Abstract
The entomopathogenic bacteria Bacillus thuringiensis serovar. israelensis (Bti) and Lysinibacillus sphaericus have successfully been used to control insects of public health relevance, including those from the genera Aedes, Anopheles, Culex, and Simulium. These bacteria display a specific mode of action that relies on unique interactions which makes them the most selective agents currently available to control Diptera larvae. They produce crystalline insecticidal proteins that act on the larval midgut through their interaction with specific receptors. L. sphaericus presents a single major larvicidal factor, the binary (Bin) protoxin, whose action relies on the binding to one class of receptors, while Bti crystals contain four main protoxins (Cry4Aa, Cry4Ba, Cry11Aa, Cyt1Aa) which display interactions with a group of distinct midgut receptor molecules. The mode of action of L. sphaericus displays a greater potential for resistance selection, compared to Bti which has no record of insect resistance to date. These major mosquitocidal toxins and their interaction with midgut target sites, as well as resistance issues related to their utilization, are summarized in this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Among the microbial control agents available, Bacillus thuringiensis serovar. israelensis (Bti) and Lysinibacillus sphaericus have been employed for the production of biolarvicides aimed at the control of dipterans of medical importance (Lacey 2007). Some strains of these bacteria produce crystalline inclusions that contain protoxins with high and selective larvicidal action against some species of Diptera. These protoxins act by ingestion and are processed into toxins in the midgut in order to target the epithelium through specific receptors. Bti was the first Bacillus thuringiensis (Bt) serovariety characterized as active against Diptera (de Barjac 1978), among several described (http://www.lifesci.sussex.ac.uk/home/Neil_Crickmore/Bt). Soon after its discovery and characterization, Bti was introduced in a large-scale program to control Simulium in the Onchocerciasis Control Program carried out in West Africa (Guillet et al. 1990), and its effectiveness led to Bti adoption in other programs worldwide (Regis et al. 2001). The major insecticidal factor in Bti-based biolarvicides is the crystal that contains both three-domain-type Cry toxins and cytolytic or Cyt toxins. These crystals have high potency and a selective spectrum for Culicidae and also target some species of Simuliidae and Chironomidae (Lacey 2007). The greatest limitation of Bti activity under field conditions is its degradation due to solar radiation and other environmental factors, and suitable formulations and application strategies are needed to achieve optimal field performance. L. sphaericus’ mosquitocidal properties were first described in 1965, in the K strain isolated in moribund Culiseta incidens larvae, by Kellen, followed by the discovery of the SSII-1 strain by Singer in 1973. However, both strains displayed low to moderate toxicity to larvae (Lacey 2007) and only in the 1980s were highly toxic strains (e.g., 1593, 2362, 2297) identified, leading to the production of commercial biolarvicides (Charles et al. 1996). The powerful action of these strains is mainly associated with the production of crystals, during bacterial sporulation, that contain the binary (Bin) protoxin which remains the major insecticidal protein produced by L. sphaericus (Berry 2012). The spectrum of L. sphaericus action is more limited than Bti, and it targets only culicids. This chapter aims to summarize current knowledge of the interaction of these insecticidal toxins with the midgut receptors of mosquito larvae and the implications for the selection of resistance and management strategies.
15.1 Mode of Action of Bacterial Toxins Employed for Mosquito Control
The larvicidal toxins produced by L. sphaericus and Bti can be defined as “bacterial disruptors of insect midgut membranes,” and they are classified as mode of action group 11 (Moa11), according to the Insect Resistance Action Committee (www.irac-online.org). As described, these proteins take the form of protoxins enclosed in crystals, and, after ingestion and midgut processing by serine proteases, they are converted into toxins. These interact with specific receptors located on the midgut epithelium, leading to cytopathological alterations and larval mortality (Charles et al. 1996). L. sphaericus strains can produce mosquito-active toxins including the binary (Bin), the group of so-called mosquitocidal toxins (Mtx1, Mtx2, Mtx3, and Mtx4), a second binary Cry48Aa-49Aa toxin and the S-layer envelope protein (Berry 2012). This chapter will focus on the mode of action of the Bin protoxin crystal, since this is the active ingredient of all L. sphaericus-based biolarvicides currently available for mosquito control. The Bin spectrum of action is limited to mosquito larvae and includes species from the genera Culex, Anopheles, Aedes/Ochlerotatus, Psorophora, and Mansonia. The most susceptible are Culex spp., in particular those from the Culex pipiens complex, followed by Anopheles species (Arredondo-Jimenez et al. 1990; Davidson 1989; Rodrigues et al. 1999). In the Aedes genus the response varies, with some species susceptible (e.g., Ochlerotatus atropalpus, Aedes vexans) and others refractory to Bin toxin, such as Aedes aegypti (Berry et al. 1993). As previously described, Bti has a broader spectrum since it is active against Culicidae, Simuliidae, and Chironomidae species (Goldberg and Margalit 1978; Lacey 2007; Rodcharoen et al. 1991). Mosquito larvae susceptibility to L. sphaericus has been reviewed by Lacey (2007) and Silva-Filha et al. (2014).
