Abstract
Electroencephalographic (EEG) data provide a direct, non-invasive measurement of neural brain activity. Nevertheless, the common assumption of EEG stationarity (i.e., time-invariant process) neglects information about the underlying neural networks connectivity. We present an approach for finding networks of brain regions, which are connected by effective associations varying over time (effective connectivity). Aiming to improve the performed connectivity analysis, brain source activity is initially reconstructed from EEG recordings, applying an inverse EEG solution with enhanced spatial resolution. Further, a time-variant effective connectivity measure is used to investigate the information flow over some predefined regions of interest. For testing purposes, validation is carried out simulated and real EEG data, promoting non-stationary dynamics. The obtained results of performance prove that inherent interpretability provided by the time-variant processes can be useful to describe the underlying neural networks flow.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
- Boundary Element Method
- Effective Connectivity
- Connectivity Measure
- Source Reconstruction
- Connectivity Direction
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
To date, the importance of measuring connectivity between spatially separate, but functionally related brain regions has become of a big interest in the study of human neural functions. Though most of the related work is designed for functional magnetic resonance imaging, Electroencephalography (EEG), which non-invasively monitors the electrical brain activity, is increasingly used because of the provided high temporal resolution at a low cost. Moreover, EEG data analysis allows exploring the dynamics and adaptability of different cognitive processes, yielding reliable connectivity estimates between the brain regions [1].
Generally speaking, EEG source connectivity analysis comprises two stages: (i) EEG signals are mapped into a source space, employing a given inversion solution method, (ii) modeling of spatio-temporal dynamics of activation patterns is performed using the predefined Regions of Interest (ROI) set [7]. In the first stage, the accuracy of brain mapping profoundly limits the interpretability of the connectivity measurements [4]. Also, the resulted connectivity measure tends to show fake connections among regions, if the mapped brain regions are not the true brain activity generators. Another aspect to consider is the static nature of most inversion solution methods that may lead to inaccurate temporal patterns of the mapped activity generators, resulting in the estimation of false connections. In the second stage, the functional or effective connections between brain regions, which mainly differ in the inclusion (effective) or not (functional) of the information flow direction, can be investigated by applying measurements of connectivity or information flow to the regions of interest [5]. Specifically, Effective connectivity is defined as the influence that one neural system exerts over another, either directly or indirectly. In contrast to functional connectivity, that is, the temporal correlations between remote neurophysiology events, the effective connectivity describes the direction of interactions between brain regions. Consequently, several measures have been proposed, commonly including space, time, and frequency domains [3]. Although most of the connectivity approaches assume that connectivity patterns remain constant at the time, there is growing evidence that brain dynamics are non-stationary [9]. As a result, there is a clear need to quantify dynamic changes in the network structure through the time [6].
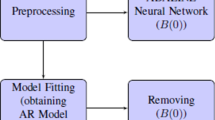
With the aim to improve identification of nonstationary brain networks, this work comprises two processing stages: Initially, brain activity is represented as a set of small spatial basis functions or patches, enforcing a compact and sparse support through the well-known approach (termed Multiple Sparse Priors). Further, some regions of interest are selected based on the recovered sources with the highest energy. Then, to accurately encode the temporal dynamics of underlying neural networks, a time variant effective connectivity measure is employed, quantifying the information flow changes over the selected regions through time. Obtained results on simulated and real EEG databases show that the proposed approach enables identifying with increased accuracy the brain activity information flow when non-stationary data is analyzed.
