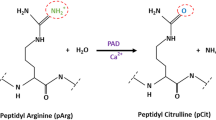
Abstract
The findings that citrullinated proteins are targeted by the most specific immune response in rheumatoid arthritis, and that citrullination also plays an important role in neurodegenerative diseases and certain cancers, have triggered many researchers to study various aspects of this form of posttranslational modification. This chapter is focused on the conditions that are needed for peptidylarginine deiminases to become active citrullinating enzymes, in particular in relation to joint inflammation as observed in rheumatoid arthritis. After briefly discussing the available methods to study the activity of peptidylarginine deiminases and their substrate specificity, the isoforms that are most relevant for citrullination during inflammation and the factors that are mediating their activation are addressed. Citrullination is crucial for one of the processes that are tightly associated with inflammation, NETosis, which is more extensively discussed in Chap. 8. Finally, peptidylarginine deiminase activation and the resulting citrullination in the context of the inflamed joints in rheumatoid arthritis are described.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
5.1 Introduction
The findings that citrullinated proteins are targeted by the most specific immune response in rheumatoid arthritis , and that citrullination also plays an important role in neurodegenerative diseases and certain cancers , have triggered many researchers to study various aspects of this form of posttranslational modification . This chapter is focused on the conditions that are needed for peptidylarginine deiminases to become active citrullinating enzymes, in particular in relation to joint inflammation as observed in rheumatoid arthritis . After briefly discussing the available methods to study the activity of peptidylarginine deiminases and their substrate specificity, the isoforms that are most relevant for citrullination during inflammation and the factors that are mediating their activation are addressed. Citrullination is crucial for one of the processes that are tightly associated with inflammation, NETosis , which is more extensively discussed in Chap. 8. Finally, peptidylarginine deiminase activation and the resulting citrullination in the context of the inflamed joints in rheumatoid arthritis are described.
Although protein deimination (citrullination) is widespread in biological systems, the finding that a major autoimmune response in rheumatoid arthritis (RA) is directed to citrullinated antigens boosted research aimed at understanding both physiological and pathophysiological aspects of deimination. Anti-citrullinated protein antibodies (ACPA) are found in up to 75% of RA patients and in less than 5% of patients with other (autoimmune) diseases and in healthy subjects. Many autoimmune diseases, including RA, are characterized by chronic inflammation . Inflammation -associated events, such as chemical or enzyme-mediated modification of proteins, may play a crucial role in the formation of neoepitopes , which can be recognized by the immune system as non-self. In genetically susceptible individuals, such neoepitopes may trigger an immune response, which in time may spread also to other epitopes of the antigens involved. The enzymes involved in citrullination, peptidylarginine deiminases (PADs), were characterized in more detail; for some of them, crystal structures were determined, substrate-based activation mechanisms were proposed, and conformational changes associated with their activation have been elucidated. New methods to study PAD activity and protein citrullination have been developed, and these have been applied in a variety of studies addressing the activation of PADs and the extent of protein citrullination in various cells and tissues. More insight into the factors required for their activation and into the substrate specificity of PAD isoforms have been obtained. The finding that one of the PAD isoforms plays an essential role in NETosis , a process important for the function of neutrophils in anti-microbial responses and inflammation , emphasized the role of PADs in both health and disease. The PAD isoforms that are most relevant for citrullination in arthritis have been determined, and many proteins that are citrullinated in inflamed joints have been identified. Several PAD inhibitors have been generated, and their applicability is being explored, not only in research but also in preclinical therapeutic approaches.
So far, five PAD isotypes have been identified in mammals, (PADs 1–4 and 6). These isozymes are highly conserved, with 50–55% overall sequence homology and close to 70% identity within the catalytic domain. PAD1 is mainly expressed in epidermis and hair follicles. PAD2 is the most ubiquitously expressed PAD enzyme and is present in a.o. macrophages and astrocytes . PAD3 is primarily expressed in the upper layers of epidermis and in hair follicles, and PAD4 is mainly expressed in white blood cells (granulocytes and monocytes). PAD6 is expressed mainly in the ovary, testis and peripheral blood leukocytes. This chapter is focused on the activation of PADs in arthritis with a focus on PAD2 and PAD4 . Methods available for studying PAD activity are summarized. The evidence for the involvement of PAD isoforms in arthritis is described, as well as the factors that are important for the enzymatic activity of PADs. Finally, the importance of PAD activation for the inflammation in RA is discussed.
5.2 Methods to Determine PAD Activity
The ability to identify and quantify citrullinated proteins is the key to understanding the involvement of PADs and the role of this type of posttranslational modification in physiological and pathophysiological processes. The specific detection of peptidylcitrulline in complex biological samples is challenging due to the small chemical difference between citrulline and arginine . Moreover, many methods used to detect peptidylcitrulline do not discriminate between citrullinated and homocitrullinated proteins. Homocitrulline , which differs from citrulline by the presence of one additional carbon in the side chain, can occur in proteins as a result of the carbamylation of lysine residues, a process that can spontaneously occur in the presence of (iso)cyanate. The most widely applied method for peptidylcitrulline detection is the so-called anti-modified citrulline (AMC) approach, originally developed by Dr. Tatsuo Senshu in Japan (Senshu et al. 1992). In this method, the proteins are incubated with compounds that are specifically reactive with the ureido side chain, resulting in an adduct that can be detected with AMC antibodies (Fig. 5.1a). Many results obtained with this elegant approach need to be interpreted with some caution, because this method will detect carbamylated proteins as efficiently as citrullinated proteins. There is increasing evidence that carbamylation occurs under (patho)physiological conditions as well (Hensen and Pruijn 2014).
Chemical tools for peptidylcitrulline detection. (a) At low pH the ureido group of (homo)citrulline reacts with diacetyl monoxime and antipyrine, resulting in modified citrulline. Anti-modified citrulline antibodies can subsequently be used for visualization or isolation of the citrullinated proteins. (b) At low pH (homo)citrulline is also reactive with glyoxal, e.g., in the form of azido-phenylglyoxal, which facilitates the detection of citrullinated proteins by subsequent reactions with alkyne-containing compounds. Fluorophores or biotin may also be conjugated to phenylglyoxal prior to the reaction with peptidyl(homo)citrulline
Unfortunately, the availability of anti-modified citrulline antibodies from immunized rabbits became problematic due to several failed attempts to elicit new anti-modified citrulline antibodies. The lack of these antibodies could be solved by using antibodies that target the citrullinated isoforms of specific proteins, e.g., anti-citrullinated fibrinogen and anti-citrullinated chemokine antibodies. For studies that are aimed at the global detection of citrullinated or carbamylated proteins, such antibodies are not suitable for obvious reasons, but recently monoclonal anti-modified citrulline antibodies have been generated, which can be applied in the procedure originally developed by Dr. Senshu. An alternative antibody that is widely used in citrullination studies is the monoclonal antibody F95 , which was raised against a deca-citrullinated peptide linked to the activated carrier protein keyhole limpet hemocyanin (Nicholas and Whitaker 2002). Although this antibody appears to be reactive with a wide spectrum of citrullinated molecules, the results of a number of studies suggest that F95 displays a restricted recognition pattern (De et al. 2005; Makrygiannakis et al. 2012).
The antibody-based assay for PAD activity (ABAP) is based on the detection of citrullinated peptides by an antibody that reacts in a citrulline -dependent manner with these peptides. Arginine-containing peptides, e.g., a peptide corresponding to a citrullinated filaggrin epitope recognized by RA autoantibodies, are immobilized in the wells of a 96-well microtiter plate and incubated with PAD containing samples. The conversion of peptidylarginine to peptidylcitrulline can subsequently be detected with an antibody specifically reactive with the citrullinated peptide, followed by an ELISA-like staining procedure (Zendman et al. 2007).
An alternative method for the detection of (homo)citrulline containing proteins is based on the chemical reaction of the ureido group with phenylglyoxal under highly acidic conditions (Tutturen et al. 2010). Under these conditions, phenylglyoxal specifically reacts with (homo)citrulline (Fig. 5.1b), and fluorophore-conjugated or click-chemistry-based variants of phenylglyoxal have been used to detect (homo)citrullinated proteins in biological samples (Hensen et al. 2015; Bicker et al. 2012). Methods to detect citrullinated/carbamylated proteins in complex samples based on this compound, however, appeared to be hampered by relatively high background reactivities (Hensen and Pruijn 2014; Hensen et al. 2015).