15.1.1 Bti Toxins
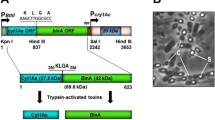
The protoxins found in Bti crystal are encoded by genes located on the pBtoxis megaplasmid (Berry et al. 2002), and the most common found are members of the Cry family, such as Cry4Aa (125 kDa), Cry4Ba (135 kDa), Cry11Aa (68 kDa), and a cytolytic toxin Cyt1Aa (28 kDa). Cry10Aa and Cyt2Ba toxins also exhibit activity against Diptera and can be detected in crystals produced by some strains. Cry and Cyt are pore-forming toxins, a family of bacterial toxins that are able to insert into the cell membrane of their hosts (de Maagd et al. 2003). Bti crystals have important larvicidal features, such as a diversity of Cry and Cyt protoxins, optimal ratio of toxins in crystals, and synergistic action of Cyt toxin, which can act as a surrogate receptor for the Cry toxins. The two toxin families display different features: Cry toxins interact with receptors to attain the pre-pore oligomeric form in order to insert themselves in cell membranes to form pores, while Cyt toxin has a cytolytic action and interacts directly with cell membranes (Soberón et al. 2007). Crystals containing both Cry and Cyt protoxins are characteristic of dipteran-active B. thuringiensis (Bt) strains.
The structure of Cry toxins shows three domains that have been characterized by crystallography and functional studies (Boonserm et al. 2005; de Maagd et al. 2003). Functionally, loops from domains II and III are responsible for interaction with specific receptors, and domain I is involved in membrane insertion, oligomerization, and pore formation (de Maagd et al. 2003). Cyt toxin has a single α-β domain, and, as described, it has cytolytic activity, acting directly on cell membrane to form pores (Bravo et al. 2007). Toxins from the Bti crystal act in synergy, and the activity of the whole crystal is far more effective than that of any individual toxins, or their combination (Crickmore et al. 1995). The Bti mode of action involves ingestion and solubilization of crystals under alkaline midgut conditions, activation of protoxins into toxins, binding to receptors, and pore formation in the cell membrane resulting in a colloid-osmotic lysis (Bravo et al. 2007; Knowles and Ellar 1987). After proteolytic cleavage at the N- and C-termini of protoxins, active Cry toxins have the ability to interact specifically with midgut microvilli (Beltrão and Silva-Filha 2007; Hofte and Whiteley 1989). Cyt toxin is able to insert itself into the cell membrane and synergizes the binding of Cry toxins, as described below.
The general Cry toxin mode of action has been explained including the hypothesis that action is based on the toxin binding to receptors followed by pore formation and a second hypothesis in which the toxins are able to activate intracellular signaling pathways that lead to cell death (Pigott and Ellar 2007; Vachon et al. 2012). A detailed outline of the Bt mode of action was presented in a previous chapter. Briefly the Bravo model, based on the action of Cry1A toxin in larvae of the lepidopteran Manduca sexta, showed that activated Cry toxins bind initially to GPI-anchored receptors such as alkaline phosphatases (ALPs) and N-aminopeptidases (APNs) with relatively low affinity, but toxins then bind with higher affinity to transmembrane cadherins (CADRs). Binding to CADRs promotes toxin oligomerization, which, under this conformational change, binds then to a second receptor, either APN or ALP again, but now with greater affinity (Bravo et al. 2004). After this binding step, Cry toxin can insert itself in the membrane and provoke pore formation. The Zhang model argues that Cry1A monomer toxin binding to the CADRs triggers a signaling mechanism that activates a cell death pathway (Zhang et al. 2006). It has been suggested that both mechanisms may occur simultaneously. CADRs, ALPs, APNs, and an α-amylase have been identified in Ae. aegypti and Anopheles larvae as Cry11Aa and Cry4Ba receptors (Bayyareddy et al. 2009; Likitvivatanavong et al. 2011). Cyt1Aa is a strategic component of Bti crystal because it can also act as a Cry toxin receptor. Cry11Aa and Cry4B can bind specifically to Cyt1Aa, subsequently enhancing Cry toxin binding to midgut microvilli receptors and inducing the formation of the pre-pore structure, which is able to insert itself in membranes and form pores in cells (Bravo et al. 2007; Cantón et al. 2011; Pérez et al. 2005, 2007). The two-receptor model proposed for Cry toxins active to Lepidoptera (Bravo et al. 2004) can also be applied to Bti Cry toxins. In this case, the Cyt toxin may play a role equivalent to that of a cadherin receptor, which is able to promote oligomer formation and lead to the subsequent binding step with high affinity to the GPI-anchored midgut receptors. Besides this set of midgut proteins that act as receptors (Likitvivatanavong et al. 2011), other molecules may also be involved in the mode of action of Bt toxins such as the immune defense involving the MAPK p38 pathway (Cancino-Rodezno et al. 2010, Torres-Martinez et al. 2016), ABC transporter proteins (Gahan et al. 2010), and other Cry-binding molecules that have been identified by proteomic approaches (Bayyareddy et al. 2009; Cancino-Rodezno et al. 2012; Stalinski et al. 2016). The Bti mode of action has been characterized by a complex set of events that do not favor the selection of resistance, as will be discussed in the next section.
