Abstract
Hemoptysis is defined as the expectoration of blood from the lower respiratory tract and could be graduated in hemoptoic sputum (sputum staining with blood streaks), and evident hemoptisis or may even present as massive hemoptysis. In the last case, it is preferable to use the term threatening hemoptysis, defined as the one that poses a risk to life for the patient.The causes of hemoptysis are multiple and varied. The disease causing hemoptysis can affect the airway, lung parenchyma, or pulmonary vessels. Although they vary according to the population studied, the most frequent causes of hemoptysis are bronchiectasis, chronic bronchitis, and bronchogenic carcinoma.Bronchoscopy plays a key role in the diagnosis and management of hemoptysis. It allows confirmation in doubtful cases, location of the bleeding point or, at least, location of the affected lung, and the determination of the cause in lesions accessible to it. It can allow the isolation of the hemorrhagic segment or lobe to avoid flooding the non-affecting bronchial tree and reduce the risk of suffocation, by selective intubation or bronchial blockade with balloon, as well as the application of local therapies that contribute to control the bleeding.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Introduction
Hemoptysis is the expectoration of blood from the lower respiratory tract. Bleeding from the upper airway is excluded from this definition.
Causes of hemoptysis are wide and varied, as well as the amount, ranging from hemoptoic expectoration (sputum stained with blood streaks) to massive or life-threatening hemoptysis (expectoration of fresh blood in important quantities).
Life-threatening hemoptysis is defined as that hemoptysis that poses risks to life for the patient. This risk is determined by the total volume of bleeding, its velocity, and the patient’s cardiopulmonary reserve [1]. In all hemoptysis and in most cases of hemoptysis, it is necessary to try to obtain both its anatomical location and etiological identification. In this sense, bronchoscopy and multidetector chest CT scan (MDCT) are complementary examinations, each with concrete advantages depending on the different clinical situations.
Bronchoscopy is indicated in order to locate the bleeding site, identifying the cause and controlling bleeding, even temporarily. Arteriography is also a very good method, since it allows to diagnose the bleeding area and treat it with embolization of bleeding vessels if possible.
Life-threatening hemoptysis thus requires two important steps to consider: first, bleeding control and location of the bleeding area and second definitive treatment of its cause.
We will define the role of bronchoscopy in both diagnosis and management of hemoptysis.
Definition
Hemoptysis is defined as the expectoration of blood from the lower respiratory tract. Bleeding from the upper airway is excluded from this definition.
In most cases the amount of bleeding is slight, the patient has hemoptoic expectoration (sputum staining with blood streaks) and hemoptysis is self-limited. In other cases the amount is higher (evident hemoptysis) or may even present massive hemoptysis (expectoration of fresh blood in important quantities).
Massive hemoptysis usually refers to the expectoration of large amounts of blood and/or the rapidity of this bleeding. The amount of expectorated blood in 24 h is usually used to differentiate between massive and non-massive hemoptysis. However, this definition varies widely in the literature, with values ranging from an expectorated blood volume of 100–600 mL, during a period of time that is also variable. Difficulty is even higher considering that hemoptysis is difficult to quantify: it could be both overestimated and underestimated by patients. Underestimation may occur when part of the blood is retained in the tracheobronchial tree.
It is therefore preferable to use the term threatening hemoptysis, defined as that which poses a risk to the life of the patient; this risk is determined by the total volume of bleeding, its velocity, and the patient’s cardiopulmonary reserve [1]. As risk indicators, the amount of hemoptysis (greater than 100 mL) and the presence of airway obstruction, respiratory failure, and hemodynamic instability should be considered [2]. Asphyxia due to clot formation along with cardiocirculatory collapse is usually the cause of death, not exsanguination. Mortality of untreated threatening hemoptysis is high, greater than 50% [3], so it is very important the immediate assessment of the patient and identification of the causes of bleeding in order to start an appropriate treatment and avoid a fatal outcome.
Etiology of Hemoptysis
The causes of hemoptysis are multiple and varied.
Before detailing, it is important to know the system of vascularization of the lung.
Vascular Origin of Hemoptysis
There are two systems by which blood reaches the lungs: pulmonary arteries and bronchial arteries. The pulmonary arteries conform a low pressure system that contains all cardiac output and are responsible for gas exchange. The bronchial arteries are part of the systemic circulation and have greater pressure and much less flow; the irrigation of the bronchi and the visceral pleura depends on them. Despite its lower contribution to pulmonary blood flow, the bronchial arteries are the source of most hemoptysis. Sometimes other systemic non-bronchial arteries may be the source of hemoptysis. In a much lower percentage, the bleeding comes from the pulmonary arteries or from the pulmonary microcirculation [4].
The vessels of the bronchial network causing bleeding are usually neoformed, usually secondary to an inflammatory disease (bronchiectasis, sarcoidosis, lung abscess, tuberculosis, etc.). The walls of these vessels are surrounded by smooth muscle fibers capable of contracting, both physically and pharmacologically. Arterial embolization is also an effective method to eliminate this neovascularization. However, the pulmonary artery network is not capable of generating a vasospasm as potent as the bronchial vessels, since its walls are thin and do not contract. Therefore, the physical and pharmacological means have only a slight effect on them. The most frequent cause of hemorrhage is ulceration of the vessel wall caused by a destructive process of the lung parenchyma (pulmonary neoplasia, bacterial necrotizing pneumonia, mycetoma). In these cases, the cessation of bleeding is usually due to the temporary sealing by a clot whose dissolution or progression of the tear can lead to a relapse with greater hemorrhage [5]. Unfortunately, it is not always feasible to differentiate the vascular network originating the hemorrhage.