Finally, the analysis of proteins by mass spectrometry provides very attractive possibilities to identify and characterize citrullinated proteins. For more information on the applicability of these methods, we refer the reader to recent review articles (Hensen and Pruijn 2014; Slade et al. 2014). Compared to other techniques, one of the main advantages of mass spectrometry is that it discriminates between citrullination (mass increase 0.98 Da) and carbamylation (mass increase 43.02 Da). In addition, the modification site can be mapped using tandem mass spectrometry. Collision-induced dissociation during tandem mass spectrometry has been shown to lead to the conversion of peptidylcitrulline to peptidyl-ornithine concomitant with the loss of 43.02 Da (Wang et al. 2016; Hao et al. 2009) which thus can be considered a citrulline signature. However, it should be noted that the loss of the carbamyl group during this procedure is also expected for homocitrulline . We have used mass spectrometric approaches to get more information on the substrate specificity of PAD enzymes and to characterize citrullinated proteins in synovial fluid samples of RA patients, which will be addressed below.
5.3 PAD Substrate Specificity
Like many other enzyme-catalyzed posttranslational modifications , citrullination is restricted to specific substrate arginines , and the amino acid context plays an important role in the selection of citrullination sites. Several studies have addressed the mechanism of action and the substrate requirements for PAD enzymes (Kearney et al. 2005; Knuckley et al. 2010; Dreyton et al. 2014), in particular for PAD2 and PAD4 . The conversion of free L-arginine appeared to be nearly undetectable, but the results of experiments with benzoylated arginines indicated that an N-terminal amide is critical and sufficient for recognition (Knuckley et al. 2010; Dreyton et al. 2014). Kinetic and mechanistic studies of PAD2 suggested that, unlike the other members of the PAD family, which were proposed to use a reverse-protonation mechanism, PAD2 uses a substrate-assisted mechanism of catalysis (Dreyton et al. 2014).
By global analyses of citrullinated proteins and by investigating the efficiency of citrullination of synthetic peptides, the influence of neighboring amino acids was assessed. Although both favored and disfavored amino acids at positions flanking the deiminated arginine have been identified, the results demonstrated that it is difficult to derive a consensus sequence for citrullination sites (Stensland et al. 2009; Assohou-Luty et al. 2014). Amino acid residues most commonly found in citrullination sites for both isotypes are glycine at +1 and tyrosine at +3 relative to the target arginine . Nevertheless, based upon the results obtained with synthetic peptide substrates and mixtures of cellular proteins, a consensus sequence comprising four amino acids flanking the human PAD4 citrullination site, two on each side, was proposed: (M/K)-(D/S)-R-(G/D)-(H/W). Neighboring lysines negatively influenced the deimination by PAD4 . In addition, it was demonstrated that the human PAD4 displays more pronounced substrate specificity than the human PAD2 (Assohou-Luty et al. 2014), which is confirmed by studies looking at PAD2 versus PAD4 deimination of specific proteins, such as fibrinogen (Nakayama-Hamada et al. 2005; van Beers et al. 2010).
5.4 PAD Isoforms Involved in Arthritis
5.4.1 PAD2 and PAD4 Expression in Hematopoietic Cells
PAD2 and PAD4 are encoded in various cells of hematopoietic origin (Vossenaar et al. 2003) and are the main PAD isoforms that have been demonstrated in the synovium of RA patients (Foulquier et al. 2007). Both isoforms are expressed by peripheral blood mononuclear cells (PBMCs), granulocytes , fibroblast-like cells, and osteoclasts . There are some discrepancies between the data obtained by mRNA analyses and protein analyses, which suggests that the expression of PADs, at least in part, may be regulated at the translational level. PADs have been reported to be active in the nucleus, the cytoplasm and the extracellular space, although there are differences between the various PAD isoforms. It was initially thought that PAD4 was solely the nuclear isoform (Nakashima et al. 2002), whereas PAD2 was cytoplasmic. Indeed, nuclear localization of PAD4 is essential for the formation of neutrophil extracellular traps (NETs; see below) (Lewis et al. 2015). However, PAD4 is also localized within cytoplasmic granules (Asaga et al. 2001), and PAD2 was found within the nucleus of macrophages (Mohanan et al. 2013) and regulates genes within mammary gland epithelial cells through nuclear functioning (Cherrington et al. 2012). Thus, it is not completely clear yet which isoform is responsible for citrullination of proteins with a particular intracellular localization; the same applies for extracellularly located citrullinated proteins (van Beers et al. 2013; Wang et al. 2016), as both PAD2 and PAD4 are found extracellularly in a synovial fluid (SF) (Kinloch et al. 2008).
PAD3 expression has been demonstrated in neutrophils in a single study (Darrah et al. 2012), and PAD3 mRNA was recently demonstrated in synovial tissue obtained from RA patients (Olivares-Martinez et al. 2016). It is unclear if PAD3 is indeed expressed as protein in the synovium and thus contributes to the production of citrullinated autoantigens in the joints.
PAD2 is expressed in mature macrophages after differentiation from monocytes (Vossenaar et al. 2004a); although using a different protocol, PAD2 has also been demonstrated in monocytes (Foulquier et al. 2007). Also the observations made for PAD4 expression differed between these two studies. The conflicting results on PAD4 and PAD2 expression at either the mRNA or protein level in monocytes and macrophages indicate that more work has to be done to clarify this situation.
Notably, in RA synovium sections, macrophage -like cells account for a substantial part of the synovial tissue (Salisbury et al. 1987) making them a considerable source for PAD2 and possibly also PAD4 . PAD2 and PAD4 are also expressed by fibroblast-like cells, which are also a dominant cell type in the inflamed synovial membrane (Chang et al. 2005; Badillo-Soto et al. 2016). With respect to granulocytes , both PAD2 and PAD4 are expressed by neutrophils (Darrah et al. 2012; Spengler et al. 2015), which are the most abundant immune cell in SF aspirated from the joint of RA patients (Bjelle et al. 1982). Neutrophils appear to be the major source of PAD4 in the inflamed joints but also importantly contribute to PAD2 levels. PAD4 is also expressed in eosinophils (Asaga et al. 2001), and PAD2 expression has been demonstrated in mast cells (Arandjelovic et al. 2012). Osteoclasts are another cell type of hematopoietic origin expressing PADs (Harre et al. 2012). PAD2 and PAD4 are expressed in osteoclast precursor cells, but PAD4 expression decreases with further differentiation into osteoclasts , while PAD2 expression increases during this process (Harre et al. 2012). Citrullinated proteins, such as vimentin , are targeted on the surface of osteoclasts by ACPAs , which are frequently found in the sera of RA patients, and this interaction induces osteoclastogenesis. This process has recently been shown to be IL-8 dependent, and PAD4 was also found to be expressed in mature osteoclasts in this latter study and thus may indeed contribute this process (Krishnamurthy et al. 2016). For a more detailed description of deamination and bone loss, we refer the reader to Chap. 6 of this volume.
5.4.2 PAD2 and PAD4 Expression in Joints
In accordance with the expression of PAD2 and PAD4 in various inflammatory cells, both isoforms have been demonstrated in the synovium of RA patients (Nakayama-Hamada et al. 2005; Foulquier et al. 2007; Kinloch et al. 2008). The presence of PAD2 and PAD4 in synovial tissue and SF is not exclusive for RA, as they are also detected in other inflammatory (joint) diseases, such as ankylosing spondylitis and psoriatic arthritis (Foulquier et al. 2007; Kinloch et al. 2008). Neither is the presence of citrullinated proteins, which are found in other forms of synovitis and also other inflammatory diseases, as exemplified by patients with inflammatory bowel disease and polymyositis (Vossenaar et al. 2004b; Raijmakers et al. 2012; Chapuy-Regaud et al. 2005; Kinloch et al. 2008; Makrygiannakis et al. 2006). Both PAD2 and PAD4 have been demonstrated in the synovium of osteoarthritis (OA) patients but at lower levels compared to RA patients (Damgaard et al. 2016b; Kinloch et al. 2008; Foulquier et al. 2007; Chang et al. 2013). Accordingly, PAD activity assays reveal significantly higher catalytic activity in SF from RA patients than from OA patients (Spengler et al. 2015; Damgaard et al. 2016b), and citrullinated proteins are almost absent in most OA samples (Chang et al. 2005; Kinloch et al. 2008). A recent study identified 200 citrullination sites, by mass spectrometr y, in SF obtained from ACPA-positive RA patients and only four sites in SF from OA patients (Wang et al. 2016). These findings probably reflect the high degree of inflammatory cell infiltration into RA joints compared to a generally non-inflammatory process in OA , in which is substantiated by the 50-fold higher synovial fluid cell count in RA (Damgaard et al. 2016b). The various inflammatory stimuli in RA are then likely to promote citrullination (Blachere et al. 2015; Arandjelovic et al. 2012). It is still unknown if one PAD isoform is predominantly responsible for citrullination of the proteins that are found in the inflamed joints of RA patients (van Beers et al. 2013; Wang et al. 2016). Certain variations in the PADI4 gene have been identified as a risk factor for the development of RA (Suzuki et al. 2003; Plenge et al. 2005). In European populations, most studies do not support this association (Barton et al. 2004; Martinez et al. 2005; Burr et al. 2010) while a few studies did observe such an association (Okada et al. 2014; Hoppe et al. 2006). A single study reported PADI2 genetic variations to be associated with RA (Chang et al. 2013). No functional studies on the reported gene variations have identified a mechanism for these increased risk ratios (Cantaert et al. 2005). The above-listed studies support the notion that PAD2 , PAD4 , and citrullinated proteins are not specifically associated with RA but instead with inflammation in general, although the intensity of citrullination may be much higher in RA than in other inflammatory conditions.
