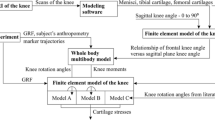
Abstract
The capability to model human joint motion is a fundamental step towards the definition of effective treatments and medical devices, with an increasing request to adapt the devised models to the specificity of each subject. We present a new approach for the definition of subject-specific models of the knee natural motion. The approach is the result of a combination of two different techniques and exploits the advantages of both. It relays upon non invasive measurements based on which a kinematic model of the natural motion is built, suitable to be extended to the definition of static and dynamic models. Comparison of the model outcomes with in vitro measurements performed on one specimen shows promising results supporting the proposed approach.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
The natural motion of the knee is the motion of the joint in unloaded conditions. It is the joint starting condition before loads are applied, thus contributing in the determination of the tibio-femoral relative position in loaded conditions. For this reason, the knowledge of the natural motion is useful for all applications which aim at replicating or restoring the natural behaviour of the knee, such as lower-limb modelling, surgical planning and prosthesis design.
The modelling of the joint natural motion can be based on mean data taken from the literature, thus providing a representation of an average joint [3, 17, 24]. However, there is an increasing request of subject-specific models that would allow personalization of treatments and prosthesis geometry to the patient needs. In these cases, the subject-specific motion would be required.
An accurate estimation of the joint motion is difficult to obtain in vivo [16]: non-invasive techniques could be inaccurate (skin-markers) or too complicated (fluoroscopy) for standard practice, while more invasive techniques (bone-pins) are not acceptable in most cases. Thus, new solutions are needed to predict the joint motion with a good accuracy, based on non-invasive measurements.
In this study a new approach is presented which exploits two techniques with complementary advantages for the modelling of the knee natural motion. The first technique (T1), was originally developed and validated for the ankle joint [4] and is here tested on the knee. T1 predicts the joint motion by optimizing the articular load distribution, assuming this condition as representative of the joint behaviour in physiological working conditions. T1 only requires a 3D representation of the articular surfaces that can be obtained from standard in vivo images of the articulation. It is however not suitable for the characterization of the joint behaviour under generic working conditions.
The second technique (T2) models the knee as a one-degree-of-freedom (1-Dof) spatial mechanism, featuring the two articular contacts and the three isometric fibres of the anterior cruciate (ACL), posterior cruciate (PCL) and medial collateral (MCL) ligaments [17, 18]. T2 was very accurate to replicate the natural motion of specimens over the full flexion arc and can be easily extended to define more complex static and dynamic models that can take into account different loading conditions [22, 23], but a reference motion is needed to adjust the model parameters.
In this study we want to exploit the advantages of both techniques by combining them into a new approach (T1\(+\)T2) which allows the definition of subject-specific models of the knee (as T2 does) from non invasive observations of its natural motion (via T1).
The aim of this work is twofold: first, to evaluate the application of T1 to the knee articulation and, second, to test the applicability of T1\(+\)T2 on the same joint. To this purpose, a leg specimen is analyzed and the knee joint motion is obtained by T1 starting from magnetic resonance imaging (MRI) data. The motion resulting from T1, together with additional information about the anatomy of the joint specimen also taken from MRI, is used as an input for the definition of T2. Finally, the results of both T1 and T1\(+\)T2 are validated against in vitro experimental measurements of the joint natural motion.
2 Materials and Methods
2.1 T1 Technique
Biologic tissues are able to modify their structure in response to the mechanical environment to which they are exposed [2, 6, 12, 19]. Experimental evidence from the literature suggests that the aim of this process is the mechanical optimization of the tissues (functional adaptation). In particular, this process produces articular surfaces that, in physiological working conditions, optimize the contact load distribution or, equivalently, maximize the joint congruence [8, 13].
It is thus possible to identify the adapted motion as the envelope of the maximum congruence configurations (i.e., positions and orientations of all bones constituting the joint). In [5] a measure of joint congruence was proposed, based on the Winkler elastic foundation contact model [14]. This measure makes it possible to estimate the peak-pressure to resultant-force ratio from the geometry of the articulating surfaces at a given configuration, i.e., from a purely geometrical perspective. As a consequence, the adapted motion can be obtained starting solely from the knowledge of the shape of the articular surfaces.
As discussed in [4], the adapted motion should also keep the isometry of the joint main ligaments. This condition is verified during the natural motion, which for this reason can be taken as a good approximation of the adapted one. In the same study, T1 was used to determine the adapted motion of ten human ankles, providing good agreement with experimental measurements of the natural motion of the same specimens. Based on these results, T1 is here applied to determine the knee natural motion.
