Abstract
Research on chromaffin cells dates back to 1856 when the venous outflow of chemical substances from the adrenal medulla into the circulation was first described. The discovery of the chromaffin granules for storage of catecholamines in 1953 was the next major break-through. Soon thereafter the co-storage of catecholamines, ATP and uniquely acidic proteins was established, together making up the isotonic storage complex within elements of the diffuse sympathoadrenal system. The core proteins constitute a family of eight genetically distinct, uniquely acidic proteins, characterized by numerous pairs of basic residues and collectively named granins. A prohormone concept was formulated when the insulin-release inhibiting peptide, pancreastatin, was identified as the mid sequence of porcine chromogranin A. Subsequently, processing resulted in a range of peptides with antifungal and antibacterial potencies, predominantly from chromogranin A, a few from chromogranin B and one from secretogranin II. A wide range of biological activites has since been documented, notably for the chromogranin A –derived peptides, affecting endothelial stability, myocardial contractility, angiogenesis, cell adhesion and tumor progression. A physiological role for full-length chromogranin A and vasostatin-I as circulating stabilizers of endothelial integrity is now evident, while the high circulating levels of chromogranin A in neuroendocrine tumors and inflammatory diseases remain an unsolved and challenging puzzle for future research.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Chromogranin A
- Chromogranin B
- Secretogranin II
- Vasostatin-I
- Vasostatin-II
- Chromofungin
- Pancreastatin
- Catestatin
- Serpinin
- Secretolytin
- Secretoneurin
- Calcium homeostasis
- Glucose homeostasis
- Endothelial stability
- Myocardial contractility
- Angiogenisis
- Cell adhesion
- Tumor progression
1 History
1.1 The First Hundred Years of the Chromaffin Cells
Research on chromaffin cells and granins can be traced back to the mid nineteenth century when Vulpian (1856) described the venous outflow of chemical substances from the adrenal medulla into the circulation. Half a century later the strong cardiovascular effects of the adrenomedullary substances (Oliver and Schäfer 1895) led to the chemical identification and synthesis of the first hormones, adrenaline and noradrenaline (Stoltz 1904). We owe the first identification of catecholamines (CA) to the function of the adrenergic neuron to Loewi, who in Loewi 1921 described the so-called Accellerans-Stoff or Sympathin and its stimulating activity on the denervated frog heart. Twenty five years later, Sympathin E was identified as noradrenaline (Von Euler 1946).
1.2 The First Decade of the Chromaffin Granules
The discovery of the subcellular organelles responsible for the storage of CA in the adrenomedullary chromaffin cells, i.e. the chromaffin granules, was a major break-through (Blaschko and Welsch 1953, Hillarp et al. 1953). Soon thereafter the chromaffin granules were shown to be electron-dense, membrane-limited granules of 150–300 mμ diameter (Lever 1955, Welzstein 1957, Hagen and Barnett 1960, Coupland 1968). In parallel, the vesicles related to the storage of noradrenaline in the adrenergic fibres (Von Euler and Hillarp 1956, Von Euler 1958, Dahlstrøm 1966) were demonstrated to be smaller and of varying size and electron density both in the axons and in the terminals (De Robertis and Pellegrino de Iraldi 1961). Biochemical studies, on the other hand, revealed that both types of organelles bore a number of similarities, such as storing the respective CA together with the energy-rich nucleotide ATP in a molar ratio of CA: ATP of close to 4:1 in the adrenomedullary (Blaschko et al. 1956, Falck et al. 1956) and of 5:1 in the adrenergic nerve granules (Schümann 1958; Banks et al. 1969). Moreover, in the adrenomedullary chromaffin cells these low molecular weight constituents were stored intragranularly at concentrations of about 0.55 and 0.13 M for CA and ATP respectively, i.e. strongly hypertonic if osmotically active. This phenomenon led Hillarp in 1959 to the postulation of a third component involved in the storage complex, possibly a protein, which could be responsible for holding CA and ATP in an isotonic, non-diffusible form until discharge from the stimulated cell.
1.3 The First Thirty Five Years of the Granins
The search for a specific macromolecule involved in the isotonic retention of CA and ATP within the storage organelles was immediately directed to the core proteins in the bovine adrenomedullary chromaffin granules (Helle 1966a, Smith and Winkler 1967, Smith and Kirshner 1967). By means of an immunological identification method (Helle 1966b) it was established that the enzymatically inactive protein, subsequently named chromogranin (Blaschko et al. 1967), was exocytotically discharged from the stimulated adrenal gland in parallel with the co-stored CA and ATP both in vitro (Banks and Helle 1965) and in vivo (Blaschko et al. 1967). Due to the easy access from local slaughterhouses the bovine adrenals soon became a convenient source of chromaffin cells and chromaffin granules (Smith and Winkler 1967), notably for research on the structural, chemical and functional properties of the family of chromogranins, i.e. the granins (Huttner et al. 1991; Winkler and Fischer-Colbrie 1992).
1.3.1 Glucose Homeostasis, Pancreastatin and the Prohormone Concept
The first chromogranin A (CgA) peptide to be recognized for its regulatory potency was named pancreastatin due to its ability to inhibit the rapid phase of insulin release from the glucose-stimulated porcine pancreas (Tatemoto et al. 1986; Efendic et al. 1987). When identified as the mid-section of porcine and human CgA (Huttner and Benedum 1987; Konecki et al. 1987), a novel concept was coined, namely of the granins as putative prohormones for biologically active peptides with regulating potentials (Eiden 1987). Subsequently, pancreastatin was shown to be involved as a regulator of insulin action not only of glucose but also of lipid and protein metabolism (Sanchez-Margalet and Gonzalez-Yanes 1998). In rat hepatoma cells also the cell growth was inhibited, depending on the availability of nitric oxide (NO) production (Sanchez-Margalet et al. 2001). The accumulated literature supports the original observation of pancreastatin as an anti-insulin agent, impairing glucose homeostasis by diminishing insulin sensitivity (see review by Valicherla et al. 2013).
1.3.2 Calcium Homeostasis and the N-Terminus of CgA
In the parathyroid gland CgA was originally described as parathyroid secretory protein-I (Cohn et al. 1981), co-secreted from the gland with the parathyroid hormone (PTH), i. e. the primary regulator of serum calcium concentrations. Peptides containing the N-terminal sequence of CgA (CgA1–76) inhibit PTH-secretion as effective as high physiological concentrations of calcium (Fasciotto et al. 1990). Pancreastatin (bCgA248–293) and parastatin (bCgA347–419) have also been shown to inhibit PTH secretion, but not yet detected in the effluents from the parathyroid cells in vivo. On the other hand, CgA1–76 was detected both in the medium of cultured parathyroid cells (Angeletti et al. 2000) and in the adrenomedullary effluents (Metz-Boutigue et al. 1993). A binding to a 78 kDa protein was identified on the parathyroid cell surface, and the blockade by pertussis toxin indicates a G-protein-coupled receptor. Moreover, the loop sequence CgA16–40 was required for inhibition of PTH secretion (Angeletti et al. 1996). Thus, inhibition of PTH secretion by CgA predominantly involves CgA1–76, occuring either by an autocrine mechanism or via the circulating concentrations of the processed peptide.
