chemokine receptor, CXCR3, from binding its ligand [154]. This intervention has proven efficacious in vitiligo mice in preventing and reversing disease [99]. CXCL10 is secreted by cytotoxic T cells in vitiligo skin [97, 98]. Though anti-CXCL10 monoclonal antibodies have been tested in treating rheumatoid arthritis and ulcerative colitis [155, 156], their efficacy has not yet been tested in vitiligo patients. It is possible that chemokine depletion will be relatively less successful due to its redundancy with, in particular, CXCL11. Overall, however, interfering with effector T cell homing offers an exciting therapeutic approach in vitiligo.
-
JAK inhibitors
Janus kinases (JAKs) are tyrosine kinases which transmit extracellular cytokine signals to the intracellular space via their association with cytokine receptors [157]. Through their activation of the STAT transcription pathway (JAK-STAT pathway) [158], the JAK kinases mediate signaling of type I cytokines and interferons [159,160,161]. This ultimately affects lymphocyte activation and proliferation [160, 162, 163]. There are four mammalian JAK kinases [160, 161]. JAK 1 and 2 are clinically relevant to vitiligo, as they mediate IFN-γ signal transduction [164, 165]. Transcription of IFN-γ-inducible genes leads to CXCL10 production [166]. A JAK 1/3 inhibitor is currently FDA-approved for the treatment of moderate to severe rheumatoid arthritis. Oral treatment has also been successfully used for alopecia areata [167] and psoriasis [168], and systemically administered treatment can prevent alopecia areata in mice [169]. Randomized controlled trials are required to further define their utility and safety, but given the mechanistic relevance of JAK inhibition to vitiligo pathogenesis, their use to treat vitiligo holds promise.
An exciting new treatment proposal entails the use of lipid-lowering statins to treat vitiligo. HMG-CoA reductase inhibitors can target a different aspect of the JAK-STAT signaling pathway than the JAK inhibitors, by inhibiting STAT1 activation [170]. Indeed, statins can prevent progression of vitiligo and promote repigmentation in mice [171] and likewise promoted repigmentation in a vitiligo patient [172].
-
Treg-based therapy
The paucity of skin-homing regulatory T cells [87] promotes a local environment whereby cytotoxic T cells may freely attack melanocytes. Cutaneous Treg recruitment fails in vitiligo patients due to decreased levels of the Treg chemokine CCL22 [87]. In fact, replenishing CCL22 was sufficient to overcome depigmentation [173]. Vitiligo-prone mice [174] exhibited reduced depigmentation following adoptive Treg transfer [107]. Animals experienced prolonged inhibition of depigmentation, while transferred Tregs migrated to the skin [107]. Likewise, vitiligo was successfully treated with rapamycin, which promoted Treg development [107, 175]. When administered with all-trans retinoic acid, rapamycin was able to confer both expansion of Tregs and biological stability of such Tregs in an inflammatory environment [176]. Rapamycin as monotherapy also conferred protection against depigmentation for up to 6 weeks after cessation of therapy in mice, highlighting its stabilizing effects on expanded Tregs [107]. Thus, creating a quantitative increase in Tregs in the skin via adoptive Treg transfer or rapamycin administration may serve as a potentially efficacious treatment modality in halting progressive vitiligo in humans.
-
Cytokine inhibition
The presence of minute immune infiltrates in depigmenting vitiligo skin warrants the application of antibodies to neutralize the effects of cytokines generated by activated lymphocytes. IFN-γ-neutralizing antibodies were shown to markedly reduce self-reactive cytotoxic T cell accumulation in the skin and to prevent depigmentation in a mouse model of vitiligo [94]. In the absence of IFN-γ, T cell activation is prevented, as is IFN-γ-induced melanocyte apoptosis [177]. Furthermore, melanocytes will downregulate the expression of MHC, including MHC class II [178]. TNF-α likely contributes to active depigmentation via its activation of CD8+ T cells. This is supported by the finding that TNF-α inhibition has been successful in halting progressive vitiligo [179,180,181], in which CD8+ T cells play an active role [25]. Paradoxically, some individuals developed de novo vitiligo when treated with anti-TNF-α agents. This may be explained by the fact that TNF-α can also increase Treg activity; the scale may be tipped in favor of depigmentation as a result of Treg depletion in these exceptional cases [182].
