Abstract
Geometric and dosimetric accuracy are two important key factors in treatment simulation and planning for advanced radiation therapy. Medical imaging, with its ability to visualize and quantify patient anatomy, plays a critical role to ensure geometric and dosimetric accuracy for radiation therapy. Among all the image modalities, Computer Tomography (CT) and Magnetic Resonance Imaging (MRI) are the most commonly used ones for patient data acquisition for treatment simulation and planning. Their clinical roles and methods by which these modalities can be used to guide treatment planning and delivery are discussed in this chapter. This Chapter also provides an overview of the guidance and issues related to effective implementation of CT and MRI in routine clinical procedures in radiation therapy.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
10.1 Introduction
Cancer is the second most common cause of death in the USA, exceeded only by heart disease, accounting for nearly one of every four deaths (American Cancer Society 2015). Radiation therapy which uses high dose of radiation to kill or slow the growth of cancer cells is one of the common treatments for cancer. Over 50% of cancer patients in the USA receive radiation therapy alone or combined with other treatment methods. Computer tomography (CT) and magnetic resonance imaging (MRI) are two major medical imaging modalities widely used to assist in accurate treatment planning and delivery of radiation treatment. The basic mathematical concepts, principles, and physics of these imaging technologies are elaborated in Chaps. 8 and 9. In this chapter, their clinical roles and methods by which these modalities can be used to improve the treatment accuracy will be discussed. This chapter will also provide an overview of the guidance and issues related to effective implementation of CT and MRI in routine clinical procedures in radiation therapy.
Before an in-depth discussion of the roles of CT and MRI in radiotherapy, it is necessary to understand the basic workflow in modern radiation therapy which is illustrated in Fig. 10.1 and explained as follows. After informed consent by radiation oncologist physician and a decision to receive radiation therapy is made, the patient will undergo treatment simulation procedure during which the patient’s body is carefully positioned so that the potential benefits of radiotherapy can be realized. The positioning is extremely important because treatment planning and treatment delivery which usually spans multiple days and weeks are all based on this position. Therefore the position needs to be designed so that it can be easily reproduced during multiple treatment fractions, and potential patient movement during the treatment is minimized. This is usually facilitated with the assistance of proper fixation and immobilization devices. Under this carefully designed position, patient’s anatomy and physiology information is acquired through volumetric imaging during the simulation procedure. Following treatment simulation, radiation oncologist delineates the treatment target on the acquired simulation images. Radiation not only kills or slows the growth of tumor cells; it can also damage the nearby healthy tissues. Therefore normal tissues and organs that might be potentially irradiated during the treatment should also be contoured on these images. A treatment plan is designed with the purpose to deliver adequate radiation dose to the target while minimizing the radiation dose to the surrounding organs at risk (OAR) based on treatment prescription made by physician. After plan evaluation and acceptance process, the treatment planning is ready for delivery once it passed appropriate quality assurance procedures. Right before treatment delivery, the patient body is carefully positioned to reproduce the same position as planned in simulation and planning phase. This is usually verified by online imaging of the patient position which is compared with initial simulation images. Position deviation detected from the image guidance will be corrected before the start of treatment delivery. Patient position may also be monitored during the treatment delivery to minimize patient motion during treatment. The treatment verification and delivery process will be repeated for multiple fractions until the entire prescribed dose is delivered. An in-depth description of the clinical workflow of radiation therapy can be found elsewhere (Khan 2007, 2010). To summarize, the key to a favorable outcome of radiation therapy includes careful positioning which can be easily and accurately reproduced during daily treatment, meticulous treatment planning, and careful implementation and delivery of the treatment plan during each treatment fraction to maximize the benefits of radiotherapy.
Medical imaging is inevitably involved in modern radiotherapy procedures. Its roles can be categorized in two main aspects: (1) acquisition of patient anatomic and physiological information for treatment planning and (2) image-guided patient setup, verification, and motion management before and during treatment delivery. The latter is usually referred as image-guided radiation therapy (IGRT) which is addressed in Chap. 5. In this chapter, the roles of CT and MRI for acquisition of patient data for treatment planning will be elaborated in detail with a brief discussion of image-guided patient setup and treatment verification.