1.4 The Granins and their Derived Peptides
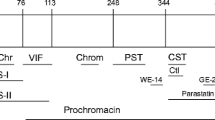
Detailed investigations of the eight members of the granin family, i.e. CgA, chromogranin B (CgB), secretogranin II (SgII) and secretogranins III-VII, have since documented that these proteins are widely distributed in distinct patterns within the diffuse neuroendocrine system of vertebrates (Helle 2004). Stimuli for release of the granins derive from a wide range of environmental and intrinsic paths, raising the concentrations of the intact prohormones and processed peptides in the extracellular space and ultimately in the circulation. The degree of processing is extensive in the adrenomedullary storage granules (Metz-Boutigue et al. 1993; Strub et al. 1995) and gives rise to a wide range of peptides with a broad spectrum of biological potencies (Helle and Angeletti 1994). The peptides derived from CgA are the vasostatins I and II, chromofungin, chromacin, pancreastatin, catestatin, WE 14, chromostatin, GE25 and parastatin and, in addition, the two most resent arrivals on the scene, serpinin (CgA403–428, Koshimizu et al. 2010) and the vasoconstriction-inhibiting factor (VIF, CgA79–113, Salem et al. 2015). Vasostatin I (VS-I, CgA1–76) and bovine catestatin (bCgA344–364) were discovered and named according to their respective inhibitory potencies, on vasodilation (Aardal and Helle 1992) and on CA secretion (Mahata et al. 1997). Since then, notably VS-I and catestatin have been shown to be involved in regulation of a wide range of mechanisms, such as endothelial permeability, angiogenesis, myocardial contractility and innate immunity, however, in many tissue exhibiting oppositely directed activities (Helle et al. 2007; Helle 2010a, b; Mahata et al. 2010).
Peptides derived from CgB, being more extensively processed than CgA in most systems and species (Strub et al. 1995), may have specific regulatory functions yet to be unravelled. SgII, on the other hand, serves a prohormone for only one conspicuously active principle, secretoneurin (Kirchmair et al. 1993, Trudeau et al. 2012), nevertheless engaged in a wide range of modulating activities related to tissue repair (Helle 2010a). Stimulated polymorphonuclear neutrophils, when accumulated in response to invading microorganism, tissue inflammation and at sites of mechanical injury, represent a non-neuroendocrine source of CgA peptides that may affect a wide range of cells involved in inflammatory responses (Lugardon et al. 2000; Zhang et al. 2009). Among them we find the vascular endothelium, the endocardium and the epithelial cells, other leucocytes, fibroblasts, cardiomyocytes, vascular and intestinal smooth muscle cells (Helle et al. 2007; Helle 2010a, b). Taken together, the release of CgA-derived peptides from gland cells, nerve terminals and immunocytes would contribute to autocrine or paracrine modulations locally while endocrine effects would result from their subsequent overflow to the circulation.
1.4.1 The Antimicrobial Peptides and Innate Immunity
Antimicrobial activities of peptides derived from the matrix of secretory granules in the bovine adrenal medulla were first reported by Metz-Boutigue and colleagues in 1998. The first three peptides found to inhibit bacteria and fungal growth were derived from the N-terminal domain of CgA (VS-I), the C-terminal end of CgB (secretolytin) and the biphosphorylated C-terminal peptide of proenkephalin-A (enkelytin). These peptides are active in a diverse range of organisms, including prokaryotes, bivalves, frogs and mammals, suggesting an important role in innate immunity, a mechanism shared by all vertebrates and present at birth as an evolutionary ancient defence mechanism (Hoffmann et al. 1999; Metz-Boutigue et al. 2000). Another CgA peptide, catestatin, derived from CgA in keratinocytes, also possess antimicrobial activity against gram-positive and gram-negative bacteria, yeast and fungi, is active notably against skin pathogens and increases in skin in response to injury and infection (Radek et al. 2008). So far, no antimicrobial activity has been assigned to SN.
The innate immunity, independent of the adaptive immune responses, is used by vertebrates as a means for short term protection against pathogenic microorganisms. The need for new antimicrobial agents is now rapidly rising due to the fast growing number of antibiotica-resistant bacteria. Accordingly, the interest in antibacterial granin-derived peptides has grown exponentially. Their therapeutic potentials are now under intensive elucidation in immunodeficient patients, in chemotherapy, in organ grafting, and against antibiotica-resistant bacterial infections (Shooshtarizodeh et al. 2010).
1.5 Functional and Clinical Aspects
At the very end of the second millennium a large body of data had accumulated on the functional and clinical aspects of the granins and their derived peptides. As assessed in a range of comprehensive reviews appearing in the first book on chromogranins (Helle and Aunis 2000a), it was evident that granins were intimately involved not only in the intracellular sorting to the secretory granules (Gerdes and Glombik 2000) and release of the isotonic amine storage complex (Borges et al. 2000), but also in their transcription, expression and secretion (Taupenot et al. 2000; Anouar et al. 2000; Kähler and Fischer-Colbrie 2000). Notably, tissue-specific processing both within the core and in the extracellular space, rendered the granins, notably CgA, CgB and Sg II as the most conspicuous prohormones with widely different effects and targets for their derived peptides (Aunis and Metz-Boutigue 2000; Metz-Boutigue et al. 2000; Parmer et al. 2000; Portela-Gomes 2000; Curry et al. 2000; Ciesielski-Treska and Aunis 2000). Accordingly, the majority of properties assigned to the granins and their peptides up to the end of the twentieth century appeared to fit into patterns of modulating strategies which might be called upon when the organism was exposed to stressful situations requiring immediate protection via the vasculature, the heart, the pancreas, parathyroid and the innate immunity system (Helle and Aunis 2000b). Moreover, since the discovery of CgA as a circulating component in patients with phochromocytoma (O’Connor and Bernstein 1984), a large body of literature implicates granins, notably CgA, as markers for a variety of diseases, such as neuroendocrine tumors, chronic heart failure and brain disorders like Parkinson’s and Alzheimer’s (O’Connor et al. 2000).
Since the turn of the century the research interest in the granins, notably in CgA and its derived peptides, has surged, as indicated by the registered 460 reviews since year 2000 of a total of 630 on CgA since 1970. Similarly, the number of papers dealing with VS-I and catestatin has grown steadily since their respective discoveries in 1992 and 1997, reaching a total of 76 and 154 for VS-I and catestatin in 2016. The major achievements will be outlined in the following sections.
1.5.1 Vasostatins, Vasodilations, the Vascular Endothelium and Angiogenesis
The human internal thoracic artery and saphenous vein were the first targets to be examined for vascular responses to the N-terminal CgA1–76 and CgA1–113 (Aardal and Helle 1992; Aardal et al. 1993). The potent contractions to endothelin-1 (ET-1) were suppressed, affecting the maximal sustained tension response but not the potency for ET-1, independent of the endothelium and extracellular calcium. Accordingly, the term vasostatins was assigned to these two N-terminal CgA peptides, numbered according to length, i.e. as VS-I and VS-II. Moreover, the arterial dilatations were independent of other constrictors over a functional range of transmural pressures, and the intrinsic and concentration-dependent dilator effects persisted at moderately elevated extracellular [K+] in both arteries (Brekke et al. 2000; Brekke et al. 2002). Thus, in pressure-activated bovine resistance arteries the naturally occurring VS-I appeared to have a direct dilator potential involving hyperpolarization, acting via the N-terminal, loop-containing domain. Moreover, as the dilator effect of CgA1–40 in the coronary artery was diminished by pertussis toxin (PTX) and abolished by antagonists to several subtypes of K+ channels, the mechanism of action seemingly involves a Gαi/o subunit and K+ channel activation in the signal pathway. Significant species differences in vasoactivity were on the other hand apparent, as neither the rat betagranin peptide rCgA7–57 nor the bovine chromofungin, bCgA47–66, had vasodilator effects in the rat cerebral artery (Mandalà et al. 2005).