5.5 PAD Activation
Members of the PAD family require the presence of Ca2+ and reducing conditions for their catalytic activity; these requirements were first demonstrated in vitro using rabbit or murine PADs with various concentrations of CaCl2 and the reducing agent dithiothreitol (DTT) (Takahara et al. 1986; Terakawa et al. 1991). PAD4 , and likely also other PAD isoforms, is more active as a dimer, although it is capable of citrullinating target substrates as a monomer (Liu et al. 2011). The normal intracellular Ca2+ concentration appears insufficient for PAD activity (Fig. 5.2), and this is also the case for the levels that can be achieved during apoptosis (Davies and Hallett 1998; Neeli et al. 2008). In contrast, the extracellular levels of Ca2+ are sufficient, but paradoxically in this environment, the levels of reducing agents , such as reduced glutathione (GSH ), are orders of magnitude lower (Griffith 1999; Jones 2002) than what is required for PAD activity (Damgaard et al. 2016a). Thus, citrullination seems to require a combination of intracellular (high levels of reducing capacity) and extracellular (high levels of Ca2+ ) conditions (Fig. 5.2). This raises the question of how PADs are activated in inflamed tissues and if unidentified activating factors inside cells or in complex body fluids may promote citrullination. Possibilities might be polyamines (Brooks 2013) and activating autoantibodies (Darrah et al. 2013).
Regulation of PAD activity in the intra- and extracellular space. Extracellular PADs are likely to exist in an inactivated form due to the oxidative extracellular environment; extracellular Ca2+ levels are sufficient for activity. Intracellular PADs exist in a reduced state but are inactive due to the low intracellular Ca2+ -levels. Upon activation of cells, e.g., by inflammatory stimuli or uncontrolled cell death , Ca2+ -influx can activate intracellular PADs followed by citrullination of intracellular proteins. Depending on the state of the stimulated/dying cells these citrullinated proteins and PADs can be released to the extracellular space. The high levels of extracellular Ca2+ concentration may lead to further activation of PADs, followed by citrullination of extracellular proteins. Extracellular PADs will in turn be inactivated by the extracellular oxidative environment
5.5.1 Calcium
Citrullination can be induced by treating cells (neutrophils , monocytes, or macrophages ) with calcium ionophores such as ionomycin (Nakashima et al. 2002; Vossenaar et al. 2004a). Ca2+ is an essential cofactor of PADs, whereas other physiologically relevant divalent cations fail to induce catalytic activity in vitro and can inhibit the activation by Ca2+ (Kearney et al. 2005). Therefore, zinc has been proposed as a physiological regulator of PAD activation (Stenberg and Roth 2015). Structural analysis of the PAD4 enzyme revealed five calcium -binding sites which are fairly conserved among all PADs except PAD6 (Arita et al. 2004). Mutation of the functional residues at the catalytic site has been shown to abolish enzymatic activity. One of these is Cys645 (Cys647 in PAD2 ), which contains an essential thiol group (Knuckley et al. 2007). Binding of Ca2+ leads to a conformational change, which positions this thiol into the active site (Arita et al. 2004). A more recent study demonstrated how a “Ca2+ switch”, initiated by the binding of Ca2+ in three of the five positions, is responsible for the proper positioning of the active site cysteine (Slade et al. 2015). This switch was found to occur at Ca2+ concentrations around 0.25 mM. Depending on the method of detection, half-maximal PAD activities have been reported at Ca2+ concentrations ranging from 40 μM (Zendman et al. 2007) to as high as 3.3 mM (Darrah et al. 2013). Most studies find the Ca2+ concentration required for half-maximal activity to be around 0.2–0.5 mM and demonstrate full enzymatic activity at Ca2+ concentrations above 2–2.5 mM—comparable to what is found in synovial fluid (Kearney et al. 2005; Damgaard et al. 2014). Inflammatory stimuli, but not apoptosis , have been shown to induce citrullination of histones and fibrinogen (Blachere et al. 2015; Neeli et al. 2008). ATP is another inflammatory signal that has been shown to induce PAD activation and robust protein citrullination (Arandjelovic et al. 2012), and this process required extracellular Ca2+ .
5.5.2 Reducing Conditions
Most studies of protein citrullination and PAD activity are based on in vitro experiments using the reducing agent DTT in the reaction buffer. DTT is a non-physiological synthetic molecule which provides the required reducing conditions for PAD activity in vitro (Terakawa et al. 1991). It is apparent that reduction of the active site thiol precedes the attack on the guanidinium carbon of arginine . In a recent study we showed that reduced glutathione (GSH ), the most abundant intracellular small molecule thiol, is capable of reducing PADs to promote their catalytic activity (Damgaard et al. 2016a) at concentrations comparable to those found within cells (Dixon et al. 2008). The extracellular GSH levels are, on the other hand, two to three orders of magnitude lower than the requirements for PAD activity (Griffith 1999). In the same study, we showed that PADs in synovial fluids are catalytically inactive, unless a reducing agent is added, and that PAD is active when 10 mM exogenous GSH is used. Interestingly, PAD released from phorbol 12-myristate 13-acetate (PMA)-stimulated leukocytes was capable of citrullinating fibrinogen without applying a reducing agent . In agreement with the findings in SFs, PAD released to leukocyte culture supernatants required a reducing agent to restore catalytic activity. The evidence for GSH being important in reducing PADs in vivo came from the observation that the glutathione reductase inhibitor 2-acetylamino-3-[4-(2-acetylamino-2-carboxyethylsulfanylthiocarbonylamino)phenylthiocarbamoylsulfanyl]propionic acid (2-AAPA), which decreases intracellular free GSH levels (Seefeldt et al. 2009), abolished the activity of the released PAD enzymes from leukocytes. Further studies will be necessary to fully dissect the role of GSH in PAD activation and to confirm its relevance. Other reducing agents such as thioredoxin may also be able to reduce and activate PADs (Yoshida et al. 1999). The balance between reducing conditions and reactive oxygen species seems important for activation/inactivation of PADs as also purposed by Dreyton and colleagues in their report on the PAD2 mechanism of action (Dreyton et al. 2014).
5.6 PAD and NETosis
During inflammation , e.g., caused by infections with bacteria , fungi, or viruses, neutrophils initiate a form of programmed cell death termed NETosis. This process results in the release of decondensed chromatin from the cells, termed neutrophil extracellular traps (NETs). Other inflammatory cells have been demonstrated to produce extracellular traps upon activation by pro-inflammatory stimuli. Most interestingly, PAD4 activity has been found to be required for NETosis, and citrullinated histones are incorporated into NETs (Muller and Radic 2015). It is likely that activated PAD4 is also released from the neutrophils during NETosis, and this may lead to the citrullination of additional proteins in the extracellular space. In addition to the association with citrullinated histones , extracellular traps are decorated with anti-microbial compounds originating from neutrophil granules (Parker and Winterbourn 2012). Thus, extracellular traps represent macromolecular assemblies, which contain citrullinated proteins, and therefore it is tempting to speculate that they play a role in the initiation of the citrulline -specific immune response in RA or in the progression of this response. Several lines of evidence support this relationship. Prominent NET autoreactivity has been detected in the sera from patients with RA, systemic lupus erythematosus (SLE) , and Felty’s syndrome (FS). Autoantibodies to citrullinated histones appeared to be produced in the majority of FS patients and in a subset of RA and SLE patients (Dwivedi et al. 2012; Dwivedi and Radic 2014; Muller and Radic 2015). Additional evidence for a functional relationship between NETosis and autoimmunity came from experiments showing that circulating neutrophils from RA or SLE patients and neutrophils in SF from RA patients display enhanced NETosis compared to neutrophils from healthy controls and from OA patients, respectively (Garcia-Romo et al. 2011; Khandpur et al. 2013). Moreover, RA sera and immunoglobulin fractions from RA patients, with high levels of ACPA and/or rheumatoid factor, significantly enhance NETosis (Khandpur et al. 2013). Finally, SLE sera were reported to have a reduced capacity to degrade NETs (Hakkim et al. 2010). Taken together, these data indicate that autoimmunity , at least in RA and SLE , is associated with changes in NET formation and/or degradation, but the involvement of NET-associated citrullinated and carbamylated proteins is yet unknown. For a more detailed description of the relationship between protein deamination and NETosis, we refer the reader to Chap. 8 of this volume .
