Abstract
Organ transplantation has emerged in recent decades as one of the most effective modalities for the treatment of end-stage organ disease. Over 100,000 transplants are performed worldwide each year; however, the supply has not been able to keep up with increasing demand. Furthermore, transplant recipients are committed to a lifelong regimen of brutal immunosuppressive medications that themselves carry significant side effect profiles, influencing clinical outcomes. Recent advances in the fields of tissue engineering and regenerative medicine are beginning to offer alternative solutions that could potentially improve the longevity, functionality, and biocompatibility profiles of transplants. Decellularization technology to produce extracellular matrix scaffolds represents one of the most promising strategies currently under investigation. Such methods can produce bioengineered, transplantable organs using autologous cells that would bypass the need for immunosuppression and its associated side effects. Furthermore, bioengineering strategies in general are not bound by supply constraints imposed by organ donation.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
In recent decades, organ transplantation technology has progressed substantially, which has paved the way for its emergence as the gold standard treatment for a myriad of clinical settings characterized by end-stage organ disease [1]. Although medical management has made impressive strides as a treatment modality, surgical transplantation remains the best and only means of permanently restoring the original function of chronically or acutely irreversibly damaged organs. The aim of this chapter is to review relevant concepts in the science and practice of modern transplantation along with related important developments in tissue engineering (TE).
Over 100,000 solid organ transplants are performed every year worldwide, including approximately 70,000 kidney and 20,000 liver transplants [2]. End-stage renal disease, for example, represents an extraordinary public health burden worldwide, which has contributed to the kidney being the most commonly transplanted organ. In 2014, about 15,700 people received kidney transplants in the United States, while over 100,000 candidates remained on the waiting list because of limited supply [3, 4].
Success rates have been improving incrementally over the years, but the challenges of donor organ supply and complications relating to immunosuppressive medications remain major obstacles in the way of further progress for the treatment of irreversible end-organ damage. Temporizing measures, such as dialysis, extends life expectancy, which predictably contributes to a growing demand for donor grafts and an ever-expanding waiting list. But candidates who do ultimately receive a transplanted organ can develop secondary neoplasms or other adverse sequelae associated with prolonged immunosuppression.
In light of these shortcomings, developing methods of tissue engineering (TE) has been very promising as a potential solution to the challenges associated with modern transplantation. TE, often touted as a subfield of regenerative medicine, refers to approaches that attempt to restore physiological or anatomical function by either regenerating cellular material or replacing diseased cells with healthy cells in tissues that might not perform this task spontaneously or sufficiently [5]. TE can be accomplished in experimental and clinical settings by administering cellular material, noncellular biomaterials, or a combination of the two [6]. Biomaterials, naturally derived or synthetic, are often employed, as are anatomically relevant “scaffolds” on which cells can be distributed or that allow for the migration and proliferation of cells in vivo as a way to replace damaged tissue.
The introduction of cellular material for the purpose of regeneration or replacement arguably can be a natural extension of the current donation system underlying modern transplantation. Indeed, it has been recognized that transplantation shares many of the same guiding principles that now guide developing TE methodologies [7]. For example, the idea of replacing diseased tissue by the direct administration of bioactive, cellular material underlies present surgical transplantation. A TE alternative may merely involve seeding cellular material within a biocompatible, organ-shaped scaffold to bioengineer a functional organ for use as a replacement [8]. Further, whether by direct healing, via administration of cellular material and biomaterials, or ex vivo fabrication of whole organs for subsequent transplantation, TE—though still its early stages—enjoys the advantage of not being limited by organ shortage because cell cultures can be expanded in the laboratory and biomaterials can be manufactured on industrial scales without donor morbidity or mortality.
8.2 Major Challenges in Transplantation
8.2.1 The Organ Shortage
The dramatic success of organ transplantation for the treatment of organ failure has stoked an overwhelming demand without a corresponding supply for donor grafts. Kidney transplantation, for example, has proven itself to be superior to dialysis as a modality of renal replacement therapy in terms of both cost-effectiveness and clinical outcomes [9, 10]. Life expectancy for dialysis patients in the United States is approximately 8 years for patients between 40 and 44 years and 4.5 years for patients between 60 and 64 years. Transplantation offers a superior life expectancy profile of 85% at 5 years, 70% at 10 years, and 44% at 20 years postoperatively.
Thus, modern transplantation can be considered a victim of its own success [11]. In 2014, only 15,700 patients received a kidney transplant, while over 100,000 remained on waiting lists at the end of the year [12, 13]. According to the Global Observatory on Donation and Transplantation (GODT), approximately 114,690 solid organs were transplanted worldwide in 2012, representing a modest increase of 1.8% from the previous year. The effort fell short, however, and met less than 10% of the global need [14].
To offset some of the growing demand, transplant operators have widened the donor pool by expanding the criteria for acceptable donors with limited success. For example, the expansion of living and deceased donor acceptance criteria to include donation after cardiac death, paired donations, altruistic donations, immunologically suboptimal donations, and donation from people with comorbidities has helped improve the probability of receiving an organ in the critical time frame while on a waiting list [15, 16]. Nevertheless, the rising incidence of chronic noncommunicable diseases such as diabetes and hypertension—leading causes of kidney disease—is projected to limit supply further and exacerbate discrepancy with demand [17].
8.2.2 A Halfway Technology
Though representing one of the greatest achievements of modern medicine, transplantation can be usefully described as a “halfway technology” in light of inherent major obstacles. Lewis Thomas, a renowned pathologist, first articulated this concept by referring to technologies that compensate for the adverse sequelae of various disease processes as opposed to actually curing them [18]. He anticipated the laborious burden of “incomplete” technologies that complicates the overall treatment regimen.
It is readily apparent how modern transplantation in its current state fits Thomas’ description of an “incomplete” technology [19]. For example, although replacing native kidneys with donor grafts reconstitutes the filtration and hormonal functions of a healthy kidney, intervention does not eradicate the baseline disease that first led to the procedure in the first place: thus the stage remains set for secondary organ failure. Preventing graft failure, moreover, requires a lifelong commitment to antirejection medications, which also cause acute and/or chronic toxicity, leading to additional clinical syndromes [20]. Consequently, meticulous long-term management is necessary to prevent side effects or graft failure and to optimize quality of life.
A clear example of the incompleteness of transplantation is seen in transplants performed for end-stage kidney disease caused by diabetes mellitus [21]. Although transplantation is a lifesaving intervention in these cases, it does not cure the baseline disease which instead will recur after the operation.
Disease recurrence after liver transplantation is, in fact, virtually inevitable. Histological evidence of infectious damage is detectable often within weeks, and progression to cirrhosis requiring another transplant occurs in 25% of cases within 5–10 years following transplantation. Consequently, the recurrence of hepatitis C presents one of the major challenges to successful modern liver transplantation and is one of the most frequent causes of recurrent disease resulting in graft failure, for all organs [22]. Naturally, the chief exacerbating factor in recurrent hepatitis C cases is immunosuppression secondary to antirejection therapy. There is, indeed, evidence to suggest that weaning from antirejection therapy in hepatitis C liver transplant recipients delays the recurrence of disease and consequently improves overall morbidity and mortality outcomes [23]. Although there are rare cases in which transplantation has been fully curative without the need for immunosuppression, that technology is entirely consistent with Thomas’s description.
Thomas’s concept of a “halfway” or “incomplete” technology is useful because it provides boundaries and benchmarks by which alternative solutions can be measured. TE, for example, can be compared to transplantation and profitably evaluated using Thomas’s conceptual framework because it shares with transplantation overlapping ancestry and methodology [24, 25]. The essential goal to restore tissue and organ function via the introduction of bioactive materials is a shared attribute, albeit with different parameters.
As will be discussed in later sections, TE allows for the use of recipient-derived cellular material to manufacture functional tissues and organs ex vivo for autologous reimplantation thereby obviating the need for toxic immunosuppressive therapy. Without transplantation’s associated side effects, a TE-derived graft would be more temporally durable and less susceptible to complications resulting in graft failure. TE methods could move transplantation closer to being a “complete” technology than organs obtained from conventional sources presently allow.
8.2.3 The Burden of Immunosuppression
Although advances in the transplantation sciences have dramatically improved morbidity and mortality outcomes, subsequent immunosuppressive protocols remain a massive burden for patients following surgery because of the need to address associated infectious, neoplastic, and end-organ complications [26, 27]. For this reason, tolerance to organ allografts has traditionally been the ultimate goal for transplant practitioners and researchers. Indeed, achieving an immunosuppression-free state (IFS) that avoids all complications and costs associated with lifelong immunosuppression is often referred to as the “Holy Grail” among affiliated clinicians [28].
Infections are among the most common causes of hospitalization following kidney transplantation and are, in fact, the major cause of hospitalization in pediatric transplant patients postoperatively [29]. Chronic immunosuppression also contributes to adverse cardiovascular events, which represent the chief cause of death in recipients [30]. Solid organ transplant recipients also have a higher risk of developing cancer because of immunosuppression and oncogenic viral infections.
Engels et al. evaluated medical data from over 175,000 transplant recipients from 1987 to 2008 (approximately 40% of all organ recipients in the United States) and found an overall doubling of cancer risk compared with the general population [31]. Standardized incidence ratios were significantly elevated for most infection-related malignancies including non-Hodgkin’s lymphoma, Kaposi sarcoma, Hodgkin lymphoma, and cancers of the stomach, oropharynx, anus, vulva, and penis in kidney, liver, heart, and lung recipients. The risk of liver cancer was only elevated among liver recipients, partly because of recurrent hepatitis B or C or diabetes—showing the efficacy of Thomas’s concept of the halfway technology.
Ultimately, the duration of immunosuppression along with the intensity of treatment is the most powerful predictor of malignancy [32]. Achieving a balance between the risk profile of antirejection therapy and the need to prevent graft rejection is a supremely difficult task of critical importance. In this context, it is no wonder that the IFS is considered the Holy Grail among transplant clinicians.
To ensure graft survival and function, patients must adhere to long-term immunosuppression. It can be estimated that over 300,000 patients in the United States alone are currently transplant recipients committed to brutal immunosuppressive regimens, with the attendant burden of close and frequent monitoring, which adversely impact health and quality of life in an unusually severe manner. There are over 1000 individuals presently living with an intestinal transplant, approximately 14,000 patients with pancreas grafts, 11,000 with lung transplants, 27,000 with heart transplants, almost 200,000 with kidney transplants, and over 59,000 with liver transplants [33,34,35,36,37,38].
Organ transplantation is also one of the most expensive medical therapies currently available and has a major impact on hospital and health system expenditures, in addition to a patient’s personal finances. Countries in which national health services are overburdened by the increasing medical needs of aging populations resort to strict cost-optimizing policies to limit access to organ transplantation and to limit expenses [39]. Thus, one of the primary challenges associated with transplantation is devising management strategies that lower the cost of the procedure without adversely impacting clinical outcomes [40].
The cost of immunosuppressant drugs and requisite frequent follow-up visits constitute an immense financial burden. For those without long-term insurance coverage, drug costs may represent a significant out-of-pocket expense that is unaffordable. Patients who depend on Medicare for health insurance face a coverage cliff at 36 months after transplant for immunosuppression drugs, a policy widely regarded as shortsighted given the massive expense incurred in the case of graft failure [41, 42]. Evans et al. surveyed kidney transplant programs in the United States and found that 70% reported that their patients have an extremely or very serious problem paying for their medications [43]. Astoundingly, 68% of the programs reported deaths and graft losses attributed to cost-related nonadherence to immunosuppressive medications.
8.3 Tissue Engineering Solutions in Transplantation
TE efforts have resulted in successful manufacture of relatively simple, hollow organs (e.g., bladder, airway, etc.) from autologous cellular sources and subsequent implantation in over 200 patients with various medical conditions without the need for subsequent antirejection therapy. (In contrast, the number of transplant recipients who have been successfully weaned off of immunosuppressive drugs in the postoperative period is much lower.) At present, the production of more complex, modular, solid organs such as the kidney, liver, pancreas, heart, and lung has not yet occurred to a clinically relevant degree. Nevertheless, methods in TE are constantly being refined and updated; consequently, the field offers a promising new arena for transplant research that could ultimately undergo a paradigm shift as it seeks to remove the obstacles described above. Proof of concept has already been established, and the transition from hollow to solid, complex organs should be the next milestone.