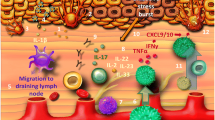
Immunotherapeutic approaches for vitiligo. Stressed melanocytes can secrete inducible HSP70. (1) Activation of dendritic cells can be inhibited by overexpressing a modified version of inducible heat shock protein, namely, HSP70iQ435A. Migratory dendritic cells transporting melanocyte-specific antigens can activate and recruit T cells from draining lymph nodes. (2) However, rapamycin can favor the development of Tregs over inflammatory IL-17-producing T cells and prevent effector T cell development and recruitment. (3) By introducing inhibitors of chemokine receptors such as anti-CXCR3, migration of effector T cells to the skin can be avoided. When encountering their target antigens presented by dendritic cells or upon arrival in the skin, T cells become activated. (4) Such T cell activation can be inhibited by JAK inhibitors. Within the skin, activated T cells will secrete cytokines that can promote ongoing autoimmunity. (5) Inhibitors of type 1 cytokines can neutralize such immune enhancement. The local environment is conducive to effector responses in the absence of sufficient regulatory T cells. (6) By topical application of steroids or calcineurin inhibitors, regulatory responses are encouraged, and autoimmune responses can be brought to a halt in early stages of disease. (7) Adoptive transfer of Tregs can similarly counterbalance ongoing effector responses. In preclinical models, most therapeutics not only halt depigmentation but also allow for subsequent repigmentation of the skin if a reservoir of stem cells is available
10 Vitiligo and Melanoma T Cell Responses
After decades without marked progress, several immune-based treatments are currently emerging for the treatment of malignant melanoma. Their efficacy is often associated with depigmentation of the skin. The development of vitiligo is a positive prognostic factor in melanoma patients [183]. This is likely the case because the presence of vitiligo denotes efficient anti-melanocyte T cell responses against shared tumor and normal melanocyte antigens [184], including MART-1 and gp100 [185]. A comparison of T cell infiltrates in vitiligo and melanoma tissue is shown in Fig. 28.2. Meanwhile, a large retrospective cohort study revealed that patients with vitiligo have a threefold decreased lifetime risk of developing melanoma [186]. Adoptive transfer of cytotoxic T cells specific for melanoma differentiation antigens has been associated with vitiligo development [31, 174, 187], again supporting a pathogenic role of autoimmune T cells in vitiligo. Interestingly, the presence of normal or increased levels of regulatory T cells in the skin of melanoma patients is associated with less effective clearing of melanoma tumors [188]; depleting these Tregs is required in order for sufficient cytotoxic T cell-mediated melanocyte destruction to ensue [93]. As vitiligo represents autoimmune melanocyte destruction in the skin, dermatologists must consider the origin of generation of these responses. While vitiligo often does not have a readily identifiable trigger by history, the appearance of this entity beyond adolescence might be a harbinger of the body generating immune responses against an underlying melanoma. This concept is similar to the clinical implications of halo nevi as a potential marker of dysplastic nevi or malignant melanocytic neoplasms [189, 190]. Therefore, new-onset vitiligo in this age group warrants a total body skin exam to screen for melanoma..
Change history
18 March 2020
Permission for using the table 11.1 had been acquired from Pigm Mel Research. Hence, caption for Table 11.1 has been corrected to “van Geel N, Speeckaert R, Taieb A et al. Koebner’s phenomenon in vitiligo: European position paper. Pigment Cell
References
Taieb A, Picardo M. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res. 2007;20:27–35.
Kim YC, Kim YJ, Kang HY, et al. Histopathologic features in vitiligo. Am J Dermatopathol. 2008;30:112–6.
Le Poole IC, van den Wijngaard RMJGJ, Westerhof W, et al. Presence or absence of melanocytes in vitiligo lesions: an immunohistochemical investigation. J Invest Dermatol. 1993;100:816–22.
Buckley WR, Lobitz WC Jr. Vitiligo with a raised inflammatory border. Arch Dermatol Syph. 1953;67:316–20.
Ishii M, Hamada T. Ultrastructural studies of vitiligo with inflammatory raised borders. J Dermatol. 1981;8:313–22.
Kemp EH, Waterman EA, Weetman AP. Immunological pathomechanisms in vitiligo. Expert Rev Mol Med. 2001;3:1–22.
Park YK, Kim NS, Hann SK, et al. Identification of autoantibody to melanocytes and characterization of vitiligo antigen in vitiligo patients. J Dermatol Sci. 1996;11:111–20.
Hara I, Takechi Y, Houghton AN. Implicating a role for immune recognition of self in tumor rejection: passive immunization against the brown locus protein. J Exp Med. 1995;182:1609–14.
Takechi Y, Hara I, Naftzger C, et al. A melanosomal membrane protein is a cell surface target for melanoma therapy. Clin Cancer Res. 1996;2:1837–42.
Venneker GT, Vodegel RM, Okada N, et al. Relative contributions of decay acceleration factor (DAF), membrane cofactor protein (MCP) and CD59 in the protection of melanocytes from homologous complement. Immunobiology. 1998;198:476–84.
Damle NK, Doyle LV, Grosmaire LS, et al. Differential regulatory signals delivered by antibody binding to the CD28 (Tp44) molecule during the activation of human T lymphocytes. J Immunol. 1988;140:1753–61.
Damle NK, Linsley PS, Ledbetter JA. Direct helper T cell-induced B cell differentiation involves interaction between T cell antigen CD28 and B cell activation antigen B7. Eur J Immunol. 1991;21:1277–82.
Lesslauer WF, Koning T, Ottenhoff M, et al. T90/44 (9.3 antigen). A cell surface molecule with a function in human T cell activation. Eur J Immunol. 1986;16:1289.