5.7 PAD Activation and Citrullination in Relation to Inflammation in RA
The levels of synovial citrullinated proteins and PAD enzymes appeared to correlate with the degree of joint inflammation (Foulquier et al. 2007; Makrygiannakis et al. 2012). In immunohistochemistry of synovial tissue sections, intracellular citrullinated proteins co-localized with PAD2 in 59% of RA samples versus 17% in controls, i.e., patients with spondyloarthropathy, OA , juvenile chronic arthritis, and villonodular synovitis (De et al. 2005). A later study detected intracellular citrullinated proteins in 85.7% of RA tissue samples and the complete absence in healthy synovium (Makrygiannakis et al. 2012). The same percentage was positive for extracellular citrullinated proteins, which were only found in one out of eight healthy donors. All RA samples stained positive for PAD2 and PAD4 , which was also the case for a vast majority of the samples from healthy controls, although their levels were significantly lower (Makrygiannakis et al. 2012). It is not surprising that PAD2 and PAD4 are also expressed in tissues from healthy individuals due to the presence of various cells of hematopoietic origin, but PAD activation appears to be specific for inflammatory conditions. Protein citrullination, as well as PAD2 expression, correlated with cell infiltration and vascularity in synovial tissue (Makrygiannakis et al. 2012), and local administration of glucocorticoids decreased cell infiltration, levels of citrullinated proteins, and PAD4 levels. A high degree of inflammation and inflammatory cell infiltration are likely to promote protein citrullination, because high levels of PAD2 and PAD4 and the activation and release of these enzymes due to cell death /activation are associated with inflammation. Recently, we found significantly higher extracellular PAD2 levels in SF from ACPA-positive than from ACPA-negative RA patients (Damgaard et al. 2016b). In agreement with other studies showing a link between citrullination and inflammation, PAD2 in SF correlated with the cell count, as well as with IL-6 , IL-8 , and IL-10 levels in SF, and with circulating levels of CRP and ACPA . Notably, the patients’ SF PAD2 levels also correlated with the disease activity, as assessed by DAS28. The cell counts were generally higher in ACPA-positive than in ACPA-negative RA patients, although the differences did not reach statistical significance (Damgaard et al. 2016b). High levels of citrullinated proteins promote persistent inflammation in patients with ACPA-positive RA (van Venrooij and Pruijn 2008) carrying HLA-DRB1 alleles containing the shared epitope (Hill et al. 2003; Scally et al. 2013). Thus, citrullinated fibrin induces a higher inflammatory response in cultured RA synovial fibroblasts than non-citrullinated fibrin, as reflected by elevated IL-6 and IL-8 production (Sanchez-Pernaute et al. 2013).
Immune complexes containing citrullinated fibrinogen stimulate macrophages to produce more TNF α than immune complexes containing native fibrinogen (Sokolove et al. 2011). Thus, ACPAs and citrullinated proteins in immune complexes may account for the enhanced stimulation of macrophages observed among ACPA-positive patients . Strong evidence for the pathogenic role of citrullinated proteins in ACPA-positive RA came from the finding that around 30% of DR4-transgenic mice develop arthritis following immunization with citrullinated fibrinogen but not after immunization with non-citrullinated fibrinogen (Hill et al. 2008). Another genetic risk factor for development of RA is the C1858T polymorphism in the gene encoding protein tyrosine phosphatase , non-receptor type 22 (PTPN22) , which results in an amino acid change from arginine to tryptophan at position 620 (Begovich et al. 2004). Conflicting data exist regarding an effect of this polymorphism on the activation of lymphocytes, which may contribute to its impact on the development of RA, but another important aspect is that the major allelic variant R620 of PTPN22 physically interacts with PAD4 and inhibits citrullination (and thereby NET formation), whereas the W620 variant of PTPN22 fails to do so (Chang et al. 2015). Citrullination of E2F-1 assists its association with chromatin , specifically with genes encoding cytokine production in granulocytes , which is mediated by the enhanced binding citrullinated E2F-1 to bromodomain-containing protein 4 (BRD4) (Ghari et al. 2016). Accordingly, the combined inhibition of PAD4 and BRD4 disrupts the chromatin-bound complex and suppresses cytokine gene expression. Disrupted chromatin association and suppression of cytokine gene expression was also observed after inhibition of PAD in dendritic cells (Jang et al. 2015).
In addition to the effects on cytokines at the transcriptional level, citrullination may also affect their function by direct modification of these signaling molecules. A number of studies have shown that cytokines and chemokines are likely to alter functionality upon citrullination, as, for example, CXCL8 and TNF α (Proost et al. 2008; Moelants et al. 2013). Citrullination of the chemokine CXCL5 appeared to be significantly higher in RA sera and SF than in normal sera and in SF from patients with other rheumatic diseases (Yoshida et al. 2014). Moreover, citrullinated CXCL5 induced more severe inflammation and recruited more monocytes than its non-citrullinated counterpart in a mouse model of inflammatory arthritis. On the other hand, citrullinated TNF α was shown less potent to stimulate cultured human fibroblasts to produce chemokines (Moelants et al. 2013). Taken together, these results indicate that citrullination, in addition to antigen production, may affect the disease process in RA at several other levels, varying from the induction of NETosis to the expression and functional activity of molecules modulating inflammation (Fig. 5.3). Each of these phenomena may be targeted therapeutically. One of the approaches, the use of PAD inhibitors , will be addressed in Chapter 25 of this volume .
PAD activation and protein citrullination in the inflamed joint. Upon infiltration of inflammatory cells, including neutrophils , in the synovial membrane, NETosis can lead to the release of PAD (and intracellular citrullinated proteins) in the extracellular space, where the relatively high Ca2+ concentrations may lead to further PAD activation and citrullination of extracellular proteins. Antigen-presenting cells (APC), e.g., macrophages (MΦ), process, and present citrullinated epitopes to the T cells (not shown), which subsequently will activate B cells , resulting in their differentiation into plasma cells and ACPA production. ACPA may also originate from activated B cells in other tissues. ACPA may form immune complexes with the citrullinated proteins, which can enhance the inflammatory response via various mechanisms, can enhance NETosis , and can activate osteoclasts (not discussed in this chapter). Immune cells and proteins can also migrate to the synovial fluid in the synovial cavity
5.8 Citrullinated Proteins in Synovial Fluid
In view of the putative pathophysiologic role of citrullinated protein-specific immune complexes in RA , it is important to obtain a comprehensive view of the citrullinated proteins present in the inflamed joints of patients with RA. A systematic analysis of citrullinated proteins present in the synovial fluid of RA patients by mass spectrometry led to the identification of 53 polypeptides containing one or more citrulline residues, which comprises 28% of all proteins identified (van Beers et al. 2013). The only partially overlapping data obtained with material from different patients suggests that this is only a minority of the citrullinated proteins occurring in the inflamed joints of RA patients, implying that many proteins will be citrullinated in these tissues. This was recently confirmed by the results of independent studies (Tutturen et al. 2014; Wang et al. 2016). Among the proteins that were found to be citrullinated were fibrinogen , vimentin , fibronectin , and histones , proteins that were reported to be modified in inflamed tissues in previous studies. When the nature and frequency of amino acids flanking the citrulline were compared with the substrate specificity data described above, this suggested that citrullination in the RA synovium is exerted by a combination of PAD enzymes, e.g., PAD2 and PAD4 . This is in agreement with the presence of both PAD2 and PAD4 in the inflamed synovium of RA patients, as already mentioned above (Foulquier et al. 2007).
