Abstract
Insects are the largest group of invertebrates having unique modalities of communication among members of the same species. Conspecific communication among insect species occurs mainly through visual, tactile, chemical, and behavioral changes. A number of studies on different insect models have been conducted by several researchers to understand the molecular, neuronal, and behavioral mechanism underlying communication among conspecifics. Though huge volume of research has been done to understand the mechanistic details of insect communication, there are a number of answered questions which require special attention. Understanding mechanisms of communication among insects has a number of potential applications in devising appropriate and sustainable control and/or management of insect population in the crop field. Pheromones are being used to effectively manage insect population since long before. Genetic basis of odor detections and interpretation of different odorants by insect species that carry message for different purposes involves several signaling receptors including G-protein-coupled receptor (GPCR) and second messenger signaling. Neuronal firing pattern following exposure to a pheromonal compound explains partially the mechanism of conspecific message delivery conspecific. However, how limited number of odorant-binding proteins that detect large spectrum of odorant species and differentiate as a different signal is not yet understood.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Communication is the process of exchange of information between two or more individuals of same or different species in which one individual transmits the message and the other receives the message, processes it, and gives appropriate response. In case of human being, communication ability is a result of a long learning process, while the same process appears to be an innate mechanism in insects. Individuals of insect species are usually born with a set of specific vocabulary which is shared only with the individuals of its own species. Communication can be an act of any part of an organism that invokes an alteration in the behavior of another organism as a response. Some emitter insects send a message using an acoustic signal to the rest of organisms by doing some action while some other insect species may do the same by developing certain physical traits such as the color pattern of wings of some butterflies. Reception of information from the emitter insect by other individual of same species occurs due to induction of some change in their receptor.
Similar to all other organisms, insects acquire information about their environment by using their five senses and exchange information among individuals of same species or other species. Some of these communication modalities may implicate contact senses such as taste and touch while other modalities may involve remote sense. Exchange of information using contact sense can occur only when two individuals come in direct physical contact with one another. Vision, olfaction, and auditory senses are the remote senses which are used frequently to promulgate information through the air or water over considerable distances. Thus, an insect may send a communication signal by making a noise, releasing a chemical, or flash a light, or the signal may simply be an inherent part of the insect’s physical makeup such as wing pattern, body color, or surface chemistry. In either case, the signal must elicit some behavioral change in responding organism.
2 Necessity of Communication
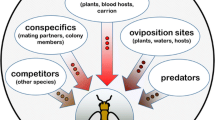
There are a number of interesting social insects which lead a group life with distinct division of labor among them. Close coordination among the members of such insect species is essential at different level for various purposes such as reproduction, search for food sources. It is well known that members of an insect species communicate frequently with organisms of the same species which is referred to as intraspecific communication. Sometimes direct or indirect communication occurs between members of one species with organisms of other species for different purposes which is referred to as interspecific communication. There are a number of reasons for communication among insect species which are enumerated as follows:
-
To search a courtship mate of same species for reproduction
-
To identify members of the same species or even to warn other organisms of its own presence
-
To convey information about the location of resources like food, nidification places, etc.
-
As an alert signal toward potential hazards to make other individuals aware of it
-
To protect territory for available resources in an area
-
As a way to camouflage or to mimic other organisms (as a defensive strategy against predators)
3 Types of Communications Among Insect Species
3.1 Visual Communication
Communication using visual signals is common among different insect species. A number of different types of visual communications are displayed by various insect species such as the color patterns and other markings on the wings of butterflies and moths. The red admiral butterfly, for instance, possesses bright, typical markings on the upper wing surface and protective coloration on the underside. Some insect species communicate by emitting light of different intensities. For example, Lampyridae (beetle Order: Coleoptera) communicate using light with individual of its own species. Another good example of use of light as communication modality is seen among fireflies. Fire flies emit pulses of light as a courtship dialogue between a male which is usually flying and a female usually perched in the vegetation. Flash pattern of emitted light and response time to light are usually unique to a species of insect. Some insects possess unique capability of communication using wavelength in the ultraviolet light. Female cabbage butterflies is one of the best examples of it which have ultraviolet reflecting scales on the dorsal wing surface. When the female cabbage butterflies fly, each down stroke of the wing creates a brief flash of UV that males of the same species can recognize for mating.
3.2 Tactile and Behavioral Communication
Despite inevitable limitation of interaction between two or just a few individuals, tactile contacts form an important modality of communication among insect species. Because of their poor vision and sound receptor, numerous insect species mostly rely on physical contact for exchange of information. The antennation between nest mates is one of the common examples of tactile communication found in insect species. The inter-individual exchange of liquid material via trophallaxis is largely based on tactile interactions with mainly the antennae and the forelegs. Both ants and termites use antennal tapping as an essential component of tactile communication though exact information exchanged in such process is still not clear. However, nest mate recognition and exchange of food through trophallaxis could be the major result of antennal tapping. Insects touch each other’s feelers to exchange messages. In case of blister beetles (Family: Meloidae) courtship usually begins with a series of antennal taps by the male on each side of the female’s body and male gets reciprocation from the female partner by lifting of wing covers and allowing to clump on the back.
Certain tree hoppers belonging to membracidae family produce vibrations in the tissue of their host plant which can be felt by all other tree hoppers residing on the same plant. Communication among bees exhibits a unique behavior similar to dance. Bees perform various types of dance to communicate the distance and direction of food sources as well as nest sites. Running in a circle popularly known as round dance is performed to indicate close sites and transitional or sickle dance for sites at an intermediate distance from the hive. This dance involves running in a semicircular or moon shape. The most complex of the dance types performed by honeybees is the waggle dance which generally performed by honey bee Apis mellifera to communicate the locations of food sources. The dance language of honey bee consists of different patterns that convey information about distance of food source from the bee hive. The number of interactions of the dance that bee performs conveys information about distance while the liveliness of dance indicates the quality of the food source. The angle of the dance provides information about the direction of the food source to other insects. Sometimes bees stop dancing and provide a food sample to other bees in the hive upon their request. Sound produced by bee during dance generally plays important role in getting attention of other bees and to keep their attention.