Etiology
The condition producing hemoptysis can affect the airway, lung parenchyma, or pulmonary vessels. Although they vary according to the population studied, the most frequent causes of hemoptysis are bronchiectasis, chronic bronchitis, and bronchogenic carcinoma [6].
-
1.
Pathology of the airway (Table 38.1)
Pathology of the airway is the most frequent cause of hemoptysis and includes:
-
Inflammatory diseases: bronchiectasis and chronic bronchitis
-
Neoplasms: bronchogenic carcinoma, carcinoid tumor, and endobronchial metastasis
-
Fistulas between the tracheobronchial tree and blood vessels, especially in the case of thoracic aorta aneurysms
-
Foreign bodies and trauma
-
Dieulafoy disease of the bronchi (presence of an abnormal bronchial artery, contiguous to the bronchial mucosa) [7, 8]
-
-
2.
Pathology of the pulmonary parenchyma (Table 38.2)
Bleeding originating from the lung parenchyma is usually due to:
-
Infections: pneumonia, tuberculosis, lung abscess, and fungal infections, mainly aspergilloma
-
Inflammatory or immunological diseases leading to diffuse alveolar hemorrhage: Goodpasture syndrome, systemic lupus erythematosus (SLE), granulomatous polyangiitis (Wegener), and microscopic polyarteritis
-
Coagulopathies: thrombopenia and administration of anticoagulant or antiaggregant drugs
-
Complications of certain techniques: transbronchial biopsy and fine needle aspiration
-
Miscellaneous: cocaine inhalation, catamenial hemoptysis, and treatment with bevacizumab (vascular endothelial-derived growth factor inhibitor, VEGF).
-
-
3.
Pathology of the pulmonary vasculature (Table 38.3)
Hemoptysis caused by diseases of the pulmonary arteries [8] may appear due to the same causes as those originating in the pulmonary parenchyma: intrinsic to the pulmonary vasculature conditions (pulmonary embolism, arteriovenous malformations) and increased pulmonary capillary pressure (mitral stenosis, pulmonary artery perforation originated by a Swan-Ganz catheter placement) [9]
-
4.
Idiopathic or cryptogenic hemoptysis
Up to 10–30% of cases, it is not possible to establish an etiological diagnosis of hemoptysis following bronchoscopy and chest CT scan [6, 10], and the patient is considered to have idiopathic or cryptogenic hemoptysis. Most of these patients are smokers and hemoptysis is usually due to inflammation of the bronchial wall produced by tobacco, rather than to an unspecified cause, known as tobacco-related hemoptysis [11]. Idiopathic hemoptysis is also related to chronic or acute bronchial inflammation, occult bronchiectasis, inactive tuberculosis, vascular pulmonary malformations, and coagulation disorders [12].
It is likely that with the use of MDCT, the proportion of cryptogenic hemoptysis will be reduced [11].
Bronchoscopy in Hemoptysis
Bronchoscopy plays a key role in the diagnosis and management of hemoptysis.
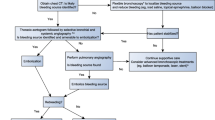
It allows confirmation in doubtful cases, location of the bleeding point or, at least, location of the affected lung, and the determination of the cause if the lesion is visible or accessible to endoscopic examination. It also allows the isolation of the hemorrhagic segment or lobe to avoid the spreading of blood to the bronchial tree and reduce the risk of suffocation. In this sense performing rigid bronchoscopy complemented by flexible bronchoscopy carries a great advantage. In cases where a rigid bronchoscope is not at hand, flexible bronchoscopy as the only endoscopic procedure can also be very useful. It can be performed at the bedside and allows selective intubation or bronchial balloon blockade, as well as the application of local therapies. It can contribute, even temporarily, to control bleeding and the application of more definitive treatments such as embolization of bronchial arteries or even, in selected cases, surgical treatment (Figs. 38.1 and 38.2) [13].
Algorithm for bronchoscopy in threatening hemoptysis. With permission. Original Source: Cordovilla R, Bollo de Miguel E, Nuñez Ares A, Cosano Povedano FJ, Herráez Ortega I, Jiménez Merchán R. Diagnosis and treatment of hemoptysis. Arch Bronconeumol. 2016;52(7):368–377. Copyright © 2016 SEPAR. Published by Elsevier España, S.L.U. All rights reserved
Nonthreatening hemoptysis algorithm. With permission. Original Source: Cordovilla R, Bollo de Miguel E, Nuñez Ares A, Cosano Povedano FJ, Herráez Ortega I, Jiménez Merchán R. Diagnosis and treatment of hemoptysis. Arch Bronconeumol. 2016;52(7):368–377. Copyright © 2016 SEPAR. Published by Elsevier España, S.L.U. All rights reserved
Diagnostic Bronchoscopy
In the event of severe hemoptysis, diagnostic bronchoscopy can help in many ways:
-
1.