2.2 T2 Technique
Many studies showed that the natural motion of the tibia with respect to the femur is represented by a complex 1-Dof spatial path, i.e. the relative position and orientation of the tibia and femur is a function of a single motion parameter, for instance the flexion angle [17, 24]. Moreover, some fibres of the ACL, PCL and MCL proved to be almost isometric during this motion. From a mechanical point of view, this means that the natural motion can be reproduced by an appropriate 1-Dof mechanism. Three-dimensional parallel mechanisms were thus defined based on this concept. One of them [17, 18] featured three rigid links representing the ACL, PCL and MCL, while the contacts between tibial and femoral condyles were replaced by the contacts between two pairs of spheres, or, equivalently, by two rigid links connecting the sphere centres at each pair. The result was a 1-Dof 5-5 spatial parallel mechanism, which features two rigid bodies (the femur and tibia) interconnected by 5 binary links.
In previous studies, the initial geometry of the mechanism, namely the attaching points and lengths of the five rigid links was determined from knee specimens. This initial geometry was then optimized in order to best-fit the experimental natural motion of the corresponding specimens [22]. This approach has been extensively validated with very good agreement between model outcomes and corresponding experimental natural motion [17]. The same approach is applied here, but the motion obtained by T1 is used as a reference for the model definition instead of the subject experimental motion.
2.3 Data Acquisition and Processing
A single fresh-frozen lower-limb specimen from a donor (female, 63 years old, weight 68 kg, height 158 cm) was analyzed. The study was approved by the donor organization, which provided written consent. A surgeon declared the leg free from anatomical defects and removed the forefoot and the soft tissues external to the joint, leaving the knee joint capsule and ligaments intact.
A stereophotogrammetric system (Vicon Motion Systems Ltd) was used to measure the tibia and femur relative motion by means of two trackers directly fixed to the bones, thus introducing no soft tissue artefacts (Fig. 1a). The specimen was mounted on a test rig for in vitro analysis of the knee joint behaviour [7] which also allows measurement of the femur-tibia relative motion when no external forces are applied. In this condition, the joint is guided only by the knee passive structures, namely ligaments and contacts, and thus the natural motion can be registered. This experimental natural motion was used only for validation purposes, but it was not used for model definition.
A MRI of the knee was acquired using an isotropic three-dimensional fast spin-echo pulse sequence T2-weighted (3D-FSE-CUBE-T2) within a 1.5 T scanner. Articular surfaces and ligament insertions were then manually segmented using the free open-source software Medical Imaging Interaction Toolkit (MITK), obtaining 3D models of the femur and tibia including bone, cartilage and ligaments (Fig. 1b). In the same way, anatomical reperi were determined on the femur and tibia models, and were used to build anatomical reference systems [22] on both bones (Fig. 2). The relative motion of these reference systems was then expressed by means of a standard convention [10], both for the computed and experimental motions.
The anatomical 3D models of the femur and tibia, comprehensive of both bone and articular cartilage, were used within T1 for the evaluation of the knee joint congruence. Flexion angle was imposed and the other five motion components were obtained by maximizing the congruence; the procedure was repeated over the full flexion arc [4].
Anatomical reference systems for the femur a and the tibia b. The tibia anatomical frame has origin in the tibia centre, i.e., the deepest point in the sulcus between the medial and lateral tibial intercondylar tubercles; x-axis orthogonal to the plane defined by the two malleoli and the tibia centre, anteriorly directed; y-axis directed from the midpoint between the malleoli to the tibia centre; z-axis as a consequence, according to the right hand rule. The femur anatomical frame has origin in the midpoint between the lateral and medial epicondyles; x-axis orthogonal to the plane defined by the two epicondyles and the hip joint centre, anteriorly directed; y-axis directed from the origin to the hip joint centre; z-axis as a consequence, according to the right hand rule
T2 definition was then performed based on the T1 motion and on the 3D femur and tibia models. The articular surfaces at the femur condyles and tibia plateaus used for congruence evaluation in T1 were approximated by best-fitting spheres in T2, and were then substituted by equivalent rigid links connecting the sphere centres. The most isometric fascicles of the ACL, PCL, MCL (i.e., the anteromedial, posteromedial, anterior fascicle respectively) were identified within the segmented ligament insertion areas [11]. The ligament isometric fibres were obtained as the pair of points (one on the femur, the other on the tibia insertion areas of isometric fascicles) that showed the minimum change in distance during the motion obtained by T1. The so-determined isometric fibres were then substituted by three rigid links. Finally, these preliminary mechanism parameters were adjusted by an optimization procedure [22] to best-fit the T1 motion. The final mechanism parameters were constrained to remain inside the experimental insertion areas and to have a maximum distance of 2 mm with respect to the preliminary parameters.