The vascular endothelium appears by itself to be a significant target for granin-derived peptides, e.g. VS-I (Ferrero et al. 2004; Blois et al. 2006a), catestatin (Theurl et al. 2010) and secretoneurin (Kähler et al. 2002). Bovine aorta endothelial cells internalizes bovine CgA (Mandalà et al. 2000) and both human CgA (Hsiao et al. 1990) and human STACgA1–78 (Roatta et al. 2011) are distributed across the vascular endothelium in two pools, a minor fraction in the blood and a major pool in the interstitium. Moreover, CgA and VS-I protect the endothelial barrier against the gap-forming, permeabilizing activity of TNFα (Ferrero et al. 2004) via a mechanism involving cytoskeletal reorganization and downregulation of the transmembrane protein intercellular VE-cadherin, responsible for the cell-cell adhesion (Ferrero et al. 2004). In contrast, catestatin (Theurl et al. 2010) as well as secretoneurin (Yan et al. 2006) impair the integrity of the endothelial barrier, however by different mechanisms. Other studies have shown that VS-1 also inhibits endothelial cell migration, motility, sprouting, invasion and capillary-like structure formation induced by vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) (Belloni et al. 2007).
The most recent newcomer among vasoactive CgA peptides corresponds to the C-terminal sequence of VS-II, CgA79–113, and has vasodilatory properties (Salem et al. 2015). This peptide, named the vasoconstriction-inhibiting factor (VIF), acts as a cofactor for the angiotensin II type 2 receptor. As the plasma concentration of VIF was significantly increased in renal patients and patients with heart failure, it seems evident that yet another CgA-derived player and yet other targets may be involved in blood pressure regulation and vascular pathophysiology.
1.5.2 Vasostatins, Catestatin and Serpinin; Myocardial Contractility and Protection against Ischemia-Induced Injury
A large body of evidence suggests that CgA, either present in circulation or produced by the heart itself, is a novel regulator of the heart. Indeed, under normal and pathophysiological conditions alike, the heart is under constant exposure not only to CA but also to the circulating CgA originating from the sympathoadrenal system. CgA may also derive locally from myocardial production, notably in ventricles of heart failure patients (Pieroni et al. 2007). The full-length CgA dilates coronaries and induce negative inotropism and lusitropism in the ex vivo perfused rat heart at 0.1–4 nM, but not at higher concentrations (Pasqua et al. 2013). Of note, analysis of the perfusates showed that exogenous CgA was not cleaved by the heart, suggesting that the myocardial effects were induced by the circulating, full-length protein. However, the same study demonstrated that physically and chemically stimulated rodent hearts could proteolytically process the intracardiac, endogenous CgA into fragments (Glattard et al. 2006). Moreover, the increased plasma levels in chronic heart failure (Ceconi et al. 2002), its over-expression in human dilated and hypertrophic cardiomyopathy (Pieroni et al. 2007) and the observation that the circulating CgA provide prognostic information on long-term mortality, independent of conventional risk markers in acute coronary syndromes (Jansson et al. 2009), all point to a significant role of CgA in human cardiovascular homeostasis. Hence, the systemic and intracardiac fates of full-length CgA and its fragments imply intriguing new aspects of the myocardial handling of CgA under normal and pathophysiological conditions.
To what extent the elevated circulating levels of CgA, VS-I and catestatin together are beneficial or detrimental to the failing heart, remains unanswered. Taking into account that an inflammatory response is caused by myocardial injury arising from ischemic reperfusion (Anaya-Prado and Toledo-Pereyra 2002), a link between plasma CgA and/or its fragments in cardioprotection seems plausible. For instance, it is well established that the human recombinant VS-1 (hrSTACgA1-78) preconditions the rat heart against myocardial necrosis arising in response to reperfusion of the ischemia-injured tissue, presumably involving the endothelial/endocardial adenosine/nitric oxide signaling pathway (Cappello et al. 2007). In contrast, catestatin, being without pre-conditioning effects, may modulate reperfusion injury during the post-ischemic reperfusion period (Penna et al. 2010; Penna et al. 2014). Hence, it seems likely that N- and C-terminal CgA fragments arising from processing of the circulating and intracardiac pools of CgA in species-specific patterns, may exert beneficial effects, not only under experimental conditions in animal models (Pasqua et al. 2013), but also in the failing human heart in situ.
Although the two structurally different CgA peptides, VS-I and catestatin, both exert negative myocardial inotropy, non-competitively inhibiting the β-adrenenoceptor on cardiomyocytes (Tota et al. 2008; Angelone et al. 2008), these apparently converging effects on the heart may be less puzzling when realizing that these two peptides may not reach peak concentrations in the same frame of time (Crippa et al. 2013). The thrombin.induced C-terminal processing of the anti-angiogenic, full length CgA into a catestatin-containing angiogenic fragment point to a functional rationale, namely maintaining protection of the heart against excessive adrenergic stimlation by CA, whether by VS-I or catestatin, regardless of the quiescent or stimulus-activated state of the vasculature.
The C-terminal peptide serpinin (CgA403-428, Koshimizu et al. 2010), is a novel CgA-derived factor in cardiovascular modulations (Tota et al. 2012). This fragment was first described for its ability to signal the increase in transcription of the serine protease inhibitor, protease nexin-1 (PN-1), a potent inhibitor of plasmin released during inflammatory processing causing cell death. Two other forms have since been identified, (pGlu)serpinin and serpinin-Arg-Arg-Gly (Koshimizu et al. 2011a). In addition to the serpinin-like effect on increasing the levels of PN-1, (pGlu)serpinin also excerts anti-apoptotic effects of relevance to protection of neurons in the central nervous system (Koshimizu et al. 2011b). Intriguingly, in the perfused rat heart both serpinin and (p-Glu)serpinin excert positive inotropic and lusitropic effects via a β1-adrenergic receptor/adenylate cyclase/cAMP/PKA pathway (Tota et al. 2012), thus contrasting the inhibitory effects of VS-I and catestatin on the cardiac β2-adrenoceptor mediated activations. It remains to be seen to what extent and at what stage in the C-terminal processing of the full-length CgA the concentrations of serpinin and pGly-serpinin may reach their functional maxima (Loh et al. 2012).
1.5.3 Angiogenesis, Cell Adhesion and Tumor Progression
CgA appears to regulate angiogenesis and tumor growth in several models of solid tumors (Corti 2010), affecting fibroblasts (Dondossola et al. 2010) and endothelial cells (Corti and Ferrero 2012) in the tumor microenvironment. Recent studies have reveled that the full-length CgA contains one anti-angiogenic site in the C-terminal region (CgA410-439) (Crippa et al. 2013), and another site in a latent form in the N-terminal domain CgA1-76. Proteolytic liberation is necessary for full activation of the anti-angiogenic property of VS-I. Intriguingly, further processing of VS-1 leads to the antimicrobial peptide CgA47-66, originally named chromofungin (Lugardon et al. 2001). Even this degradation product is able to cause negative inotropic effects and, like the unprocessed VS-I, to elicit post-conditional protection against ischemia/reperfusion damage (Filice et al. 2015).
Given the potential ability of CgA and/or its fragments to regulate tumor vessel biology, these molecules might also contribute to inhibit tumor growth, as shown in mouse lymphomas (Bianco et al. 2016) and mammary adenocarcinomas genetically engineered to release CgA locally (Colombo et al. 2002). In animal models both CgA and VS-I reduced the trafficking of tumor cells from tumor-to-blood, from blood-to-tumor and from blood-to-normal tissues (i.e. the tumor “self-seeding” and metastasis processes), by enhancing the endothelial barrier function and reducing the trans-endothelial migration of cancer cells (Dondossola et al. 2012). In certain tumor patients the CgA plasma levels may reach up to 10–100-fold. Whether these high levels of circulating CgA may also affect the growth and progression of non-neuroendocrine tumors, remains a challenging question awaiting detailed analyses of plasma concentrations of full-length CgA and VS-1 in these patients.