Confirmation of hemoptysis and exclusion of pseudohemoptysis
Although the clinical history, the characteristics of the episode, and the initial physical examination may suggest the digestive or respiratory origin of the bleeding, sometimes the aspiration of at least part of digestive bleeding content causes cough and can simulate a true hemoptysis (pseudohemoptysis) which requires an ENT examination, a high digestive endoscopy or bronchofibroscopy to differentiate.
-
2.
Diagnostic of at least the side of bleeding, in anticipation to specific treatment
Although imaging studies (chest CT scan) can identify the origin of bleeding and its cause sometimes with a superior performance than bronchoscopy [14, 15], this is still necessary. It should be indicated early, especially in massive or life-threatening hemoptysis. Bronchoscopy reveals or confirms the origin of bleeding, especially if it is performed within 48 h of the onset of the episode and in cases of significant bleeding in 73–93% of cases of massive hemoptysis [14, 16].
In the case of threatening hemoptysis, it is advisable to perform bronchoscopy as soon as possible if the patient is unstable and once the patient has been intubated [17, 18]. Endoscopy through the ET tube is safer since the airway is secure and the endoscope can be withdrawn every time oxygenation worsens or the working channel is occluded by clots.
Rigid bronchoscopy can be used for the diagnosis and initial evaluation of threatening hemoptysis, but the flexible bronchoscope has some advantages to it such as the ability to reach the distal airway more easily. It can be used in the setting more suitable for the patient: ICU, shock room, bronchoscopy room, etc., without the additional delays of having to transfer the patient to the OR to undergo rigid bronchoscopy, or the radiology room to perform angiotomography.
Bronchoscopy also proves its value in those cases of non-revealing radiological studies or those that show bilateral or non-localizing abnormalities. In any case, even in those nonthreatening episodes, it provides useful information in the event that bleeding increases dangerously in a sudden and unpredictable manner.
Location of the bleeding site requires direct visualization of active bleeding, which determines with certainty one bronchus or the responsible bronchial area. The most frequent endoscopic finding is hematic remains and clots (Fig. 38.3). Locating blood clots does not guarantee the origin of the bleeding. However, a combination of findings such as a great number of clots adhering to a particular bronchus can suggest, together with the imaging techniques, the responsible area. Blood remains should be aspirated through repeated small bronchial washes, in order to improve permeability and allow diagnostic examination of the underlying territory. However, in the presence of fresh clots adhering, it is not advisable to aspirate them given the risk of further bleeding. Subsequently, bronchoscopy can be repeated to evaluate whether they can be removed with a smaller risk of rebleeding.
A cryoprobe can be used for the removal of an adherent clot. In order to do that, a cryoprobe is placed in the center of the clot and freezing activated during 3–4 s. The clot will adhere to the end of the probe and extracted en bloc with the bronchoscope just like a foreign body would do. This procedure should be done through an ET tube or through a rigid bronchoscope in order to have complete control of the airway in the event of bleeding (Figs. 38.4 and 38.5).
-
3.
Causal diagnosis, in case of accessible bronchial lesions
Bronchoscopy allows us to perform an endobronchial inspection and evaluate mucosal changes: hypertrophic or malformed capillary vascular network, areas of inflammatory or infiltrative mucosal thickening, bronchial stenosis, endobronchial tumors, antracosis or antracoestenosis, broncholiths, etc. (Fig. 38.6). In many cases, the changes are nonspecific and, therefore, nondiagnostic [19].
In addition to the visual examination, flexible bronchoscopy allows collection of samples for cytohistological and microbiological studies: bronchial lavage and bronchoalveolar lavage in the presence of suspected alveolar hemorrhage and biopsies and/or bronchial brushing in the presence of lesions suspected of malignancy. In the case of highly vascular lesions, some authors recommend local instillation of 1–2 mL of adrenaline 1:20,000 dilution, to reduce the risk of further bleeding, although clinical evidence is low [20].
Bronchoscopy also plays a very important role in nonthreatening hemoptysis with no apparent radiological alteration.
The existence of a normal chest X-ray in the context of hemoptysis does not exclude the possibility of malignancy or other underlying pathology [4, 12, 21, 22]. The probability of malignancy in patients with hemoptysis and normal chest X-ray is low but may reach up to 10% in patients over the age of 40, with a history of smoking [23], even in patients with mild hemoptysis [24].
Bronchoscopy can detect an endobronchial lesion in 5% of patients with mild hemoptysis and normal chest X-ray [25], and HRCT detects bronchiectasis in up to 70% of cases of severe hemoptysis and normal chest X-rays [14]. Therefore, depending on the type of hemoptysis, bronchoscopy can be performed before or after the complementary radiological tests:
-
1.
Hemoptoic expectoration: If there are no risk factors for cancer, bronchoscopy is indicated when these episodes are recurrent, or when the amount of bleeding increases [25]. In the case of patients with recurrent hemoptysis, the first step is to perform a chest CT scan (HRCT or MDCTD) as it may be useful to select the most cost-effective endoscopic technique for diagnosis (flexible bronchoscopy or endobronchial ultrasound) [7, 14, 26, 27].
-
2.