3 Results
The relative motion of the tibia and femur identified by T1 was consistent with the joint constraints. All ligaments indeed showed very small length changes during T1 motion: isometric fibre length excursions were smaller than 4% of the relevant fibre maximum length for the ACL, PCL, MCL and the lateral collateral ligament (LCL), in agreement with what reported in the literature [1]. The subsequent application of T2 made the ACL, PCL, MCL perfectly isometric, while the LCL showed a length change smaller than 2%. Figure 3 shows the add-abduction (AA), the in-external rotation (IE) and the antero-posterior (AP), proximo-distal (PD) and medio-lateral (ML) translation of the tibia, plotted versus the knee flexion angle as experimentally measured and computed by T1 and T1\(+\)T2.
In Table 1, the mean absolute errors (MAE) between T1 and experimental natural motions, between T1\(+\)T2 and T1 motions and between T1\(+\)T2 and experimental natural motions are presented.
4 Discussion
The tibio-femoral motion predicted by the combination of the two techniques T1 and T2 well replicates the experimental data. There are however some differences in the IE rotation and AP translation, for which the MAEs between the model and the natural motion are about 12 and 4.5 mm, respectively. Despite these quantitative differences, computed and experimental curves show a very similar trend, in particular for the IE rotations which differ essentially by a constant offset. The typical screw-home motion of the knee is therefore correctly predicted by the model, but at each flexion angle the configuration of the tibia results less internally rotated than in the natural motion.
It is worth mentioning that, despite ligaments and contacts do guide the knee natural motion on a 1-Dof spatial path, the IE rotation is less constrained than the other motion components. As a result, the knee shows the smallest stiffness about the IE axis [9, 15], which is thus the most sensitive among the knee motion components both for experimental measure and for numerical models. For what concern the AP translations, variations in the IE rotation of the tibia are associated with AP displacements of the same bone. In fact, the tibiofemoral motion is close to a spherical one [21], whose centre does not coincide with the centre of the tibial anatomical reference system. As a result, an IE rotation of the tibia is associated with a translation of the origin of its reference system, mainly along the AP direction. It is thus reasonable that differences in the IE rotation are associated with differences in the AP translation.
Despite the above mentioned differences, both the motion computed by T1 and the experimental natural one respects the ligament isometry, producing length changes smaller than 4 and 5% respectively for the ACL, PCL, MCL and LCL. This result supports the analogy between adapted and natural motion, as validated for the ankle [4] and here hypothesized for the knee. It should also be stressed that only the ligament isometry during the T1 motion made it possible the subsequent application of T2. In fact, in general it is not possible to define a 5-5 mechanism that both follows a generic prescribed path and respects the joint anatomical constraints at the same time. A wider validation of T1 is therefore necessary in order to fully understand the relation between the natural and adapted motion of the knee joint, in terms of both the differences and analogies in terms of motion and ligament isometric behaviour shown in this study.
A similar combination of T1 and T2 was investigated in [20]. In that case however, only CT images of the knee were available, thus providing poor accuracy in the reconstruction of soft tissues that introduced some noise in the motion computed by T1. These limitations were overcome in this study by means of MRI of the articular surfaces. Moreover, the use of MRI makes the proposed approach less invasive with respect to CT images, not exposing the patient to ionizing radiation, and therefore more suitable for the in vivo clinical application.
5 Conclusion
The aim of this study was to test a new approach for the generation of subject-specific model of the natural motion of the knee joint based on non invasive measurements. This approach relays on two techniques defined as T1 and T2 that contribute to determine the final model. The advantages of both techniques are exploited: T1 provides an evaluation of the knee natural motion by non invasive measurements of the articular surfaces; then, based on this motion, T2 provides a mechanism which complies with the constraints imposed by the ligaments and articular contacts, and that can be easily extended to define more complex static and dynamic models.
The motion resulting from T1 fulfils the ligament isometry typical of the knee natural motion, thus making it possible the subsequent application of T2. The results of the combination of T1 and T2 are in good agreement with experimental data, although some differences were found.
Future work is therefore in progress on other specimens in order to further validate the proposed approach and to investigate whether the observed differences are common to all the knee joints, and in case to give a solid explanation of them.