2 Methods
2.1 Brain Source Estimation
For estimating the brain activity, we consider the following distributed solution:
where \({\varvec{Y}} \,\in \,\mathbb {R}^{C \,\times \,T}\) is the EEG data measured by C sensors at T time samples, \({\varvec{J}} \,\in \,\mathbb {R}^{D \,\times \,T}\) is the amplitude of the D current dipoles in each three-dimensional dimension distributed through cortical surface, and \({\varvec{L}} \,\in \,\mathbb {R}^{C \,\times \,D}\), commonly named lead field matrix, is the gain matrix representing the relationship between sources and EEG data. Besides, EEG measurements are assumed to be affected by zero mean Gaussian noise \({\varvec{\varXi }} \,\in \,\mathbb {R}^{C \,\times \,T}\), having a matrix covariance \({\varvec{Q}}_{{\varvec{\varXi }}} {\,=\,}\lambda _{{\varvec{\varXi }}}{\varvec{I}}_{C}\), where \({\varvec{I}}_{C}\,\in \,\mathbb {R}^{C \,\times \,C}\) is an identity matrix, and \(\lambda _{{\varvec{\varXi }}}\,\in \,\mathbb {R}^+\) is the noise variance. Under these constraints, the brain source activity can be estimated as:
where \({\varvec{Q}}\,\in \,\mathbb {R}^{D \,\times \,D}\) stands for the source covariance, constructed as a weighted sum of P available spatial solutions (or patches) \(\{{\varvec{Q}}_p,p{\,=\,}1,\ldots ,P\}\), each regarding one potentially activated cortex region weighted by its respective hyperparameter \(\lambda _p\,\in \,\mathbb {R}^+\), as follows (termed Multiple Sparse Priors – MSP) [2]:
In practice, optimization of noise variance and source covariance hyperparameters \(\{\lambda _{{\varvec{\varXi }}},\lambda _p\}\) is done using standard variational schemes as detailed in [8].
3 Time-Varying Effective Connectivity
In order to estimate the causal relation among all current dipoles, a time-variant connectivity measure, namely, full frequency adaptive directed transfer function (ffADTF), can be calculated from the coefficients of a Time-variant Autoregressive (TVAR) model, as proposed in [5]:
where \(H_{ij}(f,t)\) is the time-variant transfer matrix of the system, that describes the information flow from source j to source i, \(\forall i,j {\,=\,}1,{\ldots },D\), at frequency f and time t. As \(H_{ij}(f,t)\) may increase when there is no power in the spectrum of dipole j at that frequency and time, each term should be weighted by the autospectrum of the sending source. Thus, the modified effective connectivity measure, or spectrum-weighted adaptive directed transfer function (swADTF), is computed as follows:
As a result, the swADTF value allows analyzing the causal relation among all signals at a predefined frequency band over time.
4 Experimental Set-Up
4.1 Validation on Simulated Activity
Simulated Data Description. The simulation is designed to test whether the swADTF measure can describe the directional flow that occurs during the onset of an evoked activity. Thus, two active dipoles are simulated that promote a similar behavior to an evoked-response potential, generating the corresponding non-stationary time series by real Morlet wavelets of 1.5 s length, sampled at 200 Hz. The random central frequency of the Morlet wavelet is sampled from a Gaussian distribution with a mean of 9 Hz and standard deviation of 2 Hz. The stimulus started at \(t=0\) and the activity propagates from simulated active dipole #1 to dipole #2 at \(t=0.1\) s. Besides, the background noise of the dipole signals is set to have a 1/f spectral behavior. Therefore, each EEG is calculated by multiplying the simulated brain activity by the lead field matrix (see Eq. (1)).
For the source space modeling, we employ a tessellated surface of the gray-white matter interface with 8196 vertices (suitable source placements), having orientations fixed orthogonally to the surface. Also, the lead fields are computed using a volume conductor (calculated by boundary element method) with a mean distance between neighboring vertices adjusted to 5 mm. Thus, a synthetic EEG is generated under 128-channels configuration.
Three experimental setups, holding 100 simulations each, are performed to test the sensibility to the noise of the proposed connectivity based approach. To this end, a measurement noise is added to get signal-to-noise-ratio (SNR) levels of \(0,\ 5,\ 20\) dB. Location of active dipoles is randomly selected for each simulation. Figure 1 shows a schematic representation of the proposed testing for the simulated EEG data.
Source Level Effective Connectivity Analysis. Neural activity reconstruction is obtained by the MSP approach, using a greedy search optimization method. Then, to avoid the computation of D \(\times \) D connectivity values, the regions of interest (ROIs) are determined. To this end, a region with approximated ratio of 20 mm in the cortical surface around the dipoles with the highest energy is drawn. Consequently, the closest active dipoles are defined as the same ROI, avoiding spurious connectivity. Afterward, the averaged time series of each ROI and the connectivity measure for all the regions are extracted. Calculation the involved connectivity measure is performed under the following parameters: order of the time variant auto-regression model is set at \(p=15\), update coefficient of the Kalman filter is fixed as 0.001, and the smoother parameter is empirically adjusted to 100. Also, the connectivity is computed within the frequency interval, ranging from 0 to 30 Hz.