8.3.1 Cell-Scaffold Technology
TE approaches typically involve the use of cellular material either alone or in conjunction with a supporting scaffold [44]. The latter approach typically involves the manipulation of biomaterials into geospatially appropriate scaffolds. When seeded with cells and allowed to mature, these constructs are implanted as functional organoids with the bioengineered graft assuming the role of the transplanted organ. The use of autologous cellular material circumvents immunosurveillance, and, thus, antirejection therapy is not warranted: a theoretically supported concept that has been observed empirically [45].
Determining the cell type most suitable for regeneration and/or reseeding onto a scaffold remains a critical objective for investigators in each organ system. Experimental and clinical investigations have commonly employed fully differentiated adult cells, organ-specific progenitor cells, or pluripotent stem cells [46], all of which can be acquired from a recipient.
Natural biomaterial scaffolds can be fabricated by perfusion of detergents through animal- or human-derived organs. This method removes the cellular compartment from the organ and leaves behind an extracellular matrix (ECM). These ECM-based scaffolds are highly biomimetic because they retain their original structural architecture in three dimensions including the intricate, internal vascular networks, adhesion molecules, and cellular signaling proteins [47,48,49,50,51]. Thus, naturally derived scaffolds are particularly suited for cellular seeding.
The idea of fabricating natural ECM scaffolds was pioneered by Harvard researchers Vacanti et al. in the 1980s. In one of the earliest investigations, fetal and adult rat cells, mouse hepatocytes, pancreatic islet cells, and cells from the small intestine were seeded onto synthetically derived scaffolds [52]. The supporting materials consisted of synthetic polymers organized into fiber networks that simulated the intertwining branching networks of the connective tissue present in all organs that allows cells to remain viable by diffusion, promoting vascular ingrowth and encouraging proliferation [53]. After allowing the cells to culture on the synthetic scaffolds for 4 days, these constructs were then implanted into animals of varying species. The investigators recorded six cases of successful engraftment, each of which demonstrated viable cells, mitotic figures, and vascularization of the cell mass. This was the first report of ex vivo manufacture of implantable organoid constructs consisting of cellular material seeded on artificial supporting scaffolds. The report paved the way for further studies that ultimately led to the first clinical application: a tissue-engineered vascular graft that was used to replace an intermediate pulmonary artery in a child with right ventricular and pulmonary atresia [54].
In all successful ECM cases reported to date, autologous cells were isolated and expanded in vitro prior to seeding. In some cases, multipotent stem cells were isolated and differentiated toward specific somatic cell types prior to seeding on scaffolds [55,56,57]. Macchiarini et al. removed cells and MHC antigens from a human donor trachea and repopulated the ECM scaffold with epithelial cells and stem cell-derived chondrocytes that had been cultured from cells acquired by the recipient. This graft was then used to replace the patient’s left main bronchus. At 5-year follow-up, the tissue-engineered trachea remained open over its entire length, was well-vascularized, was completely recellularized with respiratory epithelium, and had normal ciliary function and mucus clearance [58]. Thus, the investigators provided a solid foundation for further research into potentially groundbreaking cell-scaffold technology. Comparable success using biodegradable non-ECM-based scaffolds has been reported in the urethra [59], the bladder [60], and the vagina [61].
8.3.2 ECM-Based Scaffolds
The ideal scaffold in the context of organ transplantation is biomaterial-based and approximates the three-dimensional structure of the organ to be transplanted. It should also lend biological and mechanical signals to induce cell growth and differentiation [62]. Generally, applicable design considerations include minimal immunogenicity, a degradation profile which parallels tissue regeneration, the presence of environmental factors appropriate for the seeded cell type, and appropriate mechanostructural parameters such as stiffness and tensile strength. In fact, ECM-based scaffolds are an attractive option because they include these considerations and support cells in vivo.
In recent decades, it has become increasingly clear that ECMs play a fundamental role in the viability and functionality of cells, tissues, and organs. Indeed, one of the primary goals of TE investigators is to elucidate fully the relationship between ECMs and cells to facilitate the manufacturing of better cell-scaffold grafts. Decellularized tissue matrices obtained via detergent perfusion carry the benefit of preserving the native architecture and mechanical properties of the original tissue, i.e., the ECM remains virtually intact. These frames can be obtained autologously and reseeded with autologous cells. They also have been shown to support cellular regeneration without immunologic rejection: a feature of tremendous importance to transplant investigators [63].
ECM technology is able to drive differentiation of progenitor cells into organ-specific phenotypes, indicating the potential use of stem cells for cell-scaffold technology [64]. Indeed, Remuzzi et al. recently reported on the successful recellularization of acellular rat kidney scaffolds using embryonic stem cells via infusion through the renal artery and subsequent pressure-controlled perfusion with recirculating culture medium [65].
Variations in ECM are likely to parallel the specializations of their corresponding organ systems. As such, the design of a single, all-encompassing biomaterial is not a practical design goal. To design the next generation of advanced biomaterial scaffolds, current investigators must take into consideration that body tissues and organs are highly specialized in structure and function. Decellularization, recellularization, and maturation protocols should, instead, be optimized and recent successes expanded on.
8.4 Organ Bioengineering
8.4.1 Cell Types
There are a number of potential approaches that can be used to fabricate organs ex vivo for eventual implantation into patients. Cell-scaffold technology consists of seeding cellular material on supporting scaffolds. Determining the proper cell type most suitable for regeneration and/or seeding on a scaffold remains a chief objective for each organ system investigated. Experimental and clinical investigations have commonly involved fully differentiated adult cells, organ-specific progenitor cells, or pluripotent stem cells.
Adult somatic cells hold the advantage of being well-defined and readily isolated, but, compared with progenitor and stem cells, their lifespan and regenerative potential are limited. Further, they lose function and differentiability when removed from the native environment. Their use is confined to the specific organ from which they are obtained. For example, cultured hepatocytes or renal tubular epithelial cells can be used only for liver scaffolds or kidney scaffolds, respectively, and they would likely be minimally proliferative.
Stem cells, however, possess the notable capacity of self-renewal and differentiability. Their applicability has a broader range than adult somatic cells. Within the category of stem cells, the two types most commonly utilized are adult stem cells and embryonic stem cells [66].
Embryonic stem cells (ESCs) are pluripotent stem cells derived from the inner cell mass of the embryo during the blastocyst stage that have the ability to differentiate into any cell type when induced with specific environmental cues or stimuli. Furthermore, they can self-renew indefinitely [67]. Researchers have successfully induced their differentiation into cell types from all three germ layers in vitro including cardiac cells [68], endothelial cells [69], neurons [70], insulin-producing cells [71], and renal tubular cells [72]. In the context of abdominal bioengineering, the use of embryonic stem cells would find wide applicability for experimental and clinical purposes. Nevertheless, their use is significantly limited by ethical issues along with potential teratogenicity [73, 74].
Induced pluripotent stem cells (iPSCs) are generated via special reprogramming of adult cells resulting in their dedifferentiation into pluripotent stem cells. This process does not involve manipulation or destruction of primordial embryos, thus avoiding the ethical issues posed by ESCs. iPSCs have been derived from several cell types in experimental investigations, including human keratinocytes and umbilical cord blood mononuclear cells [75, 76].
Adult stem cells, in contrast to ESCs, benefit from wide availability in the adult organism and relative ease of acquisition. They are thought to perform a major role in cellular repopulation and the replenishment process over time [77]. Indeed, adult stem cells residing in anatomical “niches” are responsible for the continual replacement of damaged or dead tissue compartments in response to microenvironmental cues [78]. Wound healing and bone remodeling are familiar examples of tissue systems with high turnover. Adult tissue systems with recognized niche populations of stem cells include the skin, fat, intestine, and kidney, among others [79,80,81,82].
Unlike somatic cells, adult stem cells can be induced to differentiate along lineages other than the organ system from which they are derived. For example, fat stem cells have been successfully differentiated into cartilage cells, hematopoietic cells, neurons, bone cells, and skeletal muscle cells [83]. Nevertheless, their differentiation potential is generally recognized to be limited to the germ layer from which they are derived, unlike the pluripotency of the ESC.
Adult cells harvested for regenerative purposes can be heterologous (e.g., porcine), allogeneic (from a donor patient), or autologous (from the patient). Autologous cells are beneficial because they are shielded from the recipient patient’s immune system because they are not recognized as nonself. Given their ability to circumvent rejection, autologous cells represent the most desirable cell type in TE investigations. Allogeneic cells are another option; acquired from another individual, their use is complicated by potential immunogenicity [84]. Heterologous cells derived from nonhuman organisms (e.g., porcine) represent another option under investigation.
Ideally, adult stem cells used for TE purposes would be expanded ex vivo and either (1) introduced directly into the patient to repair the damaged organ system or (2) seeded onto a supporting scaffold for eventual reimplantation in the patient. Isolating and expanding these cells at clinically relevant levels remain a challenge for current investigators.
8.4.2 Gastrointestinal Tract
The intestinal conduits are complex, hollow structures involved in nutrient passage and absorption, digestion, excretion, and the innate immune system. The human tract undergoes continuous turnover and renewal throughout life. This process is governed by the activity of resident stem cells in the gut wall [85].
The preexisting process of regeneration and renewal in the gastrointestinal tract underscores the unique applicability of methods in tissue engineering for repairing and regenerating segments of the GI tract. Given the microanatomical and functional characteristics of the gut, such an endeavor would require regenerating smooth muscle, specialized neuronal tissue, and a mucosal-epithelial bilayer and meeting one of the most challenging tasks—recapitulating the diverse motility patterns that are essential to proper intestinal functioning. Nevertheless, preliminary investigations have been quite promising [86].
Speer et al. isolated organoid units from mouse glandular stomach and seeded them onto biodegradable scaffolds composed of polyglycolic acid (PGA) coated with poly-l-lactic acid (PLA) and type I collagen [87]. The seeded scaffolds were implanted into the omentum of adult mice and harvested at designated time points for analysis. The constructs were found to grow and proliferate as expanding spheres with simple columnar epithelium organized into gastric glands and an adjacent muscularis. Mucous, enteroendocrine, chief, and parietal cells were all expressed in the regenerated epithelium.
Maemura et al. have employed biodegradable polymers seeded with stomach epithelial organoid units obtained from neonatal rats in various investigations [88]. They replaced recipient rat stomachs with tissue-engineered stomachs and discovered no evidence of stenosis or obstruction at the sites of anastomoses. Histological evaluation demonstrated well-developed vascularized tissue and stratified smooth muscle layers [89]. They also used their seeded scaffolds to patch gastric wall defects in rat models with considerable success [90].
The small intestine is the primary segment for nutrient absorption within the gastrointestinal tract. This process depends on the integrity of microvilli structures that line the intestinal epithelium. Early regenerative investigations employed autologous tissue patches to repair defects in the intestinal wall, but later studies underscored the potential use of absorbable biomaterials as a patch scaffold to facilitate tissue ingrowth [91]. Commonly used biomaterials have been collagen scaffolds, PLA, and PGA, among others. Fibrin hydrogels have demonstrated the sufficient mechanical rigidity to allow self-organization of circular sphincteric and intestinal smooth muscles, even in humans [92, 93].
The Vacanti group has employed intestinal organoid units acquired from the small intestine and remodeled them on biocompatible matrices [94]. These implants were able to reduce morbidity associated with massive bowel resection in rat models. Chen et al. implanted ECM-derived submucosal tubes in canine models with small bowel resections without seeding material to ascertain any regenerative potential [95]. The investigation used four dogs and concluded that this method was not efficacious, although patch repair showed potential for wall regeneration.
