Abstract
SgII is an acidic secretory which belongs to the family of chromogranins. It is present in the large-dense cored vesicles of the regulated secretory pathway of many neurons and endocrine cells and it is well conserved during evolution. Like chromogranin A, SgII can induce granulogenesis in endocrine cells but also in cells typically lacking secretory vesicles like fibroblasts. In the secretory vesicles SgII is processed to smaller peptides, e.g. secretoeneurin, EM66 and manserin. For secretoneurin several biological effects like induction of neurotransmitter release, chemotactic activity towards immune-, endothelial- and muscle cells, and potent angiogenic and vasculogenic properties have been established. Thus, SN displays potent hormonal and paracrine effects, which help to orchestrate development, maintenance, physiologic activity and repair of the surrounding tissue. In addition, SgII has been established as valuable biomarker for endocrine tumours and cardiovascular diseases.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Secretogranin II (SgII)
- Secretoneurin (SN)
- Chromogranin
- Vasculogenic Properties
- Neuron-restrictive Silencer Factor (REST)
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Secretogranin II (SgII) – also named chromogranin C - is an acidic secretory protein expressed in many neurons and endocrine tissues. It was initially isolated from rat PC12 and the soluble content of bovine chromaffin granules as third main secretory protein and belongs thus to the family of chromogranins. The chromogranins are typically widely expressed throughout neuronal and endocrine tissues. They are stored within the cell in large dense secretory vesicles and released via the regulated pathway in a calcium-dependent manner. The chromogranins represent proteins of 50–120 kD, which share several features, such as acidic nature, heat-stability and proteolytic processing to smaller peptides. The so-called granin motif (D/E-S/N-L-S/A/N-X-X-D/E-X-D/E-L) present in the C-terminus of SgII represents a moderately homologous stretch of 10 amino acids also found in chromogranins A and B. It was postulated when the primary amino acid sequence of these proteins became available (Huttner et al. 1991) and is most likely without physiological relevance. This is underlined by the absence of this motif in secretogranins III and V as well as its presence in unrelated proteins like BRCA1, BRCA2, Golgin-245 or trans-Golgi p230, an acidic protein localized to the cytosolic side of Golgi membranes (Erlich et al. 1996). The chromogranins share some physico-chemical properties like acidic pI and binding of calcium with low affinity but high capacity, which is responsible for the electron-dense core of large dense secretory vesicles. They are cleaved at pairs of consecutive basic amino acids to multiple smaller peptides and thus give rise to the characteristic pattern of multiple immunoreactive bands of intermediate size seen in immunoblots with monospecific antisera. Chromogranins have recently been shown to be ultimately involved in vesicle formation or biogenesis, to contribute to packaging and sorting of hormones and enzymes into LDVs and they can function as precursors of small peptides generated by prohormone convertases from chromogranins A, B, and SgII. Furthermore, chromogranins have been established as valuable biomarkers for the characterisation of neuroendocrine tumours and cardiovascular diseases. Several excellent reviews have recently been published, which provide further details on the structure and physiologic aspects of this protein family (Bartolomucci et al. 2011; Conlon 2010; Fischer-Colbrie et al. 2005, 1995; Helle 2010; Huttner et al. 1991; Portela-Gomes et al. 2010; Stridsberg et al. 2008; Taupenot et al. 2003).
2 Structure & Posttranslational Modifications of Secretogranin II
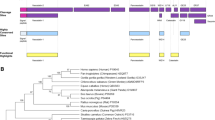
Human and mouse SgII consist of 617 amino acids (aa) whereas the bovine homolog is 4 amino acids shorter. SgII contains 20% acidic amino acids in its primary amino acid sequence (Fischer-Colbrie et al. 1990), which causes its untypical physico-chemical properties like a random-coil structure and heat-stability. Even though a molecular weight of 67 kDa can be deduced from the primary sequence, a band with an apparent Mr. of 86,000 is seen in SDS gels. This abnormal migration in SDS electrophoresis is probably due to the high amount of acidic amino acids found in the primary sequence. After co-translational cleavage of a 27 amino acid signal peptide at a typical cleavage site (Ala-X-Ala/Ala), (see (Fischer-Colbrie et al. 1990) for discussion), SgII is further posttranslationally modified in the Golgi apparatus (Fig. 1): it is sulphated at Tyr-151 and phosphorylated at Ser-532 (Lee et al. 2010). SgII is not or only marginally glycosylated. The primary amino acids sequence of bovine SgII contains no consensus sequence for N-glycosylation and SgII does not bind to concanavalin A lectin, in addition no significant O-glycosylation was detected (Fischer-Colbrie et al. 1995). In the secretory granules SgII is processed to small peptides and proteins of intermediate size by prohormone convertases PC1 and PC2, two proteases belonging to the furin-like prohormone convertase group. SgII contains 9 pairs of basic amino acids (7 KR and 2 RK sites). Five peptides (secretoneurin (SN), EM66, SL30, manserin, FA42, AM7; see Fig. 1) flanked by KR sites have been isolated from different tissues and are thus generated in vivo (Anouar et al. 1996; Kirchmair et al. 1993; Lee et al. 2010; Tilemans et al. 1994; Vaudry and Conlon 1991; Yajima et al. 2004), whereas the RK sites are probably not used for processing. In addition to these small peptides four longer processing intermediates of 20–90 kDa size have been identified by mass spectroscopy (Fournier et al. 2011; Gupta et al. 2010). These proteins include full-length SgII, two proteins cleaved at the C-terminal end at position 547 and 524 and one cleaved N-terminally at position 59. Position 527 represents dibasic cleavage site #7 (see Fig. 1) whereas cleavage sites 59 and 547 are non-dibasic sites. Cleavage site 547 is located in the manserin peptide and represents an Ala-IsoLeu site, the second non-dibasic cleavage site is located between Ala-58/Leu-59 in the N-terminal fragment of SgII (see Fig. 1). The proteolytic enzymes cleaving at these sites have not been characterized yet.
Structure and processing of secretogranin II (SgII). Human SgII contains 617 amino acids including a 27 amino acid signal peptide (SP). One potential tyrosine sulfation and phosphorylation site is shown. Sites of two consecutive basic amino acids are labelled by numbers. These sites with the exception of site 1 and 5 are used by prohormone convertases (PCs) for endoproteolytic processing. Proteins and peptides generated by PCs from the SgII precursor are shown below. Two non-dibasic cleavage sites are indicated by arrows. The name of the peptides represent their first and last amino acid in the single letter code plus the total length. SN, secretoneurin
Proteolytic processing of SgII is pronounced in most tissues, especially the central nervous system, resulting in more than 90% of SgII processed to small peptides including SN. In contrast, in the adrenal medulla, processing is more limited with the majority of SgII immunoreactivity existing as high molecular weight proteins of 20–86 kD (see Fig.1 in (Fischer-Colbrie et al. 1995). This limited processing is due to the high amount of catecholamines stored in adrenomedullary vesicles, which are potent inhibitors of prohormone convertases PC1 and PC2 (Wolkersdorfer et al. 1996). Also, in astrocytes SgII is mainly unprocessed (Fischer-Colbrie et al. 1993) reflecting the relative absence of prohormone convertase activity in these cells.