Due to the swADTF value is a time-variant measure, the averaged value over time is estimated to compare among several connectivity values directly. Besides, we describe the directional flow of the brain activity by the first two ROIs that have the highest averaged swADTF value, and that are assumed as the source and sink regions, respectively. For the subsequent analysis, the remaining ROIs are discarded. For the sake of comparison, the source and sink regions are also selected based on the energy of the reconstructed activity over each ROI (first two regions with the highest energy).
Results of Assessed Performance. As the assessment measure, the minimum Euclidean distance is computed between all dipoles of the selected ROIs and the simulated source and sink dipoles. Also, we employ as performance measure the percentage of times when the real connectivity direction is correct. Table 1 shows the error distances estimated for SNR = 0, 5, and 20 dB. As seen, the mean localization error of the connectivity-based approach is consistently lower that reduces when the SNR value increases. In the same way, the proposed method reproduces the connectivity direction in up to 88% of the times, unlike the energy-based approach that only obtain an accuracy of 50%.
Figure 2(a) presents the localization error for each of the two simulated sources assessed by both tested algorithms: connectivity (con), and energy based (en) for a fixed SNR = 5. As can be seen, the variation among simulations is significantly higher with the energy-based algorithm. This result is expected due to the different accuracy in connectivity direction provided by each algorithm. Note that the solution is robust to both kinds of simulated noise conditions, namely, neural background activity and sensor level activity, as shown by the error box-plot in Fig. 2(a), fixing SNR = 20.
4.2 Validation on Faces-Scrambled Paradigm Identification
Database Description. This EEG data collection was acquired from a subject who participated in a multimodal study of face perceptionFootnote 1. The data were recorded while making symmetry judgments of faces and scrambled faces. Either type of faces was presented for 600 ms, every 3600 ms while data were acquired on a 128-channel Active-Two system, sampled at 2048 Hz. After artifact rejection, the epochs were baseline-corrected from \(-200\) ms to 0 ms, averaged over each condition and down-sampled to 200 Hz. For modeling of the source space, the same tessellated surface with 8196 vertices, and the same model, calculated by boundary element method, was applied to build the lead fields.
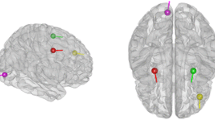
Results for Faces Conditions. As observed in Fig. 3 that displays the source reconstruction for faces condition of the faces-scrambled paradigm, the proposed methodology managed to avoid the high energy dipoles in the fusiform gyrus and focused in the frontal cortex (red lines outline the connectivity assessed). Due to the attitude of expectancy for recognizing each face, this behavior can be anticipated since the face condition study suggests that there is a controlled response besides the perceptual one.
5 Discussion and Concluding Remarks
In this work, we propose a methodology for measuring the nonstationary neural activity flow in the case of evoked response potentials. To this end, brain activity extracts from EEG recordings by solving the Multiple-Sparse-Priors inverse solution, and then, a time variant effective connectivity is used for analysis in more detail the information flow over some predefined regions of interest. However, based on the results obtained from simulated and real EEG data, the following aspects should be considered for implementation of the proposed methodology:
-
Due to the fact that the connectivity exhibits a high sensibility to the source reconstruction, the MSP-based source reconstruction is incorporated with the purpose of avoiding loss of quality of the performed accuracy. In fact, the higher the demanded localization quality of spatial-temporal patterns – the larger the needed accuracy provided by the source reconstruction solution.
-
For the sake of validation, the proposed approach is contrasted with an energy based strategy, yielding a connectivity direction that is enhanced up to 88% of the times. Also, the source localization is improved, reaching a localization error of approximately 10 mm for the values SNR = 5 and 20. Regarding the noise rejection, the proposed approach shows a stable behavior.