Numerous studies have reported the use of bone marrow-derived mesenchymal cells (MSCs) for the regeneration of intestinal tissue. Hori et al. were able to regenerate intestinal segments by seeding MSCs onto collagen scaffolds [96]. Their constructs showed a transient distribution of alpha-smooth muscle actin-positive cells but failed to regenerate a muscle layer, which is essential for peristalsis.
ESCs have also been used to regenerate functional or semi-functional intestinal tissue, under proper induction by growth factors [97, 98]. Watson et al. recently reported on the generation of human intestinal organoids procured in vitro from human embryonic stem cells or pluripotent stem cells that they were able to engraft and vascularize in mouse models [99]. In vivo transplantation resulted in marked expansion and maturation of differentiated epithelium and mesenchyme and even demonstrated digestive functions. Wieck et al. showed in a recent report that human-derived tissue-engineered colon can be populated with nervous tissue when cultured with enteric nervous system progenitor cells to reconstitute motility functions impaired in conditions such as Hirschsprung’s disease [100].
Detergent-based organ decellularization allows for the manufacture of ECM-based intestinal scaffolds. Notably, the ECM preserves the villus-crypt architecture and vasculature that supports intestinal epithelial regeneration [101]. Though clinical translation has not yet proven feasible, recent findings and ongoing studies are showing great promise.
Given the limited successful results of employing cells or biodegradable scaffolds alone, adult or stem cell seeding on ECM-based scaffolds will likely help to achieve optimal results in future investigations. Identifying the most suitable cell type remains a primary challenge for future studies, along with establishing a scaffold that recaptures the microenvironmental cues of native intestine.
8.4.3 Kidney
The kidney is a complex solid organ with important roles in endocrine, metabolic, and immunologic homeostasis. Functions include filtering the blood, maintaining adequate blood pressure and volume, and excreting toxic metabolic waste products, highlighting the crucial role of the kidney.
At present, dialysis and transplantation represent the gold standard treatment modalities for chronic kidney disease. Dialysis as a modality of renal replacement therapy does not, however, cure kidney damage. Rather, it assumes a portion of the kidney’s functions, particularly filtration. As such, dialysis leaves much to be desired.
Replacing the organ with a healthy, functioning graft, on the other hand, is a much more effective treatment. With the worldwide burden of hypertension and diabetes growing, the prevalence of chronic kidney disease is reaching epidemic proportions, and the need for functional kidneys is rising correspondingly [102, 103].
Contemporary investigators agree that significant progress for TE approaches to renal replacement therapy will require further elucidation of the native repair and regeneration processes occurring in vivo at the cellular and molecular levels [104, 105]. Because of the proportionally increased metabolic demand of the kidney itself and its waste and toxin filtration functions, renal tubular cells are constantly under the threat of acute injury and oxidative stress. It has been stipulated that, for this reason, these cell populations feature unique regenerative abilities to compensate for continuous insult. Indeed, surviving renal tubular cells have been observed to give rise to a new population of the cells following physiologic kidney damage [106].
Nagaike et al. observed that unilateral nephrectomy induces mitogenesis and hypertrophy in the contralateral kidney [107]. When Cochrane et al. created ureteral obstructions in murine models of renal injury to induce cortical tubular cell atrophy, tubular dilation, and interstitial macrophage infiltration, they observed a rapid process of reconstruction and interstitial matrix expansion upon reversal of the obstruction which ultimately restored the glomerular filtration rate [108]. However, continuous and supraphysiologic damage characteristic of chronic kidney disease overpowers the regenerative properties of these cells, and the growth of new nephrons, i.e., frank nephrogenesis, has not been shown to occur [109]. But nephron progenitor cells derived from human pluripotent stem cells are currently under investigation for TE purposes given their nephron-forming capacity [110].
Researchers have explored the potential of cell therapy to restore kidney function in the face of widespread damage. Cell-based approaches seek to achieve kidney repair and regeneration in situ upon therapeutic administration. They are based on the observation that exogenously supplied cells can stimulate and/or contribute to repair and proliferative processes [111]. Progenitor cells harvested from the proximal tubules, glomerulus, peritubules, and papillae have all demonstrated some level of regenerative capacity in recent investigations [112, 113]. Stem cells obtained from the urine have also shown some potential to reverse kidney damage and aid in the repair process [114].
A recent study, for example, demonstrated that cultured mesenchymal stem cells injected into mice with ischemia-induced kidney injuries resulted in improved renal function, promoted macrovasculature repair, attenuated kidney peritubular capillary loss, increased the proliferation of parenchymal cells, and significantly reduced overall mortality [115]. Such approaches are attractive because of the ease of isolation and expansion of mesenchymal stem cells and their potentially autologous use to reduce the risk of immunogenic rejection. Furthermore, the use of adult cells circumvents the ethical obstacles encountered when using ESCs. The results of investigations utilizing amniotic fluid-derived stem cells and induced pluripotent stem cells have been similarly encouraging [116,117,118,119].
Enhanced understanding of the regenerative properties of renal cells has led to another avenue for treatment of kidney damage: the use of embryonic kidney tissue. These primordial cells have been shown to integrate within adult organ systems, richly vascularize, and form new, mature nephrons (i.e., result in frank nephrogenesis) [120, 121]. Ureteric bud and metanephric mesenchyme cultures retain the capability to form collecting ducts through tubulogenesis and epithelization by virtue of the inherent developmental capacities of the mesonephric duct tissue.
Investigators prepared the kidney tissue in vitro and subsequently implanted it in mice models that survived for over 5 weeks. Microstructural analysis revealed glomerular vascularization in vivo, lending support for the therapeutic potential of these primordial tissues [122]. Imberti et al. implanted renal primordia under the kidney capsule of male rats with kidney injury [123]. The grafts developed glomeruli and tubuli that filtered blood and produced urine in cyst-like structures. Additionally, newly developed metanephroi initiated a process of regeneration in host tissue segments, as indicated by increased cell proliferation and vessel growth.
Preliminary and animal model investigations into kidney bioengineering using cell-scaffold technology also have been highly encouraging. ECM scaffolds produced from animal and human kidneys have been shown to retain their innate biomolecular and biophysical properties in addition to their native external anatomy [124, 125]. Orlando et al. successfully fabricated renal ECM scaffolds from porcine kidneys by pumping an aqueous detergent solution through the renal artery, thereby decellularizing the organ [126]. These scaffolds achieved total cell clearance and retained their essential architectures. The injection of contrast media through the renal artery confirmed preservation and potency of a vascular network with hierarchical branching structures without extravasation into the parenchymal compartment. Seeded endothelial cells demonstrated steady growth and adherence to structures on the bioscaffolds. Finally, unseeded scaffolds were successfully implanted in pigs to assess their in vivo biocompatibility. They were easily reperfused, sustained blood pressure, and were tolerated for 2 weeks.
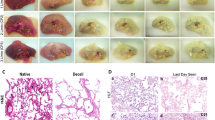
The same group identified the approximately 2600 human kidneys originally intended for transplant purposes but discarded each year due to anatomical or pathological anomalies as a platform for kidney bioengineering at clinically relevant scales. Given their human origins, the potential for use of these discarded grafts in TE investigations is monumental, at least as a stepping stone to realizable clinical translation. In 2013, they successfully produced ECM scaffolds using these discarded kidneys [127]. These human scaffolds, like their porcine analogs, were completely decellularized and maintained their structural composition. HLA antigen molecules were notably absent: an important indicator of the potential immunocompatibility of these constructs. They were able to induce vessel formation in chick chorioallantoic membranes, suggesting strong angiogenic properties. Lastly, the innate vascular system was able to resist pressure treatment at physiological levels, as a marker of compliance. Using a resin casting methodology to carry out deeper microanatomical analysis, they observed that glomerular shape and capillary width were preserved following decellularization (Fig. 8.1). Furthermore, the branching pattern and vessel integrity were unchanged. Protein analysis confirmed the retention of several growth factors implicated in renal repair and angiogenesis within the scaffolds [128].
Acellular ECM scaffolds obtained from a discarded human kidney (a–d) and related vascular corrosion cast (e–h) (From Peloso et al., Transplantation, 2015, Reprinted with permission of the American Journal of Transplantation. Copyright © 2017 American Journal of Transplantation) [128]
Investigators seeded human amniotic fluid-derived stem cells onto discarded kidney ECM scaffolds and observed their attachment and proliferation thereafter [129]. Furthermore, they synthesized and secreted various growth factors and chemokines involved in angiogenesis and matrix remodeling, indicating a dynamic, stimulatory relationship between ECM and seeded cells.
In 2013, Song et al. reported on the novel orthotopic transplantation of bioengineered kidneys in rat models [130]. Acellular rat kidneys were seeded with endothelial and epithelial cells through the renal artery and ureter. Epithelial cells showed engraftment along with organization into tubular structures expressing Na/K-ATPase and aquaporin similar to native proximal tubular epithelium. Electron microscopy showed perfused glomerular capillaries with engrafted podocytes and formation of foot processes. These bioengineered grafts were perfused by recipient circulation and produced rudimentary urine via the ureteral conduit in vivo when transplanted orthotopically.
8.4.4 Pancreas
The pancreas is functionally composed of two parts, the exocrine pancreas and endocrine pancreas. Despite deriving from the same embryologic cells, these components are structurally different and serve important functions for different physiological systems. Despite accounting for 90–95% of the pancreas mass, the exocrine pancreas is not essential for survival because of exogenous pancreatic digestive enzyme replacement therapy [131]. In contrast, the endocrine pancreas only accounts for roughly 2% of the pancreas mass while serving a vital physiological role. The endocrine pancreas is not localized to a specific region of the pancreas but is interspersed around islets of Langerhans (highly vascularized centers) over the entire tissue. Although here are five main types of endocrine pancreatic cells (alpha, beta, delta, gamma, and epsilon), the beta cell plays a particularly vital role in glucose homeostasis and energy metabolism, drawing the most attention from bioengineering research related to endocrine pancreatic deficiencies.
One of the most common pathologic endocrine deficiencies is type 1 diabetes mellitus in which beta cells are destroyed by the host’s own immune system. About 1 in 300 individuals in the United States is diagnosed with type 1 diabetes mellitus by the age of 18 [132]. Therapies for type 1 diabetes mellitus focus on the supplementation of exogenous insulin that is normally produced by the beta cells. This method requires strict patient compliance, however, and adequate patient education. Recent innovations remove the need for conscious dosing. Although exogenous insulin therapy is effective at preventing acute decompensation, less than 40% achieve and maintain therapeutic targets [133]. The most commonly accepted treatment regimens lower the incidence of diabetic emergency, but they require lifelong pharmacologic intervention without the possibility of remission. Beta cell replacement, whether by whole pancreas or individual islet cell transplantation, is currently the only method to restore long-term, stable euglycemia in type 1 diabetics.
Pancreatic transplantation is an invasive option that provides the patient an opportunity to be free from strict glycemic control by way of exogenous insulin. It is, however, typically only offered to adults in conjunction with kidney transplants because of the high risk of surgical complications and the subsequent necessity for immunosuppressant medications [134]. Nevertheless, recipients exhibit greater improvements in the micro- and macro-vascular complications of diabetes while enjoying a better quality of life. The 5-year organ survival rate for pancreatic transplantations is roughly 50% [34].
An alternative solution involves the ectopic implantation of purified islet cells and simultaneous depletion of T cells to recapture beta cell function while suppressing the autoimmune component of the disease [135, 136]. Although human islet cell purification and implantation show the same 5-year insulin independence rate as pancreatic transplantation, ectopic implantation has been found to have a lower risk of complication and represents a less invasive solution [137].
Contemporary investigations employ TE solutions for treatment of the disease without the need for lifelong pharmacologic intervention. One of the most heavily researched areas involves the induction of beta cell regeneration within host islets. There is a current debate in the research community regarding the source material of endogenous beta cell regeneration: it is still unknown whether the existing beta cells or resident stem cells are primarily responsible [138, 139]. Insulin-producing cells derived from ESCs are physiologically closer to beta cells than any adult stem cell-based beta cell. The conversion of human embryonic stem cells toward beta cell phenotypes has been achieved through the initial differentiation into endodermal cells which can be further differentiated into insulin-producing endoderm derivatives [140, 141].