Evident hemoptysis: If there is no known cause, a bronchoscopy is necessary, especially in patients with risk factors for malignancy. However, depending on the stability of the patient, it may be advisable to perform a chest CT scan first. The combined use of bronchoscopy and MDCT increases the diagnostic yield for locating the bleeding site [14].
If the patient has a normal CT scan, bronchoscopy can diagnose the cause of bleeding in up to 16% of the cases. This percentage increases up to 37% when clinical history is also taken into account [23]. If bronchoscopy does not reveal changes, the patient is considered to have cryptogenic hemoptysis. A combination of CT and negative bronchoscopy has a very low probability of malignancy (1%) after a 6-month follow-up [28].
Therapeutic Bronchoscopy
Therapeutic bronchoscopy is specifically indicated to eliminate, at least transiently, a risk situation generally in the context of massive or threatening hemoptysis. Therefore, it is an urgent action applied in combination with other life-support measures, which seek to recover and keep the patient clinically stable. Diagnosis can then be completed with imaging techniques if the status of the patient allows and apply definitive treatment. Bronchial, systemic, and/or pulmonary embolization or surgical embolization can be used according to the situation. Generally, first evaluation of the patient should be oriented to estimate the severity of the condition and decide which treatment is most convenient and where it will take place.
Hemoptoic sputum does not require hospitalization, but evident and life-threatening hemoptysis does. In the latter case, admission to the ICU is warranted. Next, a quick and accurate diagnosis should be performed in order to locate the place of bleeding and determine its cause simultaneously.
The objectives of treatment are:
-
Secure the airway.
-
Maintain adequate oxygenation.
-
Achieve hemodynamic stability.
-
Locate and stop bleeding.
-
Identify and treat the cause of hemoptysis.
Management of the patient during hospital admission includes a series of general measures:
-
1.
Rest in bed in lateral decubitus, the affected side down, with the intention of protecting the airway and avoid aspiration of blood in the unaffected lung.
-
2.
Control of clinical parameters (blood pressure, heart rate and respiratory rate, oxygen saturation) and quantification of hemoptysis.
-
3.
Supplemental oxygen supply if necessary.
-
4.
Control of cough by administering antitussives, avoiding respiratory physiotherapy techniques.
-
5.
Empirical antibiotic treatment, useful in hemoptysis associated with respiratory infections and, in general, to prevent further complications.
-
6.
Nothing per oral, to avoid aspiration to the airway, and to allow the performance of urgent tests like bronchoscopy, CT or arteriography.
-
7.
Establishment of large-caliber venous access for fluid administration, availability of a blood reserve, and, if necessary, transfusion of packed red blood cells.
-
8.
Antifibrinolytic agents: aminocaproic acid and tranexamic acid (AT) administration. They act by inhibiting the process of dissolution of the clot with the consequent reduction of hemorrhage. A Cochrane review [29] identifies two clinical trials evaluating the use of AT (Amchafibrin®), both orally and intravenously. The results indicate that they may reduce the duration of bleeding, but the number of studies is limited and there is insufficient evidence for this recommendation. However, a review of published patient series concludes that although a recommendation cannot be given with strong evidence, TA can reduce both duration and volume of the bleeding, with a low risk of short-term thromboembolic disease [30]. The recommended dose is 500 mg–1 g intravenously two or three times per day, or 1–1.5 g two to three times a day.
Aminocaproic acid (Caproamin®) has been used in isolated case series, as intracavitary instillation in aspergillomas [31, 32].
Protection of the Airway
If there is severe respiratory failure or risk of suffocation (large and rapid bleeding), orotracheal intubation is required, preferably with a thick tube (8–9 mm) to facilitate diagnostic and interventional bronchoscopy [33].
In addition, bronchial blockade may be necessary to control bleeding in order to preserve ventilation of the healthy lung [15, 34]. There are several options to accomplish this (Table 38.4):
-
1.
Perform the blockage with the orotracheal tube itself. This is possible in bleeding from the right bronchial tree, since the left main bronchus can be selectively intubated with the aid of the bronchoscope, so that the pneumatic balloon of the tube completely isolates the left lung. It should be taken into account that in tall patients, the tube may not be long enough to adequately reach the main bronchus.
-
2.
Use independent bronchial blockers that are placed through a conventional tube:
-
2.1.
Fogarty inflatable balloon catheter (n° 7 or higher). This inflatable balloon is introduced parallel to the bronchoscope and it is placed at the selected location under direct vision. This maneuver can be facilitated by rotating the head to the opposite side, in a similar way than left main bronchus intubation with the rigid bronchoscope, and bringing the end of the tube closer to the tracheal carina. This device cannot be securely anchored during long periods of time, but it may allow to block completely the bleeding site with enough time for a clot to form and adhere [33]. The introduction of the catheter independently of the bronchoscope instead of through its working channel allows continuous suctioning and improved vision.
-
2.2.
Arndt endobronchial blocker® catheter (Fig. 38.7). It can be inserted transiently attached to the end of the bronchoscope to be transported to its location (size n° [7,8,9]). It has a transparent head of three ports: one to fix the catheter of the blocking balloon, another for the introduction of the bronchoscope, and the third one for the connection to the respirator.
-
2.3.
EZ-Blocker®. It is a catheter Y-shaped at its distal end, to facilitate anchorage at the tracheal carina, and two balloons that can be inflated separately.
-
2.4.