10.2 CT based Treatment Simulation and Planning
The goal of radiation therapy is to deliver adequate dose of radiation to a predefined target area to eradicate or control the growth of tumor. This is usually realized by delivery of radiation through a medical linear accelerator (Linac). An excellent review of the mechanisms and functions of medical linear accelerator can be found elsewhere (Karzmark et al. 1993). The treatment beam generated by the medical linear accelerator consists of high-energy photons or electrons which can penetrate tissues and deposit a desired radiation dose to the tumor inside patient body. However, ionizing radiation can also damage normal tissues when it passes through patient body and cause acute or long-term side effects. The therapeutic efficacy of radiation treatment highly depends on the dose delivered to the tumor. Inadequate dose to the tumor volume may lead to treatment failure. On the other hand, excessive radiation dose will increase the probability of normal tissue complications. Modern radiation therapy techniques such as three-dimensional (3D) conformal and intensity-modulated radiation therapy (IMRT) are capable to deliver high conformal dose distribution to target while sparing the normal tissues by optimized beam angles, conformal beam aperture, and dose intensity modulation inside the treatment field (Khan 2007). Successful implementation of these advanced treatment techniques requires accurate knowledge of the location, shape, and extent of the treatment volume and normal tissues, as well as the relative geometric relationship between treatment volume and adjacent normal organs. It also requires sophisticated dose calculation algorithm for which tissue properties such as electron density and anatomic composition of tissues are essential. In summary, geometric and dosimetric accuracy are two important key factors in treatment simulation and planning for advanced radiation therapy. Medical imaging, with its ability to visualize and quantify patient anatomy, plays a critical role to ensure geometric and dosimetric accuracy for radiation therapy. Among all the image modalities, CT and MRI are the most commonly used ones for patient data acquisition for treatment simulation and planning.
10.2.1 CT Simulator
CT has a few unique features that make it a powerful tool for patient data acquisition. Most commercial CT scanners have submillimeter spatial resolution with high geometrical and spatial integrity which is ideal for accurate localization and delineation of anatomy. Volumetric imaging of patient anatomy can be obtained in a fairly short period of time using multi-slice detectors with helical scanning mode. In addition, relative linear attenuation coefficient, a function of tissue electron density and anatomic composition, can be accurately quantified by CT which is essential for accurate dose calculation.
In modern radiotherapy treatment, simulation is usually performed with a special system called CT simulator. CT simulator is very similar to conventional CT scanner used for diagnostic purpose with a few unique components (Mutic et al. 2003).
-
1.
Patient support table
In diagnostic CT scanner, the patient support couch usually has curved surface for patient’s comfort. A couch with flat top surface similar to the one used in treatment machine is implemented in CT simulator, with the desire to reduce potential position variation between simulation and treatment. This also enables patient immobilization devices to be easily fixed to the couch top through registration notches in a manner that can be reproduced on treatment machine couch.
-
2.
Large gantry bore
In treatment simulation patients are commonly set up in special positions, for example, elbows widely extended. In order to accommodate all possible treatment positions with a variety of immobilization devices, gantry opening of CT simulator is usually larger than those of diagnostic scanners. The bore size of commercial CT simulator ranges from 70 cm to 85 cm. This not only creates adequate spatial clearance for patient positioning with immobilization devices but also provides increased image field of view (FOV) allowing fully acquisition of patient external dimensions which is necessary for accurate dose calculation.
-
3.
Simulation software
In additional to image acquisition and reconstruction software, special software for treatment simulation is also incorporated in CT simulator. There are several key functions that most simulation software provides. Target definition and normal tissue contouring can be performed by using simulation software. Treatment isocenter or setup reference point can be defined by either manual placement on the acquired images or automatically position at the centroid of selected contour. The purpose of defining a reference point during simulation is to establish the origin of the treatment coordinate system in which the geometric relationship between beam isocenter, treatment target, OAR, etc. can be defined and precisely reproduced during treatment setup. Some simulation software enables placement and design of treatment beams, and secondary images such as digital reconstructed radiograph (DRR) can also be generated. In other words, advanced simulation software has the capability to perform like a treatment planning system except for the dose calculation-related functions.
-
4.
Laser positioning system
The defined treatment isocenter or reference point usually resides inside patient body and needs to be mapped and marked on the external surface of the patient so that it can be accurately visualized and localized to guide patient setup during treatment session. Most CT simulators are equipped with laser positioning system which can be used to facilitate this process. The laser system usually consists of horizontal, vertical, and sagittal lasers that interact at a virtual isocenter whose position in relative to the image isocenter is known. Once the isocenter or reference point is selected on the acquired images, the coordinates of this point will be transferred to laser system; skin markers can be placed with the assistance of lasers while patient is still on the couch.
10.2.2 CT Imaging for Treatment Planning
As noted earlier, the patient data acquired during CT simulation procedure are used in two major processes in radiation treatment planning:
-
1.
Target and normal tissue delineation
Recommendations for target delineation and definition, dose prescription, and reporting are discussed in depth in International Commission on Radiation Units and Measurement (ICRU) Report No. 62 (International Commission on Radiation Units and Measurements 1999). In general, the high spatial resolution and geometric integrity of CT are ideal for delineating treatment target and organs at risk. Figure 10.2a shows a lung tumor delineated on CT images in which the tumor and most organs are clearly visible. However, sometimes, it is difficult to fully visualize the tumor and surrounding organs due to insufficient soft tissue contrast of CT. A liver cancer tumor is shown in Fig. 10.2b which can be barely differentiated from the surrounding normal liver tissues. To help delineate the target, additional anatomic and/or functional information from other imaging modalities such as magnetic resonance imaging (MRI) and positron emission tomography (PET) are usually incorporated. For instance, functional information obtained from PET imaging is very useful to assist target delineation for the liver case as shown in Fig. 10.2b.