To avoid the controversy of ESC procurement, recent advancements in beta cell replacement have turned toward induced pluripotent stem cells. By using retroviruses to manipulate gene expressions, adult somatic cells can be reprogrammed and individualized to specific patients. In animal models, experimental therapy has already demonstrated long-term correction of hyperglycemia [142]. Though promising, further success with these cells is challenged by premature senescence, subchromosomal abnormalities, and destruction of patient-derived cells due to autoimmune phenomena.
The recellularization of ECM-based scaffolds has also been under investigation for the de novo fabrication of pancreas organs. It has been observed that islets cultured in vitro on ECM increase the longevity of insulin production in response to glucose, likely because of natural growth factors within the ECM that direct cell lines toward beta cells [143]. In addition, recent studies have hinted at the potential use of xenographic transplants. Mirmalek-Sani et al. decellularized the porcine pancreas and showed that the ECM scaffold had a patent vasculature and could be subsequently seeded with human amniotic fluid-derived stem cells [144]. The seeded islets demonstrated the ability to support normal pancreatic function by showing an increased metabolic rate and insulin secretion over isolated islets in culture. Later, similar data were confirmed by the same team on ECM scaffolds obtained from the human pancreas (Fig. 8.2) [145]. Goh et al. successfully decellularized mouse pancreata and recellularized them with acinar and beta cell lines [146]. They showed successful engraftment within the three-dimensional decellularized pancreas along with strong upregulation of insulin gene expression. Their findings supported the utility of whole pancreas ECM for enhancing pancreatic cell functionality. De Carlo et al. reported similar findings [147].
Endothelial cell seeding on acellular ECM scaffolds obtained from the human pancreas. The ECM scaffold was seeded with human pancreatic endothelial cells and cultured for 6 days in a bioreactor, consisting in a closed circuit with one chamber for organ housing, a reservoir for medium oxygenation, and a peristaltic pump (Ismatec), connected by tubing (ID 1/16″, Pharmed BPT). Pancreatic tail was surgically isolated in order to obtain a smaller volume to seed keeping at the same time an inflow (SA – red connector) and an outflow (splenic vein –SV – blue connector) (Panel a). Panel b indicates a schematic representation of the perfusion circuit for seeded pancreatic scaffold culture. Panel c shows a representative image of H&E stain showing localization of infused cells in vessels. Boxes indicate areas reported with high magnification in the panel below. Panel d illustrates representative images of H&E (left), CD31 (middle), and Ki67 (right) matrix staining (From Peloso et al., Ann Surg, 2016, Reprinted with permission of the American Journal of Transplantation. Copyright © 2017 American Journal of Transplantation) [145]
Although all of these technologies may restore the functionality of destroyed islets, the primary pathology of type 1 diabetes is autoimmune, which will remain a persistent obstacle if left unaddressed. Future strategies must incorporate adjuvant immunomodulation or autologous tissue in current technologies to terminate the chronic disease state and preserve endocrine pancreas function.
8.4.5 Liver
Liver disease morbidity and mortality outcomes are increasing worldwide. The total deaths caused by cirrhosis and liver cancer have increased by 50 million per year since 1990 [148]. At present, liver transplantation is the only curative option for patients with end-stage liver disease. Twenty percent of this patient population dies while on a waiting list, however, due to the shortage of transplantable organs [149]. Alternative modalities such as bioartificial livers and hepatocyte transplantation have been investigated, but without successful yield [150,151,152]. Given the obstacles currently facing end-stage liver patients, TE strategies for liver repair and regeneration are particularly attractive for contemporary investigators.
The liver has an innate capability to regenerate beyond the potential of other abdominal organs. In the event of injury to less than 70% of the organ, the liver can fully regenerate within 6 months of the trauma [153]. Regeneration occurs through cellular hyperplasia of the residual liver, however, and is not considered true regeneration [154]. Instead, there is an observed compensatory enlargement of the remnant liver to meet the functional demands of the body. From an evolutionary perspective, liver hyperplasia is a mechanism of repair designed to restore function, not anatomy. When damage to the liver reaches a critical threshold, compensatory regeneration is no longer sufficient, and transplantation is the usual approach in these circumstances.
Nevertheless, the regenerative properties of the liver make it uniquely amenable to methods in liver bioengineering. Current investigations involve two distinct regenerative pathways. One involves the manipulation of the existing cellular physiology in liver hepatocytes to induce regeneration. The other option, similar to approaches used in other organ systems, is use of synthetic scaffolds or ECM-based liver scaffolds obtained via decellularization technology. These scaffolds would then be reseeded with host cells and allowed to regrow either in vivo or in vitro.
Unfortunately, the actual system that regulates hepatic regeneration following injury remains poorly characterized and obscure. During the native regeneration process, cellular hyperplasia occurs spontaneously through a complex cascade of events and signals. This pathway involves inflammatory signaling, cellular proliferation, cell migration, and neoangiogenesis. If mature hepatocytes are unable to proliferate sufficiently via division, liver progenitor cells known as oval cells intervene to compensate [155, 156], thereby hinting at potential TE interventions. In addition, studies have shown that bone marrow stem cells are also involved in the regeneration and differentiation of functional hepatocytes [157, 158].
Observations on the natural regenerative capacity of injured livers led to the use of progenitor liver cells for bioengineering research. As previously mentioned, cellular hyperplasia replaces the lost volume following liver amputation, and current studies attempt to enhance the innate regenerative ability of the liver through terminal differentiation via resident bone marrow-derived or liver progenitor cells [159,160,161]. Infusing autologous bone marrow cells into patients with decompensated liver cirrhosis has already been demonstrated to be safe and, in some cases, clinically beneficial [162, 163]. Another avenue is the use of cytokine granulocyte-colony stimulating factor (G-CSF) for the activation of hematopoietic stem cell differentiation and mobilization, increasing the ability of resident progenitor cells to respond to injury [164]. Stem cells isolated from the peripheral bloodstream of liver disease patients infused with G-CSF were isolated and infused back into the patients. These patients showed significant improvements in liver enzyme levels as well as serum bilirubin when compared with the control group [165, 166]. It is thought that G-CSF is able to mobilize bone marrow cells and circulating progenitor cells while increasing hepatocyte growth factor, which is prominently involved in liver regeneration [167]. Despite these findings, researchers are unable to determine the specific progenitor cell type that is actually aiding, or is primarily responsible, in the regeneration process.
Recent work has also highlighted the use of cell-scaffold technology for liver bioengineering [168]. As in other organ systems, the chief objectives include demonstrating successful organ decellularization, successful seeding of acellular scaffolds with hepatocytes or hepatocyte progenitors, and recapitulation of original liver functions including protein synthesis/breakdown, detoxification, and bilirubin metabolism.
Investigators successfully converted the murine liver into acellular scaffolds via perfusion with SDS detergent to remove cellular material and debris. The remaining constructs retained proper collagen structure, laminin basement membranes, and internal vascular networks [169]. In the same study, the scaffold had enough structural integrity to withstand cannulation and perfusion with the reseeding cells. A follow-up study illustrated the ability of the perfused cells to leave the vasculature and distribute among the matrix [170]. Using electron microscopy, it was observed that rodent hepatocytes migrated into decellularized sinusoidal spaces and displayed levels of urea synthesis, albumin synthesis, and cytochrome P450 expression comparable to sandwiched hepatocyte cultures. This is particularly important because the liver requires a wide range of enzymes, cofactors, serum proteins, and acute phase reactants to be fully functional. This graft was able to withstand transplantation for 8 h in a rat host prior to harvesting, which showed that the hepatocytes retained their morphology, position, and integrity.
Yagi et al. recently reported on the successful decellularization of porcine livers, thereby producing ECM scaffolds at scales relevant to humans. These scaffolds were capable of supporting hepatocyte engraftment and reorganization in three dimensions [171]. Baptista et al. also observed that it is possible to decellularize whole liver in mice, rats, ferrets, rabbits, and adult pigs and also that they can be successfully seeded with human hepatocyte progenitors [172]. This study utilized the inferior vena cava for decellularization and used the portal vein, as well as the vena cava, for perfusion of cells. The decellularized vascular network was able to withstand fluid flow that entered through a central inlet vessel, branched into an extensive capillary bed, and coalesced in a single outlet vessel. Discrete populations of human fetal liver and endothelial cells were found throughout the matrix in their putative native locations, suggesting that the retention of glycosaminoglycans and collagen structure provides the necessary environmental signaling to regulate cell differentiation.
Wang et al. demonstrated that progenitor cells seeded onto a scaffold could differentiate into hepatocytes and cholangiocytes, illustrating that the scaffold could dictate the differentiation toward distinct cell lineages [173]. Barakat et al. decellularized porcine livers and successfully repopulated the scaffolds with human cells [174]. Analysis revealed that the cells were induced to differentiate into mature hepatocytes while maintaining active metabolism, the ability to withstand physiologic shear stress from blood flow, and the ability to synthesize albumin.
Most recently, Mazza et al. demonstrated the complete decellularization of discarded whole human liver and lobes to form ECM scaffolds with preserved architecture [175]. These scaffolds were repopulated with human hepatic cells that thereafter showed excellent viability, motility, and proliferation and remodeling of the ECM. The group demonstrated biocompatibility by both omental and subcutaneous xenotransplantation of liver scaffold cubes into immune-competent mice. No foreign body response was observed, dramatically underscoring the clinical potential of bioengineered organs.
8.4.6 Heart
The inability of cardiomyocytes to regenerate following infarction remains the primary cause of congestive heart failure [176]. Infarct scar tissue has long been believed to be acellular and physiologically inert. Recent studies, however, suggest that cardiac scar tissue is composed of phenotypically transformed fibroblast-like cells which behave dynamically and undergo continuous turnover. Such bioactivity suggests possible intervention using TE strategies in order to regenerate functional, healthy cardiac tissue and minimize adverse accumulation of fibrous tissue. At present, there are no available therapies which prevent or reverse cardiac damage and adverse ventricular remodeling following infarction.
The fabrication of engineered heart tissue was demonstrated for the first time over 20 years ago using embryonic chicken cardiac myocytes [177]. Since then, investigators have made enormous strides in the field of stem cell biology suggesting that widespread clinical translation may be rapidly approaching. Recent studies have demonstrated the therapeutic potential of stem cells to regenerate contractile cardiac tissue [178]. Contemporary investigators must determine the most appropriate cell type to use to achieve regeneration and choose the most efficacious engraftment technique. During the early years of cardiac restoration research, a common technique involved a direct bolus of cardiac cells suspended in saline directed into regions of infarcted tissue [179]. Other cell types used with this technique include skeletal myoblasts, neonatal cardiomyocytes, fibroblasts, smooth muscle cells, embryonic stem cells, and bone marrow progenitors with varying improvements in cardiac function [180]. Nevertheless, the proportion of cells successfully engrafting at the infarct site tends to be very low using this method [181].
Recently, investigators have improved engraftment rates by using cultured cardiomyocytes seeded on biomaterial scaffolds to create transplantable cardiac “patches.” Scaffolds are not always necessary since cardiac myocytes cultured on standard plastic dishes tend to detach as intact monolayers. Serially stacking multiple sheets on top of one another creates three-dimensional tissues that “beat” and generate force [182]. Cardiac tissue segments thus engineered in vitro demonstrate organized sarcomeres, electrical conductivity, and contractile characteristics resembling native adult myocardium [183]. These “patches” have been shown to improve left ventricular performance when implanted in doxorubicin-treated rats as models of dilated cardiomyopathy [184].
Ott et al. produced acellular ECM-based scaffolds from rodent hearts which were subsequently recellularized using neonatal rat cardiomyocytes and endothelial cells [185]. These constructs were then subjected to perfusion treatment in bioreactors for 28 days. By day 4, investigators observed macroscopic contractions, and by day 8, the maturing organoids were able to sustain pump function under physiological load and electrical stimulation.