3 Subcellular Localization
In neurons and endocrine cells SgII is localized to the so-called large dense-cored synaptic vesicles (LDV). It is not found in the cytoplasm or other subcellular organelles like lysosomes. The subcellular localisation to the LDV was established by several means including subcellular fractionation techniques, as well as by immunohistochemistry and immune electron-microscopy. In fact, due to this well-established subcellular localisation SgII is considered and has been used in numerous studies as marker molecule for LDVs. In two studies SgII-immunoreactivity was also found in the nucleus by immune-electron microscopy and immunohistochemical techniques (Yajima et al. 2008; Yoo et al. 2007). It is currently not known whether epitopes shared by a nuclear protein cross-react or whether small amounts of SgII are indeed translocated to the nucleus. This for a secreted protein untypical nuclear localisation can only unequivocally be established when tissue from SgII knock-out mice becomes available. In any case the presence of a well-defined signal peptide rather argues against a nuclear form of SgII.
The N-terminal 27 amino acids of SgII comprise a signal peptide, which cause its co-translational translocation to the rough endoplasmatic reticulum. From there SgII is sorted to the LDV of the regulated pathway. Two regions of SgII, ie a putative -helix at the very N-terminus (SgII 25–41) and 16 amino acids in the middle region (SgII 334–348) have been identified as sorting motifs (Courel et al. 2008). Sorting of SgII depends on a saturable machinery which is greatly uncharacterized still. It has been suggested that sorting occurs via binding to secretogranin III (Hotta et al. 2009), another granin. However, in secretogranin III knock-down cells SgII was still secreted in a regulated manner (Sun et al. 2013) in line with a proposed sorting mechanism operating mainly by retention (Kuliawat and Arvan 1994; Tooze 1998).
4 Tissue Expression and Secretion from Cells
SgII is constitutively and abundantly expressed throughout the endocrine and nervous system. SgII has been identified in the adrenal medulla, all 3 lobes of the pituitary, the endocrine pancreas, in C-cells of the thyroid and endocrine cells of the whole gastrointestinal tract whereas the parathyroid gland is devoid of SgII (for details see (Fischer-Colbrie et al. 1995)). In the nervous system SgII is distributed in the phylogenetically older parts of the brain with high densities of immunoreactive terminals and fibers found in the hypothalamus, extended amygdala, hippocampus, lateral septum, medial thalamic nuclei, locus coeruleus, nucleus tractus solitarii and substantiae gelatinosae of the spinal cord (Fischer-Colbrie et al. 1995). In the peripheral nervous system SgII is expressed in sensory as well as sympathetic and parasympathetic neurons. In the eye SgII/SN immunoreactivity is expressed in amacrine cells of the retina (Overdick et al. 1996) and capsaicin-sensitive sensory neurons innervating the iris/ciliary complex (Troger et al. 2005).
Under pathological conditions SgII is induced in tissues, which per se do not express SgII. Ischaemia induces SgII expression in muscle cells of the hindlimb (Egger et al. 2007; Theurl et al. 2015) and the heart (Røsjø et al. 2012; Theurl et al. 2015). A similar phenomenon was seen in animal models of cerebral ischemia for neurons of the central nervous system (Kim et al. 2002; Marti et al. 2001). Furthermore, epithelial cells from adenocarcinomas of the prostate and the gastrointestinal tract can acquire a so-called endocrine phenotype by the induction of SgII synthesis (Pruneri et al. 1998).
Like other neuroendocrine secretory proteins SgII is released en bloc with co-stored classical transmitters, other granins and neuropeptides and biosynthetic enzymes from endocrine cells and neurons into circulation or the synaptic cleft, respectively. This secretory cocktail, however, contains only small amounts of intact, full-length SgII whereas the majority of SgII immunoreactivity comprises small SgII-derived peptides, i.e. SN or EM66, generated in the vesicles by proteolytic processing prior to release. Following depolarisation of cells by action potentials or hormonal signals SgII-derived peptides are exocytotically released in a calcium-dependent manner (Troger et al. 1994).
SN released from endocrine cells and neurons was detected in several body fluids. Serum steady-state SN levels of 22 fmol/ml originate from enterochromaffin cells of the gastrointestinal tract since other sources like the adrenal medulla, pituitary and the endocrine pancreas contribute only little (Ischia et al. 2000a). This is corroborated by the fact that high-emetogenic chemotherapy, which potently stimulates secretion from gastro-intestinal enterochromaffin cells, leads to a 50% increase of SN serum levels (Ischia et al. 2000a). Similarily, serum chromogranin A, which is co-stored together with SgII in these vesicles, is increased 2.5-fold (Cubeddu et al. 1995). SN levels are elevated 5-fold in childhood and are positively correlated with serum creatinine suggesting an influence of renal clearance on SN serum levels. This can be readily explained by the low molecular weight of SN. The half-life of SN was established experimentally as 2.5 h by analysing its decline in serum following removal of SN secreting tumors (Stridsberg et al. 2008). In the urine 80 fmol/ml have been found.
SN is furthermore released in significant amounts into the cerebro-spinal fluid (1500 fmol/ml (Eder et al. 1998; Miller et al. 1996), the aqueous humor (500 fmol/ml (Stemberger et al. 2004)), synovia (16 fmol/ml (Eder et al. 1997)) and faeces (Wagner et al. 2013).
5 Phylogenetic Conservation of Secretogranin II
SgII is well conserved during evolution. It is expressed in the entire vertebrate lineage including mammalia, birds, reptilia, amphibia and fish. SN is even found in agnatha like the lamprey (Trudeau et al. 2012) but has not been detected in any invertebrates so far. In concordance with the teleost-specific whole-genome duplication, teleost bony fish contain two moderately different SgII gene products. In some teleost fish like salmon even 4 SgII gene products exist (unpublished observation).
To date, SgII sequence information of up to 300 species has been deposited in the GenBank sequence database. The entire SgII precursor is highly conserved between mammalia, whereas between mammalia and fish only the region of SgII comprising the SN peptide is significantly homolog (see Kähler and Fischer-Colbrie 2000). Apart from SN only three additional regions with stretches of 5 or more amino acids conserved between human and zebrafish SgII can be identified (Fig. 2) suggesting that SN is indeed the physiological relevant peptide present within the SgII precursor.
Phylogenetic conservation of secretogranin II (SgII). The primary amino acid sequence of human and zebrafish SgII were compared. Homologous regions with a minimum of 5 identical amino acids are given in the single letter code. Only the region of SgII comprising the secretoneurin (SN) peptide is significantly conserved
Table 1 presents the high degree of phylogenetic conservation of SN peptide with 16 out of its 33 amino acids identical between human, mammalian, bird, reptile, amphibian, cartilage fish and pre-teleost bony fish. In teleost bony fish still 12 (gene A) and 10 (gene B) are identical with human homolog and SN is even found in agnatha like the lamprey. The middle part of SN comprises the best-conserved region, whereas the C-terminus varies greatly (see Table 1). It is interesting to note that the highly conserved part of SN is composed of 2 contiguous -helices as determined by two-dimensional 1NMR analysis (Oulyadi et al. 1997).