Cohen Flexitip Endobronchial®. It is a balloon catheter curved at its distal end to facilitate placement.
-
2.1.
-
3.
Perform intubation and blockage with a special orotracheal tube:
-
3.1.
Torque Control Blocker Univent Tube®, which has a bronchial blocker that prolongs the tube itself, designed to occlude any major bronchi with the tube located inside the trachea (Fig. 38.8).
-
3.2.
Broncoflex Tub®. This particular tube has a catheter on the outside, through which a Fogarty or similar tool can be inserted, and also provides an external fixation system. Its advantage is that it fully preserves the internal gauge of the tube and facilitates the location of the balloon in any of the main bronchi by rotating the orotracheal tube on its major axis.
-
3.3.
Double-lumen tube: this particular tube allows the blockade of the bleeding site performing selective intubation. Given its reduced caliber, it is not possible to introduce the standard bronchoscope through it, and it is also difficult to anchor since the bleeding site is not directly visible.
-
3.1.
Therapeutic Bronchoscopy
In our experience, flexible bronchoscopy is the first procedure indicated when a patient presents with life-threatened hemoptysis and hemodynamic instability. It can be performed in the intensive care setting or any other critical area. When a rigid bronchoscope is available, it is advisable to intubate with the rigid tube and through it introduce the flexible endoscope. They can complement each other taking advantage of both instruments:
-
Ventilate the patient properly.
-
Ensure airway permeability by aspiration of blood and clots with large-caliber probes.
-
Perform direct hemostasis on bleeding areas, pressing with the external wall of the distal end of the rigid bronchoscope or by the application of vasoconstrictors or endobronchial coagulant therapies.
-
Access the distal bronchial tree.
Therefore, the rigid bronchoscope supplemented with the flexible bronchoscope is the most complete and safe procedure in life-threatening hemoptysis [34, 35]. However, flexible bronchoscopy remains the most used procedure in these cases, given its broad availability. Rigid bronchoscopy is less available, it requires a special training that not many pulmonary physicians have, and it has to be used in the operating room under general anesthesia or conscious sedation. That implies moving an unstable patient, a risk that may not be affordable in a life-threatening situation.
Once the origin of the bleeding has been identified, if a lung blockade is not necessary, local measures can be applied. Their clinical efficacy is limited, as well as the published evidence.
In addition to the methods described above, other interventional procedures can be performed:
-
1.
Bronchial blockade with the flexible bronchoscope and sustained aspiration in order to cause segmental collapse and stop the bleeding.
-
2.
Selective bronchial blockage through the working channel of the bronchoscope:
-
2.1.
Fogarty n° 5 (5 Fr.) [36] or a similar type of balloon catheter (Olympus B5-2C® and B7-2C® balloon).
-
2.2.
Longer catheters such as the Olympus Multi-3V Plus B-V232P-A® balloon catheter. This one is a 190 cm catheter that can be inserted through a working channel of 2.8 mm and insufflated up to 15 mm in diameter. Without deflating the balloon, it can be clamped and cut to stay in place and finally removing the bronchoscope.
-
2.1.
-
3.
Selective bronchial blockade using a guide wire: a guide is inserted through the working channel to the chosen bronchus, and after removal of the bronchoscope, a balloon catheter is placed through the guide. Although this procedure is technically more complicated, it allows the balloon catheter to be located and the bronchoscope removed [37].
The balloon can be inflated for up to 24–48 h to allow clot formation, although it can be maintained in the airway for up to several days. To prevent mucosal ischemia, it is necessary to deflate it periodically, at least three times a day [36], always under endoscopic vision in order to re-inflate immediately if bleeding persists. If the patient does not bleed again after several hours, the catheter balloon is withdrawn.
-
4.
Washing of the bronchus with cold saline serum (4 °C) using aliquots of 50 mL until bleeding is suppressed, without exceeding 500 mL total volume [38]. The mechanism of action is local vasoconstriction although there are no controlled studies that demonstrate its effectiveness [39].
-
5.
Instillation of hemostatic drugs:
-
5.1.
Vasoconstrictors : adrenaline diluted to 1: 20,000 and applied through the working channel in 1 mL aliquots. Its effect has not been compared in controlled trials and only clinical experience supports its use. In order to minimize its cardiovascular effects in patients at risk, it has been suggested to substitute it for some antidiuretic hormone derivatives such as terlipressin or ornipressin, although reports are anecdotic [40, 41].
-
5.2.
Tranexamic acid can be instilled undiluted on the bleeding site, with an initial dose of 500 mg [42, 43].
-
5.3.
Fibrinogen-thrombin (Tissucol®) . It has been used in two case series in hemoptysis cases that could not be controlled which other endoscopic procedures [44].
Topical hemostatics are not useful in fast and severe hemoptysis, since the blood washes out the hemostatic agent diminishing of abolishing its efficacy.
-
5.1.
-
6.
Other bronchial blockade systems that have been used successfully in case series:
-
6.1.
Regenerated oxidized cellulose mesh (Surgicel®) . A report by Valipour et al. [45] describes how fragments of this hemostatic and resorbable mesh were introduced into the segmental or subsegmental bronchi causing the hemorrhage to stop. They were previously introduced through the working channel of a standard bronchoscope by pulling them with a flat blade forceps. Once the bleeding site was located, they were pushed into the segmental bronchus with the same forceps. In total, four–ten fragments of 3 × 4 cm were introduced, until hemostasis was achieved. As it was a resorbable material, it was not necessary to extract it later, and the absence of bronchial sequelae was later verified.