1.5.4 A Physiological Role for the Circulating CgA
Fifty years ago when the exocytotic release of CgA into the effluents from the stimulated adrenal medulla was first reported (Banks and Helle 1965; Blaschko et al. 1967), no functional significance was assigned to the released protein. Nearly twenty years lapsed before the enzymatically inactive CgA was detected in the circulation of pheochromocytoma patients (O’Connor and Bernstein 1984). After another thirty years the N- and C-terminal domains in CgA have finally been quantified in normal plasma, thanks to highly refined immunochemical analyses, revealing subnanomolar levels of both full length CgA (0.1 nM) and VS-1 (0.4 nM) (Crippa et al. 2013). This report was also the first to show that full-length CgA and VS-1 exerted potent anti-angiogenic activity when performed with biologically relevant concentrations in the various in vitro and in vivo assays. Rather unexpectedly, the anti-angiogenic property of the intact CgA was converted to a potent pro-angiogenic fragment corresponding to the catestatin-containing fragment CgA1-373 upon blood coagulation in a thrombin-dependent manner (Crippa et al. 2013). Thus, the full length CgA, VS-1 and the catestatin-containing peptide seemingly form a balance of anti- and pro-angiogenic factors tightly regulated by proteolysis as a functional response to tissue injury when repair of the damaged tissue is called for (Crippa et al. 2013; Helle and Corti 2015). Hence, a physiological role is finally apparent for the anti-angiogenic, full-length CgA and its N-terminal peptide VS-I when circulating at normal concentrations, namely in maintaining the vascular endothelium in a quiescent state by protecting its structural integrity and, in addition, protecting the myocardium against excessive β-adrenergic stimulation and detrimental effects of ischemia-induced injury.
1.5.5 Circulating CgA as a Marker for Inflammatory Diseases
Inflammatory processes, in particular those involving the cardiovascular system, pose clinical challenges in diagnosing and therapy. For instance, the elevated plasma CgA in chronic heart disease is a strong indicator of a relationship between high plasma CgA and pro-inflammatory markers (Corti et al. 2000) as well as an independent marker of mortality (Pieroni et al. 2007). Vascular inflammation may induce pathological arterial changes and variable blood pressure. Moreover, endothelial dysfunction is now recognized as a crucial factor in hypertension, with endothelial NO production as essential for maintenance of vascular tone, being compromised as a result of systemic and localized inflammatory responses (Watson et al. 2008).
1.6 Putative Receptors for CgA and CgA-Derived Peptides
Classical, high-affinity cell surface receptors have not yet been identified for most of the CgA-derived peptides. The exception is the nicotinic acetylcholine receptor for catestatin in the sympatoadrenal system mediating the autocrine inhibitory effect of catestatin on CA secretion (Mahata et al. 1997). On the other hand, binding studies have shown that VS-I and chromofungin engage in electrostatic and hydrophobic interactions with membrane-relevant phospholipids at physiological conditions, particularly with phosphatidylserine (Blois et al. 2006b). Moreover, binding to membrane proteins with molecular weights 74 and 78 kDa were early findings for VS-I both in cultured calf smooth muscle and parathyroid cells, respectively (Angeletti et al. 1994; Russell et al. 1994). Similarly, a 70 kDa glycoprotein coupled to two different G-proteins was detected as the receptor for pancreastatin in adipocytes and hepatocytes (Sanchez-Margalet et al. 1996; Sanchez-Margalet et al. 2000). Also catestatin, eliciting histamine-release from rat mast cells, does so via its cationic and amphipatic properties (Krüger et al. 2003). Thus, analogous to the cell penetrating properties of cationic and amphipatic peptides in microorganisms (Metz-Boutigue et al. 2004), both VS-I and catestatin have been postulated to interact with and penetrate into mammalian cells via their cationic and amphipathic properties (Helle et al. 2007; Helle 2010b). Consistent with this hypothesis VS-I was reported to activate PI3K-dependent e-NOS phosphorylation via binding to a heparin sulphate proteoglycan, leading to caveolae endocytosis in bovine aortic endothelial cells (Ramella et al. 2010). A role for heparin sulphate proteoglycan as a cell surface endocytosis receptor entry of macromolecules in mammalian cells has recently gained strong support (Christianson and Belting 2014). A selective binding of CgA and VS-I to the epithelial integrin αvß6 was also recently demonstrated in a study of would healing in injured mice (Curnis et al. 2012). Integrin αvß6 belongs to a large family of heterodimeric transmembrane glycoproteins that attach cells to extracellular matrix proteins of the basement membrane. Notably, the interaction of the RGD/α-helix motif of CgA with avß6-integrin could regulate keratinocyte physiology in wound healing (Curnis et al. 2012). Although αvß6 is upregulated in tissue repair and in cancer (Bandyopadhyay and Raghavan 2009) it remains to be seen whether circulating CgA and VS-I bind to this integrin also in cancerous tissues. An indirect involvement of integrins was observed for VS-I via the phospholipid-binding amphiphilic α-helix within the chromofungin sequence CgA47-66 and the hydrophilic C-terminus CgA67-78 in murine and human dermal fibroblasts (Dondossola et al. 2010). This adhesion mechanism required cytoskeleton rearrangement but not protein synthesis, enhancing fibroblast adhesion to solid-phases.
G-protein-regulated signalling pathways coupled to Gαi/o subunits are commonly identified by their activation by PTX, the Bordetella pertussis toxin. So far, most reports on binding of CgA-derived peptides to membrane proteins refer on PTX-sensitive effects, suggesting coupling to G-proteins containing Gαi-subunits. Intriguingly, not only the glycoprotein receptor for parastatin in adipocytes and hepatocytes was sensitive to PTX (Sanchez-Margalet et al. 1996), but also the dilator effect of CgA1-40 in the coronary artery (Brekke et al. 2002) and the inhibitory effect of VS-I on gap-formation via a blockade of the activation of p38MAPK by PTX in pulmonary and coronary arterial endothelial cells (Blois et al. 2006a). Likewise, the catestatin induced release from rat mast cells was sensitive to PTX (Krüger et al. 2003). On the other hand, catestatin as well as VS-1 signal via AKT/PKB to eNOS mediating their inhibitory effects in the rat heart (Angelone et al. 2008; Tota et al. 2008). Thus, in the rat heart different G-proteins may be involved in the NO-production by VS-I and catestatin, both serving as non-competitive inhibitors of the β-adrenoceptor. Hence, not only pancreastatin, but also VS-I and catestatin appear to interact with membrane constituents via their membrane-penetrating properties, coupling to distinctly different G-protein-coupled pathways, in some tissues involving PTX-sensitive Gαi/o subunits (Helle 2010b).
2 Conclusions
While the research history of the adrenal chromaffin cells dates back to the mid 1850ies, our knowledge of the granins reflects research from the most recent six decades. The accumulated literature has unraveled that these unique proteins serve as prohormones for a range of regulatory peptides with widely different effects and target tissues. Moreover, their properties seemingly fit into patterns of functionally protective activities, e.g. in calcium, glucose and vascular homeostasis, in angiogenesis, tissue repair and heart physiology, with implications also for the diagnosis and treatment of a wide range of neuroendocrine tumors, inflammatory pathologies and cardiovascular diseases. Thus, the co-release of granins with CA and other biogenic amines opens for a novel concept for the diffuse sympathoendocrine system, namely that of buffering and counterbalancing the immediate responses to the stress-activated system.