5.9 Concluding Remarks
Many studies have provided evidence for the involvement of citrullination in arthritis. In addition to citrullination of proteins that are directly involved in inflammation , such as the histones in NETosis , many other proteins are citrullinated as a result of PAD activation in inflamed tissues. This varies from very specific citrullination events, like those that enhance the expression of pro-inflammatory cytokines , to the citrullination of many proteins upon the release of activated PAD enzymes in the extracellular space. The factors involved in PAD activation are still not completely understood. The requirement for calcium has been well established, but it is not completely clear yet how intracellular calcium levels can be changed (locally) to levels that are needed for PAD activation, e.g., within nucleus. Up to now, the requirement for reducing conditions has received much less attention, and this is particularly relevant for extracellular PAD activity. Glutathione released from dying cells, possibly concomitant with PADs, may at least temporarily provide a (local) reducing environment allowing PAD to citrullinate extracellular proteins. More research will be required to fully understand how the necessary conditions for PAD activity are generated, both in arthritic tissues and in other cells/tissues. The specific immune response to citrullinated proteins in RA , in combination with the elevated levels of citrullination and the citrullination-dependent immune complexes formed, has opened several avenues for therapeutic strategies aimed at interference with protein citrullination and PAD activation. New methods to determine PAD activity and more insight in the substrate specificity of the most relevant PAD isoforms will be helpful to assess whether such strategies will be utilizable.
References
Arandjelovic, S., McKenney, K. R., Leming, S. S., & Mowen, K. A. (2012). ATP induces protein arginine deiminase 2-dependent citrullination in mast cells through the P2X7 purinergic receptor. Journal of Immunology, 189(8), 4112–4122.
Arita, K., Hashimoto, H., Shimizu, T., Nakashima, K., Yamada, M., & Sato, M. (2004). Structural basis for Ca(2+)-induced activation of human PAD4. Nature Structural & Molecular Biology, 11(8), 777–783.
Asaga, H., Nakashima, K., Senshu, T., Ishigami, A., & Yamada, M. (2001). Immunocytochemical localization of peptidylarginine deiminase in human eosinophils and neutrophils. Journal of Leukocyte Biology, 70(1), 46–51.
Assohou-Luty, C., Raijmakers, R., Benckhuijsen, W. E., Stammen-Vogelzangs, J., de, R. A., van Veelen, P. A., Franken, K. L., Drijfhout, J. W., & Pruijn, G. J. (2014). The human peptidylarginine deiminases type 2 and type 4 have distinct substrate specificities. Biochimica et Biophysica Acta, 1844(4), 829–836.
Badillo-Soto, M. A., Rodríguez-Rodríguez, M., Pérez-Pérez, M. E., Daza-Benitez, L., Bollain-y-Goytia, J. J., Carrillo-Jiménez, M. A., Avalos-Díaz, E., & Herrera-Esparza, R. (2016). Potential protein targets of the peptidylarginine deiminase 2 and peptidylarginine deiminase 4 enzymes in rheumatoid synovial tissue and its possible meaning. European Journal of Rheumatology, 3(2), 44–49. doi:10.5152/eurjrheum.2015.0055.
Barton, A., Bowes, J., Eyre, S., Spreckley, K., Hinks, A., John, S., & Worthington, J. (2004). A functional haplotype of the PADI4 gene associated with rheumatoid arthritis in a Japanese population is not associated in a United Kingdom population. Arthritis and Rheumatism, 50(4), 1117–1121.
Begovich, A. B., Carlton, V. E., Honigberg, L. A., Schrodi, S. J., Chokkalingam, A. P., Alexander, H. C., Ardlie, K. G., Huang, Q., Smith, A. M., Spoerke, J. M., Conn, M. T., Chang, M., Chang, S. Y., Saiki, R. K., Catanese, J. J., Leong, D. U., Garcia, V. E., McAllister, L. B., Jeffery, D. A., Lee, A. T., Batliwalla, F., Remmers, E., Criswell, L. A., Seldin, M. F., Kastner, D. L., Amos, C. I., Sninsky, J. J., & Gregersen, P. K. (2004). A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. American Journal of Human Genetics, 75(2), 330–337.
Bicker, K. L., Subramanian, V., Chumanevich, A. A., Hofseth, L. J., & Thompson, P. R. (2012). Seeing citrulline: Development of a phenylglyoxal-based probe to visualize protein citrullination. Journal of the American Chemical Society, 134(41), 17015–17018.
Bjelle, A., Norberg, B., & Sjogren, G. (1982). The cytology of joint exudates in rheumatoid arthritis. Morphology and preparation techniques. Scandinavian Journal of Rheumatology, 11(2), 124–128.
Blachere, N. E., Parveen, S., Fak, J., Frank, M. O., & Orange, D. E. (2015). Inflammatory but not apoptotic death of granulocytes citrullinates fibrinogen. Arthritis Research & Therapy, 17(1), 369.
Brooks, W. H. (2013). Increased polyamines alter chromatin and stabilize autoantigens in autoimmune diseases. Frontiers in Immunology, 4, 91.
Burr, M. L., Naseem, H., Hinks, A., Eyre, S., Gibbons, L. J., Bowes, J., Wilson, A. G., Maxwell, J., Morgan, A. W., Emery, P., Steer, S., Hocking, L., Reid, D. M., Wordsworth, P., Harrison, P., Thomson, W., Worthington, J., & Barton, A. (2010). PADI4 genotype is not associated with rheumatoid arthritis in a large UK Caucasian population. Annals of the Rheumatic Diseases, 69(4), 666–670.
Cantaert, T., Coucke, P., De, R. L., Veys, E. M., De, K. F., & Baeten, D. (2005). Functional haplotypes of PADI4: Relevance for rheumatoid arthritis specific synovial intracellular citrullinated proteins and anticitrullinated protein antibodies. Annals of the Rheumatic Diseases, 64(9), 1316–1320.
Chang, X., Yamada, R., Suzuki, A., Sawada, T., Yoshino, S., Tokuhiro, S., & Yamamoto, K. (2005). Localization of peptidylarginine deiminase 4 (PADI4) and citrullinated protein in synovial tissue of rheumatoid arthritis. Rheumatology (Oxford), 44(1), 40–50.
Chang, X., Xia, Y., Pan, J., Meng, Q., Zhao, Y., & Yan, X. (2013). PADI2 is significantly associated with rheumatoid arthritis. PloS One, 8(12), e81259.
Chang, H. H., Dwivedi, N., Nicholas, A. P., & Ho, I. C. (2015). The W620 polymorphism in PTPN22 disrupts its interaction with peptidylarginine deiminase type 4 and enhances citrullination and NETosis. Arthritis & Rhematology, 67(9), 2323–2334.
Chapuy-Regaud, S., Sebbag, M., Baeten, D., Clavel, C., Foulquier, C., De, K. F., & Serre, G. (2005). Fibrin deimination in synovial tissue is not specific for rheumatoid arthritis but commonly occurs during synovitides. Journal of Immunology, 174(8), 5057–5064.
Cherrington, B. D., Zhang, X., McElwee, J. L., Morency, E., Anguish, L. J., & Coonrod, S. A. (2012). Potential role for PAD2 in gene regulation in breast cancer cells. PloS One, 7(7), e41242.
Damgaard, D., Senolt, L., Nielsen, M., Pruijn, G., & Nielsen, C. H. (2014). Demonstration of extracellular peptidylarginine deiminase (PAD) activity in synovial fluid of patients with rheumatoid arthritis using a novel assay for citrullination of fibrinogen. Arthritis Research & Therapy, 16(6), 498.
Damgaard, D., Bjorn, M. E., Steffensen, M. A., Pruijn, G. J., & Nielsen, C. H. (2016a). Reduced glutathione as a physiological co-activator in the activation of peptidylarginine deiminase. Arthritis Research & Therapy, 18(1), 102.
Damgaard, D., Senolt, L., & Nielsen, C. H. (2016b). Increased levels of peptidylarginine deiminase 2 in synovial fluid from anti-CCP-positive rheumatoid arthritis patients: Association with disease activity and inflammatory markers. Rheumatology (Oxford), 55(5), 918–927.
Darrah, E., Rosen, A., Giles, J. T., & Andrade, F. (2012). Peptidylarginine deiminase 2, 3 and 4 have distinct specificities against cellular substrates: Novel insights into autoantigen selection in rheumatoid arthritis. Annals of the Rheumatic Diseases, 71(1), 92–98.