6 Gene Organisation and Gene Regulation
The secretogranin II gene is organized into 2 exons only, which are separated by a 3 kb intron (Schimmel et al. 1992). It is located as a single copy gene on human chromosome 2q35-q36 and mouse chromosome 1, respectively (Mahata et al. 1996). The entire open reading frame plus 15 nt of the 5′ untranslated region are located on exon 2, exon 1 contains only 5′ untranslated region.
In the SgII promoter region several regulatory elements have been identified. A functional cyclic AMP response element (CRE) is located 74 bp upstream of the transcription site (Scammell et al. 2000). This site is conserved during evolution and found in the human, rat and mouse SgII promoter and confers induced gene-expression in response to several stimuli including nicotine and PACAP (Mahata et al. 1999), histamine (Bauer et al. 1993), gonadotropin-releasing hormone (GnRH) (Song et al. 2003) and NO (Li et al. 2008). In addition, the SgII promoter contains 2 TRE-like elements (Li et al. 2008) and a serum-response element (SRE) (Mahata et al. 1999), which is present in the mouse and rat promoter but not conserved in human. Nevertheless, the inactivation of this SRE decreased SgII expression in response to nicotine and PACAP (Mahata et al. 1999). Recent studies demonstrated that SgII is a genuine target gene for the RE-1 silencing transcription factor (REST) (Hohl and Thiel 2005; Watanabe et al. 2004). Since REST is significantly repressed by hypoxia (Liang et al. 2014; Lin et al. 2016) this might explain the potent up-regulation of SgII under ischemic conditions (Egger et al. 2007) despite the absence of a hypoxia-response element (HRE) in the SgII promoter.
7 Biomarker and Disease
7.1 Tumors
Twenty years after the detection of chromogranin A as main secretory protein of adrenergic chromaffin cells its pan-endocrine expression was discovered (Cohn et al. 1982; O’Connor et al. 1983). This led to its rapid application as marker for an endocrine phenotype of normal and malign tissues. In the following years this concept was extended to other members of the chromogranin family, namely chromogranin B and SgII.
SgII has been identified in pituitary adenomas, gastro-enterohepatic carcinomas, pheochromocytomas, Merckel cell carcinoma, midgut carcinoids and oat cell lung and prostate carcinomas (Conlon 2010; Guillemot et al. 2006; Ischia et al. 2000a, b; Portela-Gomes et al. 2010; Wiedenmann et al. 1988). In general, the expression of chromogranin B and SgII in tumors comprising an endocrine phenotype is more restricted than that of chromogranin A. Only in the appendix the majority of carcinoids (94%) stained positive for SgII-immunoreactivity, whereas chromogranin A was expressed less frequent there (83%, (Prommegger et al. 1998)).
In the serum, elevated levels of SgII can be expected if tumors secrete actively. In general however, serum levels vary greatly between patients and tumor types depending on their renal clearance and the degree of proteolytic processing and secretory capacity of the individual tumor cells. For two peptides generated from SgII, i.e. SN and EM66 data are available. In gut carcinoids and endocrine pancreatic tumors SN serum levels were elevated 20- and 15-fold, in pheochromocytomas, oat cell carcinomas of the lung and neuroblastomas up to 2.5–4.5-fold (Guillemot et al. 2006; Ischia et al. 2000a).
7.2 CNS Diseases
In the CSF SN levels are on average 70-fold higher compared to serum. However, there is a marked inter-individuality limiting its usefulness as potential biomarker for neurological and psychiatric diseases. On average, SgII levels are decreased by 15% in patients with multiple sclerosis (Mattsson et al. 2007) and not altered in schizophrenic patients (Miller et al. 1996) and patients with Parkinson’s or Alzheimer’s disease (Eder et al. 1998). Post-mortem studies demonstrated that SgII levels are decreased in brains of patients with tauopathies (Lechner et al. 2004) and amyotrophic laterals sclerosis due to a loss of presynaptic large dense core vesicles (Schrott-Fischer et al. 2009).
7.3 Heart Failure
Serum levels of SN were increased 2.4-fold in patients with cardiac arrest in the first 7 days and back to normal levels after another week (Hasslacher et al. 2014). These findings were corroborated in patients with acute heart failure although in this study the increase was less pronounced (1.2-fold, (Ottesen et al. 2015; Hasslacher et al. 2014)). In both studies SN serum levels were significantly correlated with a poor clinical outcome. The source of increased serum SN levels after heart failure has not unequivocally been established. It has been proposed that under severe ischemic conditions SN leeks from the cerebrospinal fluid into circulation due to an impaired tightness of the blood brain barrier (Hasslacher et al. 2014). In accordance, SN levels in umbilical cord blood was elevated in neonates with hypoxic-ischaemic encephalopathy (Wechselberger et al. 2016).
8 Physiologic Function of Secretogranin II
8.1 Biogenesis of Secretory Granules
Chromogranins induce biogenesis of secretory granule in neuronal but also non-neuronal cells. After the initial discovery that gene ablation of chromogranin A leads to a reduced granule biosynthesis (Kim et al. 2005) a similar function was established for SgII. Expression of full-length SgII stimulated biogenesis of secretory granules in fibroblast cells (Beuret et al. 2004) and a secretory-deficient PC12 cell-line (Courel et al. 2010). From these newly formed secretory granules cargo was released in a calcium dependent manner (Beuret et al. 2004). Vice versa, silencing of SgII expression led to a decrease in the number and size of large dense vesicles (Courel et al. 2010). Thus, induction of SgII in non-neuronal tissues typically lacking secretory granules sorting of SgII into the trans-Golgi network might first induce granule biogenesis whereas later on after storage and proteolytic processing in these vesicles SgII might function in addition as a neuropeptide precursor protein. It seems justified to speculate that this scenario is initiated by ischemic conditions in skeletal muscle cells (Egger et al. 2007) or under endocrine differentiation of various adenocarcinomas (Courel et al. 2014).
8.2 Sorting and Release of Secretory Proteins
SgII facilitates sorting of proopiomelanocortin (POMC) to secretory vesicles and its release from AtT-20 cells (Sun et al. 2013). It remains to be demonstrated whether SgII promotes sorting of LDV secretory proteins like POMC per se or if its established granulogenic activity alone is sufficient to mediate this effect. Also, SgII alters release of viral particles from infected cells. Depending on the type of virus, shRNA mediated down-regulation of SgII can either decrease or increase the amount of viral particles excreted into medium (Berard et al. 2015).
8.3 Precursor of the Neuropeptide/Cytokine Secretoneurin
In recent years, numerous important physiological functions of SN in the nervous, immune and endocrine system as well as on blood vessels were unravelled (for a review see (Fischer-Colbrie et al. 2005)). In the nervous system SN stimulates dopamine release from rat striatal slices and basal ganglia in vivo. It stimulates neurite outgrowth and survival of cerebellar granules cells (Fujita et al. 1999; Gasser et al. 2003). In concordance, SgII following up-regulation by REST promotes differentiation and maturation of adult hippocampal progenitor cells (Kim et al. 2015). In the immune system SN displays potent chemotactic activity toward monocytes, eosinophils and dendritic cells, which has also been shown for smooth muscle cells and fibroblasts (Dunzendorfer et al. 1998, 2001; Kähler et al. 1997a, b; Reinisch et al. 1993). In the endocrine system SN inhibits melatonin release from melanocytes and stimulates gonodotropin II release from goldfish pituitary as well as food intake (Blázquez et al. 1998; Mikwar et al. 2016).