Ezzedine K, Lim HW, Suzuki T, et al. Reviewed classification/nomenclature of vitiligo and related issues: the Vitiligo Global Issues Consensus Conference. Pigment Cell Melanoma Res. 2012;25:E1–E13.
Van Geel NA, Mollet IG, De Schepper S, et al. First histopathological and immunophenotypic analysis of early dynamic events in a patient with segmental vitiligo associated with halo nevi. Pigment Cell Melanoma Res. 2010;23:375–84.
Taieb A, Morice-Picard F, Jouary T, et al. Segmental vitiligo as the possible expression of cutaneous somatic mosaicism: implications for common non-segmental vitiligo. Pigment Cell Melanoma Res. 2008;21:646–52.
Jin Y, Birlea SA, Fain PR, et al. Variant of TYR and autoimmunity susceptibility loci in generalized vitiligo. N Engl J Med. 2010;362:1686–97.
Liu JB, Li M, Chen H, et al. Association of vitiligo with HLA-A2: a meta-analysis. J Eur Acad Dermatol Venereol. 2007;21:205–13.
Foley LM, Lowe NJ, Misheloff E, et al. Association of HLA-DR4 with vitiligo. J Am Acad Dermatol. 1983;8:39–40.
Fernando MM, Stevens CR, Walsh EC, et al. Defining the role of the MHC in autoimmunity: a review and pooled analysis. PLoS Genet. 2008;4:e1000024.
Akay BN, Bozkir M, Anadolu Y, et al. Epidemiology of vitiligo, associated autoimmune diseases and audiological abnormalities: Ankara study of 80 patients in Turkey. J Eur Acad Dermatol Venereol. 2010;24:1144–50.
Daneshpazhooh M, Behjati J, Akhyani M, et al. Anti-thyroid peroxidase antibody and vitiligo: a controlled study. BMC Dermatol. 2006;6:3.
Hegedüs L, Heidenheim M, Gervil M, et al. High frequency of thyroid dysfunction in patients with vitiligo. Acta Derm Venereol. 1994;74:120–3.
Le Poole IC, van den Wijngaard RMJGJ, Westerhof W. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am J Pathol. 1996;148:1219–28.
Van den Wijngaard R, Wankowicz-Kalinska A, Le Poole C, et al. Local immune response in skin of generalized vitiligo patients. Destruction of melanocytes is associated with the prominent presence of CLA+ T cells at the perilesional site. Lab Investig. 2000;80:1299–309.
Wu J, Zhou M, Wan Y, et al. CD8+ T cells from vitiligo perilesional margins induce autologous melanocyte apoptosis. Mol Med Rep. 2013;7:237–41.
Steitz J, Wenzel J, Gaffal E, et al. Initiation and regulation of CD8+ T cells recognizing melanocytic antigens in the epidermis: implications for the pathophysiology of vitiligo. Eur J Cell Biol. 2004;83:797–803.
Wańkowicz-Kalińska A, van den Wijngaard RM, Tigges BJ, et al. Immunopolarization of CD4+ and CD8+ T cells to Type-1-like is associated with melanocyte loss in human vitiligo. Lab Investig. 2003;83:683–95.
Ogg GS, Dunbar PR, Romero P, et al. High frequency of skin-homing melanocyte-specific cytotoxic T lymphocytes in autoimmune vitiligo. J Exp Med. 1998;188:1203–8.
Van den Boorn JG, Konijnenberg D, Dellemijn TA, et al. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J Invest Dermatol. 2009;129:2220–32.
Yee C, Thompson JA, Roche P, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of T cell mediated vitiligo. J Exp Med. 2000;192:1637–44.
Mathers AR, Larrgenia AT. Professional antigen-presenting cells of the skin. Immunol Res. 2006;36:127–36.
Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–8.
Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711.
Akbari O, Panjwani N, Garcia S, et al. DNA vaccination: transfection and activation of dendritic cells as key events for immunity. J Exp Med. 1999;189:169–78.
Bonifaz L, Bonnyay D, Mahnke K, et al. Efficient targeting of protein antigens to the dendritic cell receptor DEC205 in the steady state leads to antigen presentation on MHC class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002;196:1627–38.
Hawiger D, Inaba K, Dorsett Y, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001;194:769–80.
Liu K, Iyoda T, Saternus M, et al. Immune tolerance following delivery of dying cells to dendritic cells in situ. J Exp Med. 2002;196:1091–7.
Sparwasser T, Vabulas RM, Villmow B, et al. Bacterial CpG-DNA activates dendritic cells in vivo: T helper cell-independent cytotoxic T cell responses to soluble proteins. Eur J Immunol. 2000;30:3591–7.
Guermonprez P, Valladeau J, Zitvogel L, et al. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67.
Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811.
Nestle FO, Banchereau J, Hart D. Dendritic cells: on the move from bench to bedside. Nat Med. 2001;7:761–5.
Grimes PE, Sevall JS, Vojdani A. Cytomegalovirus DNA identified in skin biopsy specimens of patients with vitiligo. J Am Acad Dermatol. 1996;35:21–6.
Klechevsky E, Morita R, Liu M, et al. Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity. 2008;29:497–510.
Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–53.
