Abstract
In this chapter we report the case of a 51-year-old male, without comorbidities, with a history of infection of the lower airways with evolution to severe acute respiratory syndrome and acquired pneumonia with an etiological diagnosis of H1N1 infection. After the diagnosis, the patient was in refractory hypoxemia despite the mechanical ventilation with SaO2 80% and FiO2 100%, using a low dose of norepinephrine and presenting asynchronous breathing. The patient’s hypoxemia worsened, also developing a pulmonary dysfunction with high ventilatory parameters installed besides taking methylprednisolone. Thus, doctors decided to install ECMO veno-venosa (extracorporeal membrane oxygenation) in order to maintain tissue oxygenation, while the lungs were pulped for better recovery of lung function. Soon after the installation of the support, the ventilatory and laboratory parameters improved. There were several attempts to wean VV-ECMO after the sixth day undergoing circulatory support, but the patient was not responding satisfactorily. During this period, five hemorrhagic events occurred, at the drain and venipuncture sites. A few days later, there was no response to nociceptive stimuli, with mydriatic pupils and corneal reflex absent bilaterally; Glasgow 3 and the CT scan showed right massive temporal-parietal bleeding associated with hemoventricle; Duret hemorrhage, diffuse brain swelling, and a great deviation from the midline structures were noted. There was no indication of surgical treatment; the protocol of brain death was started, with confirmation of brain death by cerebral arteriography. After reporting the evolution of the patient during his ICU stay, we also discussed some points about ECMO, such as its indications, advantages, and disadvantages, among others.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Clinical Presentation
Patient WCV, masculine, 51 years old, without comorbidities, went to the hospital complaining of a sore throat, fever (38.5 °C), and nonproductive cough. There were no alterations at the chest radiography and sinus radiograph, and the patient was discharged. Three days later, the patient returned with the same symptoms, and the chest radiography revealed a discrete pulmonary infiltration at the right lung base. Therapy was initiated with levofloxacin 750 mg once a day, for 3 days. However, even after the proposed treatment, the patient continued with a fever and dry cough, presenting malaise in the morning, cold extremities, fatigue, and SpO2 70%. Treatment with oseltamivir, ceftriaxone, and clarithromycin was started, but the patient remained with intermittent noninvasive ventilation with SaO2 96%. When using high-flow oxygen mask, the SaO2 dropped down to less than 90%.
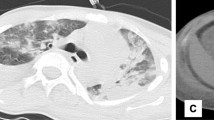
After 3 days, the patient was admitted to the ICU with the diagnosis of severe acute respiratory syndrome and severe community-acquired pneumonia caused by H1N1. The next day, the patient was in refractory hypoxemia despite the mechanical ventilation with SaO2 80% and FiO2 100%, using a low dose of norepinephrine and presenting asynchronous breathing. Doctors increased the sedation and performed three alveolar recruitments with good results, getting SaO2 92%, and maintaining minimum PEEP (11 cmH2O) to keep a SaO2 of at least 90%. After 2 days, the patient was still in refractory hypoxemia, so the doctors decided to start the ARDS (acute respiratory distress syndrome) protocol, with volume control and FiO2 50%. Five days after, the patient developed pneumomediastinum, cervical emphysema, and progressive subcutaneous emphysema with decreased ventilation and laminated pneumothorax on the left side (Fig. 1). Besides that, the doctors decreased the PEEP and increased the FiO2. A thoracic drainage catheter at the left side, beneath the lung, to drain the serous liquid from the thorax was installed, and cervical tracheostomy was performed.
The next day, the patient’s hypoxemia worsened, also developing a pulmonary dysfunction with high ventilatory parameters installed (PEEP 18, FiO2 100%, PaO2/FiO2 ratio 66) besides taking methylprednisolone.
Diagnosis, Assessment, and Treatment
After several conventional interventions like antibiotics and antiviral drugs to control the causal factor, noninvasive ventilation, mechanic ventilation, sedation, the use of corticosteroid, and other interventions, the patient remained in refractory hypoxemia. Therefore, doctors decided to install the veno-venous ECMO (extracorporeal membrane oxygenation), by puncturing the right femoral vein and right jugular internal vein, to begin with a membrane oxygenation support, using a blood and oxygen flow of 6 liters per minute and FiO2 94%. Right after the support installation, the parameters improved (Table 1).
At the next day, there was a significant improvement of hypoxemia, with pH of 7.56 (range 7.35–7.45), PaO2 96 mmHg (range 80–95 mmHg), PaCO2 31 mmHg (range 35–45 mmHg), and SatO2 98% (range 95–99%). Weaning VV-ECMO attempt was performed on the sixth day under circulatory support with good evolution, returning to the protection parameters and re-evaluating in 24 h. One day later, the patient showed an increase of urea, with hemodialysis without loss being indicated. Three days later, there was another attempt to weaning VV-ECMO, but the patient presented hypercapnia and acidosis, and only after 24 h it was possible to start weaning by reducing the blood flow and the gas to 2 L/min with FiO2 30% and mechanical ventilation with a respiratory rate of 26 breaths/min and FiO2 50%. The patient maintained PaO2 > 60 and PaCO2 > 45 without acidosis. However, hypoxia detected and hemodynamic instability led to the need to increase the parameters of VV-ECMO. The patient was responding unsatisfactorily to weaning VV-ECMO, and during this period, there were five bleeding events, at the drain and venipuncture sites.