Fig. 10.2 (a) Axial and sagittal CT images showing a lung tumor delineated by red contour and major organs at risk outlined. (b) Left: a liver lesion outlined by the blue contour which can be barely differentiated from the surrounding normal liver tissues due to the low soft tissue contrast of CT image. Right: the liver lesion can be better delineated on PET image
-
2.
Dose optimization and calculation
Most modern treatment planning software requires tissue electron density information for accurate dose calculation. A unique feature of CT is that it directly measures the linear attenuation property of tissues which is a function of tissue electron density and anatomic composition. The measured linear attenuation coefficient is usually presented as CT Hounsfield (HU) number which is a linear transformation of the linear attenuation coefficient by the following equation:
$$ \mathrm{CT}\ \mathrm{Number}\left(\mathrm{HU}\right)=\frac{\mu_{\mathrm{tissue}}-{\mu}_{\mathrm{water}}}{\mu_{\mathrm{water}}}\times 1000 $$(10.1)where μtissue and μwater are the linear attenuation coefficients of the tissue of interest and water, respectively.
The conversion from linear attenuation to electron density can be simply established by calibration using a special phantom which includes multiple inserts made of various tissue materials with known electron and physical densities. The HU number of each insert can be directly measured from the acquired CT images of the phantom. A calibration curve can be established based on the measured CT numbers and known electron densities as demonstrated in Fig. 10.3. This calibration curve which is typically scanner dependent can be incorporated in the treatment planning software and used to convert volumetric CT images into electron density information for dose calculation.
-
3.
Generation of reference image dataset for patient treatment setup
An important product of CT simulation and treatment planning process is the generation of reference images for patient treatment setup. Prior to radiation treatment, multiple radiograph images are usually acquired by the image detector and X-ray tube mounted on the treatment machine or by the megavoltage beam directly irradiated from the treatment head. These online images can be used to ensure that the relative positioning of the patient and the treatment machine agrees with the simulation position used for treatment planning. Digitally reconstructed radiograph (DRR) can be calculated from the simulation CT dataset based on treatment geometry and used as reference images for online image-guided verification and correction of patient position. The quality of DRR can affect the physician’s ability to verify and adjust patient position. As the direct input of the DRR generation, the quality of the CT images has a strong impact on the quality of DRR. For instance, the spatial resolution of DRR is affected by the slice thickness and spacing of the CT dataset. Two DRRs generated from CT images of different slice thickness are compared in Fig. 10.4 where the DRR calculated from CT with thinner slice thickness allows better visualization of anatomical details. Image artifacts in the CT dataset can translate into DRR and degrade the quality and integrity of DRR. In addition to secondary images as DRR, the simulation CT dataset itself can be directly used as reference for cone-beam CT (CBCT)-based patient setup. Similarly, the geometric accuracy and image contrast of the simulation CT can directly impact the patient position verification through online volumetric imaging. Details of the onboard image-guided patient setup will be discussed in depth in Chap. 9.
To achieve acceptable geometric and dosimetry accuracy for treatment planning, the technical factors for CT acquisition need to be carefully considered and selected. First, the image field of view (FOV) should be large enough to outline the patient body with CT couch top in the axial plane. Most CT simulators have large bore size, thus extended image FOV allowing patient scanned with immobilization devices in treatment position. The scan range in the longitudinal direction should include target, organs at risk, and enough tissues allowing accurate calculation of dose scattering. Secondly, technical factors such as tube voltage, tube current, collimation, and filtration are important parameters affecting image quality and patient dose. Details of optimization of imaging acquisition parameters for best image quality are discussed in Chap. 8. Finally image artifacts can significantly impair tissue delineation and dose calculation. For example, artifacts caused by metal objects such as dental implant, surgical clips, and prosthesis are commonly seen on CT image as shown in Fig. 10.5. The streaks and dark bands across the image not only hinder visualization and delineation of anatomical structures but also corrupt the integrity of CT numbers and electron density, resulting in errors in dose calculation. Advanced correction algorithms have been developed to overcome the issues associated with metal artifacts and shown promising results (Axente et al. 2015).
10.2.3 Four-Dimensional CT (4DCT)
Thoracic and upper abdominal cancer remains a primary challenge in radiation therapy because of respiratory-induced motion of tumor and organs. Regular CT scan suffers from geometrical distortion and artifacts caused by the respiratory-induced motion which can hinder the ability to visualize anatomical details and reduce the accuracy in delineation of target and organs at risk. Four-dimensional CT (4DCT) has become an important tool in evaluation and assessment of respiratory motion to reduce the uncertainties associated with motion in radiation therapy. 4DCT, as its name suggests, provides temporal information of patient anatomy in addition to the 3D volumetric data acquired by regular CT. The basic principle of 4DCT is that image data are over-sampled over multiple respiration cycles at every position of interest along the patient’s longitudinal axis. Each image or projection is tagged with respiratory signal obtained from an external measuring device. The image or projection data are then retrospectively sorted based on the corresponding respiratory signal resulting in multiple 3D CT datasets. Each of these 3D CT datasets represents the patient anatomy at a particular respiratory phase. Overall these 3D CT data constitute a 4DCT dataset over the entire respiration cycle. The respiratory signal can be obtained by optical tracking of surrogate placed on patient thorax or monitoring of abdominal expansion and contraction by an elastic belt. The retrospective sorting can be done for images or projections based on either amplitude or phase of the tagged respiratory signals.