A dual role for the circulating full-length CgA is now apparent, protecting the vascular endothelium by inhibiting angiogenesis under normal conditions, yet accelerating local angiogenesis in response to tissue damage, e.g. after C-terminal cleavage of the prohormone by thrombin. In additiom, the circulating pool of VS-I, which seemingly contributes to preservation of endothelial cell quiescence, may also serve to counter-balance the pro-angiogenic activity of catestatin-containing fragments when released in the systemic circulation from various sites of injury.
Although classical members of the high-affinity, transmembrane-spanning classes of receptors have yet to be linked to the effects of most of the CgA-derived peptides, other receptor classes have been implicated; in addition to G-proteins for VS-I, pancreastatin and catestatin, for VS-I also the cell surface endocytosis receptor heparin sulphate proteoglycan and the epithelial, transmembrane glycoprotein, the integrin αvβ6.
3 Perspectives
The few reports on CgA processing in patients so far published, indicate complex and disease-related patterns of fragments. For instance, decreased levels of plasma catestatin are characteristic of patients with essential hypertension and also in normotensive subjects with a family history of hypertension and increased epinephrine secretion (O’Connor et al. 2002). On the other hand, elevated plasma levels of VS-I occur in critically ill patients (Schneider et al. 2012) and of catestatin in patients with coronary heart disease and after acute myocardial infarction (Liu et al. 2013; Meng et al. 2013). On the other hand, in patients with chronic kidney disease and heart failure a new fragment derived from VS-II (VIF), is elevated (Salem et al. 2015) while VS-I and fragments, lacking the anti-angiogenic C-terminal region of CgA were increased in patients suffering from a rare form of systemic, inflammatory large vessel vasculitis although the levels of the CgA fragments did not reflect disease activity or extent (Tombetti et al. 2016). Hence, research into the pathophysiological patterns of CgA and its processing in cardiovascular and inflammatory diseases and in tumors emerges as a major challenge in order to assess whether a given pattern of circulating CgA fragments is beneficiary or detrimental to the survival of the afflicted patient.
Abbreviations
- bCgA:
-
bovine CgA1–431
- CA:
-
Catecholamines
- CgA:
-
Chromogranin A
- CgB:
-
Chromogranin B
- GE-25:
-
bCgA367–391
- PN-1:
-
Protease nexin-1
- PTH:
-
Parathyroid hormone
- PTX:
-
Pertussis toxin
- SgII:
-
Secretogranin II
- VIF:
-
Vasoinhibitory factor – CgA79–113
- VS-I:
-
Vasostatin I (CgA1–76)
- VS-II:
-
Vasostatin II (CgA1–113)
- WE-14:
-
bCgA316–330
References
Aardal S, Helle KB (1992) The vasoinhibitory activity of bovine chromogranin A fragment (vasostatin) and its independence of extracellular calcium in isolated segments of human blood vessels. Regul Pept 41:9–18
Aardal S, Helle KB, Elsayed S, Reed RK, Serch-Hanssen G (1993) Vasostatins, comprising the N-terminal domain of chromogranin A, suppress tension in isolated human blood vessel segments. J Neuroendocrinol 5:105–112
Anaya-Prado R, Toledo-Pereyra LH (2002) The molecular events underlying ischemia/reperfusion injury. Transplant Proc 34:2518–2519
Angeletti RH, D’Amico T, Russell J (2000) Regulation of parathyroid secretion. Adv Exp Med Biol 482:217–223
Angeletti RH, Aardal S, Serch-Hanssen G, Gee P, Helle KB (1994) Vasoinhibitory activity of synthetic peptides from the amino terminus of chromogranin A. Acta Physiol Scand 132:11–19
Angeletti RH, Mints L, Aber C, Russell J (1996) Determination of residues in chromogranin A (16–40) required for inhibition of parathyroid hormone secretion. Endocrinology 137:2918–2922
Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, Cerra MC (2008) The antihypertensive chromogranin A peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology 149:4780–4793
Anouar Y, Desmoucelles C, Vaudry H (2000) Neuroendocrine cell-specific expression and regulation of the human secretogranin II gene. Adv Exp Med Biol 482:113–124
Aunis D, Metz-Boutigue M-H (2000) Chromogranins: current concepts. Structural and functional aspects. Adv Exp Med Biol 482:21–38
Bandyopadhyay A, Raghavan S (2009) Defining the role of integrin alphavbeta6 in cancer. Curr Drug Targets 10:645–652
Banks P, Helle KB (1965) The release of protein from the stimulated adrenal medulla. Biochem J 97:40C–41C
Banks P, Helle KB, Major D (1969) Evidence for the presence of a chromogranin-like protein in bovine splenic nerve granules. Mol Pharmacol 5:210–212
Belloni D, Scabini S, Foglieni C, Vescini L, Giazzon A, Colombo B et al (2007) The vasostatin-I fragment of chromogranin A inhibits VEGF-induced endothelial cell proliferation and migration. FASEB J 21:3052–3062
Bianco H, Gasparri A, Generoso L, Assi E, Colombo B, Scarfò L, Bertilaccio MT, Scieizo C, Ranghetti P, Dondossola E, Ponzoni M, Caligaris-Cappio F, Ghia P, Corti A (2016) Inhibition of chronic lymphocytic leucemia progession by full length chromogranin A and its N-terminal fragment in mouse models. Oncotarget 7:41725–41736. 9407.