Darrah, E., Giles, J. T., Ols, M. L., Bull, H. G., Andrade, F., & Rosen, A. (2013). Erosive rheumatoid arthritis is associated with antibodies that activate PAD4 by increasing calcium sensitivity. Science Translational Medicine, 5(186), 186ra65.
Davies, E. V., & Hallett, M. B. (1998). High micromolar Ca2+ beneath the plasma membrane in stimulated neutrophils. Biochemical and Biophysical Research Communications, 248(3), 679–683.
De, R. L., Nicholas, A. P., Cantaert, T., Kruithof, E., Echols, J. D., Vandekerckhove, B., Veys, E. M., De, K. F., & Baeten, D. (2005). Synovial intracellular citrullinated proteins colocalizing with peptidyl arginine deiminase as pathophysiologically relevant antigenic determinants of rheumatoid arthritis-specific humoral autoimmunity. Arthritis and Rheumatism, 52(8), 2323–2330.
Dixon, B. M., Heath, S. H., Kim, R., Suh, J. H., & Hagen, T. M. (2008). Assessment of endoplasmic reticulum glutathione redox status is confounded by extensive ex vivo oxidation. Antioxidants & Redox Signaling, 10(5), 963–972.
Dreyton, C. J., Knuckley, B., Jones, J. E., Lewallen, D. M., & Thompson, P. R. (2014). Mechanistic studies of protein arginine deiminase 2: Evidence for a substrate-assisted mechanism. Biochemistry, 53(27), 4426–4433.
Dwivedi, N., & Radic, M. (2014). Citrullination of autoantigens implicates NETosis in the induction of autoimmunity. Annals of the Rheumatic Diseases, 73(3), 483–491.
Dwivedi, N., Upadhyay, J., Neeli, I., Khan, S., Pattanaik, D., Myers, L., Kirou, K. A., Hellmich, B., Knuckley, B., Thompson, P. R., Crow, M. K., Mikuls, T. R., Csernok, E., & Radic, M. (2012). Felty’s syndrome autoantibodies bind to deiminated histones and neutrophil extracellular chromatin traps. Arthritis and Rheumatism, 64(4), 982–992.
Foulquier, C., Sebbag, M., Clavel, C., Chapuy-Regaud, S., Al, B. R., Mechin, M. C., Vincent, C., Nachat, R., Yamada, M., Takahara, H., Simon, M., Guerrin, M., & Serre, G. (2007). Peptidyl arginine deiminase type 2 (PAD-2) and PAD-4 but not PAD-1, PAD-3, and PAD-6 are expressed in rheumatoid arthritis synovium in close association with tissue inflammation. Arthritis and Rheumatism, 56(11), 3541–3553.
Garcia-Romo, G. S., Caielli, S., Vega, B., Connolly, J., Allantaz, F., Xu, Z., Punaro, M., Baisch, J., Guiducci, C., Coffman, R. L., Barrat, F. J., Banchereau, J., & Pascual, V. (2011). Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Science Translational Medicine, 3(73), 73ra20.
Ghari, F., Quirke, A. M., Munro, S., Kawalkowska, J., Picaud, S., McGouran, J., Subramanian, V., Muth, A., Williams, R., Kessler, B., Thompson, P. R., Fillipakopoulos, P., Knapp, S., Venables, P. J., & La Thangue, N. B. (2016). Citrullination-acetylation interplay guides E2F-1 activity during the inflammatory response. Science Advances, 2(2), e1501257.
Griffith, O. W. (1999). Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radical Biology & Medicine, 27(9–10), 922–935.
Hakkim, A., Furnrohr, B. G., Amann, K., Laube, B., Abed, U. A., Brinkmann, V., Herrmann, M., Voll, R. E., & Zychlinsky, A. (2010). Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proceedings of the National Academy of Sciences of the United States of America, 107(21), 9813–9818.
Hao, G., Wang, D., Gu, J., Shen, Q., Gross, S. S., & Wang, Y. (2009). Neutral loss of isocyanic acid in peptide CID spectra: A novel diagnostic marker for mass spectrometric identification of protein citrullination. Journal of the American Society for Mass Spectrometry, 20(4), 723–727.
Harre, U., Georgess, D., Bang, H., Bozec, A., Axmann, R., Ossipova, E., Jakobsson, P. J., Baum, W., Nimmerjahn, F., Szarka, E., Sarmay, G., Krumbholz, G., Neumann, E., Toes, R., Scherer, H. U., Catrina, A. I., Klareskog, L., Jurdic, P., & Schett, G. (2012). Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. The Journal of Clinical Investigation, 122(5), 1791–1802.
Hensen, S. M., & Pruijn, G. J. (2014). Methods for the detection of peptidylarginine deiminase (PAD) activity and protein citrullination. Molecular & Cellular Proteomics, 13(2), 388–396.
Hensen, S. M., Boelens, W. C., Bonger, K. M., van Cruchten, R. T., van Delft, F. L., & Pruijn, G. J. (2015). Phenylglyoxal-based visualization of citrullinated proteins on Western blots. Molecules, 20(4), 6592–6600.
Hill, J. A., Southwood, S., Sette, A., Jevnikar, A. M., Bell, D. A., & Cairns, E. (2003). Cutting edge: The conversion of arginine to citrulline allows for a high-affinity peptide interaction with the rheumatoid arthritis-associated HLA- DRB1*0401 MHC class II molecule. Journal of Immunology, 171(2), 538–541.
Hill, J. A., Bell, D. A., Brintnell, W., Yue, D., Wehrli, B., Jevnikar, A. M., Lee, D. M., Hueber, W., Robinson, W. H., & Cairns, E. (2008). Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. The Journal of Experimental Medicine, 205(4), 967–979.
Hoppe, B., Haupl, T., Gruber, R., Kiesewetter, H., Burmester, G. R., Salama, A., & Dorner, T. (2006). Detailed analysis of the variability of peptidylarginine deiminase type 4 in German patients with rheumatoid arthritis: A case-control study. Arthritis Research & Therapy, 8(2), R34.
Jang, B., Kim, H. W., Kim, J. S., Kim, W. S., Lee, B. R., Kim, S., Kim, H., Han, S. J., Ha, S. J., & Shin, S. J. (2015). Peptidylarginine deiminase inhibition impairs Toll-like receptor agonist-induced functional maturation of dendritic cells, resulting in the loss of T cell-proliferative capacity: A partial mechanism with therapeutic potential in inflammatory settings. Journal of Leukocyte Biology, 97(2), 351–362.
Jones, D. P. (2002). Redox potential of GSH/GSSG couple: Assay and biological significance. Methods in Enzymology, 348, 93–112.
Kearney, P. L., Bhatia, M., Jones, N. G., Yuan, L., Glascock, M. C., Catchings, K. L., Yamada, M., & Thompson, P. R. (2005). Kinetic characterization of protein arginine deiminase 4: A transcriptional corepressor implicated in the onset and progression of rheumatoid arthritis. Biochemistry, 44(31), 10570–10582.
Khandpur, R., Carmona-Rivera, C., Vivekanandan-Giri, A., Gizinski, A., Yalavarthi, S., Knight, J. S., Friday, S., Li, S., Patel, R. M., Subramanian, V., Thompson, P., Chen, P., Fox, D. A., Pennathur, S., & Kaplan, M. J. (2013). NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Science Translational Medicine, 5(178), 178ra40.
Kinloch, A., Lundberg, K., Wait, R., Wegner, N., Lim, N. H., Zendman, A. J., Saxne, T., Malmstrom, V., & Venables, P. J. (2008). Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis and Rheumatism, 58(8), 2287–2295.
Knuckley, B., Bhatia, M., & Thompson, P. R. (2007). Protein arginine deiminase 4: Evidence for a reverse protonation mechanism. Biochemistry, 46(22), 6578–6587.
Knuckley, B., Causey, C. P., Jones, J. E., Bhatia, M., Dreyton, C. J., Osborne, T. C., Takahara, H., & Thompson, P. R. (2010). Substrate specificity and kinetic studies of PADs 1, 3, and 4 identify potent and selective inhibitors of protein arginine deiminase 3. Biochemistry, 49(23), 4852–4863.