Biomaterials used to enhance engraftment and regeneration have also been an important parameter in cardiac tissue engineering. Recent investigators have incorporated solidifying gel polymers such as fibrin glue and PEGylated fibrinogen hydrogels [186, 187]. The advantage of this technique consists in the ability to fabricate any three-dimensional architecture and manipulate the structure prior to implantation. Furthermore, certain biomaterials are able to protect seeded cells from host inflammation and facilitate functional integration within native myocardium at the injury site.
8.4.7 Airway
Approximately 2000 lung transplants were performed in 2013 to treat various diseases including alpha-1 antitrypsin deficiency, primary pulmonary hypertension, cystic fibrosis, emphysema, and others [188]. TE approaches for airway repair could potentially improve patient survival and reduce waiting times for transplantation. Among tissue engineering approaches to transplantation, the airway was one of the first systems in which clinical success was demonstrated. Macchiarini et al. performed the first bioengineered trachea transplantation in humans [55]. The team removed cells and MHC antigens from a human donor trachea and reseeded the resultant scaffold with epithelial cells and MSC-derived chondrocytes which were acquired autologously. The engineered graft was then used to replace the left main bronchus in a 30-year-old woman with end-stage bronchomalacia.
Progress with the bioengineered trachea has alluded to potential inroads for larynx and lung transplantation investigations. At present, only two human laryngeal allotransplants have been reported, and both patients required lifelong immunosuppression regimes thereafter. Baiguera et al. successfully decellularized human larynxes enzymatically to obtain acellular scaffolds [189]. Electron microscopy confirmed that their matrices retained the hierarchical structures of the native larynx. Mechanical testing demonstrated intact biomechanical integrity. Furthermore, chorioallantoic membrane analysis showed that their constructs induced a strong in vivo angiogenic response, underscoring their integration and engraftment potential. Lung transplantation, too, is a field particularly amenable to TE strategies for currently existing problems. Given the limited regenerative potential of the adult lung, investigators have sought to determine whether lung tissue can be regenerated in vitro for potential subsequent implantation. Petersen et al. decellularized the lungs of adult rats to obtain acellular matrix scaffolds [190]. They then used a bioreactor to culture pulmonary epithelium and vascular endothelium on the acellular matrices. Subsequent analysis showed that the seeded epithelium displayed robust hierarchical organization within the matrix. Furthermore, the seeded endothelial cells efficiently repopulated the vascular compartment of the decellularized constructs. In vitro testing demonstrated that the engineered lungs possessed biomechanical parameters similar to those of native lungs. These seeded scaffolds were then implanted in rats for in vivo testing and were observed to participate in gas exchange, remarkably.
Bioengineering strategies for the trachea and larynx differ from those for the lung given their relatively simpler, hollow architectures. Furthermore, their purpose is mainly derived from their structural characteristics (i.e., as air conduits) rather than their cellular functions, unlike complex solid organs. Synthetic scaffolds, such as those involving polyester urethane, polypropylene mesh, alginate gel, or PEGylated hydrogels, were used initially to integrally replace the trachea as they benefit from not requiring a donor and being easily modified to conform to recipient anatomy. Nevertheless their use is limited due to their having different biomechanical properties, being more susceptible to infection, and not vascularizing [191]. Vascularization is critical to maintain the vitality of seeded or recruited cells and subsequent proliferation [192].
Recellularization strategies in these contexts seek to recover the chondrocyte and epithelial compartments of the native airway. Stem cells, appropriately selected, have the potential to differentiate into both of these cell types. Berg et al. repopulated a detergent-decellularized human cadaveric donor trachea with autologous stem cells and implanted the resulting airway construct into a 76-year-old patient with tracheal stenosis [193]. Within a week the graft was compromised by a thick fungal infection which nevertheless controlled with local and systemic antifungal therapy. The patient eventually died after 3 weeks due to cardiac arrest, but the airway graft was found to be patent, open, and stable with intact anastomoses. Histopathological analysis during autopsy showed squamous but not ciliated epithelium, neovascularization, nerve fibers in the submucosa, and intact chondrocytes in the cartilage. Investigators have also experimented with other stem cell types including iPSCs [194], marrow-derived MSCs [195], human ESCs [196], and amniotic fluid-derived stem cells [197] for regeneration of airway tissue.
8.5 Conclusion
The use of autologous cellular material has the potential to obviate the need for lifelong antirejection therapies. De novo organ fabrication using cell-scaffold technology could, theoretically, provide a limitless supply of transplantable organs for waiting list patients, thus circumventing the challenge of organ shortage.
Large-scale clinical translation of abdominal organ bioengineering remains a distant possibility, however, because substantial work remains to be done in the laboratory. The chief obstacles posed by current medical and surgical modalities (particularly transplantation) are quite concerning and have created a pocket of necessity within which TE investigations are being conducted.
Further research is needed to identify the most suitable cell type for regenerative investigations in each organ system. Although adult somatic cells specific to the organ system being treated have shown promise, stem cells have the advantage of being multipotent and renewable, which are particularly attractive qualities in the context of TE. The risks associated with their use, however, need to be further characterized before they can be used safely in patients on a large scale.
The immunogenicity of decellularized scaffolds is another issue that needs to be assessed. Though their tolerability has been recognized both empirically and theoretically, suboptimal decellularization protocols can leave behind residual antigen activity, triggering an immune response in the host.
Investigators must also seek to understand better the interaction between ECM and cells, both endogenous and exogenous. The improved knowledge base would likely result in improved cellular engraftment, improved migration, and improved differentiation within the acellular matrix. The source of new understanding could very well lie in developmental biology and organogenesis, in which ECM-guided differentiation is paramount.
Though TE solutions for abdominal organ engineering show great promise, current researchers must also keep an eye toward research funding limitations and the role of commercial entities in bringing their innovations from bench to bedside. With these last obstacles addressed, TE solutions for transplantation may very well bring about a paradigm shift in the modern era for the treatment of end-stage organ diseases.
Abbreviations
- ECM:
-
Extracellular matrix
- ESC:
-
Embryonic stem cell
- iPSC:
-
Induced pluripotent stem cell
- MSC:
-
Mesenchymal stem cell
- PEG:
-
Polyethylene glycol
- TE:
-
Tissue engineering
References
World Health Organization. (2013). World health statistic. Geneva: World Health Organization.
World Health Organization. GKT1 activity and practices. http://www.who.int/transplantation/gkt/statistics/en/. Accessed 24 June 2015.
National Kidney Foundation. Organ donation and transplant statistics. https://www.kidney.org/news/newsroom/factsheets/Organ-Donation-and-Transplantation-Stats. Accessed 24 June 2015.
Organ Procurement and Transplantation Network. http://optn.transplant.hrsa.gov/converge/latestData/rptData.asp. Accessed 24 June 2015.
Katari, R., Peloso, A., & Orlando, G. (2014). Tissue engineering and regenerative medicine: Semantic considerations for an evolving paradigm. Frontiers in Bioengineering and Biotechnology, 2, 57.
Katari, R. S., Peloso, A., & Orlando, G. (2014). Tissue engineering. Advances in Surgery, 48, 137–154.
Orlando, G., Soker, S., Stratta, R. J., & Atala, A. (2013). Will regenerative medicine replace transplantation? Cold Spring Harbor Perspectives in Medicine, 3(8).
Peloso, A., Dhal, A., Zambon, J. P., et al. (2015). Current achievements and future perspectives in whole-organ bioengineering. Stem Cell Research & Therapy, 6, 107.
Wolfe, R. A., Ashby, V. B., Milford, E. L., et al. (1999). Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. The New England Journal of Medicine, 341(23), 1725–1730.
Abecassis, M., Bartlett, S. T., Collins, A. J., et al. (2008). Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clinical Journal of the American Society of Nephrology, 3(2), 471–480.
ERA-EDTA Annual Report. 2012. http://www.era-edta-reg.org/files/annualreports/pdf/AnnRep2012.pdf. Accessed 10 Mar 2016.
National Kidney Foundation. Organ donation and transplant statistics. https://www.kidney.org/news/newsroom/factsheets/OrganDonation-and-Transplantation-Stats. Accessed 10 Mar 2016.
Organ Procurement and Transplantation Network. http://optn.transplant.hrsa.gov/converge/latestData/rptData.asp. Accessed 10 Mar 2016.
Organ donation and transplantation activity report 2012. http://www.transplant-observatory.org/Documents/dataReports/Basicslides2012.pdf. accessed 10 Mar 2016.
Shin, E., Kwon, S. W., Yang, W. S., et al. (2015). Long-term outcomes of ABO-incompatible living donor kidney transplantation: A comparative analysis. Transplantation Proceedings, 47(6), 1720–1726.
Coilly, A., & Samuel, D. (2015). Pros and Cons: Usage of organs from donors infected with hepatitis C virus – Revision in the direct acting antiviral era. Journal of Hepatology.
Perico, N., & Remuzzi, G. (2012). Chronic kidney disease: A research and public health priority. Nephrology, Dialysis, Transplantation, 27(suppl 3), iii19–iii26.
Thomas, L. (1971). Notes of a biology-watcher: The technology of medicine. The New England Journal of Medicine, 285, 1366–1368.
Brown, E. (1996). Halfway technologies. Physician Executive, 22(12), 44–45.
Zsom, L., Wagner, L., & Fülöp, T. (2015). Minimization vs tailoring: Where do we stand with personalized immunosuppression during renal transplantation in 2015? World Journal of Transplantation, 5(3), 73–80.
Nyumura, I., Honda, K., Tanabe, K., Teraoka, S., & Iwamoto, Y. (2012). Early histologic lesions and risk factors for recurrence of diabetic kidney disease after kidney transplantation. Transplantation, 94(6), 612–619.
Mitchell, O., & Gurakar, A. (2015). Management of hepatitis C post-Liver transplantation: A comprehensive review. Journal of Clinical and Translational Hepatology, 3(2), 140–148.
Manzia, T. M., Angelico, R., Ciano, P., et al. (2014). Impact of immunosuppression minimization and withdrawal in long-term hepatitis C virus liver transplant recipients. World Journal of Gastroenterology, 20(34), 12217–12225.
Heidary Rouchi, A., & Mahdavi-Mazdeh, M. (2015). Regenerative medicine in organ and tissue transplantation: Shortly and practically achievable? International Journal of Organ Transplantation Medicine, 6(3), 93–98.
Dutkowski, P., de Rougemont, O., & Clavien, P. A. (2008 Oct). Alexis Carrel: Genius, innovator and ideologist. American Journal of Transplantation : Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons, 8(10), 1998–2003.
Orlando, G., Soker, S., & Wood, K. (2009). Operational tolerance after liver transplantation. Journal of Hepatology, 50(6), 1247–1257.
Orlando, G. (2010). Finding the right time for weaning off immunosuppression in solid organ transplant recipients. Expert Review of Clinical Immunology, 6(6), 879–892.
Schroeder RA, Marroquin CE, Kuo PC. Tolerance and the “Holy Grail” of transplantation. The Journal of Surgical Research 2003;111(1):109-119.
Dharnidharka, V. R., Stablein, D. M., & Harmon, W. E. (2004). Post-transplant infections now exceed acute rejection as cause for hospitalization: A report of the NAPRTCS. American Journal of Transplantation, 4(3), 384–389.
U.S. Renal Data System. (2013). USRDS 2013 Annual Data Report: Atlas of CKD & ESRD, Volume 2; Chapter 7: Transplantation. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. http://www.usrds.org/atlas.aspx. Accessed 11 Mar 2016.
Engels, E. A., Pfeiffer, R. M., Fraumeni, J. F., et al. (2011). Spectrum of cancer risk among US solid organ transplant recipients. Journal of the American Medical Association, 306(17), 1891–1901.
Bouwes Bavinck, J. N., Hardie, D. R., Green, A., et al. (1996). The risk of skin cancer in renal transplant recipients in Queensland, Australia. A follow-up study. Transplantation, 61(5), 715–721.
Smith, J. M., Skeans, M. A., Horslen, S. P., et al. (2015). OPTN/SRTR 2013 annual data report: Intestine. American Journal of Transplantation, 15(Suppl 2), 1–16.