8.3.1 Angiogenesis and Vasculogenesis
In 2004 a potent angiogenic and vasculogenic property of SN was discovered. Due to biologic actions of SN on vascular cells such as induction of chemotaxis in endothelial or vascular smooth muscle cells (Kähler et al. 1997a, b) and the fact that SN-containing nerve fibers are closely associated with blood vessels in the uterus (Collins et al. 2000) we hypothesized that SN might induce the growth of new blood vessels out of the pre-existing vasculature, a process called angiogenesis. We observed that SN indeed induced angiogenesis in the mouse corneal neovascularization assay and that newly generated vessels are covered by smooth muscle cells what might indicate durable, stable vessels. In vitro SN induced angiogenesis in a matrigel assay, stimulated proliferation and inhibited apoptosis of endothelial cells (ECs) and activated prominent intracellular signal transduction pathways like Akt or MAPK (Kirchmair et al. 2004a). Beside angiogenesis new blood vessels might also be generated by circulating progenitor cells, a process called vasculogenesis. We could show that SN also stimulates incorporation of these cells into new blood vessels and activates these cells in vitro (Kirchmair et al. 2004b).
Regulation by Hypoxia. Angiogenic factors typically are up-regulated by hypoxia to counteract lack of oxygen by generation of new blood vessels. In the case of SN it was already shown that induction of hypoxia in the central nervous system by ligation of the carotid artery leads to up-regulation of SN in neurons of the hippocampus or the cerebral cortex (Marti et al. 2001). We could show that also muscle cells (a cell type that normally doesn’t produce SN) in the ischemic hindlimb after ligation of the femoral artery express SN. In vitro L6 myocytes also increased SN after prolonged hypoxia but this effect was indirect as the promoter region of the gene encoding the SN precursor secretogranin-II does not contain a hypoxia-responsive element and hypoxia did not increase SN in the absence of serum. We could show that SN increase by hypoxia was dependent on hypoxia-inducible factor 1 and basic fibroblast growth factor (FGF) in contrast to the direct regulation of vascular endothelial growth factor (VEGF) (Egger et al. 2007).
SN Gene Therapy. To explore if SN might possess therapeutic potential in the treatment of ischemic limb and heart disease we generated a plasmid gene therapy vector and could demonstrate biologic activity of recombinant SN by EC chemotaxis. After injection of plasmid into ischemic hindlimbs in mice SN improved clinical outcome (less limb necrosis) and increased tissue perfusion and density of capillaries and arteries compared to control plasmid. SN gene therapy additionally increased numbers endothelial progenitor cells in this ischemia model. These findings indicate induction of angiogenesis, arteriogenesis and vasculogenesis by SN gene therapy. We could also show that SN increased other potent angiogenic factors in ECs (basic fibroblast growth factor and platelet-derived growth factor B) and stimulated the nitric oxide pathway (Schgoer et al. 2009).
In the heart SN gene therapy improved systolic function and inhibited scar formation and remodelling of the left ventricle after an experimental myocardial infarction (permanent LAD ligation). Again, density of capillaries and arterioles/arteries in the infarct border zone was increased, consistent with induction of angiogenesis and arteriogenesis also in this ischemia model.
In arterial coronary ECs SN inhibited apoptosis, stimulated proliferation and in-vitro angiogenesis and activated Akt and MAPK. Interestingly, in vitro effects were blocked by a neutralizing antibody against VEGF, indicating that SN effects depend on VEGF. We indeed could demonstrate that SN stimulates activation of VEGF receptor-2 in a receptor tyrosine kinase (RTK) array. Further experiments elucidated that SN stimulates binding of VEGF to its co-receptors neuropillin-1 and heparan-sulfate proteoglycans. In RTK assays also activation of receptors for FGF and insulin-like growth factor-1 (IGF-1) was observed by SN. This activation of several potent angiogenic growth factor receptors by SN might be the reason for the robust effect of SN we observed on growth of new blood vessels as a complex biological effects like angiogenesis probably is mediated by different factors (Albrecht-Schgoer et al. 2012). A similar effect was shown recently in airway epithelial cells where it was demonstrated that SN stimulates mucus secretion by enhancing binding of epidermal growth factor (EGF) to neuropilin-1 (Xu et al. 2014).
These observations also might indicate that SN acts via binding to growth factors thereby stimulating binding of these factors to and activation of respective tyrosine kinase receptors instead of acting via an own specific cell surface receptor. Indeed, no specific SN receptor was detected so far. In this respect it is also of interest that SN was considered to act via a G-protein coupled receptor (GPCR) in cell migration experiments as effects were blocked by pertussis toxin. It will be interesting to elucidate if, in analogy to RTK, also GPCRs are activated by SN via classical chemotactic factors.
8.3.2 Wound Healing
To investigate potential effects of SN gene therapy we investigated wound healing in diabetic mice (db/db mice). Application of SN accelerated wound closure in this model and increased density of capillaries and arterioles in the wound. In microvascular dermal endothelial cells SN stimulated proliferation and in vitro angiogenesis in a basic-FGF dependent manner. We could show that FGF receptor-3 mediates SN-induced effects and that SN stimulates binding of basic FGF to heparan-sulfate proteoglycans on dermal endothelial cells (Albrecht-Schgoer et al. 2014). This finding corroborates our observation on coronary endothelial cells that SN stimulates receptors of potent angiogenic cytokines.
8.3.3 Effects of SN on Cerebral Ischemia
Systemic (intra-venous) application of SN in a rat model of cerebral ischemia reduced infarct area, enhanced motor performance and increased brain metabolic activity. In ischemic areas of the brain in this animal model as well as in human samples after stroke SN was increased in neuronal cells. In vitro SN inhibited apoptosis of neurons in cell culture after oxygen/glucose deprivation by stimulation of the Jak/Stat pathway. In this work it was also demonstrated that SN enhanced growth of blood vessels in the ischemic brain area and attracted neuronal stem cells (Shyu et al. 2008).
8.3.4 Effects of SN on Cardiomyocytes
Recently it was demonstrated that SN is taken up by cardiomyocytes and influences Calcium (Ca2+) handling in these cells by reduction of Ca2+/calmodulin (CaM)-dependent protein kinase II δ (CaMKIIδ) activity. SN binds to CaM and CaMKII and attenuates CaMKIIδ-dependent phosphorylation of the ryanodine receptor. SN also inhibits sarcoplasmic reticulum Ca2+ leak and augments sarcoplasmic reticulum Ca2+ content. SN also attenuates Ca2+ sparks and waves in cardiomyocytes (Ottesen et al. 2015). These findings indicate that SN might have a potential to inhibit arrythmias.