3.3 Acoustic Communication
Many insects have ability to produce sound though they possess no vocal chords. Insects use various other ways to produce sounds. Ways of producing sound include rubbing of body parts together. Sounds are caused by vibrations that can pass through air, water, and solid structures which insect use as a modality to convey various messages to the members of the same species or different species. Crickets sing by rubbing one wing over the other wing. Some other insects rub their legs, scratch their bodies, or rub their jaws together to make audible sound. Buzzing sound is produced by grasshoppers by rubbing the hind legs against the wings. Sound of different frequencies is produced by mosquito’s resonation of antennal hairs. Special organs are also found in different insect species to produce sound. Male cicadas have special organs to produce sound called tymbals. Membranes present inside the tymbal can vibrate to produce a “singing” sound. A tympanic membrane in the abdomen (e.g., grasshoppers and moths) or in the tibiae of the front legs (e.g., crickets and katydids) is mostly used to detect sound. Though sound produced by most of the insects is clearly audible to human being such as that crickets’ song, many insects make supersonic sounds that are above the human range of hearing. These supersonic sounds produced by insects have more than 20,000 vibrations each second. Some grasshoppers and moths have been known to produce ultrasonic sounds of 80,000 Hz.
3.4 Chemical Communication
One of the most common way of communication among insect species is the use of odor or smell. Special scent glands are present in insects that release small volatile odorant molecule from their body. These odors are popularly called as pheromones. The female insects can produce specific odorant molecules to attract partners of its own species for mating and such molecules are known as sex pheromones. Some insect species have extraordinary sensitivity toward the sex pheromone which they can perceive even at long distance. Male moths can perceive the pheromones of female moths over distances of many kilometers. Ants use odorant molecule to mark a trail, so that other ants can use the trail to get back to the nest or to find food. The special scent released by ants enables them to know the other members of their colony. Some insects use smell to notify about the danger to each other. Sense of taste or smell is sometimes exploited various insect species to detect the presence of odors. However, most insects possess specialized receptors in their feet, antennae, and ovipositors for perception of odorant signals. One of the most important organs for detecting odors in the insect species is the antennae. In species where the female produces an odor, the males often have extra big antennae which help them to find the female and the vice versa. These chemicals are divided into two groups:
-
1.
Pheromones: These are low molecular weight volatile organic compounds released from specialized gland in insect species. Pheromones act as a chemical signals and it carries information from one member of a species to another member of the same species. The pheromones play crucial role in insect communication mostly as sex attractants, alarm substance, and many other intraspecific messages.
-
2.
Allelochemicals: The chemical messages that are transmitted from an individual of one species to member of a different species occur through allelochemicals. These primarily include defensive signals such as repellents, compounds used to locate suitable host plant, and other signals to regulate interspecific behaviors.
4 Insect Hydrocarbons and Chemical Communication
A number of insect species use cuticular lipids, especially the hydrocarbons as a medium of chemical communication among themselves (Blomquist et al. 1998). Hydrocarbons released from insect species have been known to perform variety of functions such as sex attractants and aphrodisiacs, anti-aphrodisiacs, species, caste and kin recognition cues, aggregation pheromones, and kairomones. Insect chemoreceptors can distinguish hydrocarbons by the number and placement of methyl-branching groups, degree and positions of double bonds, and chain length. Apart from its role in communication, insect cuticular lipids also play crucial role in restricting water loss and prevent a lethal rate of desiccation (Nelson and Blomquist 1995). It is challenging for all terrestrial animals with high surface area to volume ratio such as insects to conserve water in their bodies. The cuticular waxes function as anti-desiccation agent and play crucial role in meeting the need of water conservation and thus cuticular lipid is the focused target for insect control.
5 Neuronal Basis of Insect Communication
Insect species are known to communicate by secreting myriads of different volatile odorous compounds detection of which are encoded neurologically by the firing patterns of Olfactory Receptor Neurons (ORNs). The differential firing patterns of ORNs on exposure to compounds simultaneously determine the odor quality (chemical type), intensity (concentration), and dynamics (fluctuations in response time). The firing patterns of ORNs can be measured by in vivo recording from either population of ORNs or individual ORNs to study peripheral olfactory perception and odor coding. A number of studies show that recordings of ORN action potentials in response to odors are comprised of a limited number of discrete functional classes. Individual classes of ORNs can exhibit a diverse array of response properties to different odors in addition to their ability to respond differently to different sets of odors. Most neurons responding to odors are excitatory in nature but some neurons are also inhibited by certain odors. Various odor-specific onset and termination of kinetics of responses have also been displayed by ORNs. The cellular basis for an olfactory code is provided by different response spectra of the ORN types and their diverse response dynamics. Studies on response spectra of Drosophila ORNs suggest that during encoding of odors in insects, a single ORN can respond to multiple odorants and a single odorant can stimulate multiple ORN classes in a combinatorial way similar to that of mammals. Molecular studies at genetic level indicate that the distinct groups of genes encoding odorant receptor proteins are the underlying players of these responses. The OR (odorant receptor) genes underlying the responses of most of the ORN classes are now well characterized. While specialized ORNs clustered together within the main olfactory epithelium of the nasal cavity or the vomeronasal organ detect odorants in vertebrates, insect ORNs and their support cells remain in distinct olfactory sensilla on both antennal and maxillary palp structures (Stocker 1994). However, neuropil structures of the central nervous system (CNS) that participate in the synaptic relay of ORNs, glomeruli, are anatomically similar in both insect and vertebrate. Approximately 1000 in case of rat to 5000 number of glomeruli in case of dog are found in vertebrate’s olfactory bulb (OB) (Hildebrand and Shepherd 1997). However, the antennal ORN axons project either ipsilaterally or bilaterally to number of glomeruli which ranges from 20 in Aedes aegypti (Anton 1996) to approximately 300 in a crustacean olfactory system in case of arthropods (Blaustein et al. 1993; Stocker 1994).