Kandaswamy, R., Skeans, M. A., Gustafson, S. K., et al. (2015). OPTN/SRTR 2013 annual data report: Pancreas. American Journal of Transplantation, 15(Suppl 2), 1–20.
Valapour, M., Skeans, M. A., Heubner, B. M., Smith, J. M., Hertz, M. I., Edwards, L. B., Cherikh, W. S., Callahan, E. R., Snyder, J. J., Israni, A. K., et al. (2015). OPTN/SRTR 2013 annual data report: Lung. American Journal of Transplantation, 15, 1–28.
Colvin-Adams, M., Smith, J. M., Heubner, B. M., Skeans, M. A., Edwards, L. B., Waller, C. D., Callahan, E. R., Snyder, J. J., Israni, A. K., & Kasiske, B. L. (2015). OPTN/SRTR 2013 annual data report: Heart. American Journal of Transplantation, 15, 1–28.
Kim, W. R., Lake, J. R., Smith, J. M., Skeans, M. A., Schladt, D. P., Edwards, E. B., Harper, A. M., Wainright, J. L., Snyder, J. J., Israni, A. K., et al. (2015). OPTN/SRTR 2013 annual data report: Liver. American Journal of Transplantation, 15, 1–28.
Matas, A. J., Smith, J. M., Skeans, M. A., et al. (2015). OPTN/SRTR 2013 annual data report: Kidney. American Journal of Transplantation, 15(Suppl 2), 1–34.
Filipponi, F., Pisati, R., Cavicchini, G., Ulivieri, M. I., Ferrara, R., & Mosca, F. (2003). Cost and outcome analysis and cost determinants of liver transplantation in a European National Health Service hospital. Transplantation, 75(10), 1731–1736.
Irwin, F. D., Wu, C., Bannister, W. M., et al. (2016). A commercial transplant network’s perspective of value in solid organ transplantation: Strategizing for value in transplant care. Transplantation Reviews (Orlando, Fla.)
United States Cong. House. Committee on Energy and Commerce, Subcommittee on Health. (2013, June 28). Examining reforms to improve the medicare part B drug program for seniors. Washington D.C. (statement of the American Society of Transplant Surgeons). Available at https://asts.org/docs/default-source/legislative/asts-statement-to-house-committee-on-energy-and-commerce-subcommittee-on-health-june-28-2013.pdf. Accessed 14 Mar 2016.
Sack K. 2009, September 13. U.S. cost-saving policy forces new kidney transplant. The New York Times.
Evans, R. W., Applegate, W. H., Briscoe, D. M., et al. (2010). Cost-related immunosuppressive medication nonadherence among kidney transplant recipients. Clinical Journal of the American Society of Nephrology, 5(12), 2323–2328.
Marx, V. (2015). Tissue engineering: Organs from the lab. Nature, 522(7556), 373–377.
He, M., & Callanan, A. (2013). Comparison of methods for whole-organ decellularization in tissue engineering of bioartificial organs. Tissue Engineering. Part B, Reviews, 19(3), 194–208.
Goldfarb, D. A. (2005). Tissue engineering stem cells, and cloning: Opportunities for regenerative medicine. The Journal of Urology, 173(4), 1431.
Orlando, G., Soker, S., & Stratta, R. J. (2013). Organ bioengineering and regeneration as the new Holy Grail for organ transplantation. Annals of Surgery, 258(2), 221–232.
Orlando, G., Wood, K. J., De Coppi, P., Baptista, P. M., Binder, K. W., Bitar, K. N., Breuer, C., Burnett, L., Christ, G., Farney, A., Figliuzzi, M., Holmes, J. H., Koch, K., Macchiarini, P., Mirmalek Sani, S.-H., Opara, E., Remuzzi, A., Rogers, J., Saul, J. M., Seliktar, D., Shapira-Schweitzer, K., Smith, T., Solomon, D., Van Dyke, M., Yoo, J. J., Zhang, Y., Atala, A., Stratta, R. J., & Soker, S. (2012). Regenerative medicine as applied to general surgery. Annals of Surgery, 255(5), 867–880.
Orlando, G., Wood, K. J., Stratta, R. J., Yoo, J. J., Atala, A., & Soker, S. (2011). Regenerative medicine and organ transplantation: Past, present, and future. Transplantation, 91(12), 1310–1317.
Orlando, G., Baptista, P., Birchall, M., De Coppi, P., Farney, A., Guimaraes-Souza, N. K., Opara, E., Rogers, J., Seliktar, D., Shapira-Schweitzer, K., Stratta, R. J., Atala, A., Wood, K. J., & Soker, S. (2011). Regenerative medicine as applied to solid organ transplantation: Current status and future challenges. Transplant International, 24(3), 223–232.
Badylak, S. F., Weiss, D. J., Caplan, A., & Macchiarini, P. (2012). Engineered whole organs and complex tissues. Lancet, 379(9819), 943–952.
Vacanti, J. P., Morse, M. A., Saltzman, W. M., et al. (1988). Selective cell transplantation using bioabsorbable artificial polymers as matrices. Journal of Pediatric Surgery, 23, 3–9.
Vacanti, J. P. (1988). Beyond transplantation. Third annual Samuel Jason Mixter lecture. Archives of Surgery, 123, 545–549.
Shinoka, T., Imai, Y., & Ikada, Y. (2001). Transplantation of a tissue-engineered pulmonary artery. The New England Journal of Medicine, 344, 532–533.
Macchiarini, P., Jungebluth, P., Go, T., et al. (2008). Clinical transplantation of a tissue-engineered airway. Lancet, 372, 2023–2030.
Jungebluth, P., Alici, E., Baiguera, S., et al. (2011). Tracheobronchial transplantation with a stem-cell-seeded bioartificial nanocomposite: A proof-of-concept study. Lancet, 378, 1997–2004.
Elliott, M. J., De Coppi, P., Speggiorin, S., et al. (2012). Stem-cell-based, tissue engineered tracheal replacement in a child: A 2-year follow-up study. Lancet, 380, 994–1000.
Gonfiotti, A., Jaus, M. O., Barale, D., et al. (2014). The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet, 383(9913), 238–244.
Raya-rivera, A., Esquiliano, D. R., Yoo, J. J., Lopez-bayghen, E., Soker, S., & Atala, A. (2011). Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet, 377(9772), 1175–1182.
Atala, A., Bauer, S. B., Soker, S., Yoo, J. J., & Retik, A. B. (2006). Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet, 367(9518), 1241–1246.
Raya-rivera, A. M., Esquiliano, D., Fierro-pastrana, R., et al. (2014). Tissue-engineered autologous vaginal organs in patients: A pilot cohort study. Lancet, 384(9940), 329–336.
Lu, L., Zhu, X., Valenzuela, R. G., Currier, B. L., & Yaszemski, M. J. (2001). Biodegradable polymer scaffolds for cartilage tissue engineering. Clinical Orthopaedics and Related Research, (391 Suppl), S251–S270.
Chen, F., Yoo, J. J., & Atala, A. (1999). Acellular collagen matrix as a possible “off the shelf” biomaterial for urethral repair. Urology, 54(3), 407–410.
Ross, E. A., Williams, M. J., Hamazaki, T., Terada, N., Clapp, W. L., Adin, C., et al. (2009). Embryonic stem cells proliferate and differentiate when seeded into kidney scaffolds. J Am Soc Nephrol, 20(11), 2338–2347.
Bonandrini, B., Figliuzzi, M., Papadimou, E., et al. (2014). Recellularization of well-preserved acellular kidney scaffold using embryonic stem cells. Tissue Engineering. Part A, 20(9–10), 1486–1498.
Gjorevski, N., Ranga, A., & Lutolf, M. P. (2014). Bioengineering approaches to guide stem cell-based organogenesis. Development, 141(9), 1794–17804.
Brivanlou, A. H., Gage, F. H., Jaenisch, R., Jessell, T., Melton, D., & Rossant, J. (2003). Stem cells. Setting standards for human embryonic stem cells. Science, 300(5621), 913–916.
Kehat, I., Kenyagin-karsenti, D., Snir, M., et al. (2001). Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. The Journal of Clinical Investigation, 108(3), 407–414.
Levenberg, S., Golub, J. S., Amit, M., Itskovitz-eldor, J., & Langer, R. (2002). Endothelial cells derived from human embryonic stem cells. Proceedings of the National Academy of Sciences of the United States of America, 99(7), 4391–4396.
Reubinoff, B. E., Itsykson, P., Turetsky, T., et al. (2001). Neural progenitors from human embryonic stem cells. Nature Biotechnology, 19(12), 1134–1140.
Assady, S., Maor, G., Amit, M., Itskovitz-eldor, J., Skorecki, K. L., & Tzukerman, M. (2001). Insulin production by human embryonic stem cells. Diabetes, 50(8), 1691–1697.
Narayanan, K., Schumacher, K. M., Tasnim, F., et al. (2013). Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney International, 83(4), 593–603.
Denker, H. W. (2006). Potentiality of embryonic stem cells: An ethical problem even with stem cell source. Journal of Medical Ethics, 32, 665–671.
Aldahmash, A., Atteya, M., Elsafadi, M., Al-Nbaheen, M., Al-Mubarak, H. A., Vishnubalaji, R., Al-Roalle, A., Al-Harbi, S., Manikandan, M., Matthaei, K. I., & Mahmood, A. (2013). Teratoma formation in immunocompetent mice after syngeneic and allogeneic implantation of germline capable mouse embryonic stem cells. Asian Pacific Journal of Cancer Prevention, 14, 5705–5711.
Aasen, T., Raya, A., Barrero, M. J., et al. (2008). Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nature Biotechnology, 26(11), 1276–1284.
Wang, J., Gu, Q., Hao, J., et al. (2013). Generation of induced pluripotent stem cells with high efficiency from human umbilical cord blood mononuclear cells. Genomics, Proteomics & Bioinformatics, 11(5), 304–131.
Spradling, A., Drummond-barbosa, D., & Kai, T. (2001). Stem cells find their niche. Nature, 414(6859), 98–104.
Li, L., & Xie, T. (2005). Stem cell niche: Structure and function. Annual Review of Cell and Developmental Biology, 21, 605–631.
Fuchs, E. (2008). Skin stem cells: Rising to the surface. The Journal of Cell Biology, 180(2), 273–284.
Sousa, B. R., Parreira, R. C., Fonseca, E. A., Amaya, M. J., Tonelli, F. M., Lacerda, S. M., Lalwani, P., Santos, A. K., Gomes, K. N., Ulrich, H., Kihara, A. H., & Resende, R. R. (2014). Human adult stem cells from diverse origins: An overview from multiparametric immunophenotyping to clinical applications. Cytometry. Part A, 85, 43–77.
Van der flier, L. G., & Clevers, H. (2009). Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annual Review of Physiology, 71, 241–260.
Bussolati, B., Bruno, S., Grange, C., et al. (2005). Isolation of renal progenitor cells from adult human kidney. The American Journal of Pathology, 166(2), 545–555.
Lindroos, B., Suuronen, R., & Miettinen, S. (2011). The potential of adipose stem cells in regenerative medicine. Stem Cell Reviews, 7(2), 269–291.
Strom, T. B., Field, L. J., & Ruediger, M. (2002). Allogeneic stem cells, clinical transplantation and the origins of regenerative medicine. Current Opinion in Immunology, 14(5), 601–605.
Simons, B. D., & Clevers, H. (2011). Stem cell self-renewal in intestinal crypt. Experimental Cell Research, 317(19), 2719–2724.
Bitar, K. N., & Raghavan, S. (2012). Intestinal tissue engineering: Current concepts and future vision of regenerative medicine in the gut. Neurogastroenterology and Motility, 24(1), 7–19.
Speer, A. L., Sala, F. G., Matthews, J. A., & Grikscheit, T. C. (2011). Murine tissue-engineered stomach demonstrates epithelial differentiation. The Journal of Surgical Research, 171(1), 6–14.