8.3.5 Effects of SN Gene Therapy on Animal Models of High Vascular Risk
Over the last two decades a variety of endothelial growth factors have been investigated as promising novel therapeutic agents for the treatment of peripheral arterial disease or myocardial ischemia. Unfortunately, the promising results obtained in animal models could not be verified in human trials so far. One reason might be that, in pre- clinical models, often young and healthy animals were studied, whereas in clinical trials patients with severe, long-lasting atherosclerosis and impaired vascular response were treated. Nevertheless, available data from clinical trials suggest that intramuscular injection of DNA might be safe and not associated with an increased rate of cancer. Due to promising results of SN gene therapy in hindlimb and myocardial ischemia we investigated the efficacy of SN gene transfer in two animal models (type I diabetes mellitus and hypercholesterolemia) with impaired angiogenic response (Schgoer et al. 2013; Theurl et al. 2015). In both animal models therapy of hindlimb ischemia with a SN-encoding plasmid resulted in significant improvement of limb reperfusion. Moreover, animals treated with the SN-plasmid showed a significant reduction in tissue defects and amputation rate. In the Apo E −/− mice, a model of severe hypercholesterolemia, we also evaluated the effect of SN-gene therapy in myocardial ischemia induced by permanent ligation of the left anterior descending artery. Similar to our data in rats SN treatment resulted in significant improvement of cardiac function as shown by echocardiographic parameters. Immunofluorescence staining revealed a significant increase of capillary and arteriole density as possible underlying mechanism for the favourable outcome of SN-treated animals.
Despite the negative influence of hypercholesterolemia and hyperglycemia on vascular cells, SN showed beneficial effects on EC function like proliferation, in vitro angiogenesis, or activation of the MAPK-ERK1/2 signaling cascade.
The local injection of the SN-plasmid did not result in elevated SN-serum levels and did not influence plaque progression as described for systemic administration of VEGF. Therefore, to our current knowledge, SN-gene therapy seems to be an effective and safe treatment strategy for hind limb and myocardial ischemia. Large animal models should be the next step to bring SN-gene therapy from bench to bedside.
References
Albrecht-Schgoer K, Schgoer W, Holfeld J, Theurl M, Wiedemann D, Steger C, Gupta R, Semsroth S, Fischer-Colbrie R, Beer AG, Stanzl U, Huber E, Misener S, Dejaco D, Kishore R, Pachinger O, Grimm M, Bonaros N, Kirchmair R (2012) The angiogenic factor secretoneurin induces coronary angiogenesis in a model of myocardial infarction by stimulation of vascular endothelial growth factor signaling in endothelial cells. Circulation 126:2491–2501
Albrecht-Schgoer K, Schgoer W, Theurl M, Stanzl U, Lener D, Dejaco D, Zelger B, Franz WM, Kirchmair R (2014) Topical secretoneurin gene therapy accelerates diabetic wound healing by interaction between heparan-sulfate proteoglycans and basic FGF. Angiogenesis 17:27–36
Anouar Y, Jégou S, Alexandre D, Lihrmann I, Conlon JM, Vaudry H (1996) Molecular cloning of frog secretogranin II reveals the occurrence of several highly conserved potential regulatory peptides. FEBS Lett 394:295–299
Bartolomucci A, Possenti R, Mahata SK, Fischer-Colbrie R, Loh YP, Salton SR (2011) The extended Granin family: structure, function, and biomedical implications. Endocr Rev 32:755–797
Bauer JW, Kirchmair R, Egger C, Fischer-Colbrie R (1993) Histamine induces a gene-specific synthesis regulation of secretogranin II but not of chromogranin a and B in chromaffin cells in a calcium-dependent manner. J Biol Chem 268:1586–1589
Berard AR, Severini A, Coombs KM (2015) Differential reovirus-specific and herpesvirus-specific activator protein 1 activation of secretogranin II leads to altered virus secretion. J Virol 89:11954–11964
Beuret N, Stettler H, Renold A, Rutishauser J, Spiess M (2004) Expression of regulated secretory proteins is sufficient to generate granule-like structures in constitutively secreting cells. J Biol Chem 279:20242–20249
Blázquez M, Bosma PT, Chang JP, Docherty K, Trudeau VL (1998) G-aminobutyric acid up-regulates the expression of a novel secretogranin-II messenger ribonucleic acid in the goldfish pituitary. Endocrinology 139:4870–4880
Cohn DV, Zangerle R, Fischer-Colbrie R, Chu LLH, Elting JJ, Hamilton JW, Winkler H (1982) Similarity of secretory protein I from parathyroid gland to chromogranin a from adrenal medulla. Proc Natl Acad Sci U S A 79:6056–6059
Collins JJ, Wilson K, Fischer-Colbrie R, Papka RE (2000) Distribution and origin of secretoneurin-immunoreactive nerves in the female rat uterus. Neuroscience 95:255–264
Conlon JM (2010) Granin-derived peptides as diagnostic and prognostic markers for endocrine tumors. Regul Pept 165:5–11
Courel M, El Yamani FZ, Alexandre D, El Fatemi H, Delestre C, Montero-Hadjadje M, Tazi F, Amarti A, Magoul R, Chartrel N, Anouar Y (2014) Secretogranin II is overexpressed in advanced prostate cancer and promotes the neuroendocrine differentiation of prostate cancer cells. Eur J Cancer 50(17):3039–3049
Courel M, Soler-Jover A, Rodriguez-Flores JL, Mahata SK, Elias S, Montero-Hadjadje M, Anouar Y, Giuly RJ, O’Connor DT, Taupenot L (2010) Pro-hormone secretogranin II regulates dense core secretory granule biogenesis in catecholaminergic cells. J Biol Chem 285:10030–10043
Courel M, Vasquez MS, Hook VY, Mahata SK, Taupenot L (2008) Sorting of the neuroendocrine secretory protein Secretogranin II into the regulated secretory pathway: role of N- and C-terminal alpha-helical domains. J Biol Chem 283:11807–11822
Cubeddu LX, O’Connor DT, Parmer RJ (1995) Plasma chromogranin A: a marker of serotonin release and of emesis associated with cisplatin chemotherapy. J Clin Oncol 13:681–687
Dunzendorfer S, Kaser A, Meierhofer C, Tilg H, Wiedermann CJ (2001) Peripheral neuropeptides attract immature and arrest mature blood-derived dendritic cells. J Immunol 166:2167–2172