15.1.2 Lysinibacillus Sphaericus Binary Toxin
The binary (Bin) protoxin is a heterodimer composed of two subunits BinA (42 kDa) and BinB (51 kDa) proteins which is produced during sporulation and deposited as a parasporal crystalline inclusion within the exosporium (Kalfon et al. 1984). The subunits are produced in equimolar amounts and form a co-crystal in sporulating L. sphaericus. The first selective step of L. sphaericus is the need for ingestion of crystals by larvae, followed by their solubilization in the alkaline environment of the gut, and activation of the protoxin forms into toxins by proteolytic cleavage, mediated by midgut proteinases (Charles et al. 1996). The subunits BinA and BinB are converted into active polypeptides of 39 and 43 kDa, respectively, due to cleavage of residues from the N- and C-termini (Broadwell et al. 1990). The processing and the presence of equimolar amounts of both subunits are essential factors in achieving optimal activity of this toxin (Nicolas et al. 1993). For C. pipiens larvae, the BinB component of the toxin is responsible for binding to the receptor, while the BinAt subsequently binds to BinB or the BinB-receptor complex (Charles et al. 1997). The functional domains of these subunits have been investigated through mutagenesis to identify regions and specific amino acids involved in binding to the Cqm1 receptor, binding between the two subunits and in vivo toxicity to larvae. N- and C-termini of BinA may be involved in the interaction of the BinB subunit (Kale et al. 2013; Oei et al. 1992). The N-terminal region of BinB (residues 33–158) is needed for receptor binding, and some residues identified are critical for this interaction (Romão et al. 2011; Singkhamanan et al. 2013).
In highly susceptible species of the C. pipiens complex, Bin toxin displays a marked and regionalized binding to the gastric caeca and posterior midgut. In Anopheles gambiae larvae the binding pattern is less clearly defined, and for Ae. aegypti, which is refractory to Bin toxin, this interaction is rather nonspecific compared to the two previous species (Davidson 1989). Quantitative binding assays between the Bin toxin and midgut microvilli of C. pipiens larvae have demonstrated high affinity (K d 5–20 nM), while a lower affinity (K d 30–110 nM) has been found for An. gambiae and An. stephensi, which are, overall, five- to tenfold less susceptible (in vivo) than C. pipiens (Nielsen-Leroux and Charles 1992; Nielsen-Leroux et al. 1995, 2002; Silva-Filha et al. 1997). For the refractory Ae. aegypti larvae, only a very low level of specific Bin toxin binding to the midgut is detected (Nielsen-Leroux and Charles 1992). Toxin binding to the midgut receptors of susceptible species leads to cytopathological alterations that have been described in Bin-treated C. pipiens larvae. These include disruption of microvilli, cytoplasmic vacuolization, mitochondria swelling, and breakdown of the endoplasmatic reticulum (Charles 1987; de Melo et al. 2008; Silva Filha and Peixoto 2003; Singh and Gill 1988; Tangsongcharoen et al. 2015). Other sites can also be affected as neural tissues and muscles (Singh and Gill 1988). The mode of action of the Bin toxin, following receptor binding, remains unclear, but there is evidence that the Bin toxin can form pores in the cell membranes, like the pore-forming toxins of B. thuringiensis and other bacteria (Pauchet et al. 2005; Schwartz et al. 2001). The vacuolization of target cells accompanied by the uptake of toxins into vesicles is also a marked effect of Bin intoxication (Davidson 1988). Bin toxin induces cell autophagy and displays a mechanism that prevents toxin degradation (Opota et al. 2011). The crystal structure of the BinB subunit has revealed features that support its action through pore formation, as proposed by previous studies (Srisucharitpanit et al. 2014). A recent study that revealed the BinAB structure suggests that BinA has the capacity to interact with the complex of BinB bound to the receptor, for co-internalization (Colletier et al. 2016).