A basic assumption of 4DCT is that patient’s respiration is regular and reproducible over time. Therefore image data acquired from different respiration cycles can be combined into one dataset associated with a particular phase or amplitude. However, it is very common that patient has irregular respiration, and the datasets acquired from multiple breath cycles are inconsistent, which can lead to significant image artifact or distortion on the 4DCT images. Secondly, the respiratory signal is usually measured from external surrogate that may not correlate well with the internal tumor motion. This out-of-sync issue can cause substantial deviation and errors in treatment planning and delivery. Finally, large radiation ionizing dose is usually associated with 4DCT acquisition because of its over-sampling nature. The imaging protocol needs to be carefully reviewed and optimized to prevent excessive radiation dose to patient. In summary, 4DCT provides very useful information for tumor motion management for treatment planning and delivery. However, careful review of the image and comprehensive quality assurance of the process are critical in clinical implementation of 4DCT for radiation therapy.
10.2.4 Quality Assurance (QA) of CT Simulator
To ensure geometric and dosimetric accuracy, rigorous quality assurance (QA) program needs to be developed and implemented for CT simulator, simulation software, and the simulation process. Recommendations and guidance for development of a comprehensive QA program can be found in the task group report No. 66 of the American Association of Physicists in Medicine (AAPM) (Mutic et al. 2003). In general there are three major aspects to be considered for the CT simulator:
-
1.
Safety and radiation dosimetry
Radiation exposure from the CT scanner poses health hazard to both patients and hospital staffs. Generally speaking, the radiation doses received by patients from the CT simulation process are much lower than the treatment doses that they will receive from radiation therapy; thus the radiation exposure of the CT simulator is not a major concern for patients. However, one should note that the treatment doses are usually well collimated to be conformal to a relatively small treatment area, while the radiation exposures imposed by CT scanner are much widely spread to the normal tissues within the scanning range. It is important to evaluate and monitor the dosimetry of the scanner during initial acceptance followed by periodic QA to prevent unnecessary radiation exposure to patients. Radiation exposure to hospital staff and public must be carefully controlled to be below the regulatory limits and based on the principle of ALARA (as low as reasonably achievable) (NCPR 1993). This is usually achieved by proper radiation shielding of the CT room. The details of radiation shielding design for CT-scanner rooms can be found in in the National Council on Radiation Protection and Measurement (NCPR) report No. 147 (NCPR 2004). Emergency safety equipment such as emergency-off switches are usually installed inside the CT room and at the control consoles to allow interruption of scanner operation under emergency situation. The functionality of these switches needs to be checked during acceptance testing and at regular basis.
-
2.
Performance of electromechanical components
CT scanner itself is a very sophisticated system integrated from multiple electromechanical components such as X-ray generator, collimation, detectors, gantry, patient support couch, and laser marking and positioning device. Specific quality assurance tests need to be performed periodically to evaluate the performance of these components. For example, incorrect calibration of the X-ray generator can lead to significant degradation of the image quality and/or unnecessary imaging dose to patient. Thus the beam properties such as the energy and intensity of photons generated from the X-ray generator need to be checked with the programmed settings on the control console during acceptance testing followed by annual test. For mechanical systems such as patient support couch and laser positioning device, the geometric and motion accuracy is critical and needs to be evaluated at regular basis. The QA equipment and test methods for each electromechanical component are elaborated in detail in AAPM task group No. 66 (Mutic et al. 2003).
-
3.
Image quality performance
Image quality is the ultimate evaluation of the entire system performance of CT simulator. Suboptimal image quality may impair the ability to identify and delineate tumor target and organs at risk for treatment planning, which can lead to significant errors such as inadequate radiation dose to tumors or overdose to normal tissues. Inferior image quality can also cause degradation of the secondary image such as DRR, resulting in uncertainties and errors in patient positioning during treatment. The image quality metrics to be evaluated are similar to those for diagnostic CT scanner, including spatial resolution and integrity, imaging noise and contrast resolution, image uniformity and accuracy of CT HU numbers, etc. The evaluation of these quality metrics is usually performed by using specific QA phantom as shown in Fig. 10.6. The phantom consists of multiple sections with each section designed for a specific QA test. For instance, images of the sections designed for spatial resolution, contrast resolution, and image uniformity tests are shown, respectively, in Fig. 10.6. In general, the test methods for image quality are very similar to those of diagnostic scanner which were outlined in detail in AAPM task group reports No. 39 and 66 (Mutic et al. 2003; Lin et al. 1993).