6 Molecular and Biochemical Basis of Chemical Communication
The molecular and biochemical basis of chemical communication in insect began to be understood when a broad class of water-soluble proteins was discovered in olfactory mucosa and sensilla which later on was found to be playing vital role in the olfactory process. These water-soluble proteins are secretory in nature and are known as Odorant Binding Proteins (OBPs). The initial identification and characterization of OBPs was based on its ability to directly bind known odorants in both insect (Vogt 1987) and vertebrate (Pevsner et al. 1985) systems. OBPs have been hypothesized to facilitate the solubilization of hydrophobic odorants molecules, act as its carrier, and elevate its effective concentration for receptor binding. Insect species are now known to possess a subset of OBPs with remarkable ability to bind with pheromone known as pheromone-binding proteins (PBPs) which are expressed in male-specific, pheromone-sensitive hairs (Vogt 1987). Similar proteins are also found in both male and female moths antenna structures but are associated with general odorant-sensitive neurons and hence are designated as general odorant-binding proteins (GOBPs) (Vogt et al. 1991). Large and diverse OBP/PBP family of olfactory proteins have now been identified in vertebrates as well as in various insect species including A. mellifera, Drosophila melanogaster (Pikielny et al. 1994) and true bugs (Dickens et al. 1998). However, there are numerous conflicting reports on physiological roles played by OBPs as some OBPs bind to a broad array of ligands with no visible specificity whereas some other OBPs have tremendous specificity in recognizing and binding only one class of odorant species (Dear et al. 1991).
The physiological function of odorant-binding proteins (OBPs) that mediate chemoreception in insects still poses number of unanswered questions. Studies show that the OBPs plays pivotal role in the overall process of olfactory signal transduction and slight change at genetic level can drastically effect the signaling process involving odorants. Kim et al. (1998) demonstrated that mutations in one candidate OBP gene, lush, resulted in defective ethanol sensitivity in D. melanogaster. It has been observed in fire ant Solenopsis invicta that OBP family proteins play crucial role in regulation of complex social behaviors (Krieger and Ross 2002). Further research on OBP family proteins is warranted to reveal the importance of these highly expressed olfactory proteins in numerous other species. One of the attractive hypotheses suggests that OBPs not only serve as a shuttle proteins responsible for bringing odorant ligands in proximity to olfactory receptors, OBPs could also play important role in increasing the complexity of olfactory inventory as a result of their differential affinity for particular odorants. Thus, the multiplicative binding affinities of both ORs and OBPs could represent the diverse olfactory sensitivity of an insect.
In addition to ligand based activation of receptor, cessation or reduction of signaling in response to repeated or persistent stimuli is an important component of sensory perception known as desensitization. Desensitization is observed in all chemosensory systems in almost all organisms and can vary from complete termination of signaling to graded attenuation of agonist potency (Dohlman et al. 1991). Desensitization of GPCR-mediated signal transduction is carried out mainly through the combined activity of two classes of proteins: G-protein-coupled serine/threonine receptor kinases (GRKs) and arrestins (Freedman and Lefkowitz 1996). Second messenger-induced kinases such as cAMP-dependent protein kinase A (PKA) and protein kinase C (PKC) cause phosphorylation of specific intracellular residues on GPCRs resulting in slow desensitization, GRKs phosphorylate only the agonist-bound (activated) form of GPCRs and are responsible for rapid receptor-specific desensitization (Inglese et al. 1993). Phosphorylation by GRKs serves to promote the binding of arrestin proteins, which further uncouple GPCRs from the G-protein-based signaling cascade (Pippig et al. 1993).
Furthermore, GRKs and arrestins are also intimately involved in GPCR internalization, an integral component of GPCR resensitization (Ferguson et al. 1996). Recent studies show that visual arrestins also function in olfactory signal transduction pathways in D. melanogaster and Anopheles gambiae (Merrill et al. 2002), while huge number of ORs and OBPs are present in both these insects, only three genes encode dual-functional arrestins which make them an attractive target for reducing the olfactory sensitivity of insects of medical and economic importance. GPCRs contain seven transmembrane spanning regions of 20–25 amino acids and are most prevalent superfamily of proteins currently known and are having more than 5000 members (Gether 2000; Strader et al. 1994). These proteins link ligands and downstream effectors by transmitting, amplifying, and integrating other cellular signals (Dohlman et al. 1991). ORs being a member of the GPCR superfamily are hypothesized to function through a signal transduction pathway similar to other GPCRs and with specific components unique to olfactory tissue, such as Golf (a Gs-like protein), adenylate cyclase III, and cAMP-gated channel (Pilpel et al. 1998).
7 Pheromones in Communication Among Insect Partners
Due to the small body size of insects, their ability to produce and perceive auditory and visual signals over large distances is limited (Greenfield 2002). Social communication in insects largely depends on chemo sensation through chemicals involved in communication known as semiochemicals. Semiochemicals can be grouped into two classes: allelochemicals and pheromones. While allelochemicals are chemicals produced and secreted by one species of organism that elicit a behavioral or physiological response in a member of the other species, pheromones are those that elicit a response in a member of a same species (Wyatt 2014). Understanding the mechanisms behind chemical communications in insects using recent advances in insect genomics, molecular genetics, and neuroanatomical techniques has been a major focus because of its potent and high impact application in disease control and agriculture.
