Abstract
Although evidence suggests that cultivated soybeans (Glycine max (L.) Merr.) have been domesticated in northern China, current theories suggest that soybeans could have been domesticated in the same way in southern China in the medium or low “Yellow River valley” of central China, northeastern China, or simultaneously in multiple centers. For soybeans, centers of genetic diversity are considered important sources of genetic variability. Plant genetic resources include cultivated plants and wild species of proven value or even potential. These resources have great importance to humankind because they make possible the solutions of numerous agriculture problems in the present and future, which can be found in plant genetic resources. In the case of soybean, an annual species in the subgenus Soja and 22 perennial species within the subgenus Glycine have been reported as related wild species. Therefore, genetic resources are considered for purposes of genetic improvement of the crop. Much of the genetic variability of this crop has been maintained and conserved in Germplasm Banks (BG) in several eastern and western countries. In Brazil, the formation of the Soybean Germplasm Active Bank (BAG/Soybean) started in September 1975, at Embrapa Soybean, in order to gather pure and characterized stocks of genotypes for the use of breeders and geneticists.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Soybean is an autogamous plant that belongs to the family Leguminosae, subfamily Papilionoideae, Phaseoleae tribe, and Glycine genus. The latter is subdivided into two subgenres: the subgenus Glycine and the subgenus Soja. Within the subgenus Soja, there are two recognized species, Glycine max and Glycine soja, with 2n = 40 chromosomes. The cultivated soybean belongs to the species G. max.
This chapter is intended to describe the center of diversity and genetic resources of the genus Glycine.
Origin of Soybeans
In addition to G. max and G. soja, an intermediate form known as G. gracilis Skvortz has been described, first proposed as a new species by Skvortzow (1927). However, G. gracilis is not recognized as a new species by the “International Legume Database and Information Service” (ILDIS) and by the US Department of Agriculture—Germplasm Resources Information Network (USDA-GRIN) (Hymowitz 2004). Many authors consider G. gracilis a variant of G. max. According to the study of Chen and Nelson (2004), G. gracilis accessions can be distinguished from G. max and G. soja based on phenotypic markers and DNA, but do not necessarily support the designation of another species, and G. gracilis is more related to G. max accessions than to G. soja.
As to the origin of the genus Glycine (with 2n = 20), and based on a broad review on works involving taxonomic, cytological, cytogenetic, and molecular systematic aspects, Hymowitz (2004) hypothesized that the probable ancestor for the genus Glycine 2n = 20 would have occurred in Southeast Asia (Fig. 3.1). However, such a parent either would be extinct or had not yet been collected and identified in Cambodia, Laos, or Vietnam. This ancestral species is believed to have undergone a process of autopolyploidization or allopolyploidization (2n = 2x = 40) before or after the dissemination from the ancestral region into the Australian continent or to China.
The origin of genus Glycine. Source: Hymovitz (2004)
Perennial wild species that have adapted to the ecological niches of the Australian continent have not been domesticated. The route of migration from the ancestral region to China from a common ancestor is believed to have occurred initially through a wild perennial species (2n = 4x = 40, unknown or extinct), followed by an annual wild species (2n = 4x = 40, G. soja) until finally giving rise to the annual cultivated species (2n = 4x = 40, G. max, cultivated). Morphological data, cytogenetics, analysis of mitochondrial DNA fragments, ribosomal RNA, chloroplastic DNA, and ITS sequences of the ribosomal nuclear DNA region have considered Glycine soja as the ancestor of G. max (Chen and Nelson 2004).
All currently described species of the genus Glycine exhibit diploid-like meiosis and are generally endogamous, producing seeds by cleistogamy. Allopolyploidization (interspecific hybridization followed by chromosome duplication) has probably played an important role in the speciation of the genus Glycine, in which Glycine species with 40 chromosomes and G. tabacina and G. tomentella with 80 chromosomes are tetraploid and octoploid, respectively. The expression of four ribosomal DNA loci in G. curvata and G. cyrtoloba (Singh et al. 2001) reinforces this hypothesis of the origin of the allotetraploid.