References
Belvedere, C., Ensini, A., Feliciangeli, A., Cenni, F., D’Angeli, V., Giannini, S., Leardini, A.: Geometrical changes of knee ligaments and patellar tendon during passive flexion. J. Biomech. 45(11), 1886–1892 (2012)
Benjamin, M., Ralphs, J.R.: Fibrocartilage in tendons and ligaments-an adaptation to compressive load. J. Anat. 193(Pt 4), 481–494 (1998)
Blankevoort, L., Huiskes, R., De Lange, A.: The envelope of passive knee joint motion. J. Biomech. 21(9), 705–720 (1988)
Conconi, M., Leardini, A., Parenti-Castelli, V.: Joint kinematics from functional adaptation: A validation on the tibio-talar articulation. J. Biomech. 48(12), 2960–2967 (2015)
Conconi, M., Parenti-Castelli, V.: A sound and efficient measure of joint congruence. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 228(9), 935–941 (2014)
Eckstein, F., Hudelmaier, M., Putz, R.: The effects of exercise on human articular cartilage. J. Anat. 208, 491–512 (2006)
Forlani, M., Sancisi, N., Conconi, M., Parenti-Castelli, V.: A new test rig for static and dynamic evaluation of knee motion based on a cable-driven parallel manipulator loading system. Meccanica (2015). doi:10.1007/s11012-015-0124-1
Frost, H.M.: An approach to estimating bone and joint loads and muscle strength in living subjects and skeletal remains. Am. J. Hum. Biol. 11, 437–455 (1999)
Grood, E.S., Stowers, S.F., Noyes, F.R.: Limits of movement in the human knee. Effect of sectioning the posterior cruciate ligament and posterolateral structures. J. Bone Joint Surg. Am. 70(1), 88–97 (1988)
Grood, E.S., Suntay, W.J.: A joint coordinate systenf for the clinical description of three-dimensional motions: application to the knee. J. Biomech. Eng. 135, 136–144 (1983)
Harner, C.D., Baek, G.H., Vogrin, T.M., Carlin, G.J., Kashiwaguchi, S., Woo, S.L.: Quantitative analysis of human cruciate ligament insertions. Arthrosc. J. Arthrosc. Relat. Surg. 15(7), 741–749 (1999)
Hayashi, K.: Biomechanical studies of the remodeling of knee joint tendons and ligaments. J.Biomech. 29, 707–716 (1996)
Heegaard, J.H., Beaupre, G.S., Carter, D.R.: Mechanically modulated cartilage growth may regulate joint surface morphogenesis. J. Orthop. Res. 17, 509–517 (1999)
Johnson, K.: Contact Mechanics. Cambridge University Press, Cambridge (1985)
Markolf, K.L., Mensch, J.S., Amstutz, H.C.: Stiffness and laxity of the knee-the contributions of the supporting structures. A quantitative in vitro study. J. Bone Joint Surg. Am. 58(5), 583–594 (1976)
Masum, M.A., Pickering, M.R., Lambert, A.J., Scarvell, J.M., Smith, P.N.: A review: techniques for kinematic analysis of knee joints. In: Australian Biomedical Engineering Conference (ABEC) (2014)
Ottoboni, A., Parenti-Castelli, V., Sancisi, N., Belvedere, C., Leardini, A.: Articular surface approximation in equivalent spatial parallel mechanism models of the human knee joint. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 224(9), 1121–1132 (2010)
Parenti-Castelli, V., Di Gregorio, R.: Parallel mechanisms applied to the human knee passive motion simulation, pp. 333–344. Kluwer Academic Publishers, Pirano-Portoroz, Slovenia (2000)
Robling, A.G., Castillo, A.B., Turner, C.H.: Biomechanical and molecular regulation of bone remodeling. Ann. Rev. Biomed. Eng. 8, 455–498 (2006)
Sancisi, N., Conconi, M., Parenti-Castelli, V.: Prediction of the subject-specific knee passive motion from non-invasive measurements. In: The 25th Congress of the International Society of Biomechanics - XXV ISB. Glasgow, UK (2015)
Sancisi, N., Parenti-Castelli, V.: A 1-dof parallel spherical wrist for the modelling of the knee passive motion. Mech. Mach. Theory 45(4), 658–665 (2010)
Sancisi, N., Parenti-Castelli, V.: A novel 3d parallel mechanism for the passive motion simulation of the patella-femur-tibia complex. Meccanica 46(1), 207–220 (2011)
Sancisi, N., Parenti-Castelli, V.: On the role of passive structures in the knee loaded motion, pp. 1–8. Springer, Berlin (2012)
Wilson, D., Feikes, J., Zavatsky, A., O’Connor, J.: The components of passive knee movement are coupled to flexion angle. J. Biomech. 33(4), 465–473 (2000)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Conconi, M., Sancisi, N., Parenti-Castelli, V. (2018). Subject-Specific Model of Knee Natural Motion: A Non-invasive Approach. In: Lenarčič, J., Merlet, JP. (eds) Advances in Robot Kinematics 2016. Springer Proceedings in Advanced Robotics, vol 4. Springer, Cham. https://doi.org/10.1007/978-3-319-56802-7_27
Download citation
DOI: https://doi.org/10.1007/978-3-319-56802-7_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56801-0
Online ISBN: 978-3-319-56802-7
eBook Packages: EngineeringEngineering (R0)