Diverse classes of chemicals such as ketones, aldehydes, and fatty acids have been co-opted by several insect species to serve as pheromones over time through evolution (Yew and Chung 2015). The original function of cuticular hydrocarbons (CHCs) was as anti-desiccants but now it serves a dual role in pheromone signaling (Chung and Carroll 2015). Intrinsic properties of pheromones such as volatility vary depending upon its chemical nature. Some pheromones are volatile compounds while some are nonvolatile such as cuticular hydrocarbons. To coup with such variable volatility of pheromones, insects have evolved sophisticated pheromone-sensing organs for volatile and nonvolatile chemicals. While olfactory receptors present in the antennae and maxillary palps detect volatile pheromones like ketones, contact chemosensory receptors distributed across the body of the insect are implicated in the detection of low-volatile or nonvolatile pheromones, such as long chain CHCs (Ferveur 2005; Aquiloni et al. 2015).
Pheromones have been widely investigated as a sex attractant to drive behaviors associated with mating. Diverse classes of insect mating pheromones have been identified in numerous insect species which are secreted and perceived species specifically. Lepidopteran (butterflies and moths) are known to release volatile pheromones primarily for long-distance sexual advertisement (Greenfield 2002). On the other hand, fruit flies exploit both high-volatile and low-volatile CHCs pheromones for complex courtship behaviors (Haberer et al. 2014). Insects exhibiting dual parental care secrete pheromones to recognize mating partners (Müller et al. 2003). Beetle females are the best example that recognize their mate via nonvolatile CHC pheromones using contact chemo sensation mechanism (Wang and Anderson 2010; Carde 2014). Male–male interactions like aggression are also regulated by pheromonal signaling in many insect species. For example, a male-specific volatile pheromone 11-cis-vaccenyl acetate (cVA) is secreted by D. melanogaster that pleiotropically suppresses male–male courtship and aggression (Wertheim et al. 2006).
Apart from mating and sexual behaviors, pheromones are also used as a signal to induce the formation of groups of conspecifics and designated as aggregation pheromones (Imen et al. 2015). Aggregation pheromones are typically volatile long distance signal and are perceived by the olfactory system (van Zweden and d’Ettorre 2010). However, the cockroach, Periplaneta americana, uses both high-volatile and low-volatile CHCs as a signal for aggregation at diurnal resting site (Suh et al. 2014). Additionally, pheromone-driven social behaviors such as nest mate recognition and nest defense are independent of mating and are prevalent in social insects. Volatile alarm pheromones are mostly used for recruiting conspecifics to attack intruders (Wyatt 2014; Sakurai et al. 2014).
Insect species come across large numbers of volatile organic compounds of natural as well as anthropogenic origin. Thus it is imperative for insects to differentiate a myriad of physiologically irrelevant chemical compounds in the environment from essential semiochemical signals such as sex pheromones. The ability of pheromones in conveying message to the conspecific insects is dependent on chemical structure of the molecule and even tiny change in the pheromone molecules renders them completely inactive (Kaissling 1987). The extraordinary selectivity of the olfactory system (i.e., its ability to discriminate) is coupled with an inordinate sensitivity. To advertise their readiness to mate for reproduction, females secrete very minute quantity of sex pheromones and thus avoid being noticeable. On the other hand, detectors in males display remarkable sensitivity and perceive such small amounts of pheromone in a way that the signal-to-noise ratio of the system approaches the theoretical limit. Furthermore a dynamic process of signal inactivation is a prerequisite in case of odor oriented navigation. Males encounter pheromone molecules as flashing signals consisting of diminutive burst of high flux estranged by periods during which the flux is zero while flying toward a pheromone releasing female. The average duration of spikes within puffs of pheromones is on the millisecond scale, and it declines as the moth approaches the source of pheromone (Murlis et al. 2000). Thus, a male moth has to perceive selectively minute quantity of pheromones and reset the pheromone detectors on a millisecond timescale.
8 Molecular Mechanism of Odor Reception in Insects
Olfactory receptors (ORs) and odorant binding proteins (OBPs) have been studied extensively to understand their role in odor sensitivity and discrimination. OBPs have got special attention as regulator of dynamics of olfaction system in insects as well as in higher vertebrates which has been two strong line of evidence as below:
First, expression of a Drosophila odorant receptor in Xenopus oocytes provided direct evidence for its function, its activation was slower requiring timescale of second than normally observed millisecond timescale in in vivo function. This extreme slow response of ORs could be because of lack of OBPs in the heterologous system of xenopus olfaction process.
Second, kinetic studies demonstrated that the pH-dependent conformational change in BmPBP requires less than 4 ms. Studies on structural biology aspect of the molecules indicate that conformational change in BmPBP is an intramolecular mechanism to facilitate binding and release of pheromones by pheromone-binding proteins. Whether the remarkable selectivity of the insect’s olfactory system (Kaissling 1987) is achieved by the specificity of pheromone-binding proteins or the olfactory receptors is still unclear. When tested with a limited number of candidate ligands, OBPs bind to candidate ligands specifically (Du and Prestwich 1995; Maïbèche-Coisné et al. 1997; Maida et al. 2000; Plettner et al. 2000; Wojtasek et al. 1999). However, the number of OBPs is significantly less than the number of compounds that insects can smell. Even in the case of Drosophila, a species which has been extensively studied, only a few number of OBPs have been identified (Graham and Davies 2002). How limited number of OBPs detect unlimited numbers of different odorant species is still a matter of research. Evidences show that a Drosophila olfactory receptors are not specific to a single ligand (Wetzel et al. 2001). It can be stimulated by compounds with remarkably different chemical structures, such as cyclohexanol and cyclohexanone, benzaldehyde, and benzyl alcohol. The extraordinary specificity of insect olfactory system has been extensively explored using pioneering electroantennogram (Schneider 1957) and single sensillum recordings (Schneider and Boeckh 1962) at the Max Planck Institute. Even the generalist detectors for plant compounds have now been demonstrated to have inordinate specificity (Hansson and Christensen 1999; Nikonov et al. 2001; Nikonov et al. 2002). The mechanism of such specificity of a receptor could be based on the concept of “layers of filters” of participating OBPs that operate step by step. OBPs transport only small subset of the ligands to reach the pore tubule where each OR can be stimulated by a small number of ligands out of which only few of them reach the dendrite. Thus though neither the OBPs nor the ORs are extremely specific, the whole machinery can show remarkable selectivity by acting as two step filter.