Krishnamurthy, A., Joshua, V., Haj, H. A., Jin, T., Sun, M., Vivar, N., Ytterberg, A. J., Engstrom, M., Fernandes-Cerqueira, C., Amara, K., Magnusson, M., Wigerblad, G., Kato, J., Jimenez-Andrade, J. M., Tyson, K., Rapecki, S., Lundberg, K., Catrina, S. B., Jakobsson, P. J., Svensson, C., Malmstrom, V., Klareskog, L., Wahamaa, H., & Catrina, A. I. (2016). Identification of a novel chemokine-dependent molecular mechanism underlying rheumatoid arthritis-associated autoantibody-mediated bone loss. Annals of the Rheumatic Diseases, 75(4), 721–729.
Lewis, H. D., Liddle, J., Coote, J. E., Atkinson, S. J., Barker, M. D., Bax, B. D., Bicker, K. L., Bingham, R. P., Campbell, M., Chen, Y. H., Chung, C. W., Craggs, P. D., Davis, R. P., Eberhard, D., Joberty, G., Lind, K. E., Locke, K., Maller, C., Martinod, K., Patten, C., Polyakova, O., Rise, C. E., Rudiger, M., Sheppard, R. J., Slade, D. J., Thomas, P., Thorpe, J., Yao, G., Drewes, G., Wagner, D. D., Thompson, P. R., Prinjha, R. K., & Wilson, D. M. (2015). Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nature Chemical Biology, 11(3), 189–191.
Liu, Y. L., Chiang, Y. H., Liu, G. Y., & Hung, H. C. (2011). Functional role of dimerization of human peptidylarginine deiminase 4 (PAD4). PloS One, 6(6), e21314.
Makrygiannakis, D., af, K. E., Lundberg, I. E., Lofberg, R., Ulfgren, A. K., Klareskog, L., & Catrina, A. I. (2006). Citrullination is an inflammation-dependent process. Annals of the Rheumatic Diseases, 65(9), 1219–1222.
Makrygiannakis, D., Revu, S., Engstrom, M., af, K. E., Nicholas, A. P., Pruijn, G. J., & Catrina, A. I. (2012). Local administration of glucocorticoids decreases synovial citrullination in rheumatoid arthritis. Arthritis Research & Therapy, 14(1), R20.
Martinez, A., Valdivia, A., Pascual-Salcedo, D., Lamas, J. R., Fernandez-Arquero, M., Balsa, A., Fernandez-Gutierrez, B., de la Concha, E. G., & Urcelay, E. (2005). PADI4 polymorphisms are not associated with rheumatoid arthritis in the Spanish population. Rheumatology (Oxford), 44(10), 1263–1266.
Moelants, E. A., Mortier, A., Grauwen, K., Ronsse, I., Van, D. J., & Proost, P. (2013). Citrullination of TNF-alpha by peptidylarginine deiminases reduces its capacity to stimulate the production of inflammatory chemokines. Cytokine, 61(1), 161–167.
Mohanan, S., Horibata, S., McElwee, J. L., Dannenberg, A. J., & Coonrod, S. A. (2013). Identification of macrophage extracellular trap-like structures in mammary gland adipose tissue: A preliminary study. Frontiers in Immunology, 4, 67.
Muller, S., & Radic, M. (2015). Citrullinated autoantigens: From diagnostic markers to pathogenetic mechanisms. Clinical Reviews in Allergy & Immunology, 49(2), 232–239.
Nakashima, K., Hagiwara, T., & Yamada, M. (2002). Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. The Journal of Biological Chemistry, 277(51), 49562–49568.
Nakayama-Hamada, M., Suzuki, A., Kubota, K., Takazawa, T., Ohsaka, M., Kawaida, R., Ono, M., Kasuya, A., Furukawa, H., Yamada, R., & Yamamoto, K. (2005). Comparison of enzymatic properties between hPADI2 and hPADI4. Biochemical and Biophysical Research Communications, 327(1), 192–200.
Neeli, I., Khan, S. N., & Radic, M. (2008). Histone deimination as a response to inflammatory stimuli in neutrophils. Journal of Immunology, 180(3), 1895–1902.
Nicholas, A. P., & Whitaker, J. N. (2002). Preparation of a monoclonal antibody to citrullinated epitopes: Its characterization and some applications to immunohistochemistry in human brain. Glia, 37(4), 328–336.
Okada, Y., Wu, D., Trynka, G., Raj, T., Terao, C., Ikari, K., Kochi, Y., Ohmura, K., Suzuki, A., Yoshida, S., Graham, R. R., Manoharan, A., Ortmann, W., Bhangale, T., Denny, J. C., Carroll, R. J., Eyler, A. E., Greenberg, J. D., Kremer, J. M., Pappas, D. A., Jiang, L., Yin, J., Ye, L., Su, D. F., Yang, J., Xie, G., Keystone, E., Westra, H. J., Esko, T., Metspalu, A., Zhou, X., Gupta, N., Mirel, D., Stahl, E. A., Diogo, D., Cui, J., Liao, K., Guo, M. H., Myouzen, K., Kawaguchi, T., Coenen, M. J., van Riel, P. L., van de Laar, M. A., Guchelaar, H. J., Huizinga, T. W., Dieude, P., Mariette, X., Bridges, S. L., Jr., Zhernakova, A., Toes, R. E., Tak, P. P., Miceli-Richard, C., Bang, S. Y., Lee, H. S., Martin, J., Gonzalez-Gay, M. A., Rodriguez-Rodriguez, L., Rantapaa-Dahlqvist, S., Arlestig, L., Choi, H. K., Kamatani, Y., Galan, P., Lathrop, M., Eyre, S., Bowes, J., Barton, A., de, V. N., Moreland, L. W., Criswell, L. A., Karlson, E. W., Taniguchi, A., Yamada, R., Kubo, M., Liu, J. S., Bae, S. C., Worthington, J., Padyukov, L., Klareskog, L., Gregersen, P. K., Raychaudhuri, S., Stranger, B. E., De Jager, P. L., Franke, L., Visscher, P. M., Brown, M. A., Yamanaka, H., Mimori, T., Takahashi, A., Xu, H., Behrens, T. W., Siminovitch, K. A., Momohara, S., Matsuda, F., Yamamoto, K., & Plenge, R. M. (2014). Genetics of rheumatoid arthritis contributes to biology and drug discovery. Nature, 506(7488), 376–381.
Olivares-Martinez, E., Hernandez-Ramirez, D. F., Nunez-Alvarez, C. A., Cabral, A. R., & Llorente, L. (2016). The amount of citrullinated proteins in synovial tissue is related to serum anti-cyclic citrullinated peptide (anti-CCP) antibody levels. Clinical Rheumatology, 35(1), 55–61.
Parker, H., & Winterbourn, C. C. (2012). Reactive oxidants and myeloperoxidase and their involvement in neutrophil extracellular traps. Frontiers in Immunology, 3, 424.
Plenge, R. M., Padyukov, L., Remmers, E. F., Purcell, S., Lee, A. T., Karlson, E. W., Wolfe, F., Kastner, D. L., Alfredsson, L., Altshuler, D., Gregersen, P. K., Klareskog, L., & Rioux, J. D. (2005). Replication of putative candidate-gene associations with rheumatoid arthritis in >4,000 samples from North America and Sweden: association of susceptibility with PTPN22, CTLA4, and PADI4. American Journal of Human Genetics, 77(6), 1044–1060.
Proost, P., Loos, T., Mortier, A., Schutyser, E., Gouwy, M., Noppen, S., Dillen, C., Ronsse, I., Conings, R., Struyf, S., Opdenakker, G., Maudgal, P. C., & Van, D. J. (2008). Citrullination of CXCL8 by peptidylarginine deiminase alters receptor usage, prevents proteolysis, and dampens tissue inflammation. The Journal of Experimental Medicine, 205(9), 2085–2097.
Raijmakers, R., van Beers, J. J., El-Azzouny, M., Visser, N. F., Bozic, B., Pruijn, G. J., & Heck, A. J. (2012). Elevated levels of fibrinogen-derived endogenous citrullinated peptides in synovial fluid of rheumatoid arthritis patients. Arthritis Research & Therapy, 14(3), R114.
Salisbury, A. K., Duke, O., & Poulter, L. W. (1987). Macrophage-like cells of the pannus area in rheumatoid arthritic joints. Scandinavian Journal of Rheumatology, 16(4), 263–272.
Sanchez-Pernaute, O., Filkova, M., Gabucio, A., Klein, M., Maciejewska-Rodrigues, H., Ospelt, C., Brentano, F., Michel, B. A., Gay, R. E., Herrero-Beaumont, G., Gay, S., Neidhart, M., & Juengel, A. (2013). Citrullination enhances the pro-inflammatory response to fibrin in rheumatoid arthritis synovial fibroblasts. Annals of the Rheumatic Diseases, 72(8), 1400–1406.