Dumay O, Karam A, Vian L, et al. Ultraviolet AI exposure of human skin results in Langerhans cell depletion and reduction of epidermal antigen-presenting cell function: partial protection by a broad-spectrum sunscreen. Br J Dermatol. 2001;144:1161–8.
Birbeck MS, Breathnach AS, Everall JD. An electron microscope study of basal melanocytes and high-level clear cells (Langerhans cells) in vitiligo. J Invest Dermatol. 1961;37:51.
Kao C-H, Yu H-S. Depletion and repopulation of Langerhans cells in nonsegmental type vitiligo. J Dermatol. 1990;17:287–96.
Inaba KG, Schuler MD, Witmer J, et al. The immunologic properties of purified Langerhans cells: distinct requirements for the stimulation of unprimed and sensitized T lymphocytes. J Exp Med. 1986;164:605.
Schuler G, Steinman RM. Murine epidermal Langerhans cells mature into potent immunostimulatory dendritic cells in vitro. J Exp Med. 1985;161:526.
Krausgruber T, Blazek K, Smallie T, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231–8.
Jun HS, Yoon CS, Zbytnuik L, et al. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese diabetic mice. J Exp Med. 1999;189:347–58.
Richmond JM, Frisoli ML, Harris JE. Innate immune mechanisms in vitiligo: danger from within. Curr Opin Immunol. 2013;25:676–82.
Schroder KP, Hertzog PJ, Ravasi T, et al. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–89.
Soma T, Ogo M, Suzuki J, et al. Analysis of apoptotic cell death in human hair follicles in vivo and in vitro. J Invest Dermatol. 1998;111:948–54.
Van Furth R. Development and distribution of mononuclear phagocytes. In: Gallin JI, Goldstein IM, Snyderman R, editors. Inflammation. New York: Raven Press; 1992. p. 325.
Kurosaka K, Watanabe N, Kobayashi Y. Production of proinflammatory cytokines by resident tissue macrophages after phagocytosis of apoptotic cells. Cell Immunol. 2001;211:1–7.
Rajaiah R, Moudgil KD. Heat-shock proteins can promote as well as regulate autoimmunity. Autoimmun Rev. 2009;8:388–93.
Mosenson JA, Eby JM, Hernandez C, et al. A central role for inducible heat-shock protein 70 in autoimmune vitiligo. Exp Dermatol. 2013;22:566–9.
Määttänen P, Gehring K, Bergeron JJ, Thomas DY. Protein quality control in the ER: the recognition of misfolded proteins. Semin Cell Dev Biol. 2012;21:500–11.
Welch NJ. Heat shock proteins functioning as molecular chaperones: their roles in normal and stressed cells. In: Molecular chaperones. Netherlands: Springer; 1993. p. 71–7.
Benbrook DM, Long A. Integration of autophagy, proteasomal degradation, unfolded protein response and apoptosis. Exp Oncol. 2012;34:286–97.
Calderwood SK, Stevenson MA, Murshid A. Heat shock proteins, autoimmunity, and cancer treatment. Autoimmune Dis. 2012;2012:486069.
Binder R, Han D, Srivastava PK. CD91: a receptor for the heat shock protein gp96. Nat Immunol. 2000;1:51.
Srivastava PK, Udono H, Blachere NE, et al. Heat shock proteins transfer peptides during antigen processing and CTL priming. Immunogenetics. 1994;39:93–8.
Basu S, Binder RJ, Suto R, et al. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int Immunol. 2000;12:1539–46.
Mambula SS, Calderwood SK. Heat shock protein 70 is secreted from tumor cells by a nonclassical pathway involving lysosomal endosomes. J Immunol. 2006;177:7849–57.
Millar DG, Garza KM, Odermatt B, et al. Hsp70 promotes antigen-presenting cell function and converts T-cell tolerance to autoimmunity in vivo. Nat Med. 2003;9:1469–76.
Denman CJ, McCracken J, Hariharan V, et al. HSP70i accelerates depigmentation in a mouse model of autoimmune vitiligo. J Invest Dermatol. 2008;128:2041–8.
Mosenson JA, Zloza A, Klarquist J, et al. HSP70i is a critical component of the immune response leading to vitiligo. Pigment Cell Melanoma Res. 2012;25:88–98.
Abdou AG, Maraee AH, Reyad W. Immunohistochemical expression of heat shock protein 70 in vitiligo. Ann Diag Pathol. 2013;17:245–9.
Mosenson JA, Zloza A, Nieland JD, et al. Mutant HSP70 reverses autoimmune depigmentation in vitiligo. Sci Transl Med. 2013;5:174ra28.
Sanghavi SA, Dongre AM, Khopkar US. Koebnerization and generalized spread of vitiligo following radiotherapy. Indian Dermatol Online J. 2013;4:147.
Picardi A, Pasquini P, Cattaruzza MS, et al. Stressful life events, social support, attachment security and alexithymia in vitiligo. Psychother Psychosom. 2003;72:150–8.