9 Pheromone Detection by Olfactory Systems
The antennae and maxillary palps are the primary sensory organs in insects that detect volatile ligands. A huge array of anatomically and functionally diverse specialized structure called sensilla cover these organs. Inside the sensilla, olfactory receptor neurons (ORNs) are found in large numbers that are responsible for the detection of various chemicals (Suh et al. 2014). For example, four different types of sensilla are found on the antennae of silkworm Bombyx mori, out of which three are found to detect general, non-pheromone chemicals while the other one a long trichodea is uniquely tuned for detection of the sex pheromones such as bombykol and bombykal (Sakurai et al. 2014). D. melanogaster possess a trichoid sensilla which can specifically detect volatile pheromones like cVA and methyl laurate (ML) (Dweck et al. 2015). Chemical and molecular identities of diverse compound acting as pheromones are well characterized, however, the receptors responsible for specifically detecting such diverse pheromones in insect species are still unexplored. Though the recent advancement in Drosophila molecular genetics and in some insects has largely filled this gap, neurophysiological processes and behavioral alteration involved in pheromonal signaling require further research in numerous other insect species. It is now known that two different families of olfactory receptors (ORs) seem to detect the majority of insect volatile pheromones Kaissling (1986). The members of the olfactory receptor family were identified first of all as volatile pheromone receptors (Vosshall et al. 2000; Clyne et al. 1999). cVA, a known pheromone, was shown to activate and inhibit innate behavioral programs via the activation of Or67d expressing and Or65a-expressing neurons using neuronal and behavioral approaches (Datta et al. 2008; Liu et al. 2011). Furthermore, these neuronal and genetic architectures have been known to be evolutionarily conserved across the Drosophila species group (Dweck et al. 2015; Dekker et al. 2015; Lebreton et al. 2014). Pheromone receptor neurons synapse with central projection neurons in discrete glomeruli within the antennal lobe similar to olfactory receptor neurons.
10 Behavioral Mechanism of Communication Among Insect Species
Most of the insects live a solitary life except few conspecific contacts. Temporary aggregations among the insect species is often associated with the abundance of food materials as in case of grasshoppers and the encounter of conspecific males and females prior to copulation during breeding season. Social insects are characterized by the communities where they live in permanent association with their nest mates. In this regard, bees, bumblebees, wasps, ants, and termites have fascinated human beings due to their well-organized and impressive colonies. The social lifestyle of insects goes along with the foreseeable development of a communication system which allows the individual members of the colony to exchange information. This mode of communication occurs through various sensory channels, using visual, acoustic, tactile, sometimes magnetic, and especially chemical signals.
11 Honeybee Dancing
The best-known example of communication among social insect communication is the dancing event that is used by the honeybee workers to instruct their nest mates regarding the food sources. Karl von Frisch unraveled the significance of this dancing behavior and has been honored with Nobel Prize for this achievement (Frisch 1954; Frisch et al. 1967). Bee workers returning from a successful foraging journey enter the nest and perform peculiar dances on the vertical nest combs to communicate information about the food source to the nest mates. Subsequently, the nearby nest mates decipher the encoded information and recognize the distance and direction of the food source. This exchange of information in the form of waggle dance involves various stimuli such as visual cues, chemical cues. The returning forager bee gives visual cues to the nest mates about direction of the food source by orienting itself with respect to the position of the sun. The odor in the nest entrance that acts as a chemical cue helps the returning bees to recognize its own nest. Once the returning bee enters the bee hive, communication to the nest mate about the food sources depends mostly on the tactile cues through direct body contact to the nearby nest mate because of the darkness inside the bee hive. Through the direct body contact the dancing bee provides a chemical cue nest mates by offering some collected nectar so that they can recognize the target food source. The acoustic cues come from the buzzing sound of the dancer’s moving wings that play essential role in conveying the exact position of the food source (Michelsen et al.1989). Subsequent movement of the nest mates from bee hive to the food source mostly relies upon the sun compass as a visual cue to localize the exact position of food source. However, the visual communication using visual cues is not common in social insects because of the fact that insects possess compound eye with poor vision. For visual tracking of the foraging leading bee to the food source, a well-developed sight is an utmost necessity which the insect species lack (Nieh 2004).
In some insect species, the big compound eyes of the males facilitate in localizing the females partner before mating and also for orientation during mating. Ants because of their exceptional visual capacities detect polarized light to orient themselves for different purposes (Wehner 2003). Wood ant also uses its visual capacity for recognition of environmental patterns. The foraging workers of the wood ant can reopen the same routes accurately which they had followed in last summer after hibernation. Change in the environmental cues such as felling of tress has been reported to drastically decrease the fidelity of the reopened route (Rosengren and Pamilo 1978). However, some insect species possess no visual system such as eye and hence visual cues play no role in their case. For example, some ants and termite species because of total absence of eyes cannot use visual cues. Winged social insects use acoustic communication by producing buzzing sound through high-frequency wing movements. As in the case of honey bee, sound produced through rapid wing movement at high frequency and movement of thoracic muscle during waggle dance helps to attract attention and provide information about distance and quality of food sources to the nest mates (Nieh 2004). The queen’s tooting and quacking signals give acoustic communication about newly enclosing queens to make contact with each other (Michelsen et al. 1986). Sounds produced by knocking body parts onto the substrate called as drumming in wingless termites (Röhrig et al. 1999) and in some ants provide acoustic signals and bring about behavioral responses (Hölldobler 1999).
Stridulation behavior in some ant species such as rapid movement of the scraper situated at the posterior dorsal margin against parallel ridges of first gastral tergite plays important role in nest mate selection. Atta ants stridulate while cutting leaf fragments in order to recruit nest mates (Roces and Hölldobler 1996; Eibl-Eibesfeldt and Eibl-Eibesfeldt 1967). Stridulation activities appear to regulate ant’s species in maneuvering the leaf fragment into a carrying position (Roces and Hölldobler 1995). However, there are a number of controversial reports regarding the transmission of ant stridulatory signals through air (Hickling and Brown (2000). It is still unknown whether the ants are deaf and hence detection of sound occur through substrate-borne vibrations and not by sound produced (Roces and Tautz 2001).