Fig. 10.6 In summary, comprehensive QA program needs to be developed and implemented for the hardware, software, and process associated with CT simulation for radiation therapy. Based on the impact to patient treatment and the likelihood of quality degradation of the tested parameter over time, the QA testing is usually distributed among daily, monthly, and annual tests. For example, the misalignment of lasers to the imaging isocenter can cause catastrophic mistake in patient treatment. As a result it is recommended to be checked during daily QA. The test methods and tolerance levels vary between different scanners and need to be carefully designed based on recommendations and guidelines established by premier scientific and professional organizations such as AAPM.
10.3 Magnetic Resonance Imaging (MRI) in Radiotherapy
Magnetic resonance imaging (MRI) is an imaging technique that measures magnetic resonance signals from tissues under strong magnetic field. The basic physics of MRI can be briefly described below (Khan 2010; Hendee and Ritenour 2002). Each hydrogen nucleus behaves as a tiny magnet with a magnetic moment. Under a strong external magnetic field, the nuclei align with the direction of the magnetic field and also precess about the field at a certain frequency called resonance frequency. When a second alternating magnetic field is applied by a pulse of electromagnetic radio-frequency (RF) signal, the nuclei absorb energy from the RF pulse and then precess around the new field in the transverse direction, a process usually referred as tissue excitation. When the RF is turned off, the nuclei start to release energy by two independent relaxation processes which happen simultaneously along different directions. The nuclei return back to their original status along the longitudinal direction of the external magnetic field by releasing the absorbed energy to the surrounding tissue which is known as T1 relaxation. On the other hand, the process of relaxation of nuclei in the traverse direction is often referred as T2 relaxation. The relaxation properties and the resonance frequency for nucleus heavily depend on the characteristics of tissues and its surrounding environment, such as the presence of chemical bonds, paramagnetic ions, and even the rate of flow of fluids (Hendee and Ritenour 2002). As a result the signal induced by the relaxation processes can be different from various tissues which constitute the image contrast. The physics and image formation, as well as pulse sequences of MRI, are discussed in detail in Chap. 9. This chapter focuses on the review of clinical applications of MRI in radiation therapy field.
10.3.1 MRI for Radiation Therapy Treatment Planning
There are a few unique features of MRI which make it an ideal image tool for treatment planning and delivery verification of radiation therapy. As discussed early, MRI signals are not only sensitive to the proton density but also the environment that the protons reside in. The highly versatile MR pulse sequences can be programmed to further exploit the difference in chemical compositions of different types of tissues to provide superior soft tissue contrast and tumor conspicuity. Consequently MRI has been widely used to assist target delineation in radiation therapy. CT and MRI images of a patient with a brain tumor are compared in Fig. 10.7. The tumor outlined by the red contour is indiscernible to the normal brain tissue on the CT image due to similar X-ray attenuation properties of tumors and normal brain tissue. On the other hand, the lesion is clearly highlighted on the T1-weighted MRI image. In addition, notable edema surrounding the tumor which is usually included as treatment target can be easily identified on the T2-weighted image.
While it has become a mainstay for target delineation, anatomic image typically does not provide physiological information, which can be important biomarker suggestive of early change in tumor, or provide information characterizing microenvironment surround tumor and identifying microscopic diseases which are not easy to be detected on anatomic image. Not only does MRI provide superior soft tissue contrast for anatomic imaging, but it also can be programmed to measure physiological and functional information of tissues. Functional MRI provides a powerful tool for the detection and characterization of tumors as well as for monitoring their response to therapy. For instance, dynamic contrast-enhanced MRI (DCE-MRI) (Matsuo et al. 2014) can be used to estimate blood flow, permeability, and blood volume which are important biomarkers for tumor angiogenesis, a physiological process through which new blood vessels develop to support tumor growth (Birbrair et al. 2014). The ability of a tumor to initiate angiogenesis and divert blood flow to the tumor plays important role in tumor progression and metastasis. Quantitative imaging of tumor vascular physiology both spatially and temporally not only allows accurate detection of tumor at early stage but also provides valuable assessment of treatment response.
Another advantage of the MRI is that it does not impose ionizing radiation to patient which is ideal for continuous imaging, for example, tumor and organ motion tracking. Using a similar method as 4DCT, 4DMRI can be generated by continuous acquisition throughout breathing cycles and retrospectively sorting of the images by its associated respiration phase. Without concern of excessive radiation dose to patient, the acquisition of 4DMRI can be optimized to minimize image artifacts and distortion. In addition, the excellent soft tissue contrast of MRI makes it highly desirable for motion management for abdominal cancers which are hardly to be detected on 4DCT. Another area that MRI has significant potential is real-time dynamic tracking of tumor or organs during radiation treatment delivery.