Dunzendorfer S, Schratzberger P, Reinisch N, Kähler CM, Wiedermann CJ (1998) Secretoneurin, a novel neuropeptide, is a potent chemoattractant for human eosinophils. Blood 91:1527–1532
Eder U, Leitner B, Kirchmair R, Pohl P, Jobst KA, Smith AD, Mally J, Benzer A, Riederer P, Reichmann H, Saria A, Winkler H (1998) Levels and proteolytic processing of chromogranin a and B and secretogranin II in cerebrospinal fluid in neurological diseases. J Neural Transm 105:39–51
Eder U, Hukkanen M, Leitner B, Mur E, Went P, Kirchmair R, Fischer-Colbrie R, Polak JM, Winkler H (1997) The presence of secretoneurin in human synovium and synovial fluid. Neurosci Lett 224:139–131
Egger M, Schgoer W, Beer AG, Jeschke J, Leierer J, Theurl M, Frauscher S, Tepper OM, Niederwanger A, Ritsch A, Kearney M, Wanschitz J, Gurtner GC, Fischer-Colbrie R, Weiss G, Piza-Katzer H, Losordo DW, Patsch JR, Schratzberger P, Kirchmair R (2007) Hypoxia up-regulates the angiogenic cytokine secretoneurin via an HIF-1alpha- and basic FGF-dependent pathway in muscle cells. FASEB J 21:2906–2917
Erlich R, Gleeson PA, Campbell P, Dietzsch E, Toh BH (1996) Molecular characterization of trans-Golgi p230. A human peripheral membrane protein encoded by a gene on chromosome 6p12–22 contains extensive coiled-coil alpha-helical domains and a granin motif. J Biol Chem 271:8328–8337
Fischer-Colbrie R, Kirchmair R, Kähler CM, Wiedermann CJ, Saria A (2005) Secretoneurin: a new player in angiogenesis and chemotaxis linking nerves, blood vessels and the immune system. Curr Protein Pept Sci 6:373–385
Fischer-Colbrie R, Kirchmair R, Schobert A, Olenik C, Meyer DK, Winkler H (1993) Secretogranin II is synthesized and secreted in astrocyte cultures. J Neurochem 60:2312–2314
Fischer-Colbrie R, Gutierrez J, Hsu CM, Iacangelo A, Eiden LE (1990) Sequence analysis, tissue distribution and regulation by cell depolarization, and second messengers of bovine secretogranin II (chromogranin C) mRNA. J Biol Chem 265:9208–9213
Fischer-Colbrie R, Laslop A, Kirchmair R (1995) Secretogranin II: molecular properties, regulation of biosynthesis and processing to the neuropeptide secretoneurin. Prog Neurobiol 46:49–70
Fournier I, Gaucher D, Chich JF, Bach C, Shooshtarizadeh P, Picaud S, Bourcier T, Speeg-Schatz C, Strub JM, Van Dorsselaer A, Corti A, Aunis D, Metz-Boutigue MH (2011) Processing of chromogranins/secretogranin in patients with diabetic retinopathy. Regul Pept 167:118–124
Fujita Y, Katagi J, Tabuchi A, Tsuchiya T, Tsuda M (1999) Coactivation of secretogranin-II and BDNF genes mediated by calcium signals in mouse cerebellar granule cells. Mol Brain Res 63:316–324
Gasser MC, Berti I, Hauser KF, Fischer-Colbrie R, Saria A (2003) Secretoneurin promotes pertussis toxin-sensitive neurite outgrowth in cerebellar granule cells. J Neurochem 85:662–669
Guillemot J, Anouar Y, Montero-Hadjadje M, Grouzmann E, Grumolato L, Roshmaninho-Salgado J, Turquier V, Duparc C, Lefebvre H, Plouin PF, Klein M, Muresan M, Chow BK, Vaudry H, Yon L (2006) Circulating EM66 is a highly sensitive marker for the diagnosis and follow-up of pheochromocytoma. Int J Cancer 118:2003–2012
Gupta N, Bark SJ, Lu WD, Taupenot L, O’Connor DT, Pevzner P, Hook V (2010) Mass spectrometry-based neuropeptidomics of secretory vesicles from human adrenal medullary pheochromocytoma reveals novel peptide products of prohormone processing. J Proteome Res 9:5065–5075
Hasslacher J, Lehner GF, Harler U, Beer R, Ulmer H, Kirchmair R, Fischer-Colbrie R, Bellmann R, Dunzendorfer S, Joannidis M (2014) Secretoneurin as a marker for hypoxic brain injury after cardiopulmonary resuscitation. Intensive Care Med 40:1518–1527
Helle KB (2010) Chromogranins a and B and secretogranin II as prohormones for regulatory peptides from the diffuse neuroendocrine system. Results Probl Cell Differ 50:21–44
Hohl M, Thiel G (2005) Cell type-specific regulation of RE-1 silencing transcription factor (REST) target genes. Eur J Neurosci 22:2216–2230
Hotta K, Hosaka M, Tanabe A, Takeuchi T (2009) Secretogranin II binds to secretogranin III and forms secretory granules with orexin, neuropeptide Y, and POMC. J Endocrinol 202:111–121
Huttner WB, Gerdes HH, Rosa P (1991) The granin (chromogranin/secretogranin) family. TIBS 16:27–30
Ischia R, Gasser RW, Fischer-Colbrie R, Eder U, Pagani A, Cubeddu LX, Lovisetti-Scamihorn P, Finkenstedt G, Laslop A, Winkler H (2000a) Levels and molecular properties of secretoneurin-immunoreactivity in the serum and urine of control and neuroendocrine tumor patients. J Clin Endocrinol Metab 85:355–360
Ischia R, Hobisch A, Bauer R, Weiss U, Gasser RW, Horninger W, Bartsch G Jr, Fuchs D, Bartsch G, Winkler H, Klocker H, Fischer-Colbrie R, Culig Z (2000b) Elevated levels of serum secretoneurin in patients with therapy resistant carcinoma of the prostate. J Urol 163:1161–1164
Kähler CM, Kirchmair R, Kaufmann G, Kähler STEA, Reinisch N, Fischer-Colbrie R, Hogue-Angeletti R, Winkler H, Wiedermann CJ (1997a) Inhibition of proliferation and stimulation of migration of endothelial cells by secretoneurin in vitro. Arterioscler Thromb Vasc Biol 17:932–939
Kähler CM, Schratzberger P, Wiedermann CJ (1997b) Response of vascular smooth muscle cells to the neuropeptide secretoneurin. A functional role for migration and proliferation in vitro. Arterioscler Thromb Vasc Biol 17:2029–2035
Kähler CM, Fischer-Colbrie R (2000) Secretoneurin - a novel link between the nervous and the immune system. Conservation of the sequence and functional aspects. Adv Exp Med Biol 482:279–290
Kim HJ, Denli AM, Wright R, Baul TD, Clemenson GD, Morcos AS, Zhao C, Schafer ST, Gage FH, Kagalwala MN (2015) REST regulates non-cell-autonomous neuronal differentiation and maturation of neural progenitor cells via secretogranin II. J Neurosci 35:14872–14884
Kim T, Zhang CF, Sun Z, Wu H, Loh YP (2005) Chromogranin a deficiency in transgenic mice leads to aberrant chromaffin granule biogenesis. J Neurosci 25:6958–6961
Kim YD, Sohn NW, Kang C, Soh Y (2002) DNA array reveals altered gene expression in response to focal cerebral ischemia. Brain Res Bull 58:491–498