12 Magnetic Orientation
Magnetic orientation among few insect species such as ants with respect to earth’s magnetic field has been reported to be used as communication modalities. Several reports suggest that the magnetic nanoparticles present in the body of the insect detect the geomagnetic (Acosta-Avalos et al. 1999). In the absence of sunlight cues, leaf-cutting ants appear to be responding to the geomagnetic field during its foraging journey (Banks and Srygley 2003). The ability to perceive the earth’s magnetic field has also been demonstrated in a number of insect species such as the fire ant Solenopsis invicta (Anderson and Vander Meer 1993), bees (Gould 1980), and bumble bees (Chittka et al. 1998). However, orientation along the earth’s magnetic field is not a true mode of communication among the insect species.
Conclusion
Communication among insect species involves complex process of exchange of information encoded in semiochemicals like pheromones, sex attractants, acoustic exchange of messages through production of unique sound, complex and peculiar behavior, and highly sensitive and selective reception of signals. In spite of many years of research into the role of pheromones and other related factors regulating the behavior of insects, our understanding of the mechanisms and evolutionary processes that support these complex signals is still in their infancy. Although studies in the fruit fly D. melanogaster are paving the way for understanding the sensory, neuroethological, and genetic principles of pheromonal communication, the current lack of comparable genetic tool for other insect species hinders progress in the field. A number of studies have been undertaken in the recent past to understand the neuronal, molecular, and behavioral basis of insect communication in few insect models. Insects are the members of largest phylum arthropoda with huge numbers of insect species and unique communication modalities. Insect pest is the major threat to modern crop system that includes numerous hybrid varieties with reduced pest resistance. Modern agricultural practice in recent years has introduced large numbers of dangerous persistent pesticides to the environment which has resulted in incidence of number of diseases in the human system. Understanding the mechanism of insect communication would help in managing pest species without polluting the environment. For that purpose, identification of receptors and cells responsible for pheromonal communication in diverse insect species will enable the field to take advantage of the wealth of existing behavioral and physiological data from these species. Furthermore, as a number of insect species act as pest or as disease vectors, understanding the mechanism of pheromonal signaling in regulating behavior of these insect species can be implicated for the development of more sustainable and specific environment-friendly control methods.
References
Acosta-Avalos D, Wajnberg E, Oliveira PS, Leal I, Farina M, Esquivel DMS (1999) Isolation of magnetic nanoparticles from Pachycondyla marginata ants. J Exp Biol 202:2687–2692
Anderson JB, Vander Meer RK (1993) Magnetic orientation in the fire ant, Solenopsis invicta. Naturwissenschaften 80:568–570
Anton S (1996) Central olfactory pathways in mosquitoes and other insects. Ciba Found Symp 200:184–192. discussion 192–196, 226–232
Aquiloni L, Tricarico E, SpringerLink (Online service) Springer International Publishing (2015) In: Aquiloni L, Tricarico E (eds) Social recognition in invertebrates the knowns and the unknowns. Springer International Publishing, Berlin
Banks AN, Srygley RB (2003) Orientation by magnetic field in leaf-cutter ants, Atta Colombica (hymenoptera: Formicidae). Ethology 109:835–846
Blaustein DN, Simmons RB, Burgess MF, Derby CD, Nishikawa M, Olson KS (1993) Ultrastructural localization of 5’AMP odorant receptor sites on the dendrites of olfactory receptor neurons of the spiny lobster. J Neurosci 13:2821–2828
Blomquist GJ, Tillman JA, Mpuru S, Seybold SJ (1998) The cuticle and cuticular hydrocarbons of insects: structure, function and biochemistry. In: Vander Meer RK, Breed M, Winston M, Espelie C (eds) Pheromone communication in social insects. Westview Press, Boulder, CO, pp 34–54
Carde RT (2014) Defining attraction and aggregation pheromones: teleological versus functional perspectives. J Chem Ecol 40:519–520
Chittka L, Williams NM, Rasmussen H, Thomson JD (1998) Navigation without vision: bumblebee orientation in complete darkness. Proc R Soc Lond B 266:45–50
Chung H, Carroll SB (2015) Wax, sex and the origin of species: dual roles of insect cuticular hydrocarbons in adaptation and mating. BioEssays 37:822–830
Clyne PJ, Warr CG, Freeman MR, Lessing D, Kim J, Carlson JR (1999) A novel family of divergent seven-transmembrane proteins: candidate odorant receptors in drosophila. Neuron 22:327–338
Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R (2008) The drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature 452:473–477
Dear TN, Boehm T, Keverne EB, Rabbitts TH (1991) Novel genes for potential ligand-binding proteins in subregions of the olfactory mucosa. EMBO J 10:2813–2819
Dekker T, Revadi S, Mansourian S, Ramasamy S, Lebreton S, Becher PG, Angeli S, Rota-Stabelli O, Anfora G (2015) Loss of drosophila pheromone reverses its role in sexual communication in Drosophila suzukii. Proc Biol Sci 282:20143018