Despite the tempting benefits discussed above, MRI still remains as auxiliary image modality for radiotherapy due to a few deficiencies described below. As a result, MRI images are usually co-registered with CT images and used as complement to CT in target delineation. However, additional uncertainties can be induced by image registration which can result in geometric errors up to a few millimeters as demonstrated in a multi-institutional benchmark study for cranial CT-MRI registration (Ulin et al. 2010). Extra cost and time associated with multimodality imaging also impose obstacle to obtain high-quality MRI suitable for treatment planning. For example, most MRI images used for radiation treatment planning are from diagnostic scans in which the patient is scanned in a position very different from the CT simulation. These diagnostic scans often have limited image FOV and large slice thickness and spacing, increasing the uncertainties and difficulties in image registration. Recently there has been a growing interest of developing cost-effective MRI-based treatment simulation and planning process. The following obstacles need to be overcome in order to fully exploit the advantages of MRI for radiation treatment planning:
-
1.
Image distortion
MRI image is prone to geometric distortion which impairs the accuracy in target delineation and dose calculation for radiation treatment planning. The image distortion is caused by both system-related and patient-specific factors such as field inhomogeneity, nonlinearity of gradient field, and chemical shifts induced by magnetic susceptibility variations (Walker et al. 2014). The image distortion can be significant even for simple geometry, such as the sphere phantom shown in Fig. 10.8a. The magnitude of distortion varies across the image field of view and usually increases toward the periphery of the FOV. A number of post-processing correction methods have been developed to mitigate the distortions (Karger et al. 2006; Doran et al. 2005; Reinsberg et al. 2005). However, residual distortions can still impose uncertainties for target delineation and dose calculation, and rigorous quality assurance needs to be implemented to ensure the accuracy of using MRI for radiation therapy. The spatial integrity of MRI images can be quantified by scanning of a geometric phantom as shown in Fig. 10.8b. The phantom is made from a grid of cylindrical landmarks whose geometric locations are preciously known. From the acquired image, the locations of these grids can be detected, and the deviation from its known locations can be used to quantify the spatial distortion as shown in Fig. 10.8b. With the improvement of MRI system hardware design and rigorous quality assurance of image performance, it is possible to maintain the spatial integrity within 1–2 mm across the imaging field of view (Walker et al. 2014).
Fig. 10.8 (a) MRI image of a sphere phantom showing significant geometric distortion. (b) Image of a spatial integrity phantom consisting of a grid of cylindrical landmarks. The location of each grid point is detected from the image and compared with its known position. Deviations greater than 2 mm are identified by the red crosses
-
2.
Lack of information for dose calculation
As noted early, most modern treatment planning software requires tissue electron density information to account for inhomogeneity in dose calculation which can be directly converted from HU numbers of CT images via a calibration curve or look-up table. The signals measured from MRI do not directly correlate with tissue electron density and thus cannot be used solely for dose calculation. One simple approach is to ignore the heterogeneous anatomy and consider the entire body as water in dose calculation. This simplification has minimal impact to dose calculation in the regions where tissue density is relatively homogenous such as the brain. The dose calculations based on MRI have been reported to be less than 2% for brain tumors compared with CT-based planning (Kristensen et al. 2008). However large dose calculation errors can occur in the region with a large amount of bone, lung tissues, and air cavity if everything is simplified as water in calculation. A second approach is to segment various tissues from MRI images and assign bulky density to these segmented structures. This method improves the accuracy of dose calculation in regions such as the lung but can be labor intensive and time consuming. Efforts have been made to develop atlas- or voxel-based automatic segmentation methods, and the optimal bulky density values of different tissues were also investigated in many studies. It has been shown that the dose calculation based on the bulk density assignment approach achieves reasonable accuracy for treatment planning for various disease sites (Jonsson et al. 2010).
-
3.
Lack of information for patient setup
Daily patient treatment setup heavily relies on the reference images such as DRR generated from treatment planning. Bony anatomy on the DRR is commonly used to guide patient position adjustment during treatment setup. However the low signal intensity of bones on MRI makes it difficult to generate reference images with bony anatomic features. This has become a limiting factor to implement MRI-based treatment simulation and planning. To overcome this issue, imaging sequences to depict bones on MRI have been investigated, and one of the promising methods is based on the ultrashort echo time (UTE) technique. The low signal intensity of the bone is resulted from the extremely short T2 relaxation time of cortical bones. To capture the short T2 signals from the bone, the UTE method acquires images at two different echo times, resulting in different T2 weighting. As the cortical bone has shorter transverse relaxation time (T2) compared with other tissues, subtraction of the two acquired images results in enhancement of bony anatomy. Excellent depiction of bony anatomy details can be observed on the UTE-MRI volumetric images which allow to generate high-quality DRRs solely based on MRI as demonstrated in Fig. 10.9 (Yang 2016).
Fig. 10.9 To facilitate MRI-based treatment simulation, dedicated MRI system – MR simulator – has been developed which incorporates similar components as CT simulator. In addition to the laser position system, flat tabletop, and enlarged bore size, the MR simulator is also equipped with software for distortion correction and special imaging sequences for bony anatomy and functional imaging as discussed earlier. Special considerations also need to be paid for the patient immobilization devices for MR simulation. The powerful magnetic field of MRI system may pose a safety risk to patient and staff because it can attract ferromagnetic objects and cause a sudden movement of these objects. In addition, the metal objects can also cause signal loss and distortion and generate artifacts on MRI images. Therefore the patient immobilization devices need to be carefully designed to minimize the metal components. In general, great care has to be taken to ensure the safety of patient, staff, and equipment during the entire MRI simulation process.