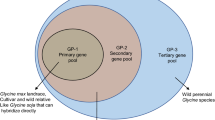
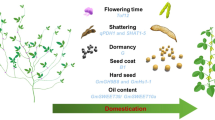
As for the origin of the cultivated species [Glycine max (L.) Merr.], linguistic, geographical, and historical evidences suggest that soybean [Glycine max (L.) Merr.] was domesticated during the Zhou dynasty, in the Eastern China around the eleventh century BC. Around the first century, soybeans probably expanded through Central and Southern China and also through Korea. The movement of soybean germplasm within this region, primary diversity center, is associated with the development and consolidation of the territories and the degeneration of the Chinese dynasty. From the first century until the mid-fifteenth century AD, soybeans were introduced in Japan, Indonesia, the Philippines, Vietnam, Thailand, Malaysia, Myanmar, Nepal, and Northern India, where many landraces (name given to cultivars prior to the date of scientific improvement) were developed. These regions comprise the secondary diversity center.
Despite evidences that soybeans were domesticated in Northern China, current theories show that this legume could have been domesticated in the same way in Southern China, in the middle or lower “Yellow River Valley” of Central China (Zhou et al. 1999), Northeast China, or simultaneously in multiple centers (Lu 1977). However, there is no consensus on this issue, since wild soybeans commonly grow in Eastern China in the latitude range 24–53 N, Japan, Korea, and the Far East of Russia. Theoretically, soybean domestication could have occurred in any region of China. However, many researchers accept the hypothesis that soybean was domesticated in the “Yellow River Valley” or “Yangtze River” valleys of Central or Southern China.
Two recent studies illustrate the arguments for these areas as centers of origin. In the first one, DNA markers were used to compare landraces and accessions of wild soybean in order to infer patterns of domestication using germplasm from the South, Central, and Northwest Regions of China. The premise of the study was that G. max should be more related to the gene pool of G. soja, from which it was domesticated. Gai et al. (1999) evaluated chloroplasts and mitochondria of 200 G. max accessions and 200 of G. soja using molecular markers such as restriction fragment length polymorphism (RFLP). The authors showed that Glycine max accessions from all regions of China were more related to those of Glycine soja from the southern region of the “Yangtze River” valley than to accessions of G. soja from other regions. These data support the idea that G. max was domesticated from G. soja from the southern region and then disseminated into other regions. It was also possible to infer that the beginning of the expansion of domesticated soybean to the Central and Northeast Region of China may have favored the significant soybean variability regarding the total cycle within these geographic regions, which has allowed the adaptation of the crop to the spring, summer, and fall planting system. The fact is that the diversity among the DNA markers of the three regions of China was much broader than among the different maturity groups within a region.
In a second study, Zhou et al. (1999) used the Vavilov concept that the greatest genetic diversity for a species should be that of the domestication center. They evaluated 15 morphological and biological traits of 22,695 accessions of G. max from China and concluded that the soybean diversity center resides within a corridor between Southwest and Northeast China, which includes the provinces of Sichuan, Shaanxi, Shanxi, Hebei, and Shandong. This corridor connects two agriculture cradles in China, the “Yellow River Valley” and “Yangtze River Valley.” These two ancient centers of agriculture have a long history of soybean and millet farming, and the agricultural exchange between these two areas was intense (Gai and Guo 2001).
Genetic Variability
In the case of soybeans, genetic diversity centers are considered important sources of genetic variability. Accessions were grouped into four major groups: the first formed by China (primary diversity center), the second formed by Korea and Japan (secondary diversity center), the third formed by the other countries of South Asia (Asia, India, Indonesia, and Vietnam) and Russia (secondary diversity center), and the fourth formed by all non-Asian countries where soybean cultivation is recent, when compared to the millennium data of the other groups (Africa, Americas, Europe). Such grouping was based on several studies carried out by several authors, who compare the geographical origin of soybean accessions, as well as the genetic distance between the accessions, which suggests the importance of this criterion (Griffin and Palmer 1995; Li and Nelson 2001). The consistency of the results provides strong evidence that accessions from Japan and South Korea are genetically similar and distinct from China’s accessions. These studies also indicate that the soybean gene pool from Japan and South Korea was probably derived from few introductions from China. Based on these results, one observes that Japan and South Korea are secondary sources of soybean germplasm, but distinct from China’s gene pool. They also show that germplasm accessions from various regions of Asia were categorized into four groups: Korea and Japan, China and Eastern Russia, Southeast Asia, and Central South Asia.