Dickens JC, Callahan FE, Wergin WP, Murphy CA, Vogt RG (1998) Odorant binding proteins of true bugs. Generic specificity, sexual dimorphism, and association with subsets of chemosensory sensilla. Ann N Y Acad Sci 855:306–310
Dohlman HG, Thorner J, Caron MG, Lefkowitz RJ (1991) Model systems for the study of seven-transmembrane-segment receptors. Annu Rev Biochem 60:653–688
Du G, Prestwich GD (1995) Protein structure encodes the ligand binding specificity in pheromone binding proteins. Biochemistry 34:8726–8732
Dweck HK, Ebrahim SA, Thoma M, Mohamed AA, Keesey IW, Trona F, Lavista-Llanos S, Svatos A, Sachse S, Knaden M et al (2015) Pheromones mediating copulation and attraction in drosophila. Proc Natl Acad Sci U S A 112:E2829–E2835
Eibl-Eibesfeldt I, Eibl-Eibesfeldt E (1967) Das Parasitenabwehren der Minima- Arbeiterinnen der Blattschneider-Ameise (Atta cephalotes). Z Tierpsychol 24:278–281
Ferguson SS, Downey WER, Colapietro AM, Barak LS, Menard L, Caron MG (1996) Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271:363–366
Ferveur JF (2005) Cuticular hydrocarbons: their evolution and roles in drosophila pheromonal communication. Behav Genet 35:279–295
Freedman NJ, Lefkowitz RJ (1996) Desensitization of G protein-coupled receptors. Recent Prog Horm Res 51:319–351. discussion 352–353
Frisch K (1954) The dancing bees: an account of the life and senses of the honey bee, 1st edn. Springer-Verlag, Wien, pp XIV–183
Frisch K, Wenner AM, Johnson DL (1967) Honeybees: do they use direction and distance information provided by their dancers? Science 158:1072–1077
Gether U (2000) Uncovering molecular mechanisms involved in activation of G protein coupled receptors. Endocr Rev 21:90–113
Gould JL (1980) The case for magnetic sensitivity in birds and bees (such as it is): surprising concentrations of magnetite in the tissues of some animals may explain their sensitivity to the earth’s magnetic field. Am Sci 68:256–267
Graham LA, Davies PL (2002) The odorant-binding proteins of Drosophila melanogaster. Annotation and characterization of a divergent gene family. Gene 292:43–55
Greenfield MD (2002) Signalers and receivers: mechanisms and evolution of arthropod communication. Oxford University Press, Oxford
Haberer W, Steiger S, Müller JK (2014) Dynamic changes in volatile emissions of breeding burying beetles. Physiol Entomol 39:153–164
Hansson BS, Christensen TA (1999) Functional characteristics of the antennal lobe. In: Hansson BS (ed) Insect olfaction. Springer, Berlin, pp 125–161
Hickling R, Brown RL (2000) Analysis of acoustic communication by ants. J Acoust Soc Am 108:1920–1929
Hildebrand JG, Shepherd GM (1997) Mechanisms of olfactory discrimination: converging evidence for common principles across phyla. Annu Rev Neurosci 20:595–631
Hölldobler B (1999) Multimodal signals in ant communication. J Comp Physiol 184A:129–141
Imen S, Christian M, Virginie D, Colette R (2015) Intraspecific signals inducing aggregation in Periplaneta americana (Insecta: Dictyoptera). Environ Entomol 44:713–723
Inglese J, Freedman NJ, Koch WJ, Lefkowitz RJ (1993) Structure and mechanism of the G protein-coupled receptor kinases. J Biol Chem 268:23735–23738
Kaissling K-E (1986) Temporal characteristics of pheromone receptor cell responses in relation to orientation behaviour of moths. In: Payne TL, Birchm MC, Kennedy CEJ (eds) Mechanisms in insect olfaction. Oxford University Press, Oxford, pp 193–200
Kaissling K-E (1987) In: Colbow K (ed) R. H. Wright lectures on insect olfaction. Simon Fraser University, Burnaby, BC
Kim MS, Repp A, Smith DP (1998) LUSH odorant-binding protein mediates chemosensory responses to alcohols in Drosophila melanogaster. Genetics 150:711–721
Krieger MJ, Ross KG (2002) Identification of a major gene regulating complex social behavior. Science 295:328–232
Lebreton S, Grabe V, Omondi AB, Ignell R, Becher PG, Hansson BS, Sachse S, Witzgall P (2014) Love makes smell blind: mating suppresses pheromone attraction in drosophila females via Or65a olfactory neurons. Sci Rep 4:7119
Liu W, Liang X, Gong J, Yang Z, Zhang YH, Zhang JX, Rao Y (2011) Social regulation of aggression by pheromonal activation of Or65a olfactory neurons in drosophila. Nat Neurosci 14:896–902
Maïbèche-Coisné M, Sobrio F, Delaunay T, Lettere M, Dubroca J, Jacquin-Joly E, Nagnan-LeMeillour P (1997) Pheromone binding proteins of the moth mamestra brassicae: specificity of ligand binding. Insect Biochem Mol Biol 27:213–221
Maida R, Krieger J, Gebauer T, Lange U, Ziegelberger G (2000) Three pheromone binding proteins in olfactory sensilla of the two silkmoth species Antheraea polyphemus and Antheraea Pernyi. Eur J Biochem 267:2899–2908
Merrill CE, Riesgo-Escovar JR, Pitts RJ, Kafatos FC, Carlson JR, Zwiebel LJ (2002) Visual arrestins in olfactory pathways of drosophila and the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci U S A 99:1633–1638
Michelsen A, Kirchner WH, Andersen BB, Lindauer M (1986) The tooting and quacking vibration signals of honeybee queens: a quantitative analysis. J Comp Physiol 158A:605–611
Michelsen A, Andersen BB, Kirchner WH, Lindauer M (1989) Honeybees can be recruited by a mechanical model of a dancing bee. Naturwissenschaften 76:277–280
Müller JK, Eggert A-K, Elsner T (2003) Nestmate recognition in burying beetles: the “breeder’s badge” as a cue used by females to distinguish their mates from male intruders. Behav Ecol 14:212–220
Murlis J, Willis MA, Cardé RT (2000) Spatial and temporal structures of pheromone plumes in fields and forests. Physiol Entomol 25:211–222