Similar to CT simulator, comprehensive quality assurance program needs to be established to ensure the performance of MR simulator and the entire clinical simulation workflow. The QA program should include acceptance tests prior to MR system clinical operation followed by periodic QA procedures to evaluate the system and image performance as well as MR safety. Details of the QA procedures for MRI systems can be found in the practical guidelines published by American Association of Physicists in Medicine (AAPM) (Jackson et al. 2010).
10.3.2 MRI-Guided Radiation Therapy (MRIgRT)
The advent of the integrated imaging system with radiotherapy machine provides online image guidance to minimize setup errors and refine target localization following the initial patient positioning. The ability to visualize patient anatomy by either 2D projection or 3D volumetric image allows comprehensive assessment of patient positioning uncertainties prior to every treatment delivery. Currently most clinical imaging guidance systems are based on X-ray technology, such as radiograph and onboard cone-beam CT (CBCT), which can clearly depict bony anatomy details, but suffer with limited ability to differentiate soft tissues. Patient positioning based on bony anatomy only is certainly not ideal if tumor targets cannot be directly visualized. In addition, the position of tumor and organs relative to the treatment beam may change during treatment due to physiological motions such as breathing, cardiac, and intestine movement as well as swallowing. Real-time tracking of tumor motion can be made by online X-ray fluoroscopic imaging; however, it also suffers poor soft tissue contrast and often requires implanted fiducial marker as surrogate. Radiation dose from continuous fluoroscopic imaging also poses a major concern and makes it less practical to be implemented in daily clinical practice.
With the superior soft tissue contrast and nonionizing properties of MRI, it is natural to strive for MRI-based patient setup and treatment delivery. In addition to the issues described in the last section, there are a few more challenges to overcome when integrating a MRI system with radiotherapy machine. First, electromagnetic interference between the two systems needs to be minimized. The radio-frequency (RF) signals generated from tissues for MR imaging are very weak, and any external RF noise can easily destroy the fidelity of the signal and corrupt the image. Modem medical linear accelerator (Linac) is a very complicated system consisting of sophisticated mechanical and electromagnetic components which constitute significant sources of RF noises. Even the motor driving the multileaf collimator can generate enough RF noise to totally corrupt the MRI image. On the other hand, the strong magnetic field of MRI can affect the performance of the medial linear accelerator significantly through Lorentz force. Under external magnetic field, the trajectory of a moving charged particle will deviate from its original direction and result in change in dose distribution. As a result, the dose point spread kernel becomes asymmetrical in homogenous medium, leading to reduced buildup and asymmetrical beam penumbra. The dose perturbation due to magnetic field is more severe at the tissue-air interface where significant dose increase occurs due to secondary electrons being forced back into the tissue by the Lorentz force (Raaijmakers et al. 2008). The dosimetric disturbance due to the magnetic field needs to be carefully considered when designing MRI-guided radiotherapy machine and accurately modeled in the treatment planning system.
In addition to the challenges related to basic physics, there are a few practical issues to be considered for clinical implementation of MRI-guided radiotherapy. Diagnostic MRI imaging usually takes about 10–20 min or longer, making it impractical to be incorporated into daily radiation treatment session which is usually less than 20 min. It is imperative to develop fast MRI image acquisition protocols for patient setup and positioning. Site planning is another area that needs special attenuation when integrating MRI with radiotherapy system. The RF shielding for MRI is necessary to prevent RF noise from corrupting the image signal. Retrofitting of existing treatment vault to accommodate RF shielding in additional to the radiation shielding can be challenging. One should also consider the potential interference of magnetic field on treatment machines, patient, and staff next to the MRI room which may require additional magnetic shielding. Most quality assurance equipment and devices are either incompatible or not safe to be used under strong magnetic field. Development of new QA equipment for MRI-guided radiotherapy is an important area which warrants additional resources and investment. Finally, physicians, physicists, and technicians in radiation oncology field may have relatively limited experiences with MRI. Staff training is a major component in clinical implementation of MRI-guided radiotherapy.
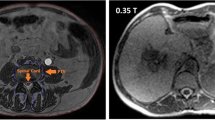
MRI-guided radiotherapy systems are in the very early stage with most systems still under development. Recently a Cobalt radiotherapy-based system – ViewRay – has become clinically available (Mutic and Dempsey 2014). Instead of integrating a linear accelerator with MRI, radioisotope Cobalt-60 is used as radiation source which effectively mitigates the interference issues between magnetic field and radiotherapy machine. Three Cobalt-60 sources are mounted on a rotating gantry 120 degrees apart, providing a dose rate around 600 cGy/min. Each source head is equipped with an individual multileaf collimator consisting of 30 pair of leafs for intensity-modulated radiation therapy (IMRT). The gantry is sandwiched by a split superconductor magnet with a bore size of 70 cm. A low-strength magnetic field of 0.35 Tesla is chosen aimed to reduce the image geometric distortion and minimize the Lorentz force effect. A 3D volumetric image with 1.5 mm isotropic resolution can be acquired within 3 min. As shown in Fig. 10.10, the soft tissue contrast on the MRI is much superior to the CBCT, which not only helps improve the accuracy of patient treatment setup, but it also facilitates better assessment of treatment response for timely adaptive therapy. In addition to static imaging, it also allows 2D real-time imaging on the sagittal plane with a rate of four frames per second. The dynamic imaging capability enables real-time tumor motion tracking for gated radiotherapy.