According to the same authors, within China the diversity of the groups formed generally reflects the geographical origin of accessions. In the study of Li and Nelson (2001), the degree of genetic variation revealed by RAPDs within China was more extensive than within Japan and South Korea. As a result, accessions from China were divided into four subgroups, according to the provincial origin. The first subgroup was composed of accessions from the Northeast region of China; the second, by the accessions of the Huanghe-Huaihe-Haihe region; the third, by the accessions of the South region of China; and the fourth group by accessions whose province data are unknown.
Soybean variability for physiological, morphological, and agronomic features is considered quite broad. This allows the use thereof in breeding and selection programs of cultivars with high adaptability and stability to the most diverse edaphoclimatic conditions and production systems. Chinese farmers were responsible for this genetic variability, since, well before history was written, they used this genetic diversity so that the soybean crop could reach high levels, by increasing yield and resistance to diseases and pests and by adapting the crop to extreme climates. Historical data reveal the importance of farmers for the development of phenotypically diverse varieties during the domestication process. These varieties that preceded scientific breeding were defined as landraces, which are irreplaceable genetic material, inasmuch as they represent the genetic diversity gathered by farmers over 3000 years, which included the conversion of wild soybeans to modern crop. By the beginning of the twentieth century, Chinese farmers had already grown between 20,000 and 45,000 different soybean genotypes. Many of these landraces have been collected by germplasm curators and breeders and are preserved in extensive germplasm collections, and make up the largest source of genetic diversity within soybean germplasm collections. The best breeders in the world use such diversity as a basis for soybean breeding (Singh and Hymowitz 1999; Carter et al. 2004).
Traditionally, the evaluation of genetic diversity in soybean has been based on differences in morphological and agronomic traits or genealogy information, which has provided important information for germplasm management and evaluation. Phenotypic diversity in soybean is extensive and is under the genetic control of qualitative and quantitative traits. Qualitative traits provide tags for the identification of accessions and classification of differences. Most economic characters and plant traits have quantitative inheritance.
Brazil is among the countries with the greatest diversity of species in the world, and yet it is extremely dependent on germplasm from other countries, as most cultivated species have their centers of origin in other continents. With grounds on this assumption, the area of genetic resources stands out for its importance in the search for new species not native to Brazil, but cultivated in the world, in the conservation of these introductions and multiplications and in the search for genetic variability of introduced and native species.
Genetic Resources
Plant genetic resources include plants cultivated and wild species of proven value or even potential. These resources are of great importance to humankind, for it is possible that solutions to numerous agriculture problems, both current and future, may be found in plant genetic resources. Therefore, conservation and rational use of genetic resources can contribute to the eradication of hunger and poverty.
Despite the great diversity, the number of plants used by man is minimal when compared to that of plant species in nature. Throughout its history, man has known about 300,000 edible species, but only 300 have been used as food. Currently, only 15 species contribute with 90% of human nutrition, in that, eight of them are responsible for 75% of the vegetal contribution to human energy (Nass 2001). The dependence on a limited number of species shows the risk of food safety and points out the importance of the management, conservation, and use of genetic resources.
The maintenance of these resources is done by means of the establishment of environmental protection areas and through the collection and maintenance of these materials, which are now known as germplasm. Germplasm is defined by Allard (1971) as the sum total of the materials of each species. Thus, these may be in the form of plants, anthers, pollen, seeds and tissues (meristem, callus), cells, or even simple structure.
Each germplasm unit should represent a single copy of the genetic material and the living organism of current or potential interest. Consequently, germplasm is the element of genetic resources that represents the inter- and intraspecific genetic variability, to conserve and use it in research in general, especially in breeding programs. Thus, genetic resources comprise the diversity of the genetic material contained in primitive, obsolete, traditional, or modern varieties, in wild relatives of the target species (wild species or primitive lines) that can be used now or in the future for food, agriculture, and other purposes.
Germplasm collections were proposed to preserve the genetic diversity of cultivated species, due to the adoption of modern cultivars as a replacement to primitive ones.