Kirchmair R, Egger M, Walter DH, Eisterer W, Niederwanger A, Woell E, Nagl M, Pedrini M, Murayama T, Frauscher S, Hanley A, Silver M, Brodmann M, Sturm W, Fischer-Colbrie R, Losordo DW, Patsch JR, Schratzberger P (2004a) Secretoneurin, an angiogenic neuropeptide, induces postnatal vasculogenesis. Circulation 110:1121–1127
Kirchmair R, Gander R, Egger M, Hanley A, Silver M, Ritsch A, Murayama T, Kaneider N, Sturm W, Kearny M, Fischer-Colbrie R, Kircher B, Gaenzer H, Wiedermann CJ, Ropper AH, Losordo DW, Patsch JR, Schratzberger P (2004b) The neuropeptide secretoneurin acts as a direct angiogenic cytokine in vitro and in vivo. Circulation 109:777–783
Kirchmair R, Hogue-Angeletti R, Gutierrez J, Fischer-Colbrie R, Winkler H (1993) Secretoneurin--a neuropeptide generated in brain, adrenal medulla and other endocrine tissues by proteolytic processing of secretogranin II (chromogranin C). Neuroscience 53:359–365
Kuliawat R, Arvan P (1994) Distinct molecular mechanisms for protein sorting within immature secretory granules of pancreatic beta-cells. J Cell Biol 126:77–86
Lechner T, Adlassnig C, Humpel C, Kaufmann WA, Maier H, Reinstadler-Kramer K, Hinterholzl J, Mahata SK, Jellinger KA, Marksteiner J (2004) Chromogranin peptides in Alzheimer’s disease. Exp Gerontol 39:101–113
Lee JE, Atkins N, Hatcher NG, Zamdborg L, Gillette MU, Sweedler JV, Kelleher NL (2010) Endogenous peptide discovery of the rat circadian clock: a focused study of the suprachiasmatic nucleus by ultrahigh performance tandem mass spectrometry. Mol Cell Proteomics 9:285–297
Li L, Hung AC, Porter AG (2008) Secretogranin II: a key AP-1-regulated protein that mediates neuronal differentiation and protection from nitric oxide-induced apoptosis of neuroblastoma cells. Cell Death Differ 15:879–888
Liang H, Studach L, Hullinger RL, Xie J, Andrisani OM (2014) Down-regulation of RE-1 silencing transcription factor (REST) in advanced prostate cancer by hypoxia-induced miR-106b~25. Exp Cell Res 320:188–199
Lin TP, Chang YT, Lee SY, Campbell M, Wang TC, Shen SH, Chung HJ, Chang YH, Chiu AW, Pan CC, Lin CH, Chu CY, Kung HJ, Cheng CY, Chang PC (2016) REST reduction is essential for hypoxia-induced neuroendocrine differentiation of prostate cancer cells by activating autophagy signaling. Oncotarget 7:26137–26151
Mahata SK, Kozak CA, Szpirer J, Szpirer C, Modi WS, Gerdes HH, Huttner WB, O’Connor DT (1996) Dispersion of chromogranin/secretogranin secretory protein family loci in mammalian genomes. Genomics 33:135–139
Mahata SK, Mahata M, Livsey CV, Gerdes HH, Huttner WB, O’Connor DT (1999) Neuroendocrine cell type-specific and inducible expression of the secretogranin II gene: crucial role of cyclic adenosine monophosphate and serum response elements. Endocrinology 140:739–749
Marti E, Ferrer I, Blasi J (2001) Differential regulation of chromogranin a, chromogranin B and secretoneurin protein expression after transient forebrain ischemia in the gerbil. Acta Neuropathol 101:159–166
Mattsson N, Ruetschi U, Podust VN, Stridsberg M, Li S, Andersen O, Haghighi S, Blennow K, Zetterberg H (2007) Cerebrospinal fluid concentrations of peptides derived from chromogranin B and secretogranin II are decreased in multiple sclerosis. J Neurochem 103:1932–1939
Mikwar M, Navarro-Martin L, Xing L, Volkoff H, Hu W, Trudeau VL (2016) Stimulatory effect of the secretogranin-ll derived peptide secretoneurin on food intake and locomotion in female goldfish (Carassius Auratus). Peptides 78:42–50
Miller C, Kirchmair R, Troger J, Saria A, Fleischhacker WW, Fischer-Colbrie R, Benzer A, Winkler H (1996) CSF of neuroleptic-naive first-episode schizophrenic patients: levels of biogenic amines, substance P, and peptides derived from chromogranin a (GE-25) and secretogranin II (secretoneurin). Biol Psychol 39:911–918
O’Connor DT, Burton D, Deftos LJ (1983) Chromogranin a: immunohistology reveals its universal occurrence in normal polypeptide hormone producing endocrine glands. Life Sci 33:1657–1663
Ottesen AH, Louch WE, Carlson CR, Landsverk OJ, Kurola J, Johansen RF, Moe MK, Aronsen JM, Hoiseth AD, Jarstadmarken H, Nygard S, Bjoras M, Sjaastad I, Pettila V, Stridsberg M, Omland T, Christensen G, Rosjo H (2015) Secretoneurin is a novel prognostic cardiovascular biomarker associated with cardiomyocyte calcium handling. J Am Coll Cardiol 65:339–351
Oulyadi H, Davoust D, Vaudry H (1997) A determination of the solution conformation of secretoneurin, a neuropeptide originating from the processing of secretogranin II, by 1H-NMR and restrained molecular dynamics. Eur J Biochem 246:665–673
Overdick B, Kirchmair R, Marksteiner J, Fischer-Colbrie R, Troger J, Winkler H, Saria A (1996) Presence and distribution of a new neuropeptide, secretoneurin, in human retina. Peptides 17:1–4
Portela-Gomes GM, Grimelius L, Wilander E, Stridsberg M (2010) Granins and granin-related peptides in neuroendocrine tumours. Regul Pept 165:12–20
Prommegger R, Obrist P, Ensinger C, Schwelberger HG, Wolf C, Fischer-Colbrie R, Mikuz G, Bodner E (1998) Secretoneurin in carcinoids of the appendix – immunohistochemical comparison with chromogranins a, B and secretogranin II. Anticancer Res 18:3999–4002
Pruneri G, Galli S, Rossi RS, Roncalli M, Coggi G, Ferrari A, Simonato A, Siccardi AG, Carboni N, Buffa R (1998) Chromogranin a and B and secretogranin II in prostatic adenocarcinomas: neuroendocrine expression in patients untreated and treated with androgen deprivation therapy. Prostate 34:113–120
Reinisch N, Kirchmair R, Kähler CM, Hogue-Angeletti R, Fischer-Colbrie R, Winkler H, Wiedermann CJ (1993) Attraction of human monocytes by the neuropeptide secretoneurin. FEBS Lett 334:41–44
Røsjø H, Stridsberg M, Florholmen G, Stenslokken KO, Ottesen AH, Sjaastad I, Husberg C, Dahl MB, Oie E, Louch WE, Omland T, Christensen G (2012) Secretogranin II; a protein increased in the myocardium and circulation in heart failure with cardioprotective properties. PLoS One 7:e37401
Scammell JG, Reddy S, Valentine DL, Coker TN, Nikolopoulos SN, Ross RA (2000) Isolation and characterization of the human secretogranin II gene promoter. Mol Brain Res 75:8–15