Blaschko H, Welsch AD (1953) Localization of adrenaline in cytoplasmic particles of the bovine adrenal medulla. Arch Exp Path Pharmak 219:17–22
Blaschko H, Born GVR, D’Iorio A, Eade NR (1956) Observations on the distribution of catecholamines and adenosine phosphate in the bovine adrenal medulla. J Physiol Lond 183:548–557
Blaschko H, Comline RS, Schneider FH, Silver M, Smith AD (1967) Secretion of a chromaffin granule protein, Chromogranin, from the adrenal gland after splanchnic stimulation. Nature (Lond) 215:58–59
Blois A, Srebro B, Mandalà M, Corti A, Helle KB, Serck-Hanssen G (2006b) The chromogranin A peptide vasostatin-I inhibits gap formation and signal transduction mediated by inflammatory agents in cultured bovine pulmonary and coronary arterial endothelial cells. Regul Pept 135:78–84
Blois A, Holmsen H, Martino G, CortiA M-BM-H, Helle KB (2006a) Interactions of chromogranin A-derived vasostatins and monolayers of phosphatidyl serine, phosphatidylcholine and phosphatidylethanolamine. Regul Pept 134:30–37
Borges R, Machado JD, Alonso C, Brioso MA, Goméz JF (2000) Functional role of chromogranins: the intragranular matrix in the last phase of exocytosis. Adv Exp Med Biol 482:69–82
Brekke JF, Kirkeleit J, Lugardon K, Helle KB (2000) Vasostatins. Dilators of bovine resistance arteries. Adv Exp Med Biol 482:239–246
Brekke JF, Osol GJ, Helle KB (2002) N-terminal chromogranin derived peptides as dilators of bovine coronary resistance arteries in vitro. Regul Pept 105:93–100
Cappello S, Angelone T, Tota B, Pagliaro P, Penna C, Rastaldo R, Corti A, Losano G, Cerra MC (2007) Human recombinant chromogranin A-derived vasostatin-1 mimics preconditioning via an adenosine/nitric oxide signaling mechanism. Am J Physiol Heart Circul Physiol 293:H719–H727
Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, Parrinello G, Corti A (2002) Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J 23:967–974
Christianson HC, Belting M (2014) Heparan sulphate proteoglycan as a cell surface endocytosis receptor. Matrix Biol 35:51–55
Ciesielski-Treska J, Aunis D (2000) Chromogranin A introduces a neurotoxic phenotype in brain microglial cells. Adv Exp Med Biol 482:291–298
Cohn DV, Morrisey JJ, Hamilton JW, Shofstall RE, Smardo FL, Chu LLH (1981) Biochemistry 20:4135–4140
Colombo B, Curnis F, Foglieni C, Monno A, Arrigoni G, Corti A (2002) Chromogranin a expression in neoplastic cells affects tumor growth and morphogenesis in mouse models. Cancer Res 62:941–946
Corti A (2010) Chromogranin A and the tumor microenvironment. Cell Mol Neurobiol 30:1163–1170
Corti A, Ferrero E (2012) Chromogranin A and the endothelial barrier function. Curr Med Chem 19:4051–4058
Corti A, Ferrari R, Ceconi C (2000) Chromogranin A and tumor necrosis factor-alpha (TNF) in chronic heart failure. Adv Exp Med Biol 482:351–359
Coupland RE (1968) Determining sizes and distribution of sizes of spherical bodies such as chromaffin granules in tissue sections. Nature (Lond) 217:384–388
Crippa L, Bianco M, Colombo B, Gasparri AM, Ferrero E, LohYP CF, Corti A (2013) A new chromogranin A-dependent angiogenic switch activated by thrombin. Blood 121:392–402
Curnis F, Gasparri A, Longhi R, Colombo B, D’Alessio S, Pastorino F, Ponzoni M, Corti A (2012) Chromogranin A binds to avb6-integrin and promotes wound healing in mice. Cell Mol Life Sci 69:2791–2803
Curry WJ, Norlen P, Barkatullah SC, Johnston CF, Håkanson R, Hutton JC (2000) Chromogranin A and its derived peptides in the rat and porcine gastro-entero-pancreatic system: expression, localization and characterization. Adv Exp Med Biol 482:205–213
Dahlstrøm A (1966) The intraneuronal distribution of noradrenaline and the transport and lifespan of aqmine storage granules in the sympathetic adrenergic neuron, a histological and biochemical study. Ivar Högströms Trykkeri AB, Stockholm
De Robertis E, Pellegrino de Iraldi A (1961) Plurovesicular secretory processes and nerve endings in the pineal gland of the rat. J Biophys Biochem Cytol 10:361–372
Dondossola E, Gasparri A, Bachi A, Longhi R, Metz-Boutigue MH, Tota B, Helle KB, Curnis F, Cort A (2010) Role of vasostatin-1 C-terminal region in fibroblast cell adhesion. Cellular and molecular life sciences : Cell Mol Life Sci 67:2107–2118
Dondossola E, Crippa L, Colombo B, Ferrero E, Corti A (2012) Chromogranin a regulates tumor self-seeding and dissemination. Cancer Res 72:449–459
Efendic S, Tatemoto K, Mutt V, Quan C, Chang D, Ostenson CG (1987) Pancreastatin and islet hormone release. Proc Natl Acad Sci U S A 84:7257–7260
Eiden L (1987) Is chromogranin A a prohormone? Nature 325:301
Von Euler US (1946) A specific sympathomimetic ergone in adrenergic nerve fibres (Sympathin) and its relations to adrenaline and noradrenaline. Acta Physiol Scand 12:73–97
Von Euler US (1958) The presence of the adrenergic neurotransmitter in intraaxonal structures. Acta Physiol Scand 43:155–166
Von Euler US, Hillarp N-Å (1956) Evidence of the presence of noradrenaline in submicroscopic structures of adrenergic axons. Nature (Lond) 177:44–45
Falck B, Hillarp N-Å, Högberg B (1956) Content and intracellular distribution of adenosine triphosphate in cow adrenal medulla. Acta Physiol Scand 36:360–376
Fasciotto BH, Gorr S-U, Bourdeau AM, Cohn DV (1990) Autocrine regulation of patathyroid secretion: inhibition of secretion by chromogranin A (secretory protein-I) and potentiation of secretion by chromogranin A and pancreastatin antibodies. Endocrinology 133:461–466
Ferrero E, Scabini S, Magni E, Foglieni C, Belloni D, Colombo B, Curnis F, Villa A, Ferrero ME, Corti A (2004) Chromogranin A protects vessels against tumor necrosis factor alpha-induced vascular leakage. FASEB J 18:554–555
Filice E, Pasqua T, Quintieri AM, Cantafio P, Scavello F, Amodio N, Cerra MC, Marban C, Schneider F, Metz-Boutigue M-H, Angelone T (2015) Chromofungin, CgA 47–66 –derived peptide, produces basal cardiac effects and postconditioning cardioprotective action during ischemia/reperfusion injury. Peptides 71:40–48
Gerdes H-H, Glombik MM (2000) Signal-mediated sorting of secretory granules. Adv Exp Med Biol 482:41–54
Glattard E, Angelone T, Strub JM, Corti A, Aunis D, Tota B, Metz-Boutigue MH, Goumon Y (2006) Characterization of natural vasostatin-containing peptides in rat heart. FEBS J 273:3311–3321
Hagen P, Barnett RJ (1960) The storage of amines in the chromaffin cell. In: Wolstenholme GEW, O’Connor MO (eds) Adrenergic Mechanisms. Ciba Found Symp, Churchill, London, pp 83–99
Helle KB (1966a) Some chemical and physical properties of the soluble protein fraction of bovine adrenal cgromaffin granules. Mol Pharmacol 2:298–310
Helle K (1966b) Antibody formation against soluble protein from bovine adrenal chromaffin granules. Biochim Biophys Acta 117:107–110
Helle KB (2004) The granin family of uniquely acidic proteins of the diffuse neuroendocrine asystem: comparative and functional aspects. Biol Rev Camb Philos Soc 79:769–794
Helle KB (2010a) Regulatory peptides from chromogranin A and secretogranin II. Putative modulators of cells and tissues involved in inflammatory conditions. Regul Pept 165:45–51