Scally, S. W., Petersen, J., Law, S. C., Dudek, N. L., Nel, H. J., Loh, K. L., Wijeyewickrema, L. C., Eckle, S. B., van, H. J., Pike, R. N., McCluskey, J., Toes, R. E., La Gruta, N. L., Purcell, A. W., Reid, H. H., Thomas, R., & Rossjohn, J. (2013). A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. The Journal of Experimental Medicine, 210(12), 2569–2582.
Seefeldt, T., Zhao, Y., Chen, W., Raza, A. S., Carlson, L., Herman, J., Stoebner, A., Hanson, S., Foll, R., & Guan, X. (2009). Characterization of a novel dithiocarbamate glutathione reductase inhibitor and its use as a tool to modulate intracellular glutathione. The Journal of Biological Chemistry, 284(5), 2729–2737.
Senshu, T., Sato, T., Inoue, T., Akiyama, K., & Asaga, H. (1992). Detection of citrulline residues in deiminated proteins on polyvinylidene difluoride membrane. Analytical Biochemistry, 203(1), 94–100.
Slade, D. J., Subramanian, V., Fuhrmann, J., & Thompson, P. R. (2014). Chemical and biological methods to detect post-translational modifications of arginine. Biopolymers, 101(2), 133–143.
Slade, D. J., Fang, P., Dreyton, C. J., Zhang, Y., Fuhrmann, J., Rempel, D., Bax, B. D., Coonrod, S. A., Lewis, H. D., Guo, M., Gross, M. L., & Thompson, P. R. (2015). Protein arginine deiminase 2 binds calcium in an ordered fashion: Implications for inhibitor design. ACS Chemical Biology, 10(4), 1043–1053.
Sokolove, J., Zhao, X., Chandra, P. E., & Robinson, W. H. (2011). Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcgamma receptor. Arthritis and Rheumatism, 63(1), 53–62.
Spengler, J., Lugonja, B., Jimmy, Y. A., Zubarev, R. A., Creese, A. J., Pearson, M. J., Grant, M. M., Milward, M., Lundberg, K., Buckley, C. D., Filer, A., Raza, K., Cooper, P. R., Chapple, I. L., & Scheel-Toellner, D. (2015). Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis & Rhematology, 67(12), 3135–3145.
Stenberg, P., & Roth, B. (2015). Zinc is the modulator of the calcium-dependent activation of post-translationally acting thiol-enzymes in autoimmune diseases. Medical Hypotheses, 84(4), 331–335.
Stensland, M. E., Pollmann, S., Molberg, O., Sollid, L. M., & Fleckenstein, B. (2009). Primary sequence, together with other factors, influence peptide deimination by peptidylarginine deiminase-4. Biological Chemistry, 390(2), 99–107.
Suzuki, A., Yamada, R., Chang, X., Tokuhiro, S., Sawada, T., Suzuki, M., Nagasaki, M., Nakayama-Hamada, M., Kawaida, R., Ono, M., Ohtsuki, M., Furukawa, H., Yoshino, S., Yukioka, M., Tohma, S., Matsubara, T., Wakitani, S., Teshima, R., Nishioka, Y., Sekine, A., Iida, A., Takahashi, A., Tsunoda, T., Nakamura, Y., & Yamamoto, K. (2003). Functional haplotypes of PADI4, encoding citrullinating enzyme peptidylarginine deiminase 4, are associated with rheumatoid arthritis. Nature Genetics, 34(4), 395–402.
Takahara, H., Okamoto, H., & Sugawara, K. (1986). Calcium-dependent properties of peptidylarginine deiminase from rabbit skeletal muscle. Agricultural and Biological Chemistry, 50, 2899–2904.
Terakawa, H., Takahara, H., & Sugawara, K. (1991). Three types of mouse peptidylarginine deiminase: Characterization and tissue distribution. Journal of Biochemistry, 110(4), 661–666.
Tutturen, A. E., Fleckenstein, B., & de Souza, G. A. (2014). Assessing the citrullinome in rheumatoid arthritis synovial fluid with and without enrichment of citrullinated peptides. Journal of Proteome Research, 13(6), 2867–2873.
Tutturen, A. E., Holm, A,, Jorgensen, M,, Stadtmuller, P., Rise, F., & Fleckenstein, B. (2010). A technique for the specific enrichment of citrulline-containing peptides. Analytical Biochemistry, 403, 43–51.
van Beers, J. J., Raijmakers, R., Alexander, L. E., Stammen-Vogelzangs, J., Lokate, A. M., Heck, A. J., Schasfoort, R. B., & Pruijn, G. J. (2010). Mapping of citrullinated fibrinogen B-cell epitopes in rheumatoid arthritis by imaging surface plasmon resonance. Arthritis Research & Therapy, 12(6), R219.
van Beers, J. J., Schwarte, C. M., Stammen-Vogelzangs, J., Oosterink, E., Bozic, B., & Pruijn, G. J. (2013). The rheumatoid arthritis synovial fluid citrullinome reveals novel citrullinated epitopes in apolipoprotein E, myeloid nuclear differentiation antigen, and beta-actin. Arthritis and Rheumatism, 65(1), 69–80.
van Venrooij, W. J., & Pruijn, G. J. (2008). An important step towards completing the rheumatoid arthritis cycle. Arthritis Research & Therapy, 10(5), 117.
Vossenaar, E. R., Zendman, A. J., van Venrooij, W. J., & Pruijn, G. J. (2003). PAD, a growing family of citrullinating enzymes: Genes, features and involvement in disease. BioEssays, 25(11), 1106–1118.
Vossenaar, E. R., Radstake, T. R., van der Heijden, A., van Mansum, M. A., Dieteren, C., de Rooij, D. J., Barrera, P., Zendman, A. J., & van Venrooij, W. J. (2004a). Expression and activity of citrullinating peptidylarginine deiminase enzymes in monocytes and macrophages. Annals of the Rheumatic Diseases, 63(4), 373–381.
Vossenaar, E. R., Smeets, T. J., Kraan, M. C., Raats, J. M., van Venrooij, W. J., & Tak, P. P. (2004b). The presence of citrullinated proteins is not specific for rheumatoid synovial tissue. Arthritis and Rheumatism, 50(11), 3485–3494.
Wang, F., Chen, F. F., Gao, W. B., Wang, H. Y., Zhao, N. W., Xu, M., Gao, D. Y., Yu, W., Yan, X. L., Zhao, J. N., & Li, X. J. (2016). Identification of citrullinated peptides in the synovial fluid of patients with rheumatoid arthritis using LC-MALDI-TOF/TOF. Clinical Rheumatology, 35(9), 2185–2194.
Yoshida, S., Katoh, T., Tetsuka, T., Uno, K., Matsui, N., & Okamoto, T. (1999). Involvement of thioredoxin in rheumatoid arthritis: Its costimulatory roles in the TNF-alpha-induced production of IL-6 and IL-8 from cultured synovial fibroblasts. Journal of Immunology, 163(1), 351–358.
Yoshida, K., Korchynskyi, O., Tak, P. P., Isozaki, T., Ruth, J. H., Campbell, P. L., Baeten, D. L., Gerlag, D. M., Amin, M. A., & Koch, A. E. (2014). Citrullination of epithelial neutrophil-activating peptide 78/CXCL5 results in conversion from a non-monocyte-recruiting chemokine to a monocyte-recruiting chemokine. Arthritis & Rhematology, 66(10), 2716–2727.
Zendman, A. J., Raijmakers, R., Nijenhuis, S., Vossenaar, E. R., Tillaart, M., Chirivi, R. G., Raats, J. M., van Venrooij, W. J., Drijfhout, J. W., & Pruijn, G. J. (2007). ABAP: Antibody-based assay for peptidylarginine deiminase activity. Analytical Biochemistry, 369(2), 232–240.
Acknowledgments
The authors would like to thank Claus H. Nielsen (Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark) for the critical reading of and helpful comments on this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Damgaard, D., Pruijn, G.J.M. (2017). PAD Activation in Arthritis. In: Nicholas, A., Bhattacharya, S., Thompson, P. (eds) Protein Deimination in Human Health and Disease. Springer, Cham. https://doi.org/10.1007/978-3-319-58244-3_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-58244-3_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58243-6
Online ISBN: 978-3-319-58244-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)