Five days later, left anisocoria was noted, and a computed tomography (CT) was performed with the patient heparinized. There was no answer to nociceptive stimuli, mydriatic pupils, corneal reflex absent bilaterally, Glasgow 3 and Ramsay 6. They turned off sedation. In the CT scan, right massive temporal-parietal bleeding associated with hemoventricle, Duret hemorrhage, diffuse brain swelling, and a great deviation from the midline structures were noted (Fig. 2). There was no indication of surgical treatment; therefore ECMO veno-venous weaning and blood replacement were made. The next day the protocol of brain death secondary to severe intracerebral hemorrhage with tetraventricular flood was started, with confirmation of brain death by cerebral arteriography.
Questions
1. What is ECMO?
The extra corporeal membrane oxygenation system (ECMO) – a set of cannulae, an artificial oxygenation membrane, and a pump – is an extracorporeal circulation closed circuit. It provides pulmonary, heart, or cardiorespiratory support. When used for cardiorespiratory assistance, it is in the occurrence of heart failure, pulmonary failure, or both. Deoxygenated, carbon dioxide-rich blood is drained from the venous system and pumped through an artificial oxygenation membrane, to then return to the arterial system after oxygenation. It is a continuous flow. The aim is to keep tissue perfusion with oxygenated blood while waiting for the recovery of an impaired organ: the heart, lungs, or both.
2. What are the criteria for ECMO installation statement? Does patient in question had these criteria?
The mandatory criteria are:
-
Tracheal intubation and mechanical ventilation
-
Acute onset lung disease
-
Bilateral pulmonary infiltrate
-
PaO2/FiO2 ratio < 200 with positive end-expiratory pressure ≥ 10 cmH2O
-
Reversible lung injury
And the complementary criteria are (at least one must be met):
-
PaO2/FiO2 ratio ≤ 50 with a FiO2 = 1, for at least 1 h, with or without the use of rescue therapies (alveolar recruitment, inhaled NO, and prone position)
-
Hypercapnia with pH remaining ≤ 7.20 using an RR ≥ 35 breaths/min (whenever possible), a tidal volume = 4–6 mL/Kg, and a plateau pressure ≤ 30 cmH2O
-
Murray lung injury score > 3, with worsening of the clinical status
-
PaO2/FiO2 ratio ≤ 50 with a FiO2 ≥ 0.8 for at least 3 h, despite the use of rescue therapies
It is important to remember that there are contraindications: mechanical ventilation for more than 7 days with plateau pressures > 30 and FiO2 > 90%, severe immunosuppression, and recent brain hemorrhage.
The patient had all mandatory criteria and one complementary criterion (PaO2/FiO2 ratio ≤ 50 with a FiO2 ≥ 0.8 for at least 3 h, despite the use of rescue therapies).
3. What benefits ECMO brings to the patient?
The circulatory support made by VV-ECMO promotes appropriate oxygenation of the blood, as the patient cannot do this due to severe lung injury. Thus, membrane oxygenation improves tissue perfusion, significantly reducing the ventilatory parameters, which saves work for the lungs and allowing their recovery.
If the patient had a heart failure (e.g., due to congenital diaphragmatic hernia, pulmonary hypertension refractory, cardiomyopathy, or heart failure), instead of respiratory failure, ECMO-VA would be the best intervention option because it provides cardiac support, promotes excellent oxygen delivery, and allows rapid stabilization.
The benefits of ECMO in adult patients with cardiac failure or refractory acute respiratory distress syndrome (ARDS) are still debated; ECMO was initially associated with poor survival rates. However, recent technological advances in the ECMO circuit have led to a reduction in the rate of technical issues and complications. Moreover, improved understanding of the benefits of ECMO has emerged from its widespread use as a rescue therapy for ARDS and refractory hypoxemia associated with H1N1/2009 infection (“swine flu”).
4. In which other situations was the use of mechanical circulatory assist devices considered/indicated?
Circulatory assist devices can be used in patients with terminal heart failure since the heart transplant is a limited option for the insufficient number of donors to meet the demand of patients with terminal heart failure. The devices can also be used as a bridge to recovery, bridge to decision (when it is not yet clear whether the patient is or is not eligible for transplant), bridge to transplantation, or destination therapy.
5. What are the possible complications of implanted ECMO?
Hemorrhage and infection are the two main complications related to ECMO. Most patients require continuous anticoagulation, and more than 50% of them will suffer at least one hemorrhagic complication. Hemorrhage can occur in any organ, with intracranial bleeding being the most devastating. Other complications such as multiple transfusions, thromboembolism, limb ischemia, per-ECMO hemodialysis, and right ventricular dysfunction are also common.