-
The considered nonstationarity of the underlying networks in the EEG recordings is clearly affecting the performance achieved by the proposed and comparison approaches. Thus, Table 1 shows that the energy-based methodology (that does not take connectivity into account) fails in detecting the real connectivity flow accurately. Consequently, even the brain activity sources have been rightly identified by the mapping algorithm, the temporal dynamic analysis must be incorporated to improve the activity flow detection.
-
Since the location accuracy is improved, a better interpretability of results may be supplied. Thus, in the case of tested faces-scrambled database, the connectivity avoids the hippocampus and points out to the frontal lobe, suggesting that there is a controlled response due to the attitude of expectancy for recognizing each face.
As a concluding remark, the performed validation on real and simulated data prove that the proposed approach enables identification of the information flow over regions of interest drawn over the brain cortex. To this end, we discuss a more detailed analysis of the temporal patterns, extracted from EEG recordings, in a predefined frequency band. Consequently, even all experiments are carried out for the case of evoked response potentials, the methodology can be readily extrapolated to other types of brain dynamics, such as epileptic activity. Also, the inclusion of a brain mapping method that encourages a set of temporal constraints will be considered as future work, attempting to improve the accuracy of the connectivity analysis.
Notes
- 1.
freely available at http://www.fil.ion.ucl.ac.uk/spm/data/mmfaces/.
References
Brookes, M.J., O’Neill, G.C., Hall, E.L., Woolrich, M.W., Baker, A., Corner, S.P., Robson, S.E., Morris, P.G., Barnes, G.R.: Measuring temporal, spectral and spatial changes in electrophysiological brain network connectivity. NeuroImage 91, 282–299 (2014)
Friston, K., Harrison, L., Daunizeau, J., Kiebel, S., Phillips, C., Trujillo-Barreto, N., Henson, R., Flandin, G., Mattout, J.: Multiple sparse priors for the M/EEG inverse problem. NeuroImage 39(3), 1104–1120 (2008)
Greenblatt, R., Pflieger, M., Ossadtchi, A.: Connectivity measures applied to human brain electrophysiological data. J. Neurosci. Methods 207(1), 1–16 (2012)
Grosse-wentrup, M.: Understanding brain connectivity patterns during motor imagery for brain-computer interfacing. In: Koller, D., Schuurmans, D., Bengio, Y., Bottou, L. (eds.) Advances in Neural Information Processing Systems 21, pp. 561–568. Curran Associates, Inc (2009)
van Mierlo, P., Carrette, E., Hallez, H., Raedt, R., Meurs, A., Vandenberghe, S., Van Roost, D., Boon, P., Staelens, S., Vonck, K.: Ictal-onset localization through connectivity analysis of intracranial EEG signals in patients with refractory epilepsy. Epilepsia 54(8), 1409–1418 (2013)
Monti, R.P., Hellyer, P., Sharp, D., Leech, R., Anagnostopoulos, C., Montana, G.: Estimating time-varying brain connectivity networks from functional MRI time series. NeuroImage 103, 427–443 (2014)
Schoffelen, J.M., Gross, J.: Source connectivity analysis with MEG and EEG. Hum. Brain Mapp. 30(6), 1857–1865 (2009)
Wipf, D., Nagarajan, S.: A unified bayesian framework for MEG/EEG source imaging. NeuroImage 44(3), 947–966 (2009)
Woolrich, M.W., Baker, A., Luckhoo, H., Mohseni, H., Barnes, G., Brookes, M., Rezek, I.: Dynamic state allocation for MEG source reconstruction. NeuroImage 77, 77–92 (2013)
Acknowledgments
This work was supported by the research project 11974454838 founded by COLCIENCIAS.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Martinez-Vargas, J.D. et al. (2017). Identification of Nonstationary Brain Networks Using Time-Variant Autoregressive Models. In: Ferrández Vicente, J., Álvarez-Sánchez, J., de la Paz López, F., Toledo Moreo, J., Adeli, H. (eds) Natural and Artificial Computation for Biomedicine and Neuroscience. IWINAC 2017. Lecture Notes in Computer Science(), vol 10337. Springer, Cham. https://doi.org/10.1007/978-3-319-59740-9_42
Download citation
DOI: https://doi.org/10.1007/978-3-319-59740-9_42
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-59739-3
Online ISBN: 978-3-319-59740-9
eBook Packages: Computer ScienceComputer Science (R0)