Helle KB (2010b) The chromogranin A-derived peptides vasostatin.I and catestatin as regulatory peptides for cardiovascular functions. Cardiovasc Res 85:9–16
Helle KB, Angeletti RH (1994) Chromogranin A: a multipurpose prohormone? Acta Physiol Scand 152:1–10
Helle KB, Aunis D (2000b) A physiological role for the granins as prohormones for homeostatically important regulary peptides? Adv Exp Med Biol 482:389–397
Helle KB, Aunis D (2000a) Chromogranins. Functional and Clinical Aspects. Adv. Exp. Med. Boil 482:1–405
Helle KB, Corti A (2015) Chromogranin A: a paradoxical player in angiogenesis and vascular biology. Cell Mol Life Sci 72:339–348
Helle KB, Corti A, Metz-Boutigue MH, Tota B (2007) The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci 64:2863–2886
Hillarp N-Å, Lagerstedt S, Nilson B (1953) The isolation of a granular fraction from the subrarenal medulla, containing the sympathomimetic catecholamines. Acta Physiol Scand 29:251–263
Hillarp N-Å (1959) Further observations on the state of the chatecholamines stored in the adrenal medullary granules. Acta Physiol Scand 47:271–279
Hoffmann JA, Kafator FC, Janeway CA Jr, Ezkowitz RAB (1999) Phylogenetic perspectives in innate immunity. Science 284:1313–1318
Hsiao RJ, Seerger RC, Yyu A, O’Connor DT (1990) Chromogranin A in children with neuroblastoma: plasma concentration parallels disease stage and predicts survival. J Clin Invest 85:1555–1559
Huttner WB, Benedum UM (1987) Chromogranin A and pancreastatin. Nature 325:305
Huttner WB, Gerdes H-H, Rosa P (1991) Chromogranins/secretogranins-widespread consttituets of the secretory granule matrix in endocrine cells and neurons. In: Gratzl M, Langley K (eds) Markers for neural and endocrine cells. VCH, Weinheim, pp 93–133
Jansson AM, Rosjo H, Omland T, Karlsson T, Hartford M, Flyvbjerg A, Caidahl K (2009) Prognostic value of circulating chromogranin A levels in acute coronary syndromes. Eur Heart J 30:25–32
Kähler CM, Fischer-Colbrie R (2000) A novel link between the nervous and the immune system. Adv Exp Med Biol 482:279–290
Kähler CM, Kaufmann G, Kähler ST, Wiedermann CJ (2002) The neuropeptide secretoneurin stimulates adhesion of human monocytes to arterial and venous endothelial cells in vitro. Regul Pept 110:65–73
Kirchmair R, Hogue-Angeletti R, Guitirrez J, Fischer-Colbrie R, Winkler H (1993) Secretoneurin – a neuropeptide generated in brain, adrenal medulla, and other endocrine tissues by proteolytic processing of secretoneurin II (Chromogranin C). Neuroscience 53:359–365
Konecki DS, Benedum UM, Gerdes HH, Huttner WB (1987) The primary structure o human chromogranin A and pancreastatin. J Biol Chem 261:17026–17030
Koshimizu H, Kim T, Cawley NX, Loh YP (2010) Chromogranin A: a new proposal for traffiking, processing and induction of granule biogenesis. Regul Pept 160:153–159
Koshimizu H, Cawley NX, Kim T, Yergey AL, Loh YP (2011a) Serpinin: a novel chromogranin A-derived, secreted peptide up-regulates protease nex-1 expression and granule biogenesis in endocrine cells. Mol Endocrinol 25:732–744
Koshimizu H, Cawley NX, Yergey AL, Loh YP (2011b) Role of pGlu-serpinin; a novel chromogranin A-derived peptide in inhibition of cell death. J Mol Neurosci 45:294–303
Krüger P-G, Mahata SK, Helle KB (2003) Catestatin (CgA344–364) stimulates rat mast cell release of histamine in a manner comparable to mastoparan and other cationic charged neuropeptides. Regul Pept 114:29–35
Lever JD (1955) Electron microscopic observations on the normal and denervated adrenal medulla of the rat. Endocrinol 57:621–635
Liu L, Ding W, Zhao F, Shi L, Pang Y, Tang C (2013) Plasma levels and potential roles of catestatin in patients with coronary heart disease. Scand Cardiovasc J 47:217–224
Loewi O (1921) Über Humorale Übertragbarkeit der Herznervenwirkung. Pflüg Arch Ges Physiol 189:239–241
Loh YP, Koshimizu H, Cawley NX, Tota B (2012) Serpinins: role in granule biogenesis, inhibition of cell death and cardiac functions. Curr Med Chem 19:4086–4092
Lugardon K, Raffner R, Goumon Y, Corti A, Delmas A, Bulet P, Aunis D, Metz-Boutigue M-H (2000) Antibacterial and antifungal activities of vasostatin-1, the N-terminal fragment of chromogranin A. J Biol Chem 275:10745–10753
Lugardon K, Chasserot-Golaz S, Kiefler AE, Maget-Dana R, Nullans G, Kiefler B, Aunis D, Metz-Boutigue M-H (2001) Structural and biochemical characterization of chromofungin, the antifungal chromogranin A-(47–68)-derived peptide. J Biol Chem 276:35875–35882
Mandalà M, Stridsberg M, Helle KB, Serck-Hanssen G (2000) Endothelial handling of chromogranin A. Adv Exp Med Biol 482:167–178
Mandalà M, Brekke JF, Serck-Hanssen G, Metz-Boutigue MH, Helle KB (2005) Chromogranin A-derived peptides: interaction with rat posterior cerebral artery. Regul Pept 124:73–80
Mahata SK, O’Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ (1997) Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest 100:1623–1633
Mahata SK, Mahata M, Fung M, O’Connor DT (2010) Catestatin: a multifunctional peptide from chromogranin A. Regul Pept 162:33–43
Meng L, Wang J, Ding WH, Han P, Yang Y, Qi LT, Zhang BW (2013) Plasma catestatin level in patients with acute myocardial infarction and its correlation with ventricular remodelling. Postgrad Med J 89:193–196
Metz-Boutigue M-H, Garcia-Sablone P, Hogue-Angeletti R, Aunis D (1993) Intracellular and extracellular processing of chromogranin A: determination of cleavage sites. Europ J Biochem 217:247–257
Metz-Boutigue M-H, Goumon Y, Lugardon K, Stub JM, Aunis D (1998) Antibacterial peptides are present in chromaffin cell secretory granules. Cell Mol Neurobiol 18:249–266
Metz-Boutigue M-H, Lugardon K, Goumon Y, Raffner R, Strub J-M, Aunis D (2000) Antibacterial and antifungal peptides derived from chromogranins and proenkephalin.A: from structural to biological aspects. Adv Exp Med Biol 482:299–315
Metz-Boutigue M-H, Helle KB, Aunis A (2004) The innate immunity: roles for antifungal and antibacterial peptides secreted by chromfaffin granules from the adrenal medulla. Curr Med Chem-Immun, Endoc Metab Agents 4:169–177
O’Connor DT, Bernstein KN (1984) Radioimmunoassay of chromogranin A in plasma as a measure of exocytotic sympathoadrenal activity in normal subjects and patients with pheochromocytoma. N Engl J Med 311:764–770
O’Connor DT, Mahata SK, Taupenot L, Mahata M, Taylor CVL (2000) Chromogranin in human disease. Adv Exp Med Biol 482:377–388
O’Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ (2002) Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens 20:1335–1345
Oliver G, Schäfer EA (1895) The physiological effects of extracts of the supraadrenal capsules. J Physiol Lond 18:230
Parmer RJ, Mahata M, Gong Y, Jiang Q, O’Connor DT, Xi XP, Miles LA (2000) Processing of chromogranin A by plasmin provides a novel mechanism for regulating catecholamine secretion. J Clin Invest 106:907–915
Pasqua T, Corti A, Gentile S, Pochini L, Bianco M, Metz-Boutigue MH, Cerra MC, Tota B, Angelone T (2013) Full-length human Chromogranin-A cardioactivity: myocardial, coronary and stimulus-induced processing evidence in normotensive and hypertensive male rat hearts. Endocrinology 154:3353–3365