Frankel and Brown (1984) described three phases that have characterized genetic resource activities over the past 60 years: the first emphasized biogeography, taxonomy, and evolution; the second, the collection and conservation of germplasm; and the third, the characterization and use of germplasm. In the first two phases, the strategy was to collect and preserve as much of the allelic diversity as possible. Higher-value alleles have been found to be rare and often restricted to specific geographic areas. This has resulted in farms to ensure perfect collection with the maximum diversity of germplasm. Accordingly, for many species, germplasm collections have become large and diffuse. The size of the collection and the limit of financial resources available have reduced the efficiency of germplasm evaluation, which has discouraged the enrichment and use thereof.
Genetic resources can be conserved by maintaining the species in their habitats (in situ) or outside them (ex situ). The conservation method to be adopted depends on the needs, the possibility of conservation, and the target species. The purpose of ex situ conservation is to maintain accessions without alterations in their genetic constitutions. Thus, the use of germplasm banks ensures conservation, making accessions genetically stable and at the reach of users.
Gene banks around the world maintain collections of plant genetic resources for long-term conservation and to facilitate accession for plant breeders, researchers, and other users. In recent decades there has been remarkable progress in gathering and conserving these resources (van Hintun et al. 2000). However, in order to efficiently use the genetic potential maintained in the germplasm collections, detailed knowledge of the collection, including characterization and evaluation, is required. This contributes to the prevention of possible genetic losses, such as those that may occur during multiplications of the accessions collected, and allows for the establishment of sites or collection areas that contain greater variability, thus helping in the planning of new collections.
In the case of soybeans, an annual species within the subgenus Soja and 22 perennial species within the subgenus Glycine have been reported as related wild species (Table 3.1). Out of the 22 species, seven were described before 1981, and 15 additional ones after this period. These species are, therefore, considered as genetic resources for breeding purposes (Hymowitz 2004). Much of the genetic variability of this crop has been maintained and conserved in germplasm banks (GB) in several eastern and western countries. According to data collected and updated by the International Plant Genetic Resources Institute (IPGRI), over 170,000 accessions of Glycine max are maintained by more than 160 institutions in approximately 70 countries. China has the largest soybean germplasm collection in the world with nearly 26,000 accessions of G. max and 6200 accessions of G. soja, located at the Institute of Crop Germplasm Resources of the Chinese Academy of Agricultural Science in Beijing (Carter et al. 2004). The US Department of Agriculture (USDA) germplasm collection is the second largest, with 18,570 G. max accessions, 1116 G. soja accessions, and 919 accessions of perennial species of Glycine. All perennial species are native to Australia, which has the largest collection in the world, with over 2100 accessions. Currently, more than 3500 accessions of the 22 perennial species of Glycine have been maintained in nine collections worldwide. Most of the accessions of Glycine max and Glycine soja available in the world were collected in China and Japan.
Prior to 1949, no effort was made to preserve soybean germplasm in the United States, and many domestic introductions and varieties were wasted. The Soybean Germplasm Collection was established in 1949 to collect and maintain the entire soybean variability in the world, with an emphasis on East Asian landraces, where soybeans originated. When the collection was established in 1949, all available lines in the USDA and Canada were pooled and, thus, the collection started. Each accession entered into the USDA system is identified with the prefix PI and a number from the Plant Introduction Office. Most of the collection refers to the introduction of plants (16,981 accessions). Accessions of the US Soybean Germplasm Bank, after being characterized, evaluated, and multiplied, are made available to the scientific community and can be requested through the website [http://www.ars-grin.gov/npgs/orders.html]. All accesses of Glycine max are kept as pure lines. Each access in the collection is descended from a single seed of the original seed lot, and multiple accesses are preserved from samples of heterogeneous introductions. The only genetic variation that can exist within an access is a result of the heterogeneity of the original seed. As Glycine max is autogamous, accession within the collection can be considered homogeneous and homozygous. To maintain such integrity, extensive numbers of descriptors have been used to allow most contaminants to be easily detected and removed.
In addition to these germplasms, other institutions working in soybean breeding maintain smaller, but genetically important, collections to develop their own programs. Some of the collections in South America are also large, but they have been established more recently. Records indicate that accessions that belong to these collections derive, virtually exclusively, from other collections. European collections are smaller but may be genetically important because they contain landraces or their derivatives that were introduced from Asia over 100 years ago.