-
6.2.
Endobronchial valves, designed for endoscopic volume reduction and used for other purposes such as persistent air leakage or bronchopleural fistula. Isolated cases of their application in the treatment of hemoptysis have also been described [46].
-
6.3.
Silicone plugs (Watanabe spigots) [47, 48]. Initially introduced by Watanabe for endoscopic treatment of bronchopleural fistulas, they have demonstrated their efficacy in the transient tamponade of hemorrhagic segmental bronchi. The insertion and removal is performed by apprehending them with a biopsy forceps and transporting them at the end of the bronchofibroscope.
-
6.1.
-
7.
Laser coagulation: in cases of accessible, endoscopically visible tumor causing bleeding:
-
7.1.
Laser photocoagulation (Nd: YAG, Nd: YAP, diode laser): the efficacy in stopping bleeding ranges from 60 to 74%, although a reduction is achieved in up to 94% of cases [49, 50]. If the bleeding is important, results are not so favorable [51]. Laser can be effective causing photocoagulation in depth. Very good results have been reported when applied on bleeding endobronchial tumors [49], but little is achieved on severe hemoptysis caused by laser application itself. In this context, the results have not been so favorable [51]. In fact, in highly vascular tumors causing severe hemoptysis, there is a tendency to avoid laser treatments unless an obstruction can be solved with the treatment, and the risks are justify.
-
7.2.
Electrocoagulation with argon plasma. Argon plasma is an electrocoagulant method that does not require tissue contact and acts rapidly superficially. It is less effective than laser in coagulating in depth, and mechanical debridement is more difficult. But it can be very effective, at least transiently, in mucosal lesions whenever cough can be effectively inhibited and there is no significant active bleeding at the time of application. In that case, free blood is coagulated and the treatment does not reach the actual site of bleeding. Increasing the argon flow can facilitate its effect, risking the possibility of gas embolism. In a series of patients with endobronchial lesions responsible for active bleeding, argon plasma coagulation immediately stopped bleeding in 100% of cases (Fig. 38.9)[52].
-
7.1.
Summary
Hemoptysis is defined as the expectoration of blood from the lower respiratory tract. In most cases the amount of bleeding is slight, the patient has hemoptoic sputum (sputum staining with blood streaks), and hemoptysis is self-limited. In other cases the amount is more important (evident hemoptysis) or may even present as massive hemoptysis (expectoration of fresh blood in important quantities). However, it is preferable to use the term threatening hemoptysis, defined as the one that poses a risk to life for the patient.
The causes of hemoptysis are multiple and varied. The disease causing hemoptysis can affect the airway, lung parenchyma, or pulmonary vessels. Although they vary according to the population studied, the most frequent causes of hemoptysis are bronchiectasis, chronic bronchitis, and bronchogenic carcinoma. On most occasions bleeding comes from the bronchial arteries, sometimes other systemic non-bronchial arteries may be the source of hemoptysis. In a much lower percentage, the bleeding comes from the pulmonary arteries or from the pulmonary microcirculation.
Bronchoscopy plays a key role in the diagnosis and management of hemoptysis. It allows confirmation in doubtful cases, location of the bleeding point or, at least, location of the affected lung, and the determination of the cause in lesions accessible to it. It can allow the isolation of the hemorrhagic segment or lobe to avoid flooding the non-affecting bronchial tree and reduce the risk of suffocation, by selective intubation or bronchial blockade with balloon, as well as the application of local therapies that contribute to control the bleeding.
Recommendations
-
1.
In all patients with hemoptysis, a bronchoscopy is indicated unless the patient no longer has active bleeding and the cause of hemoptysis is known or when hemoptoic expectoration is self-limited in a patient without risk factors for lung cancer.
-
2.
The first objective of bronchoscopy is to confirm hemoptysis and assess its severity and location.
-
3.
Bronchoscopy should be performed during active bleeding within the first 24–48 h.
-
4.
In threatening hemoptysis bronchoscopy should be performed immediately in order to control bleeding.
-
5.
Location of the source of bleeding requires visualization to determine the bronchus or responsible bronchial area with certainty.
-
6.
In the presence of a fresh clot, its immediate withdrawal should not be performed. It is preferable a subsequent examination to reduce the risk of rebleeding.
-
7.
Previous instillation with 1: 20,000 epinephrine in vascularized endobronchial lesions may be effective in diminishing bleeding.
-
8.
The use of tranexamic acid is recommended to reduce the duration and volume of bleeding in threatening hemoptysis.
-
9.
Intubation in patients with threatening hemoptysis should be performed with endotracheal tubes of 8 mm or larger.
References
Roig Cutillas J, Llorente Fernández JL, Ortega Morales FJ, Orriols Martínez R, Segarra Medrano A. Manejo de la hemoptisis amenazante. Arch Bronconeumol. 1997;33:31–40.
Ibrahim WH. Massive haemoptysis: the definition should be revised. Eur Respir J. 2008;32:1131.