Maresca V, Roccella M, Roccella F, et al. Increased sensitivity to peroxidative agents as a possible pathogenic factor of melanocyte damage in vitiligo. J Invest Dermatol. 1997;109:310–3.
Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208–14.
Kroll TM, Bommiasamy H, Boissy RE, et al. 4-Tertiary butyl phenol exposure sensitizes human melanocytes to dendritic cell-mediated killing: relevance to vitiligo. J Invest Dermatol. 2005;124:798–806.
Van den Boorn JG, Picavet DI, van Swieten PF, et al. Skin-depigmenting agent monobenzone induces potent T-cell autoimmunity toward pigmented cells by tyrosinase hapentization and melanosome autophagy. J Invest Dermatol. 2011;131:1240–51.
Strbo N, Podack ER. Secreted heat shock protein 96-Ig: an innovative vaccine approach. Am J Reprod Immunol. 2008;59:407–16.
Asea A. Mechanisms of HSP72 release. J Biosci. 2007;32:579–84.
Vega VL, Rodríguez-Silva M, Frey T, et al. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180:4299–307.
Mosenson JA, Flood K, Klarquist J, et al. Preferential secretion of inducible HSP70 by vitiligo melanocytes under stress. Pigment Cell Melanoma Res. 2014;27:209–20.
Dwivedi M, Laddha NC, Arora P, et al. Decreased regulatory T-cells and CD4+/CD8+ ratio correlate with disease onset and progression in patients with generalized vitiligo. Pigment Cell Melanoma Res. 2013;26:586–91.
Mandelcorn-Monson RL, Shear NH, Yau E, et al. Cytotoxic T lymphocyte reactivity to gp100, MelanA/MART-1, and tyrosinase, in HLA-A2-positive vitiligo patients. J Invest Dermatol. 2003;121:550–6.
Palermo B, Campanelli R, Garbelli S, et al. Specific cytotoxic T lymphocyte responses against Melan-A/MART1, tyrosinase and gp100 in vitiligo by the use of major histocompatibility complex/peptide tetramers: the role of cellular immunity in the etiopathogenesis of vitiligo. J Invest Dermatol. 2001;117:326–32.
Klarquist J, Eby JM, Henning SW, et al. Functional cloning of a gp100-reactive T-cell receptor from vitiligo patient skin. Pigment Cell Melanoma Res. 2016;29(3):379–84.
Klarquist J, Denman CJ, Hernandez C, et al. Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res. 2010;23:276–86.
Lili Y, Yi W, Ji Y, et al. Global activation of CD8+ cytotoxic T lymphocytes correlates with an impairment in regulatory T cells in patients with generalized vitiligo. PLoS One. 2012;7:e37513.
Lin M, Zhang BX, Shen N, et al. Regulatory T cells from active non-segmental vitiligo exhibit lower suppressive ability on CD8+ CLA+ T cells. Eur J Dermatol. 2014;24:676–82.
Cvetanovich GL, Hafler DA. Human regulatory T cells in autoimmune diseases. Curr Opin Immunol. 2010;22:753–60.
Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–16.
Boniface K, Dessarthe B, Vernisse C, et al. Vitiligo is enriched with population of skin T cells expressing a resident memory phenotype. J Invest Dermatol. 2015;135:S76.
Byrne KT, Cote AL, Zhang P, et al. Autoimmune melanocyte destruction is required for roust CD8+ memory T cell responses to mouse melanoma. J Clin Invest. 2011;121:1797–809.
Harris JE, Harris TH, Weninger W, et al. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869–76.
Luster AD, Ravetch JV. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J Exp Med. 1987;166:1084–97.
Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. 2011;89:207–15.
Biddison WE, Taub DD, Cruikshank WW, et al. Chemokine and matrix metalloproteinase secretion by myelin proteolipid protein-specific CD8+ T cells: potential roles in inflammation. J Immunol. 1997;158:3046–53.
Gattass CR, King LB, Luster AD, et al. Constitutive expression of interferon gamma-inducible protein 10 in lymphoid organs and inducible expression in T cells and thymocytes. J Exp Med. 1994;179:1373–8.
Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23.
Kimura S, Tanimoto A, Wang KY, et al. Expression of macrophage-derived chemokine (CCL22) in atherosclerosis and regulation by histamine via the H2 receptor. Pathol Int. 2012;62:675–83.
Gregg RK, Nichols L, Chen Y, et al. Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J Immunol. 2010;184:1909–17.
Antonelli A, Ferrari SM, Fallahi P. The role of the Th1 chemokine CXCL10 in vitiligo. Ann Transl Med. 2015;3:S1.
Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–86.
Moser B, Wolf M, Walz A, et al. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004;25:75–84.
Nakajima C, Mukai T, Yamaguchi N, et al. Induction of the chemokine receptor CXCR3 on TCR-stimulated T cells: dependence on the release from persistent TCR-triggering and requirement for IFN-γ stimulation. Eur J Immunol. 2002;32:1792–801.
Caretto D, Katzman SD, Villarino AV, et al. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol. 2010;184:30–4.
Chatterjee S, Eby JM, Al-Khami AA, et al. A quantitative increase in regulatory T cells controls development of vitiligo. J Invest Dermatol. 2014;134:1285–94.