Accessions that precede scientific breeding are probably the greatest resource of genetic diversity in G. max collections and are good measure of the effective size of the collection. Not all G. max germplasm currently preserved in Eastern Asian countries precedes scientific breeding, but it is estimated that approximately 40,000 of the 93,000 soybean accessions in Asia may fall into this category. Accessions falling within this description originated in Eastern Asia, in the region that comprises India in the West, Japan in the East, Indonesia in the South, and Russia in the North. In the center of this region is China, the most important source of accessions.
In Brazil, the Soybean Active Germplasm Bank (BAG/Soja) was established in September 1975, at Embrapa Soja, with the purpose of gathering pure and characterized genotype stocks for the use of breeders and geneticists, in order to incorporate all the germplasm in the country and for acquisition of new accessions of agronomic importance. Initially, the bank received genotypes from UEPAE/Pelotas, FECOTRIGO, and IPAGRO, totaling 1200 new accessions, which were multiplied and characterized in the 1975/1976 agricultural season. In 1976, genotypes from the IAPAR collection and from the collection of UEPAE, in Ponta Grossa, were incorporated into the BAG/Soja. In 1977, cultivars from Delta Branch Experimentation Station, Stoneville, Mississippi, were introduced in Brazil. In 1979, 118 genotypes were collected from the UEPAE of Pelotas, which constitute the collection of genetic types. In 1980, at least 28 genotypes were introduced from the US Regional Soybean Laboratory, Urbana, Illinois. Eight other genotypes were introduced in Brazil in 1981, derived from IITA Nigeria. In 2007, the process of importing the soybean collection from the US Germplasm Bank began, with 20,614 accessions. The vast majority of these accessions were introduced mainly from China, Japan, and other countries where diversification of species had occurred. These accessions are identified by the acronym PI, initials for plant introduction. In addition to the PI accessions, the BAG/Soja germplasm collection also has public domain lines and cultivars developed in Brazil and the North American collection of genetic types (identified by the “T”) of all genes already studied and described.
Currently, Embrapa has two germplasm collections, totaling more than 25,200 soybean accessions, consisted mainly of plant introductions (PIs), national improved lines, and cultivars adapted to tropical and subtropical areas (Table 3.2). An active collection—Active Germplasm Bank—is maintained for short and medium periods at Embrapa Soja (Londrina, PR), where morphological and agronomic characterization, evaluation, and multiplication are also carried out, as well as the activities of regeneration and increase of stocks and the accessions are stored in cold rooms to maintain a temperature of 5 °C and 25% RH. The second, the soybean base collection (CBS), is maintained at Embrapa Recursos Genéticos e Biotecnologia—CENARGEN (Brasília, DF) under long-term storage conditions. The introduction, exchange, and quarantine of postentry are carried out exclusively by CENARGEN, in the case of material coming from outside or from regions not allowed in the country. The base collection is stored in aluminized packages, at a temperature of −18 °C.
Expansion to the Western World: Soybeans in Brazil
Soybean was introduced in the western world as of the eighteenth century when, in 1739, soybean seeds from the Paris Botanical Garden were experimentally planted in Europe. In the American continent, its introduction occurred in 1765 by Samuel Bowen in Savannah, Georgia, and the earliest account of its behavior dates back to 1804. Despite being known and intensely exploited in Eastern diet, for over 5000 years, the West ignored its cultivation until the second decade of the twentieth century, when the United States began its commercial exploitation—first as forage and then as grain. In 1940, at the peak of soybean cultivation as forage, about two million hectares were cultivated in that country for that purpose. Beginning in 1941, the planted grain area surpassed that cultivated for forage, which declined fast until it disappeared in the mid-1960s, while the area planted to grain production grew exponentially, not only in the United States but also in Brazil and Argentina, especially after the 1970s (Bonato and Bonato 1987).
Bonato and Bonato (1987) presented a complete review of the introduction and of the first experiments with soybeans in Brazil. The first experimental soybean planting reference in Brazil dates back to 1882, when cultivars were introduced and tested by Gustavo D’Utra in Bahia. The germplasm was brought from the United States and was not adapted to the low latitude conditions of that state (latitude 12°S), so, the crop was unsuccessful in the region. Ten years later the first studies were carried out in São Paulo and, in 1901, in Rio Grande do Sul (RS).