Chun JY, Morgan R, Belli AM. Radiological management of hemoptysis: a comprehensive review of diagnostic imaging and bronchial arterial embolization. Cardiovasc Intervent Radiol. 2010;33:240–50.
Bruzzi JF, Rémy-Jardin M, Delhaye D, Teisseire A, Khalil C, Rémy J. Multidetector row CT of hemoptysis. Radiographics. 2006;26:3–22.
Jougon J, Ballester M, Delcambre F, Bride TM, Valat P, Gómez F, et al. Massive hemoptysis what place for medical and surgical treatment. Eur J Cardiothorac Surg. 2002;22:345–51.
Ketai LH, Mohammed TL, Kirsch J, Kanne JP, Chung JH, Donnelly EF, et al. ACR appropriateness criteria hemoptysis. J Thorac Imaging. 2014;29:W19–22.
Kolb T, Gilbert C, Fishman EK, Terry P, Pearse D, Feller-Kopman D, et al. Dieulafoy’s disease of the bronchus. Am J Respir Crit Care Med. 2012;186:1191.
Khalil A, Parrot A, Nedelcu C, Fartoukh M, Marsault C, Carette MF. Severe hemoptysis of pulmonary arterial origin: signs and role of multidetector row CT angiography. Chest. 2008;133:212–9.
Nellaiyappan M, Omar HR, Justiz R, Sprenker C, Camporesi EM, Mangar D. Pulmonary artery pseudoaneurysm after Swan-Ganz catheterization: a case presentation and review of literature. Eur Heart J Acute Cardiovasc Care. 2014;3:281–8.
Savale L, Parrot A, Khalil A, Antoine M, Théodore J, Carette MF, et al. Cryptogenic hemoptysis: from a benign to a life-threatening pathologic vascular condition. Am J Respir Crit Care Med. 2007;175:1181–5.
Menchini L, Remy-Jardin M, Faivre JB, et al. Cryptogenic haemoptysis in smokers: angiography and results of embolization in 35 patients. Eur Respir J. 2009;34:1031–9.
Herth F, Ernst A, Becker HD. Long-term outcome and lung cancer incidence in patients with hemoptysis of unknown origin. Chest. 2001;120:1592–4.
Cordovilla R, Bollo de Miguel E, Nuñez A, Cosano JF, Herráez I, Jiménez Merchán R. Diagnosis and treatment of hemoptysis. Arch Bronconeumol. 2016;52:368–77.
Revel MP, Fournier LS, Hennebicque AS, Cuenod CA, Meyer G, Reynaud P, et al. Can CT replace bronchoscopy in the detection of the site and cause of bleeding in patients with large or massive hemoptysis? Am J Roentgenol. 2002;179:1217–24.
Müller NL. Hemoptysis: high-resolution CT vs bronchoscopy. Chest. 1994;105:982–3.
Hsiao EI, Kirsch CM, Kagawa FT, Wehner JH, Jensen WA, Baxter RB. Utility of fiberoptic bronchoscopy before bronchial artery embolization for massive hemoptysis. Am J Roentgenol. 2001;177:861–7.
Jean-Baptiste E. Clinical assessment and management of massive hemoptysis. Crit Care Med. 2000;28:1642–7.
Dweik R, Stoller JK. Role of bronchoscopy in massive hemoptysis. Clin Chest. 1999;20:89–105.
Patel SR, Stoller JK. The role of bronchoscopy in hemoptysis. In: Wang KP, Mehta AC, editors. Flexible bronchoscopy. Cambridge: Blackwell Science; 1995. p. 298–321.
Prakash UBS, Freitag L. Hemoptysis and bronchoscopy-induced hemorrage. In: Prakash UBS, editor. Bronchoscopy. New York: Raven Press; 1994. p. 227–49.
Khalil A, Soussan M, Mangiapan G, Fartoukh M, Parrot A, Carette MF. Utility of high-resolution chest CT scan in the emergency management of haemoptysis in the intensive care unit: severity, localization and aetiology. Br J Radiol. 2007;80:21–5.
Lee YJ, Lee SM, Park JS, Yim JJ, Yang SC, Kim YW, et al. The clinical implications of bronchoscopy in hemoptysis patients with no explainable lesions in computed tomography. Respir Med. 2012;106:413–9.
Thirumaran M, Sundar R, Sutcliffe IM, Currie DC. Is investigation of patients with haemoptysis and normal chest radiograph justified? Thorax. 2009;64:854–6.
O’Neil KM, Lazarus AA. Hemoptysis. Indications for bronchoscopy. Arch Intern Med. 1991;151:171–4.
Pramanik B. Hemoptysis with diagnostic dilemma. Expert Rev Respir Med. 2013;7:91–7.
Tak S, Ahluwalia G, Sharma SK, Mukhopadhya S, Guleria R, Pande JN. Haemoptysis in patients with a normal chest radiograph: bronchoscopy-CT correlation. Australas Radiol. 1999;43:451–5.
McGuinness G, Beacher JR, Harkin TJ, Garay SM, Rom WM, Naidich DP. Hemoptysis: prospective high-resolution CT/bronchoscopic correlation. Chest. 1994;105:1155–62.
Colice GL. Detecting lung cancer as a causa of hemoptysis in patients with a normal chest radiograph: bronchoscopy vs CT. Chest. 1997;111:877–84.