Ranges GE, Figari IS, Espevik T, et al. Inhibition of cytotoxic T cell development by transforming growth factor beta and reversal by recombinant tumor necrosis factor alpha. J Exp Med. 1987;166:991–8.
Scheurich P, Thoma B, Ricer U, et al. Immunoregulatory activity of recombinant human tumor necrosis factor (TNF)-alpha: induction of TNF receptors on human T cells and TNF-alpha-mediated enhancement of T cell responses. J Immunol. 1987;138:1786–90.
Swope VB, Abdel-Malek Z, Kassem LM, et al. Interleukins 1α and 6 and tumor necrosis factor α are paracrine inhibitors of human melanocyte proliferation and melanogenesis. J Invest Dermatol. 1991;96:180–5.
Kim NH, Jeon S, Lee HJ, et al. Impaired PI3K/Akt activation-mediated NF-κB inactivation under elevated TNF-α is more vulnerable to apoptosis in vitiliginous keratinocytes. J Invest Dermatol. 2007;127:2612–7.
Froelich CJ, Dixit VM, Yang X. Lymphocyte granule-mediated apoptosis: matters of viral mimicry and deadly proteases. Immunol Today. 1998;19:30–6.
Lowin B, Hahne M, Mattmann C, et al. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathway. Nature. 1994;370:650–2.
Nagata S. Apoptosis by death factor. Cell. 1997;88:355–65.
Rivoltini L, Radrizzani M, Accornero P, et al. Human melanoma-reactive CD4+ and CD8+ CTL clones resist Fas ligand-induced apoptosis and use Fas/Fas ligand-independent mechanisms for tumor killing. J Immunol. 1998;161:1220–30.
Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–98.
Bassiouny DA, Shaker O. Role of interleukin-17 in the pathogenesis of vitiligo. Clin Exp Dermatol. 2011;36:292–7.
Wang CQ, Cruz-Inigo AE, Fuentes-Duculan J, et al. Th17 cells and activated dendritic cells are increased in vitiligo lesions. PLoS One. 2011;6:e18907.
Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–73.
Boissy RE, Liu YY, Medrano EE, et al. Structural aberration of the rough endoplasmic reticulum and melanosome compartmentalization in long-term cultures of melanocytes from vitiligo patients. J Invest Dermatol. 1991;97:395–404.
Koca R, Armutcu F, Altinyazar HC, et al. Oxidant-antioxidant enzymes and lipid peroxidation in generalized vitiligo. Clin Exp Dermatol. 2004;29:406–9.
Schallreuter KU, Moore J, Wood JM, et al. In vivo and in vitro evidence for hydrogen peroxide (H2O2) accumulation in the epidermis of patients with vitiligo and its successful removal by a UVB-activated pseudocatalase. J Investig Dermatol Symp Proc. 1999;4:91–6.
Shalbaf M, Gibbons NC, Wood JM, et al. Presence of epidermal allantoin further supports oxidative stress in vitiligo. Exp Dermatol. 2008;17:761–70.
Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–37.
Gastpar R, Gehrmann M, Bausero MA, et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–47.
Yu R, Broady R, Huang Y, et al. Transcriptome analysis reveals markers of aberrantly activated innate immunity in vitiligo lesional and non-lesional skin. PLoS One. 2012;7:e51040.
Richmond JM, Frisoli ML, Harris JE. Innate immune mechanisms in vitiligo: danger from within. Curr Opin Immunol. 2013;25:676–82.
Shi F, Kong BW, Song JJ, et al. Understanding mechanisms of vitiligo development in Smyth line of chickens by transcriptomic microarray analysis of evolving autoimmune lesions. BMC Immunol. 2012;13:18.
Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90–100.
Norris DA, Kissinger RM, Naughton GM, et al. Evidence for immunologic mechanisms in human vitiligo: patients’ sera induce damage to human melanocytes in vitro by complement-mediated damage and antibody-dependent cellular cytotoxicity. J Invest Dermatol. 1988;90:783–9.
Chandra S, Kumar A, Singh KK, et al. Congenital vitiligo. Indian J Dermatol Venereol Leprol. 1992;58:339.
Kedward AL, Gawkrodger DJ. Congenital stable symmetrical type vitiligo in a patient whose mother developed vitiligo during pregnancy. Eur J Dermatol. 2008;18:353.
Yashar SS, Gielczyk R, Scherschun L, et al. Narrow-band ultraviolet B treatment for vitiligo, pruritus, and inflammatory dermatoses. Photodermatol Photoimmunol Photomed. 2003;19:164–8.
Ullrich SE. Mechanism involved in the systemic suppression of antigen-presenting cell function by UV irradiation: keratinocyte-derived IL-10 modulates antigen-presenting cell function of splenic adherent cells. J Immunol. 1994;152:3410–6.
Rivas JM, Ullrich SE. The role of IL-4, IL-10, and TNF-alpha in the immune suppression induced by ultraviolet radiation. J Leukoc Biol. 1994;56:769–75.