Similar to what happened in the United States, during the 1920s and 1940s, the first soybean cultivars introduced in Brazil were studied mostly intended for the evaluation of their performance as forage than as grain-producing plants for the meal and vegetable oil industries. However, commercial farming began much later. The first official statistics began to be reported in 1941 in RS, and the first soybean processing industry was set up, in 1945, in the State of São Paulo. The real drive to large-scale production in Brazil occurred in the mid-1950s, with the official decision to provide tax incentives for wheat production, which also benefited soybeans due to the perfect combination of the two crops, considering a technical viewpoint such as, for instance, the economic one.
In the 1960s, the crop initially expanded in latitudes between 30° S and 20° S, mainly in the States of Rio Grande do Sul, Santa Catarina, Paraná, and São Paulo. At the same time, about 200 North American lines were introduced and began to be tested in Ponta Grossa, Londrina, and Maringá, cities located in the State of Paraná. From this introduction originated the recommendations of cultivars Viçoja and Mineira, and the launch of Campos Gerais, Paraná, Florida and Sant’Ana. Moreover, it allowed the recommendation of American cultivars Bragg, Davis, Hardee, Hill, and Hood and of the Brazilian cultivars Santa Rosa and Industrial (Kaster et al. 1981). Given the identification of the cultivars with better adaptation, breeders began to combine the traits of these cultivars through hybridization.
Despite the significant production growth during the 1960s, it was in the following decade that soybean production grew further and was consolidated as the main crop of Brazilian agribusiness, having gone from 1.5 million tons (1970) to more than 15 million tons (1979). Such growth was due not only to the increase in cultivated area (1.3–8.8 million hectares) but also to the significant increase in yield (1140–1730 kg/ha). More than 80% of Brazilian soybean production in the late 1970s was still concentrated in the three southern states, but the central region of Brazil already showed signs of becoming a major producer. Until 1970, commercial soybean crops worldwide were restricted to temperate and subtropical climates whose latitudes were close to or above 30°. Its expansion toward the low latitudes of Brazil was limited in those days, due to the response of the crop to the photoperiod, since it is classified among short-day plants, as it will delay flowering until the day length is shorter than a critical photoperiod, which is specific for each cultivar. In the 1970s, plant size, dependent on the varietal response to the photoperiod, became the main limiting factor in the expansion of soybean farming in Brazilian tropics (Bonetti 1981).
Only after the 1970s was it possible to obtain genotypes featuring the late-blooming trait in lower latitudes, as a result of hybridizations between cultivars adapted to high-latitude regions and sources of genes for late flowering under short-day conditions. These genes known as genes for long juvenile period were present in genotype PI 240664 from the Philippines. With the breaking of the too early flowering barrier, Brazilian breeding programs developed germplasm adapted to the tropical conditions and enabled soybean cultivation in any region of the national territory (Kiihl et al. 1985).
In 1970, less than 2% of the national soybean production was harvested in regions with latitudes below 20°S. In 1980, such percentage rose to 20%, in 1990 it already exceeded 40%, and in 2004 it reached 64%. Such transformation upheld and consolidated the State of Mato Grosso as the national leader in soybean production.
Final Considerations
The inter- and intraspecific genetic variability of the genus Glycine conserved in germplasm banks in Brazil and in the world is essential to be used in research in general, especially in breeding programs.