Penna C, Alloatti G, Gallo MP, Cerra MC, Levi R, Tullio F, Bassino E, Dolgetta S, Mahata SK, Tota B et al (2010) Catestatin improves post-ischemic left ventricular function and decreases ischemia/reperfusion injury in heart. Cell Mol Neurobiol 30:1171–1179
Penna C, Pasqua T, Amelio D, Perrelli M-G, Angotti C, Tullio F, Mahata SK, Tota B, Pagliaro P, Cerra MC, Angelone T (2014) Catestatin increases the expression of anti-apoptotic and proangiogenetic factors in the post-ischemic hypertrophied heart of SHR. PLoS One 9:e102536. pp1–11
Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, Cerra MC, Bellocci F, Crea F, Maseri A (2007) Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J 28:1117–1127
Portela-Gomes GM (2000) Chromogranin A immunoreactivity in neuroendocrine cells in the human gastrointestinal tract and pancreas. Adv Exp Med Biol 482:193–204
Radek KA, Lopez-Garcia B, Hupe M, Niesman IR, Elias PM, Taupenot L, Mahata SK, O’Connor DT, Gallo RL (2008) The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J Invest Dermatol 2008 128:1525–1534
Ramella R, Boero O, Alloatti G, Angelone T, Levi R, Gallo MP (2010) Vasostatin-1 activates eNOS in endothelial cells through a proteoglycan-dependent mechanism. J Cell Biochem 110:70–79
Roatta S, Passatore M, Novello M, Colombo B, Dondossola E, Mohammed M, Losano G, Corti A, Helle KB (2011) The chromogranin A-derived peptide vasostatin-I: in vivo effects on cardiovascular variables in the rabbit. Regul Pept 168:10–20
Russell J, Gee P, Liu SM, Angeletti RH (1994) Stimulation of parathyroid hormone secretion by low calcium is inhibited by amino terminal chromogranin peptides. Endocrinology 135:337–342
Salem S, Jankowski V, Asare Y, Liehn E, Welker P, Raya-Bermudez A, Pineda-Martos C, Rodrigues M, Muñoz-Castrañeda JR, Bruck H, Marx N, Machado FB, Straudt M, Heinze G, Zidek W, Jankowski J (2015) Identification of the vasoconstriction-inhibiting factor (VIF), a potent endogenous cofactor of angiotensin II acting on the angiotensin II type receptor. Circulation 131:1426–1434
Sanchez-Margalet V, Gonzalez-Yanes C (1998) Pancreastatin inhibits insulin action in rat adipocytes. Am J Phys 275:E1055–E1060
Sanchez-Margalet V, Gonzalez-Yanes C, Najib S (2001) Pancreastatin, a chromogranin A-derived peptide, inhibits DNA and protein synthesis by producing nitric oxide in HTC rat hepatpma cells. J Hepatol 35:80–85
Sanchez-Margalet V, Gonzalez-Yanes C, Santos-Alvarez J, Najib S (2000) Pancreastatin. Biological effects and mechanism of action. Adv Exp Med Biol 482:247–262
Sanchez-Margalet V, Lucas M, Goberna R (1996) Pancreastatin action in the liver: dual coupling to different G-proteins. Cell Signal 8:9–12
Schneider F, Bach C, Chung H, Crippa L, Lavaux T, Bollaert PE, Wolff M, Corti A, Launoy A, Delabranche X et al (2012) Vasostatin-I, a chromogranin A-derived peptide, in non-selected critically ill patients: distribution, kinetics, and prognostic significance. Intensive Care Med 38:1514–1522
Schümann HJ (1958) Über den Noradrenaline und ATP-gehalt sympatischer Nerven. Arch Exp Path Pharmak 233:296–300
Shooshtarizodeh P, Zhang D, Chich JF, Gasnier C, Schneider F, Aunis D, Metz-Boutique M-H (2010) The antimicrobial peptides derived from chromogranin/secretogranin family, new actors of innate immunity. Regul Pept 165:102–110
Smith AD, Winkler H (1967) Purification and properties of an acidic protein from chromaffin granules of bovine adrenal medulla. Biochem J 103:483–492
Smith WJ, Kirshner A (1967) A specific soluble protein from the catecholamine storage vesicles of bovine adrenal medulla. Mol Pharmacol 3:52–62
Stoltz F (1904) Über Adrenaline und Alkylamineacetobrenzcathechin. Ber Deutsch Chem Gesellsch 37:4149–4154
Strub JM, Garcia-Sablone P, Lønning K, Taupenot L, Hubert P, van Dorsselar A, Aunis D, Metz Boutigue MH (1995) Processing of chromogranin B in bovineadrenal medulla; identification of secretolytin, the endotenous C-terminal fragment of residues 614–626 with antibacterial activity. Eur J Biochem 229:356–368
Tatemoto K, Efendic S, Mutt V, Makk G, Feistner GJ, Barchas JD (1986) Pancreastatin, a novel pancreatic peptide that inhibits insulin secretion. Nature 324:476–478
Taupenot L, Mahata M, Mahata SK, Wu H, O’Connor DT (2000) Regulation of chromogranin A transcription and catecholamine secretion by the neuropeptide PACAP. Adv Exp Med Biol 482:97–112
Theurl M, Schgoer W, Albrecht K, Jeschke J, Egger M, Beer AG, Vasiljevic D, Rong S, Wolf AM, Bahlmann FH et al (2010) The neuropeptide catestatin acts as a novel angiogenic cytokine via a basic fibroblast growth factor-dependent mechanism. Circ Res 107:1326–1335
Tombetti E, Colombo B, Di Chio MC, Sartorelli S, Papa M, Salerno A, Bozzolo EP, Tombolini E, Benedetti G, Godi C, Lanzani C, Rovere-Querini P, Del Maschio A, Ambrosi A, De Cobelli F, Sabbadini MG, Baldisera E, Corti A, Manfredi AA (2016) Chromogranin-A production and fragmentation in patients with Takayasy arteritis. Artheritis Res Ther 18:187–200
Tota B, Angelone T, Mazza A, Cerra CM (2008) The chromogranin A derived vasostatins: new players in the endocrine heart. Curr. Med. Chem 15:1444–1451
Tota B, Gentile S, Pasqua T, Bassino E, Koshimizu H, Cawley NX, Cerra MC, Loh YP, Angelone T (2012) The novel chromogranin A-derived serpinin and pyroglutaminated serpinin peptides are positive cardiac beta-adrenergic-like inotropes. FASEB J 26:2888–2898
Trudeau VL, Martyniuk CJ, Zhao E, Hu H, Volkoff H, Decatur WA, Basak A (2012) Is secretoneurin a new hormone? Gen Comp Endocrinol 175:10–18
Valicherla GR, Hossain Z, Mahata SK, Gayen JR (2013) Pancreastatin is an endogenous peptide that regulates glucose homeostasis. Physiol Genomics 45:1060–1071
Vulpian M (1856) Note sur quelque reactions proper a la substances des capsules surrénales. C R Acad Sci (Paris) 43:663
Watson T, Goon PK, Lip GY (2008) Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal 10:1079–1088
Welzstein R (1957) Electronenmichroscopische Untersuchungen aus Nebennierenmark von Maus, Meehrschweinchen und Katze. Z Zellforsch 46:517–576
Winkler H, Fischer-Colbrie R (1992) The chromogranins A and B: the first 25 years and future perspectives. Neuroscience 49:497–528
Yan S, Wang X, Wang H, Yao Q, Chen C (2006) Secretoneurin increases monolayer permeability in human coronary artery endothelial cells. Surgery 140:243–251
Zhang D, Shooshtarizadeh P, Laventie B-J, Colin DA, Chich J-F, Vidic J, de Barry J, Chasserot-Golaz S, Delalande F, Van Dorsselaer A, Schneider F, Helle K, Aunis D, Prevost G, Metz-Boutigue M-H (2009) Two chromogranin A-derived peptides induce calcium entry in human neutrophils by calmodulin-regulated alcium independent phospholipase A2. PLoS One 4:e4501. pp1–14
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Helle, K.B. (2017). History and Perspectives. In: Angelone, T., Cerra, M., Tota, B. (eds) Chromogranins: from Cell Biology to Physiology and Biomedicine. UNIPA Springer Series. Springer, Cham. https://doi.org/10.1007/978-3-319-58338-9_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-58338-9_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58337-2
Online ISBN: 978-3-319-58338-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)