Nelson DR, Blomquist GJ (1995) Insect waxes. In: Hamilton RJ, Christie WW (eds) Waxes: chemistry, molecular biology and functions. The Oily Press, England, pp 1–90
Nieh JC (2004) Recruitment communication in stingless bees (hymenoptera, Apidae, Meliponinae). Apidologie 35:159–182
Nikonov AA, Valiyaveettil JT, Leal WS (2001) A photoaffinity-labeled green leaf volatile compound “tricks” highly selective and sensitive insect olfactory receptor neurons. Chem Senses 26:49–54
Nikonov AA, Peng G, Tsurupa G, Leal WS (2002) Unisex pheromone detectors and pheromone-binding proteins in scarab beetles. Chem Senses 27:495–504
Pevsner J, Trifiletti R, Strittmatter SM, Synder SH (1985) Isolation and characterization of an olfactory receptor protein for odorant pyrazines. Proc Natl Acad Sci U S A 82:3050–3054
Pikielny CW, Hasan G, Rouyer F, Rosbash M (1994) Members of a family of drosophila putative odorant-binding proteins are expressed in different subsets of olfactory hairs. Neuron 12:35–49
Pilpel Y, Sosinsky A, Lancet D (1998) Molecular biology of olfactory receptors. Essays Biochem 33:93–104
Pippig S, Andexinger S, Daniel K, Puzicha M, Caron MG, Lefkowitz RJ, Lohse MJ (1993) Overexpression of beta-arrestin and beta-adrenergic receptor kinase augment desensitization of beta 2-adrenergic receptors. J Biol Chem 268:3201–3208
Plettner E, Lazar J, Prestwich EG, Prestwich GD (2000) Discrimination of pheromone enantiomers by two pheromone binding proteins from the gypsy moth Lymantria dispar. Biochemistry 39:8953–8962
Roces F, Hölldobler B (1995) Vibrational communication between hitchhikers and foragers in leaf-cutting ants (Atta cephalotes). Behav Ecol Sociobiol 37:297–302
Roces F, Hölldobler B (1996) Use of stridulation in foraging leaf-cutting ants: mechanical support during cutting or short-range recruitment signal? Behav Ecol Sociobiol 39:293–299
Roces F, Tautz J (2001) Ants are deaf. J Acoust Soc Am 109:3080–3082
Röhrig A, Kirchner WH, Leuthold RH (1999) Vibrational alarm communication in the African fungus-growing termite genus Macrotermes (Isoptera, Termitidae). Insect Soc 46:71–77
Rosengren R, Pamilo P (1978) Effect of winter timber felling on behaviour of foraging wood ants (Formica rufagroup) in early spring. Memorabilia Zool 29:143–155
Sakurai T, Namiki S, Kanzaki R (2014) Molecular and neural mechanisms of sex pheromone reception and processing in the silkmoth Bombyx mori. Front Physiol 5:125
Schneider D (1957) Elektrophysiologische untersuchungen von chemo- und mechanorezeptoren der antenne des seidenspinners Bombyx mori L. Z Vergl Physiol 40:8–41
Schneider D, Boeckh J (1962) Rezeptorpotential und nervenimpulse einzelner olfaktorischer sensillen der insektenantenne. Z Vergl Physiol 45:405–412
Stocker RF (1994) The organization of the chemosensory system in Drosophila melanogaster: a review. Cell Tissue Res 275:3–26
Strader CD, Fong TM, Tota MR, Underwood D (1994) Structure and function of G protein-coupled receptors. Annu Rev Biochem 63:101–132
Suh E, Bohbot JD, Zwiebel LJ (2014) Peripheral olfactory signaling in insects. Curr Opin Insect Sci 6:86–92
van Zweden JS, d’Ettorre P (2010) Nestmate recognition in social insects and the role of hydrocarbons. In: Insect hydrocarbons: biology, biochemistry and chemical ecology, vol 11. Cambridge University Press, Cambridge, pp 222–243
Vogt RG (1987) The molecular basis of pheromone reception: its influence on behavior. In: Prestwich GD, Blomquis GJ (eds) Pheromone biochemistry. Academic Press, New York, pp 385–431
Vogt RG, Rybczynski R, Lerner MR (1991) Molecular cloning and sequencing of general odorant-binding proteins GOBP1 and GOBP2 from the tobacco hawk moth Manduca sexta: comparison with other insect OBPs and their signal peptides. J Neurosci 11:2972–2984
Vosshall LB, Wong AM, Axel R (2000) An olfactory sensory map in the fly brain. Cell 102:147–159
Wang L, Anderson DJ (2010) Identification of an aggression-promoting pheromone and its receptor neurons in drosophila. Nature 463:227–231
Wehner R (2003) Desert ant navigation: how miniature brains solve complex tasks. J Comp Physiol 189:579–588
Wertheim B, Allemand R, Vet LEM, Dicke M (2006) Effects of aggregation pheromone on individual behaviour and food web interactions: a field study on drosophila. Ecol Entomol 31:216–226
Wetzel CH, Behrendt H-J, Gisselmann G, Störtkuhl KF, Hovemann B, Hatt H (2001) Functional expression and characterization of a drosophila odorant receptor in a heterologous cell system. Proc Natl Acad Sci 98:9377–9380
Wojtasek H, Picimbon JF, Leal WS (1999) Identification and cloning of odorant binding proteins from the scarab beetle Phyllopertha diversa. Biochem Biophys Res Commun 263:832–837
Wyatt TD (2014) Pheromones and animal behavior: chemical signals and signatures, 2nd edn. Cambridge University Press, Cambridge
Yew JY, Chung H (2015) Insect pheromones: an overview of function, form, and discovery. Prog Lipid Res 59:88–105
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Baitharu, I., Shroff, S., Sahu, J.K. (2018). Molecular, Neuronal, and Behavioral Mechanism of Communication Among Insect Species: A Review. In: Kumar, D., Gong, C. (eds) Trends in Insect Molecular Biology and Biotechnology. Springer, Cham. https://doi.org/10.1007/978-3-319-61343-7_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-61343-7_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-61342-0
Online ISBN: 978-3-319-61343-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)