References
Allard, R.W.: Princípios do melhoramento genético de plantas, p. 331. USAID, Rio de Janeiro (1971)
Bonato, E.R., Bonato, A.L.V.: A soja no Brasil: história e estatística, p. 61. EMBRAPA-CNPSo, Londrina (1987). (EMBRAPA-CNPSo. Documentos, 21)
Bonetti, L.P.: Distribuição da soja no mundo. In: Miyasaka, S., Medina, J.C. (eds.) A Soja no Brasil, pp. 1–6. ITAL, Campinas (1981)
Carter, T.E., Nelson, R.L., Sneller, C.H., Cui, Z.: Genetic diversity in soybean. In: Boerma, H.R., Specht, J.E. (eds.) Soybeans: Improvement, Production and Uses, 3rd edn, pp. 303–416. ASA, Madison, (2004). (Agronomy Monograph 16)
Chen, Y., Nelson, R.L.: Genetic variation and relationships among cultivated, wild, and semiwild soybean. Crop Sci. 44, 316–325 (2004)
Frankel, O.H., Brown, A.H.D.: Plant genetic resources today: a critical appraisal. In: Holden, J.H.W., Williams, J.T. (eds.) Crop Genetic Resources: Conservation and Evaluation, pp. 249–257. G. Allen, London (1984)
Gai, J., Guo, W.: History of Maodou production in China. In: Proceedings of Second International Vegetable Soybean Conference, pp. 41–47. Washington State University, Tacoma (2001)
Gai, J., Xu, D., Gao, Z., Zhao, T., Shimamoto, Y., Abe, J., Fukushi, H., Kitajima, S.: Genetic diversity of annual species of soybeans and their evolutionary relationship in China. In: Proceedings of the World Soybean Research Conference VI, p. 515. University of Illinois/Soybean Research & Development Council, Chicago (1999)
Griffin, J.D., Palmer, R.G.: Variability of thirteen isozyme loci in the USDA soybean germplasm collections. Crop Sci. 35, 897–904 (1995)
van Hintum, T.J.L., Brown, A.H.D., Spillane, C., Hodgkin, T.: Core Collection of Plant Genetic Resources, p. 48. IPGRI, Rome (2000). (IPGRI. Technical Bulletin, 3)
Hymowitz, T.: Speciation and cytogenetics. In: Boerma, H.R., Specht, J.E. (eds.) Soybeans: Improvement, Production and Uses, 3rd edn, pp. 97–136. ASA, Madison (2004). (Agronomy Monograph 16)
Kaster, M., Queiroz, E.F., Teresawa, F.: Introdução da soja nas principais regiões agrícolas. In: Miyasaka, S., Medina, J.C. (eds.) A soja no Brasil, pp. 22–24. ITAL, Campinas (1981)
Kiihl, R.A.S., Bays, I.A., Almeida, L.A.: Soybean breeding for the Brazilian tropics. In: Proceedings of the Symposium [On] Soybean in Tropical and Subtropical Cropping Systems, 1983, pp. 141–143. AVRDC, Shanhua (1985)
Li, Z., Nelson, R.L.: Genetic diversity among soybean accessions form three countries measured by RAPDs. Crop Sci. 41, 1337–1347 (2001)
Lu, S.L.: The origin of cultivated soybean (G. max). In: Wang, J.L. (ed.) Soybean. Shanxi People’s Press, Shanxi (1977)
Nass, L.L.: Capítulo 2: Utilização de recursos genéticos vegetais no melhoramento. In: Nass, L.L., Valois, A.C.C., de Melo, I.S., Valadares-Inglis, M.C. (eds.) Recursos Genéticos e Melhoramento-Plantas, pp. 29–56. Fundação MT, Rondonópolis (2001)
Singh, R.J., Hymowitz, T.: Soybean genetic resources and crop improvement. Genome. 42, 605–616 (1999)
Singh, R.J., Kim, H.H., Hymowitz, T.: Distribution of rDNA loci in the genus Glycine Willd. Theor. Appl. Genet. 103, 212–218 (2001)
Skvortzow, B.V.: The soybean-wild and cultivated in Eastern Asia. Proc. Manchurian Res. Soc. Pub. Ser. A Nat. History Sec. 22, 1–8 (1927)
Zhou, X., Peng, Y., Wang, G., Chang, R.: Studies on the center of genetics diversity and origin of cultivated soybean. In: Proceedings of the Sixth World Soybean Research Conference, p. 510. University of Illinois/Soybean Research & Development Council, Chicago (1999)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
de Oliveira, M.F., Arias, C.A.A. (2017). Center for Diversity and Genetic Resources. In: Lopes da Silva, F., Borém, A., Sediyama, T., Ludke, W. (eds) Soybean Breeding. Springer, Cham. https://doi.org/10.1007/978-3-319-57433-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-57433-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57432-5
Online ISBN: 978-3-319-57433-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)