Weichenthal M, Schwarz T. Phototherapy. How does UV work? Photodermatol Photoimmunol Photomed. 2005;21:260–6.
Aragane Y, Kulms D, Luger TA, et al. Downregulation of interferon-g-activated STAT1 by ultraviolet light. Proc Natl Acad Sci U S A. 1997;94:11490–5.
Hegazy RA, Fawzy MM, Gawdat HI, et al. T helper 17 and Tregs: a novel proposed mechanism for NB-UVB in vitiligo. Exp Dermatol. 2014;23:283–6.
Scherschun L, Kim JJ, Lim HW. Narrow-band ultraviolet B is a useful and well-tolerated treatment for vitiligo. J Am Acad Dermatol. 2001;44:999–1003.
Cooper KD, Oberhelman L, Hamilton T, et al. UV exposure reduces immunization rates and promotes tolerance to epicutaneous antigens in humans: relationship to dose, CD1a-DR+ epidermal macrophage induction, and Langerhans cell depletion. Proc Natl Acad Sci U S A. 1992;89:8497–501.
Fisher MS, Kripke ML. Systemic alteration induced in mice by ultraviolet light irradiation and its relationship to ultraviolet carginogenesis. Proc Natl Acad Sci U S A. 1977;74:1688–92.
Hersey P, Haran G, Hasic E, et al. Alteration of T cell subsets and induction of suppressor T cell activity in normal subjects after exposure to sunlight. J Immunol. 1983;131:171–4.
Noonan FP, Fabo EC, Kripke ML. Suppression of contact hypersensitivity by UR radiation and its relationship to UV-induced suppression of tumor immunity. Photochem Photobiol. 1981;34:683–9.
Ullrich SE, Azizi E, Kripke ML. Suppression of the induction of delayed-type hypersensitivity reactions in mice by a single exposure to ultraviolet radiation. Photochem Photobiol. 1986;43:633–8.
Virador VM, Muller J, Wu X, et al. Influence of α-melanocyte-stimulating hormone and ultraviolet radiation on the transfer of melanosomes to keratinocytes. FASEB J. 2002;16:105–7.
Wu CS, Yu CL, Wu CS, et al. Narrow-band ultraviolet-B stimulates proliferation and migration of cultured melanocytes. Exp Dermatol. 2004;13:755–63.
Cui J, Shen LY, Wang GC. Role of hair follicles in the repigmentation of vitiligo. J Invest Dermatol. 1991;97:410–6.
Casacci M, Thomas P, Pacifico A, et al. Comparison between 308-nm monochromatic excimer light and narrowband UVB phototherapy (311–313 nm) in the treatment of vitiligo–a multicentre controlled study. J Eur Acad Dermatol Venereol. 2007;21:956–63.
El-Zawahry BM, Bassiouny DA, Sobhi RM, et al. A comparative study on efficacy of UVA1 vs. narrow-band UVB phototherapy in the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2012;28:84–90.
Hook RR Jr, Berkelhammer J, Oxenhandler RW. Melanoma: Sinclair swine melanoma. Am J Pathol. 1982;108:130–3.
Logomasini MA, Stout RR, Marcinkowski R. Jet injection for the needle-free administration of compounds, vaccines, and other agents. Int J Pharm Compd. 2013;17:270–80.
Mohan KE, Cordeiro M, Vaci C, et al. CXCR3 is required for migration to dermal inflammation by normal and in vivo activated T cells: differential requirements by CD4 and CD8 memory subsets. Eur J Immunol. 2005;35:1702–11.
Wang XX, Wang QQ, Wu JQ, et al. Increased expression of CXCR3 and its ligands in patients with vitiligo and CXCL10 as a potential clinical marker for vitiligo. Br J Dermatol. 2016;174(6):1318–26.
Wijtmans M, Verzijl D, Leurs R, et al. Towards small-molecule CXCR3 ligands with clinical potential. ChemMedChem. 2008;3:861–72.
Mayer L, Sandborn WJ, Stepanov Y, et al. Anti-IP-10 antibody (BMS-936557) for ulcerative colitis: a phase II randomised study. Gut. 2014;63:442–50.
Yellin M, Paliienko I, Balanescu A, et al. A phase II, randomized, double-blind, placebo-controlled study evaluating the efficacy and safety of MDX-1100, a fully human anti-CXCL10 monoclonal antibody, in combination with methotrexate in patients with rheumatoid arthritis. Arthritis Rheum. 2012;64:1730–9.
Di Lernia V. Targeting the IFN-gamma/CXCL10 pathway in lichen planus. Med Hypotheses. 2016;92:60–1.
O’Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: new surprises in the Jak/Stat pathway. Cell. 2002;109:S121–31.
Darnell JE Jr. STATs and gene regulation. Science. 1997;277:1630–5.
Leonard WJ, O’Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322.
Leonard WJ. Type I cytokines and interferons and their receptors. In: Paul WE, editor. Fundamental immunology. 4th ed. Philadelphia, PA: Lippincott Raven; 1999. p. 741–74.