Superior soft tissue contrast was achieved on the online MRI setup images (top row) where tumor and normal organs such as the rectum and bladder can be easily delineated for accurate patient setup and adaptive planning. In contrast, the image quality was significantly inferior on the cone-beam CT (bottom row)
Reference
American Cancer Society (2015) Cancer Facts & Figures 2015. American Cancer Society, Atlanta
Axente M et al (2015) Clinical evaluation of the iterative metal artifact reduction algorithm for CT simulation in radiotherapy. Med Phys 42(3):1170–1183
Birbrair A et al (2014) Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol 307(1):C25–C38
Doran SJ et al (2005) A complete distortion correction for MR images: I. Gradient warp correction. Phys Med Biol 50(7):1343–1361
Hendee WR, Ritenour ER (2002) Medical imaging physics, 4th edn. Wiley-Liss. xix, New York, p 512
International Commission on Radiation Units and Measurements (1999) Prescribing, recording, and reporting photon beam therapy. ICRU report, Bethesda, Md.: International Commission on Radiation Units and Measurements. 52 p
Jackson EF et al (2010) Acceptance testing and quality assurance procedures for magnetic resonance imaging facilities: report of the AAPM MR subcommittee task group No. 100
Jonsson JH et al (2010) Treatment planning using MRI data: an analysis of the dose calculation accuracy for different treatment regions. Radiat Oncol 5:62
Karger CP et al (2006) Accuracy of device-specific 2D and 3D image distortion correction algorithms for magnetic resonance imaging of the head provided by a manufacturer. Phys Med Biol 51(12):N253–N261
Karzmark CJ, Nunan CS, Tanabe E (1993) Medical electron accelerators. McGraw-Hill, Inc., Health Professions Division. xiv, New York, p 316
Khan FM (2007) Treatment planning in radiation oncology, 2nd edn. Lippincott Williams & Wilkins, Philadelphia. xv, 527 p., 16 p. of plates
Khan FM (2010) The physics of radiation therapy, 4th edn. Lippincott Williams & Wilkins, Philadelphia. x, 531, 30
Kristensen BH et al (2008) Dosimetric and geometric evaluation of an open low-field magnetic resonance simulator for radiotherapy treatment planning of brain tumours. Radiother Oncol 87(1):100–109
Lin P-JP et al (1993) Specification and acceptance testing of computed tomography scanners: report of the AAPM diagnostic X-ray imaing committee task group No. 2
Matsuo M et al (2014) Magnetic resonance imaging of the tumor microenvironment in radiotherapy: perfusion, hypoxia, and metabolism. Semin Radiat Oncol 24(3):210–217
Mutic S, Dempsey JF (2014) The view ray system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol 24(3):196–199
Mutic S et al (2003) Quality assurance for computed-tomography simulators and the computed-tomography-simulation process: report of the AAPM radiation therapy committee task group no. 66. Med Phys 30(10):2762–2792
NCRP, (1993) Recommendations on limits for exposure to ionizing radiation: recommendations of the National Council on Radiation Protection and Measurements. NCRP report., Bethesda
NCPR, (2004) Structural shielding design for medical X-Ray imaging facilities. National Council on Radiation Protection and Measurement. NCRP Report 147., Bethesda
Raaijmakers AJ, Raaymakers BW, Lagendijk JJ (2008) Magnetic-field-induced dose effects in MR-guided radiotherapy systems: dependence on the magnetic field strength. Phys Med Biol 53(4):909–923
Reinsberg SA et al (2005) A complete distortion correction for MR images: II. Rectification of static-field inhomogeneities by similarity-based profile mapping. Phys Med Biol 50(11):2651–2661
Ulin K, Urie MM, Cherlow JM (2010) Results of a multi-institutional benchmark test for cranial CT/MR image registration. Int J Radiat Oncol Biol Phys 77(5):1584–1589
Walker A et al (2014) MRI distortion: considerations for MRI based radiotherapy treatment planning. Australas Phys Eng Sci Med 37(1):103–113
Yang Y et al (2016) Accuracy of UTE-MRI-based patient setup for brain cancer radiation therapy. Med Phys 43(1):262–267
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Cao, M. (2017). CT and MRI in Radiotherapy. In: Maqbool, M. (eds) An Introduction to Medical Physics. Biological and Medical Physics, Biomedical Engineering. Springer, Cham. https://doi.org/10.1007/978-3-319-61540-0_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-61540-0_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61538-7
Online ISBN: 978-3-319-61540-0
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)