Maemura, T., Shin, M., Kinoshita, M., et al. (2008). A tissue-engineered stomach shows presence of proton pump and G-cells in a rat model, resulting in improved anemia following total gastrectomy. Artificial Organs, 32(3), 234–239.
Maemura, T., Shin, M., Sato, M., Mochizuki, H., & Vacanti, J. P. (2003). A tissue-engineered stomach as a replacement of the native stomach. Transplantation, 76(1), 61–65.
Maemura, T., Kinoshita, M., Shin, M., et al. (2012). Assessment of a tissue-engineered gastric wall patch in a rat model. Artificial Organs, 36(4), 409–417.
Chen, M. K., & Beierle, E. A. (2004). Animal models for intestinal tissue engineering. Biomaterials, 25(9), 1675–1681.
Hecker, L., Baar, K., Dennis, R. G., & Bitar, K. N. (2005). Development of a three-dimensional physiological model of the internal anal sphincter bioengineered in vitro from isolated smooth muscle cells. American Journal of Physiology. Gastrointestinal and Liver Physiology, 289(2), G188–G196.
Somara, S., Gilmont, R. R., Dennis, R. G., & Bitar, K. N. (2009). Bioengineered internal anal sphincter derived from isolated human internal anal sphincter smooth muscle cells. Gastroenterology, 137(1), 53–61.
Grikscheit, T. C., Siddique, A., Ochoa, E. R., et al. (2004). Tissue-engineered small intestine improves recovery after massive small bowel resection. Annals of Surgery, 240(5), 748–754.
Chen, M. K., & Badylak, S. F. (2001). Small bowel tissue engineering using small intestinal submucosa as a scaffold. The Journal of Surgical Research, 99(2), 352–358.
Hori, Y., Nakamura, T., Kimura, D., et al. (2002). Experimental study on tissue engineering of the small intestine by mesenchymal stem cell seeding. The Journal of Surgical Research, 102(2), 156–160.
Finkbeiner, S. R., & Spence, J. R. (2013). A gutsy task: Generating intestinal tissue from human pluripotent stem cells. Digestive Diseases and Sciences, 58(5), 1176–1184.
Spence, J. R., Mayhew, C. N., Rankin, S. A., et al. (2011). Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature, 470(7332), 105–109.
Watson, C. L., Mahe, M. M., Múnera, J., et al. (2014). An in vivo model of human small intestine using pluripotent stem cells. Nature Medicine, 20(11), 1310–1314.
Wieck, M. M., El-nachef, W. N., Hou, X., et al. (2016). Human and murine tissue-engineered colon exhibit diverse neuronal subtypes and can be populated by enteric nervous system progenitor cells when donor colon is aganglionic. Tissue Engineering. Part A, 22(1–2), 53–64.
Resende, R. R., Fonseca, E. A., Tonelli, F. M. P., Sousa, B. R., Santos, A. K., Gomes, K. N., Guatimosim, S., Kihara, A. H., & Ladeira, L. O. (2014). Scale/topography of substrates surface resembling extracellular matrix for tissue engineering. Journal of Biomedical Nanotechnology, 10, 1157–1193.
Eckardt, K. U., Coresh, J., Devuyst, O., et al. (2013). Evolving importance of kidney disease: From subspecialty to global health burden. Lancet, 382(9887), 158–169.
Hoerger, T. J., Simpson, S. A., Yarnoff, B. O., Pavkov, M. E., Ríos Burrows, N., Saydah, S. H., Williams, D. E., & Zhuo, X. (2015). The future burden of CKD in the United States: A simulation model for the CDC CKD initiative. American Journal of Kidney Diseases, 65, 403–411.
Zambon, J. P., Magalhaes, R. S., Ko, I., et al. (2014). Kidney regeneration: Where we are and future perspectives. World Journal of Nephrology, 3(3), 24–30.
Salvatori, M., Peloso, A., Katari, R., & Orlando, G. (2014). Regeneration and bioengineering of the kidney: Current status and future challenges. Current Urology Reports, 15(1), 379.
Rinkevich, Y., Montoro, D. T., Contreras-trujillo, H., et al. (2014). Vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Reports, 7(4), 1270–1283.
Nagaike, M., Hirao, S., Tajima, H., et al. (1991). Renotropic functions of hepatocyte growth factor in renal regeneration after unilateral nephrectomy. The Journal of Biological Chemistry, 266(34), 22781–22784.
Cochrane, A. L., Kett, M. M., Samuel, C. S., et al. (2005). Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. Journal of the American Society of Nephrology, 16(12), 3623–3630.
Davidson, A. J. (2011). Uncharted waters: Nephrogenesis and renal regeneration in fish and mammals. Pediatric Nephrology, 26(9), 1435–1443.
Lam, A. Q., & Bonventre, J. V. (2015). Regenerating the nephron with human pluripotent stem cells. Current Opinion in Organ Transplantation, 20(2), 187–192.
Little, M. H. (2006). Regrow or repair: Potential regenerative therapies for the kidney. Journal of the American Society of Nephrology, 17(9), 2390–2401.
Pleniceanu, O., Harari-Steinberg, O., & Dekel, B. (2010). Concise review: Kidney stem/progenitor cells: Differentiate, sort out, or reprogram? Stem Cells, 28(9), 1649–1660.
Romagnani, P., Lasagni, L., & Remuzzi, G. (2013). Renal progenitors: An evolutionary conserved strategy for kidney regeneration. Nature Reviews. Nephrology, 9(3), 137–146.
Zhang, Y., McNeill, E., Tian, H., Soker, S., Andersson, K.-E., Yoo, J. J., & Atala, A. (2008). Urine derived cells are a potential source for urological tissue reconstruction. The Journal of Urology, 180, 2226–2233.
Xing, L., Cui, R., Peng, L., et al. (2014). Mesenchymal stem cells, not conditioned medium, contribute to kidney repair after ischemia-reperfusion injury. Stem Cell Research & Therapy, 5(4), 101.
Hauser, P. V., de Fazio, R., Bruno, S., et al. (2010). Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery. The American Journal of Pathology, 177, 2011–2021.
Sedrakyan, S., da Sacco, S., Milanesi, A., et al. (2012). Injection of amniotic fluid stem cells delays progression of renal fibrosis. Journal of the American Society of Nephrology, 23, 661–673.
Lee, P. Y., Chien, Y., Chiou, G. Y., Lin, C. H., Chiou, C. H., & Tarng, D. C. (2012). Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via downregulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell Transplantation, 21, 2569–2585.
Taguchi, A., Kaku, Y., Ohmori, T., et al. (2014). Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell, 14(1), 53–67.
Abrahamson, D. R., St John, P. L., Pillion, D. J., & Tucker, D. C. (1991). Glomerular development in intraocular and intrarenal grafts of fetal kidneys. Laboratory Investigation, 64(5), 629–639.
Woolf, A. S., Palmer, S. J., Snow, M. L., & Fine, L. G. (1990). Creation of a functioning chimeric mammalian kidney. Kidney International, 38(5), 991–997.
Rosines, E., Johkura, K., Zhang, X., Schmidt, H. J., De Cambre, M., Bush, K. T., & Nigam, S. K. (2010). Constructing kidney-like tissues from cells based on programs for organ development: Toward a method of in vitro tissue engineering of the kidney. Tissue Engineering Part A, 16, 2441–2455.
Imberti, B., Corna, D., Rizzo, P., et al. (2015). Renal primordia activate kidney regenerative events in a rat model of progressive renal disease. PLoS ONE, 10(3), e0120235.
Nakayama, K. H., Batchelder, C. A., Lee, C. I., & Tarantal, A. F. (2010). Decellularized rhesus monkey kidney as a three-dimensional scaffold for renal tissue engineering. Tissue Engineering. Part A, 16(7), 2207–2216.
Sullivan, D. C., Mirmalek-sani, S. H., Deegan, D. B., et al. (2012). Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials, 33(31), 7756–7764.
Orlando, G., Farney, A. C., Iskandar, S. S., et al. (2012). Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Annals of Surgery, 256(2), 363–370.
Orlando, G., Booth, C., Wang, Z., et al. (2013). Discarded human kidneys as a source of ECM scaffold for kidney regeneration technologies. Biomaterials, 34, 5915–5925.
Peloso, A., Petrosyan, A., Da Sacco, S., et al. (2015). Renal extracellular matrix scaffolds from discarded kidneys maintain Glomerular Morphometry and vascular resilience and retains critical growth factors. Transplantation, 99(9), 1807–1816.
Petrosyan, A., Orlando, G., Peloso, A., Wang, Z., Farney, A. C., Rogers, G., Katari, R., Da Sacco, S., Sedrakyan, S., De Filippo, R. E., Stratta, R. J., Soker, S., & Perin, L. (2015). Understanding the bioactivity of stem cells seeded on extracellular matrix scaffolds produced from discarded human kidneys: A critical step towards a new generation bio-artificial kidney. CellR4., 3(1), e1401.
Song, J. J., Guyette, J. P., Gilpin, S. E., Gonzalez, G., Vacanti, J. P., & Ott, H. C. (2013). Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nature Medicine, 19(5), 646–651.
D’Haese, J. G., Ceyhan, G. O., Demir, I. E., Layer, P., Uhl, W., Löhr, M., Rychlik, R., Pirilis, K., Zöllner, Y., Gradl, B., et al. (2014). Pancreatic enzyme replacement therapy in patients with exocrine pancreatic insufficiency due to chronic pancreatitis: A 1-year disease management study on symptom control and quality of life. Pancreas, 43, 834–841.
Tao, Z., Shi, A., & Zhao, J. (2015). Epidemiological perspectives of diabetes. Cell Biochemistry and Biophysics, 1–5.
Orlando, G., Stratta, R. J., & Light, J. (2011). Pancreas transplantation for type 2 diabetes mellitus. Current Opinion in Organ Transplantation, 16(1), 110–115.
Bottino, R., & Trucco, M. (2015). Clinical implementation of islet transplantation: A current assessment. Pediatric Diabetes, 16(6), 393–401.
Bellin, M. D., Kandaswamy, R., Parkey, J., Zhang, H.-J., Liu, B., Ihm, S. H., Ansite, J. D., Witson, J., Bansal-Pakala, P., Balamurugan, A. N., et al. (2008). Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. American Journal of Transplantation, 8, 2463–2470.
Ricordi, C., Lacy, P. E., Finke, E. H., Olack, B. J., & Scharp, D. W. (1988). Automated method for isolation of human pancreatic islets. Diabetes, 37, 413–420.
Bellin, M. D., Barton, F. B., Heitman, A., Harmon, J. V., Kandaswamy, R., Balamurugan, A. N., Sutherland, D. E. R., Alejandro, R., & Hering, B. J. (2012). Potent induction immunotherapy promotes long-term insulin independence after islet transplantation in type 1 diabetes. American Journal of Transplantation, 12, 1576–1583.
Dor, Y., Brown, J., Martinez, O. I., & Melton, D. A. (2004). Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature, 429, 41–46.
Xu, X., D’Hoker, J., Stangé, G., Bonné, S., De Leu, N., Xiao, X., Van De Casteele, M., Mellitzer, G., Ling, Z., Pipeleers, D., et al. (2008). β cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell, 132, 197–207.
D’Amour, K. A., Agulnick, A. D., Eliazer, S., Kelly, O. G., Kroon, E., & Baetge, E. E. (2005). Efficient differentiation of human embryonic stem cells to definitive endoderm. Nature Biotechnology, 23, 1534–1541.
D’Amour, K. A., Bang, A. G., Eliazer, S., Kelly, O. G., Agulnick, A. D., Smart, N. G., Moorman, M. A., Kroon, E., Carpenter, M. K., & Baetge, E. E. (2006). Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nature Biotechnology, 24, 1392–1401.
Alipio, Z., Liao, W., Roemer, E. J., et al. (2010). Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proceedings of the National Academy of Sciences of the United States of America, 107(30), 13426–13431.
De Carlo, E., Baiguera, S., Conconi, M. T., Vigolo, S., Grandi, C., Lora, S., Martini, C., Maffei, P., Tamagno, G., Vettor, R., et al. (2010). Pancreatic acellular matrix supports islet survival and function in a synthetic tubular device: In vitro and in vivo studies. International Journal of Molecular Medicine, 25, 195–202.