Ghoreschi K, Gadina M. JAKpot! New small molecules in autoimmune and inflammatory. Exp Dermatol. 2014;23:7–11.
Lindstrom TM, Robinson WH. A multitude of kinases – which are the best targets in treating rheumatoid arthritis? Rheum Dis Clin N Am. 2010;36:367–83.
O’Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161–70.
Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–83.
Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–91.
Craiglow BG, King BA. Killing two birds with one stone: oral tofacitinib reverses alopecia universalis in a patient with plaque psoriasis. J Invest Dermatol. 2014;134:2988–90.
Mamolo C, Harness J, Tan H, et al. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, improves patient-reported outcomes in a phase 2b, randomized, double-blind, placebo-controlled study in patients with moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2014;28:192–203.
Xing L, Dai Z, Jabbari A, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–9.
Zhao Y, Gartner U, Smith FJ, et al. Statins downregulate K6a promoter activity: a possible therapeutic avenue for pachyonychia congenita. J Invest Dermatol. 2011;131:1045–52.
Agarwal P, Rashighi M, Essien KI, et al. Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo. J Invest Dermatol. 2015;135:1080–8.
Noel M, Gagne C, Bergeron J, et al. Positive pleiotropic effects of HMG-CoA reductase inhibitor on vitiligo. Lipids Health Dis. 2004;3:7.
Eby JM, Kang HK, Tully ST, et al. CCL22 to activate treg migration and suppress depigmentation in vitiligo. J Invest Dermatol. 2015;135:1574–80.
Mehrotra S, Al-Khami AA, Klarquist J. A coreceptor-independent transgenic human TCR mediates anti-tumor and anti-self-immunity in mice. J Immunol. 2012;189:1627–38.
Daniel C, Wennhold K, Kim HJ, et al. Enhancement of antigen-specific Treg vaccination in vivo. Proc Natl Acad Sci U S A. 2010;107:16246–51.
Scotta C, Esposito M, Fazekasova H, et al. Differential effects of rapamycin and retinoic acid on expansion, stability and suppressive qualities of human CD4(+)CD25(+)FOXP3(+) T regulatory cell subpopulations. Haematologica. 2013;98:1291–9.
Yang L, Wei Y, Sun Y, et al. Interferon-gamma inhibits melanogenesis and induces apoptosis in melanocytes: a pivotal role of CD8+ cytotoxic T lymphocytes in vitiligo. Acta Derm Venereol. 2015;95:664–71.
Al Badri AM, Foulis AK, Todd PM, et al. Abnormal expression of MHC class II and ICAM-1 by melanocytes in vitiligo. J Pathol. 1993;169:203–6.
AlGhamdi KM, Khurrum H, Rikabi A. Worsening of vitiligo and onset of new psoriasiform dermatitis following treatment with infliximab. J Cutan Med Surg. 2011;15:280–4.
Kim NH, Torchia D, Rouhani P, et al. Tumor necrosis factor-α in vitiligo: direct correlation between tissue levels and clinical parameters. Cutan Ocul Toxicol. 2011;30:225–7.
Rigopoulos D, Gregoriou S, Larios G, et al. Etanercept in the treatment of vitiligo. Dermatology. 2007;215:84–5.
Webb KC, Tung R, Winterfield LS, et al. Tumour necrosis factor-α inhibition can stabilize disease in progressive vitiligo. Br J Dermatol. 2015;173:641–50.
Quaglino P, Marenco F, Osella-Abate S, et al. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: results from a single-institution hospital-based observational study. Ann Oncol. 2010;21:409–14.
Das PK, van den Wijngaard RM, Wankowicz-Kalinska A, et al. A symbiotic concept of autoimmunity and tumour immunity: lessons from vitiligo. Trends Immunol. 2001;22:130–6.
Irvine DJ, Purbhoo MA, Krogsgaard M, et al. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–9.
Teulings HE, Overkamp M, Ceylan E, et al. Decreased risk of melanoma and nonmelanoma skin cancer in patients with vitiligo: a survey among 1307 patients and their partners. Br J Dermatol. 2013;168:162–71.
Sakai C, Kawakami Y, Law LW, et al. Melanosomal proteins as melanoma-specific immune targets. Melanoma Res. 1997;7:83–95.
Turk MJ, Guevara-Patiño JA, Rizzuto GA, et al. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–82.
Pellegrini JR, Wagner RF Jr, Nathanson L. Halo nevi and melanoma. Am Fam Physician. 1984;30:157–9.
Reed RJ, Webb SV, Clark WH, et al. Minimal deviation melanoma (halo nevus variant). Am J Surg Pathol. 1990;14:53–68. Ficabore ndipidias ilis unt
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Webb, K.C., Henning, S.W., Le Poole, I.C. (2019). Immunity/Immunopathology. In: Picardo, M., Taïeb, A. (eds) Vitiligo. Springer, Cham. https://doi.org/10.1007/978-3-319-62960-5_28
Download citation
DOI: https://doi.org/10.1007/978-3-319-62960-5_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-62958-2
Online ISBN: 978-3-319-62960-5
eBook Packages: MedicineMedicine (R0)