Schgoer W, Theurl M, Albrecht-Schgoer K, Jonach V, Koller B, Lener D, Franz WM, Kirchmair R (2013) Secretoneurin gene therapy improves blood flow in an ischemia model in type 1 diabetic mice by enhancing therapeutic neovascularization. PLoS One 8:e74029
Schgoer W, Theurl M, Jeschke J, Beer AG, Albrecht K, Gander R, Rong S, Vasiljevic D, Egger M, Wolf AM, Frauscher S, Koller B, Tancevski I, Patsch JR, Schratzberger P, Piza-Katzer H, Ritsch A, Bahlmann FH, Fischer-Colbrie R, Wolf D, Kirchmair R (2009) Gene therapy with the angiogenic cytokine secretoneurin induces therapeutic angiogenesis by a nitric oxide-dependent mechanism. Circ Res 105:994–1002
Schimmel A, Bräunling O, Rüther U, Huttner WB, Gerdes H-H (1992) The organisation of the mouse secretogranin II gene. FEBS Lett 3:375–380
Schrott-Fischer A, Bitsche M, Humpel C, Walcher C, Maier H, Jellinger K, Rabl W, Glueckert R, Marksteiner J (2009) Chromogranin peptides in amyotrophic lateral sclerosis. Regul Pept 152:13–21
Shyu WC, Lin SZ, Chiang MF, Chen DC, Su CY, Wang HJ, Liu RS, Tsai CH, Li H (2008) Secretoneurin promotes neuroprotection and neuronal plasticity via the Jak2/Stat3 pathway in murine models of stroke. J Clin Invest 118:133–148
Song SB, Rhee M, Roberson MS, Maurer RA, Kim KE (2003) Gonadotropin-releasing hormone-induced stimulation of the rat secretogranin II promoter involves activation of CREB. Mol Cell Endocrinol 199:29–36
Stemberger K, Pallhuber J, Doblinger A, Troger J, Kirchmair R, Kralinger M, Fischer-Colbrie R, Kieselbach G (2004) Secretoneurin in the human aqueous humor and the absence of an effect of frequently used eye drops on the levels. Peptides 25:2115–2118
Stridsberg M, Eriksson B, Janson ET (2008) Measurements of secretogranins II, III, V and proconvertases 1/3 and 2 in plasma from patients with neuroendocrine tumours. Regul Pept 148:95–98
Sun M, Watanabe T, Bochimoto H, Sakai Y, Torii S, Takeuchi T, Hosaka M (2013) Multiple sorting systems for secretory granules ensure the regulated secretion of peptide hormones. Traffic 14:205–218
Taupenot L, Harper KL, O’Connor DT (2003) The chromogranin-secretogranin family. N Engl J Med 348:1134–1149
Theurl M, Schgoer W, Albrecht-Schgoer K, Lener D, Wolf D, Wolf M, Demetz E, Tymoszuk P, Tancevski I, Fischer-Colbrie R, Franz WM, Marschang P, Kirchmair R (2015) Secretoneurin gene therapy improves hind limb and cardiac ischaemia in Apo E−/− mice without influencing systemic atherosclerosis. Cardiovasc Res 105:96–106
Tilemans D, Jacobs GFM, Andries M, Proost P, Devreese B, Van Damme J, Van Beeumen J, Denef C (1994) Isolation of two peptides from rat gonadotroph-conditioned medium displaying an amino acid sequence identical to fragments of secretogranin II. Peptides 15:537–545
Tooze SA (1998) Biogenesis of secretory granules in the trans-Golgi network of neuroendocrine and endocrine cells. Biochim Biophys Acta 1404:231–244
Troger J, Doblinger A, Leierer J, Laslop A, Schmid E, Teuchner B, Opatril M, Philipp W, Klimaschewski L, Pfaller K, Gottinger W, Fischer-Colbrie R (2005) Secretoneurin in the peripheral ocular innervation. Invest Ophthalmol Vis Sci 46:647–654
Troger J, Kirchmair R, Marksteiner J, Seidl CV, Fischer-Colbrie R, Saria A, Winkler H (1994) Release of secretoneurin and noradrenaline from hypothalamic slices and its differential inhibition by calcium channel blockers. Naunyn-Schmied Arch Pharmacol 349:565–569
Trudeau VL, Martyniuk CJ, Zhao E, Hu H, Volkoff H, Decatur WA, Basak A (2012) Is secretoneurin a new hormone? Gen Comp Endocrinol 175:10–18
Vaudry H, Conlon JM (1991) Identification of a peptide arising from the specific post-translation processing of secretogranin II. FEBS Lett 284:31–33
Wagner M, Stridsberg M, Peterson CG, Sangfelt P, Lampinen M, Carlson M (2013) Increased fecal levels of chromogranin a, chromogranin B, and secretoneurin in collagenous colitis. Inflammation 36:855–861
Watanabe Y, Kameoka S, Gopalakrishnan V, Aldape KD, Pan ZZ, Lang FF, Majumder S (2004) Conversion of myoblasts to physiologically active neuronal phenotype. Genes Dev 18:889–900
Wechselberger K, Schmid A, Posod A, Hock M, Neubauer V, Fischer-Colbrie R, Kiechl-Kohlendorfer U, Griesmaier E (2016) Secretoneurin serum levels in healthy term neonates and neonates with hypoxic-ischaemic encephalopathy. Neonatology 110:14–20
Wiedenmann B, Waldherr R, Buhr H, Hille A, Rosa P, Huttner WB (1988) Identification of gastroenteropancreatic neuroendocrine cells in normal and neoplastic human tissue with antibodies against synaptophysin, chromogranin a, secretogranin I (chromogranin B), and secetogranin II. Gastroenterology 95:1364–1374
Wolkersdorfer M, Laslop A, Lazure C, Fischer-Colbrie R, Winkler H (1996) Processing of chromogranins in chromaffin cell culture: effects of reserpine and a-methyl p-tyrosine. Biochem J 316:953–958
Xu R, Li Q, Zhou J, Zhou X, Perelman JM, Kolosov VP (2014) Secretoneurin induces airway mucus hypersecretion by enhancing the binding of EGF to NRP1. Cell Physiol Biochem 33:446–456
Yajima A, Narita N, Narita M (2008) Recently identified a novel neuropeptide manserin colocalize with the TUNEL-positive cells in the top villi of the rat duodenum. J Pept Sci 14:773–776
Yajima A, Ikeda M, Miyazaki K, Maeshima T, Narita N, Narita M (2004) Manserin, a novel peptide from secretogranin II in the neuroendocrine system. Neuroreport 15:1755–1759
Yoo SH, Chu SY, Kim KD, Huh YH (2007) Presence of secretogranin II and high-capacity, low-affinity Ca2+ storage role in nucleoplasmic Ca2+ store vesicles. Biochemistry 46:14663–14671
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Fischer-Colbrie, R., Theurl, M., Kirchmair, R. (2017). Secretogranin II: Novel Insights into Expression and Function of the Precursor of the Neuropeptide Secretoneurin. In: Angelone, T., Cerra, M., Tota, B. (eds) Chromogranins: from Cell Biology to Physiology and Biomedicine. UNIPA Springer Series. Springer, Cham. https://doi.org/10.1007/978-3-319-58338-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-58338-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58337-2
Online ISBN: 978-3-319-58338-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)