ECMO circuit failure or breakage may lead to catastrophic failure, but this is unusual as long as all components are secure. Bedside staffs are trained to check the circuit integrity regularly to prevent problems and to react promptly in the case of acute failure. Cannula displacement or malposition is a major issue as this affects blood flow and ECMO efficiency.
6. When should we start the mechanical assistance weaning?
It is not recommended that the patient remains supported by more than 8–10 days. However, to start weaning it is necessary to meet the following criteria:
-
pH – 7,5
-
PaO2 – 136 mmHg
-
PaCO2 – 57 mmHg
-
PaO2/FiO2 – 388
-
PEEP – 6 cmH2O
-
FiO2 – 35%
-
Respiratory rate – 15 breaths/min
-
Tidal volume/ideal weight – 5.8 mL/Kg
-
Plateau pressure – 22 cmH2O
-
Peak inspiratory pressure – 26 cmH2O
-
Static lung compliance – 20 mL/cmH2O
-
Norepinephrine – 0,00 μg/kg.min
-
Mean arterial pressure – 90 mmHg
-
Heart rate – 80 bpm
-
Lactate – 0,9 mmol/L
-
Base excess – 17 mmol/L
-
C-reactive protein – 17 mg/L
-
Hemoglobin – 9 g/dL
-
Midazolam – 0,00 mg/kg.h
-
Fentanyl – 2 μg/kg.h
-
Atracurium – 0,00 mg/kg.h
-
Sequential Organ Failure Assessment Score – 7
-
Murray score – 1,2
7. What are the types of ECMO?
There are three ways to set up an ECMO circuit: venoarterial ECMO (VA-ECMO), veno-venous ECMO (VV-ECMO), and arteriovenous ECMO (AV-ECMO). The VA-ECMO allows gas exchange and hemodynamic support while blood is pumped from the venous to the arterial side. VV-ECMO facilitates gas exchange removing the blood from the venous side and then pumping back into it, but without providing hemodynamic support. AV-ECMO facilitates gas exchange by using the patient’s own arterial pressure to pump the blood from the arterial to the venous side.
8. Why was it chosen VV-ECMO in this patient?
When the cardiac function is preserved, VV-ECMO is the best option to improve gas exchange. The patient did not need any hemodynamic support because his cardiac function was preserved. The only dysfunction was a respiratory one; he had a severe lung injury, which was caused by ARDS, making a poor oxygenation. Therefore, VV-ECMO was the best choice and assistance for this patient.
Review About the Addressed Disease or Treatment
As seen in the case, H1N1 infection can generate severe inflammation in the body, thereby compromising the respiratory function of the patient. This inflammatory process generated by this infection caused serious damage to the patient progressively, reaching a point where refractory hypoxemia remained even after several interventions, making ECMO an option.
In order to recover the organ, it is recommended to use ECMO as therapy, since it maintains a good tissue perfusion in a patient in whom this function is being prevented due to some lung injury, which was the case. This process is performed by oxygenating the venous blood through the artificial oxygenation membrane present in the system, and since the patient in the case had preserved cardiac function, VV-ECMO was the best choice.
Bibliography
Roncon-Albuquerque R Jr. ECMO (extracorporeal membrane oxygenation) como opção terapêutica no ARDS grave. Rev Port Med Intensiva. 2010;17(1):43–6.
Martinez G, Vuylsteke A. Extracorporeal membrane oxygenation in adults. BJA Educ. 2012;12(2):57–61.
Saueressig MG, Schwarz P, Schlatter R, Moreschi AH, Wender OCB, Macedo-Neto AV. Extracorporeal membrane oxygenation for postpneumonectomy ARDS. Braz J Pulmonol. 2014;40(2):203–6.
Azevedo LCP, Park M, Costa ELV, Santos EV, Hirota A, Taniguchi LU, Schettino GPP, Amato MBP, Carvalho CRR. Extracorporeal membrane oxygenation in severe hypoxemia: time for reappraisal? Braz J Pulmonol. 2012;38(1):7–12.
Colafranceschini AS, Monteiro AJO, Canale LS, Campos LAA, Montera MW, Silva PRD, Fernandes MR, Pinto AA, Molas SM, Mesquita ET. Adult extracorporeal life support: a failed or forgotten concept? Arq Bras Cardiol. 2008;91(1):36–41.
Peura JL, Colvin-Adams M, Francis GS, Grady KL, Hoffman TM, Jessup M, John R, Kiernan MS, Mitchell JE, O'Connell JB, Pagani FD, Petty M, Ravichandran P, Rogers JG, Semigran MJ, Toole JM. Recommendations for the use of mechanical circulatory support: device strategies and patient selection: a scientific statement from the American Heart Association. Circulation. 2012;126(22):2648–67.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Tomazelli, A.D., Piaz, M.R.D., Falqueto, N.S., Lima, M.L. (2019). Mechanical Circulatory Support in Patient with Pulmonary Dysfunction. In: Almeida, R., Jatene, F. (eds) Cardiovascular Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-57084-6_26
Download citation
DOI: https://doi.org/10.1007/978-3-319-57084-6_26
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-57083-9
Online ISBN: 978-3-319-57084-6
eBook Packages: MedicineMedicine (R0)