Prutsky G, Domecq J, Salazar CA, Accinelli R. Antifibrinolytic therapy to reduce haemoptysis from any cause. Cochrane Database Syst Rev. 2012;4:CD008711.
Moen CA, Burrell A, Dunning J. Does tranexamic acid stop haemoptysis? Interact Cardiovasc Thorac Surg. 2013;17:991–4.
Shapiro MJ, Albelda SM, Mayock RL, McLean GK. Severe hemoptysis associated with pulmonary aspergilloma. Percutaneous intracavitary treatment. Chest. 1988;94:1225–31.
Ortiz de Saracho J, Pérez-Rodríguez E, Zapatero J, Sánchez J, Navío P, Flores J. Therapeutic alternatives in complicated nonsurgical pulmonary aspergillomas. Arch Bronconeumol. 1995;31:83–5.
Haas AR. Management of massive hemoptysis. In: Ernst A, Herth FJF, editors. Principles and practice of interventional pulmonology. New York: Springer; 2013. p. 455–62.
Lordan J, Gascoigne A, Corris PA. The pulmonary physician in critical care. Illustrative case 7: assessment and management of massive hemoptysis. Thorax. 2003;58:814–9.
Sakr L, Dutau H. Massive hemoptysis: an update on the role of bronchoscopy in diagnosis and management. Respiration. 2010;80:38–58.
Freitag L, Tekolf E, Stamatis G, Montag M, Greschuchna D. Three years experience with a new balloon catheter for the management of haemoptysis. Eur Respir J. 1994;7:2033–7.
Kato R, Sawafuji M, Kawamura M, Kikuchi K, Kobayashi K. Massive hemoptysis successfully treated by modified bronchoscopic balloon tamponade technique. Chest. 1996;109(3):842.
Conlan AA, Hurwitz SS, Krige L, Nicolaou N, Pool R. Massive hemoptysis. Review of 123 cases. J Thorac Cardiovasc Surg. 1983;85:120–4.
Cahill BC, Ingbar DH. Massive hemoptysis. Assessment and management. Clin Chest Med. 1994;15:147–67.
Sharkey AJ, Brennen MD, O’Neill MP, et al. A comparative study of the haemostatic properties and cardiovascular effects of adrenaline and ornipressin in children using enflurane anaesthesia. Acta Anaesthesiol Scand. 1982;26:368–70.
Tuller C, Tuller D, Tamm M, Brutsche MH. Hemodynamic effects of endobronchial application of ornipressin versus terlipressin. Respiration. 2004;71:397–401.
Solomonov A, Fruchter O, Zuckerman T, Brenner B, Yigla M. Pulmonary hemorrhage: a novel mode of therapy. Respir Med. 2009;103:1196–200.
Márquez-Martín E, González Vergara D, Martín-Juan J, Romero Flacón A, López-Campos JL, Rodríguez-Panadero F. Endobronchial administration of tranexamic acid for controlling pulmonary bleeding. A pilot study. J Bronchol Interv Pulmonol. 2010;17:122–5.
de Gracia J, de la Rosa D, Catalán E, Álvarez A, Bravo C, Morell F. Use of endoscopic fibrinogen-thrombin in the treatment of severe hemoptysis. Respir Med. 2003;97:790–5.
Valipour A, Kreuzer A, Koller H, Loessler W, Burghuber OC. Bronchoscopy-guided topical hemostatic tamponade therapy for the management of life-threatening hemoptysis. Chest. 2005;127:2113–8.
Koegelenberg C, Bruwer JW, Bolliger CT. Endobronchial valves in the management of recurrent haemoptysis. Respiration. 2014;87:84–8.
Dutau H, Palot A, Haas A, Decamps I, Durieux D. Endobronchial embolization with a silicone spigot as temporary treatment for massive hemoptysis: a new bronchoscopic approach of the disease. Respiration. 2006;73:830–2.
Bylicki O, Vandemoortele T, Laroumagne S, Astoul P, Dutou H. Temporary endobronchial with silicone spigots for moderate hemoptysis: a retrospective study. Respiration. 2012;84:225–30.
Han CC, Prasetyo D, Wright GM. Endobronchial palliation using Nd:YAG laser in associated with improved survival when combined with multimodal adjuvant treatments. J Thorac Oncol. 2007;2:59–64.
Hetzel MR, Smith SG. Endoscopic palliation of tracheobronchial malignancies. Thorax. 1991;46:325–33.
Shankar S, George PJ, Hetzel MR, Goldstraw P. Elective resection of tumours of the trachea and main carina after endoscopic laser therapy. Thorax. 1990;45:493–5.
Morice RC, Ece T, Ece F, Keus L. Endobronchial argon plasma coagulation for treatment of haemoptysis and neoplastic airway obstruction. Chest. 2001;119:781–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Cordovilla, R., de Miguel, E.B., Cascón Hernández, J.A. (2018). Hemoptysis, Endoscopic Management. In: Díaz-Jimenez, J., Rodriguez, A. (eds) Interventions in Pulmonary Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-58036-4_38
Download citation
DOI: https://doi.org/10.1007/978-3-319-58036-4_38
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58035-7
Online ISBN: 978-3-319-58036-4
eBook Packages: MedicineMedicine (R0)