Mirmalek-Sani, S. H., Orlando, G., McQuilling, J., Pareta, R., Mack, D., Salvatori, M., Farney, A. C., Stratta, R. J., Atala, A., Opara, E. C., & Soker, S. (2013). Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials, 34(22), 5488–5495.
Peloso, A., Urbani, L., Katari, R., Maghsoudlou, P., Alvarez Fallas, M. E., Sordi, V., Citro, A., Niu, G., Zambon, J. P., Farney, A. C., Iskandar, S. S., Rogers, J., Stratta, R. J., Opara, E. C., Piemonti, L., Furdui, C. M., Soker, S., De Coppi, P., & Orlando, G. (2016). The human pancreas as a source of ECM scaffold for a new generation bio-artificial endocrine pancreas. Annals of Surgery, 264(1), 169–179.
Goh, S. K., Bertera, S., Olsen, P., Candiello, J., Halfter, W., Uechi, G., Balasubramani, M., Johnson, S., Sicari, B., Kollar, E., Badylak, S. T., & Banerjee, I. (2013). Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials, 34(28), 6760–6772.
De Carlo, E., Baiguera, S., Conconi, M. T., et al. (2010). Pancreatic acellular matrix supports islet survival and function in a synthetic tubular device: In vitro and in vivo studies. International Journal of Molecular Medicine, 25(2), 195–202.
Murray, C. J., & Lopez, A. D. (2013). Measuring the global burden of disease. The New England Journal of Medicine, 369(5), 448–457.
Dutkowski, P., Oberkofler, C. E., Béchir, M., et al. (2011). The model for end-stage liver disease allocation system for liver transplantation saves lives, but increases morbidity and cost: A prospective outcome analysis. Liver Transplantation, 17(6), 674–684.
Fox, I. J., Chowdhury, J. R., Kaufman, S. S., et al. (1998). Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. The New England Journal of Medicine, 338(20), 1422–1426.
Dhawan, A., Mitry, R. R., Hughes, R. D., et al. (2004). Hepatocyte transplantation for inherited factor VII deficiency. Transplantation, 78(12), 1812–1814.
Struecker, B., Raschzok, N., & Sauer, I. M. (2014). Liver support strategies: Cutting-edge technologies. Nature Reviews. Gastroenterology & Hepatology, 11(3), 166–176.
Minuk, G. Y. (2003). Hepatic regeneration: If it ain’t broke, don’t fix it. Canadian Journal of Gastroenterology, 17, 418–424.
Kay, M. A., & Fausto, N. (1997). Liver regeneration: Prospects for therapy based on new technologies. Molecular Medicine Today, 3, 108–115.
Meng, F., Francis, H., Glaser, S., et al. (2012). Role of stem cell factor and granulocyte colony-stimulating factor in remodeling during liver regeneration. Hepatology, 55(1), 209–221.
Cardinale, V., Wang, Y., Carpino, G., et al. (2011). Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology, 54(6), 2159–2172.
Petersen, B. E., Bowen, W. C., Patrene, K. D., et al. (1999). Bone marrow as a potential source of hepatic oval cells. Science, 284(5417), 1168–1170.
Alison, M. R., Poulsom, R., Jeffery, R., et al. (2000). Hepatocytes from non-hepatic adult stem cells. Nature, 406(6793), 257.
Schmelzer, E., Wauthier, E., & Reid, L. M. (2006). The phenotypes of pluripotent human hepatic progenitors. Stem Cells, 24, 1852–1858.
Sugimoto, S., Harada, K., Shiotani, T., Ikeda, S., Katsura, N., Ikai, I., Mizuguchi, T., Hirata, K., Yamaoka, Y., & Mitaka, T. (2005). Hepatic organoid formation in collagen sponge of cells isolated from human liver tissues. Tissue Engineering, 11, 626–633.
Zhang, L., Theise, N., Chua, M., & Reid, L. M. (2008). The stem cell niche of human livers: Symmetry between development and regeneration. Hepatology, 48, 1598–1607.
Terai, S., Takami, T., Yamamoto, N., et al. (2014). Status and prospects of liver cirrhosis treatment by using bone marrow-derived cells and mesenchymal cells. Tissue Engineering. Part B, Reviews, 20(3), 206–210.
Pan, X. N., Zheng, L. Q., & Lai, X. H. (2014). Bone marrow-derived mesenchymal stem cell therapy for decompensated liver cirrhosis: A meta-analysis. World Journal of Gastroenterology, 20(38), 14051–14057.
Liu, F., Pan, X., Chen, G., et al. (2006). Hematopoietic stem cells mobilized by granulocyte colony-stimulating factor partly contribute to liver graft regeneration after partial orthotopic liver transplantation. Liver Transplantation, 12(7), 1129–1137.
Duan, X. Z., Liu, F. F., Tong, J. J., et al. (2013). Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World Journal of Gastroenterology, 19(7), 1104–1110.
Tsolaki, E., Athanasiou, E., Gounari, E., et al. (2014). Hematopoietic stem cells and liver regeneration: Differentially acting hematopoietic stem cell mobilization agents reverse induced chronic liver injury. Blood Cells, Molecules & Diseases, 53(3), 124–132.
Piscaglia, A. C., Shupe, T. D., SH, O., Gasbarrini, A., & Petersen, B. E. (2007). Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology, 133(2), 619–631.
Soto-Gutierrez, A., Zhang, L., Medberry, C., Fukumitsu, K., Faulk, D., Jiang, H., Reing, J., Gramignoli, R., Komori, J., Ross, M., et al. (2011). A whole-organ regenerative medicine approach for liver replacement. Tissue Engineering Part C: Methods, 17, 677–686.
Shupe, T., Williams, M., Willenberg, B., & Petersen, B. (2010). Method for the decellularization of intact rat liver. Organogenesis, 6, 134–136.
Uygun, B. E., Soto-Gutierrez, A., Yagi, H., Izamis, M. L., Guzzardi, M. A., Shulman, C., Milwid, J., Kobayashi, N., Tilles, A., Berthiaume, F., Hertl, M., Nahmias, Y., Yarmush, M. L., & Uygun, K. (2010). Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nature Medicine, 16, 814–820.
Yagi, H., Fukumitsu, K., Fukuda, K., et al. (2013). Human-scale whole-organ bioengineering for liver transplantation: A regenerative medicine approach. Cell Transplantation, 22(2), 231–242.
Baptista, P. M., Siddiqui, M. M., Lozier, G., Rodriguez, S. R., Atala, A., & Soker, S. (2011). The use of whole organ decellularization for the generation of a vascularized liver organoid. Hepatology, 53(2), 604–617.
Wang, Y., Cui, C. B., Yamauchi, M., Miguez, P., Roach, M., Malavarca, R., Costello, M. J., Cardinale, V., Wauthier, E., Barbier, C., Gerber, D. A., Alvaro, D., & Reid, L. M. (2011). Lineage restriction of human hepatic stem cells to mature fates is made efficient by tissue-specific biomatrix scaffolds. Hepatology, 53, 293–305.
Barakat, O., Abbasi, S., Rodriguez, G., et al. (2012). Use of decellularized porcine liver for engineering humanized liver organ. The Journal of Surgical Research, 173(1), e11–e25.
Mazza, G., Rombouts, K., Rennie Hall, A., et al. (2015). Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Scientific Reports, 5, 13079.
Sun, Y., & Weber, K. T. (2000). Infarct scar: A dynamic tissue. Cardiovascular Research, 46(2), 250–256.
Eschenhagen, T., Wakatsuki, T., & Elson, E. L. (1995, August 19–22). A new method to measure isometric force of contraction in embryonic cardiac myocytes. Report No. 95-17 on the second international conference on Cellular Engineering, La Jolla (Abstract).
Kehat, I., Kenyagin-karsenti, D., Snir, M., et al. (2001). Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. The Journal of Clinical Investigation, 108(3), 407–414.
Fazel, S., Tang, G. H., Angoulvant, D., et al. (2005). Current status of cellular therapy for ischemic heart disease. The Annals of Thoracic Surgery, 79(6), S2238–S2247.
Murry, C. E., Field, L. J., & Menasché, P. (2005). Cell-based cardiac repair: Reflections at the 10-year point. Circulation, 112(20), 3174–3183.
Qian, H., Yang, Y., Huang, J., et al. (2007). Intracoronary delivery of autologous bone marrow mononuclear cells radiolabeled by 18F-fluoro-deoxy-glucose: Tissue distribution and impact on post-infarct swine hearts. Journal of Cellular Biochemistry, 102(1), 64–74.
Hirt, M. N., Hansen, A., & Eschenhagen, T. (2014). Cardiac tissue engineering: State of the art. Circulation Research, 114(2), 354–367.
Zimmermann, W. H., Schneiderbanger, K., Schubert, P., et al. (2002). Tissue engineering of a differentiated cardiac muscle construct. Circulation Research, 90(2), 223–230.
Leontyev, S., Schlegel, F., Spath, C., et al. (2013). Transplantation of engineered heart tissue as a biological cardiac assist device for treatment of dilated cardiomyopathy. European Journal of Heart Failure, 15(1), 23–35.
Ott, H. C., Matthiesen, T. S., Goh, S. K., et al. (2008). Perfusion-decellularized matrix: Using nature's platform to engineer a bioartificial heart. Nature Medicine, 14(2), 213–221.
Christman, K. L., Vardanian, A. J., Fang, Q., Sievers, R. E., Fok, H. H., & Lee, R. J. (2004). Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. Journal of the American College of Cardiology, 44(3), 654–660.
Habib, M., Shapira-schweitzer, K., Caspi, O., et al. (2011). A combined cell therapy and in-situ tissue-engineering approach for myocardial repair. Biomaterials, 32(30), 7514–7523.
Organ Procurement and Transplantation Network. http://optn.transplant.hrsa.gov. Accessed April 2016.
Baiguera, S., Gonfiotti, A., Jaus, M., et al. (2011). Development of bioengineered human larynx. Biomaterials, 32(19), 4433–4442.
Petersen, T. H., Calle, E. A., Zhao, L., et al. (2010). Tissue-engineered lungs for in vivo implantation. Science, 329(5991), 538–541.
Grillo, H. C. (2002). Tracheal replacement: A critical review. The Annals of Thoracic Surgery, 73(6), 1995–2004.
Novosel, E. C., Kleinhans, C., & Kluger, P. J. (2011). Vascularization is the key challenge in tissue engineering. Advanced Drug Delivery Reviews, 63(4–5), 300–311.
Berg, M., Ejnell, H., Kovács, A., et al. (2014). Replacement of a tracheal stenosis with a tissue-engineered human trachea using autologous stem cells: A case report. Tissue Engineering. Part A, 20(1–2), 389–397.
Wong, A. P., Bear, C. E., Chin, S., et al. (2012). Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nature Biotechnology, 30(9), 876–882.
Seguin, A., Baccari, S., Holder-espinasse, M., et al. (2013). Tracheal regeneration: Evidence of bone marrow mesenchymal stem cell involvement. The Journal of Thoracic and Cardiovascular Surgery, 145(5), 1297–1304.e2.
Mcintyre, B. A., Alev, C., Mechael, R., et al. (2014). Expansive generation of functional airway epithelium from human embryonic stem cells. Stem Cells Translational Medicine, 3(1), 7–17.
Li, Y., Xu, W., Yan, J., et al. (2014). Differentiation of human amniotic fluid-derived mesenchymal stem cells into type II alveolar epithelial cells in vitro. International Journal of Molecular Medicine, 33(6), 1507–1513.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Katari, R., Edgar, L., Enck, K., Peloso, A., Tamburrini, R., Orlando, G. (2017). Tissue Bioengineering in Transplantation. In: Nadig, S., Wertheim, J. (eds) Technological Advances in Organ Transplantation. Springer, Cham. https://doi.org/10.1007/978-3-319-62142-5_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-62142-5_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-62141-8
Online ISBN: 978-3-319-62142-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)