The availability of midgut molecules that act as receptors for the Bin toxin is crucial for determining the susceptibility status of mosquito species for this toxin. Furthermore, Bin toxin resistance mechanisms found to date are associated with the failure of Bin toxin binding to those midgut receptors, as presented in Sect. 15.2. The receptors of the Bin toxin, which have been characterized in three susceptible species, are α-glucosidases bound to the midgut epithelium and named Cpm1 (C. pipiens maltase 1) (Darboux et al. 2001, Silva-Filha et al. 1999), Cqm1 (C. quinquefasciatus maltase 1) (Romão et al. 2006), and Agm3 (An. gambiae maltase 3) (Opota et al. 2008). Ae. aegypti displays the Aam1 protein (Aedes aegypti maltase 1), which is an ortholog with 74% identity to the Cqm1 receptor; however, Aam1 is not able to bind to the Bin toxin (Ferreira et al. 2010, 2014). These α-glucosidases (EC 3.2.1.20), belonging to the α-amylase family that plays a role in digestion, have the ability to hydrolyze α-1-4 links between glucose residues of carbohydrates (Krasikov et al. 2001). The Cpm1 α-glucosidase was the first receptor characterized for the Bin toxin in C. pipiens larvae (Darboux et al. 2001), and it shares 97% and 66% identity with the Cqm1 and Agm3 orthologs, respectively. These genes are organized in three exons and two introns, and their open reading frames encode the Cpm1/Cqm1 and Agm3 proteins with 580 and 588 residues, respectively. They display four conserved α-glucosidase domains, predicted consensus N-glycosylation sites, and a conserved sequence for a glycosylphosphatidylinositol (GPI) anchor (Darboux et al. 2001; Opota et al. 2008; Romão et al. 2006). The expression of the receptors as membrane-bound proteins is essential for the activity of Bin toxin, and mutations in their genes, which prevents the expression of these molecules as GPI-bound proteins to the midgut, are the most important resistance mechanism found in C. pipiens larvae. Ae. aegypti refractoriness seems to be based on the lack of ability of Aam1 to bind Bin toxin (Ferreira et al. 2010), although this protein is correctly located in the midgut through a GPI anchor. Minor differences in the amino acids of the Cqm1 and Aam1 protein sequence seem to be responsible for their capacity to interact or not with the Bin toxin. The N-terminal segment of Cqm1 (S129–A312) is responsible for binding to the Bin toxin, and a group of six amino acids within this region is critical for the ability of Cqm1 to bind the Bin toxin. These amino acids are not conserved in Aam1 and may be responsible for the refractoriness of this species (Ferreira et al. 2010, 2014).
15.2 Resistance Reports, Mechanisms, and Diagnosis
15.2.1 Investigation of Bti Resistance
Bacillus thuringiensis (Bt)-based biolarvicides have been used for pest control since the 1960s (Bravo et al. 2011), and field resistance to Bt toxins has already been reported for some species (Bravo et al. 2011). On the other hand, resistance to Bti biolarvicides has not been recorded to date. In the 1980s, very soon after its discovery, Bti was introduced for simulid and culicid control in a number of countries (Margalit and Dean 1985). In some areas of Germany, Switzerland, and France, Bti has been employed, mainly for Aedes spp. control, for more than 30 years without reports of resistance as reviewed by Ferreira and Silva-Filha (2013). The screening of the susceptibility of mosquito populations to Bti, before the introduction of this biolarvicide, has also provided baseline data for the natural variations occurring in several areas. Culex pipiens populations, without previous Bti exposure, have shown susceptibility variations ranging from less than 3- to 12.5-fold (Vasquez et al. 2009; Wirth et al. 2001). Aedes aegypti, Aedes albopictus, and Aedes rusticus populations of different origins and never exposed to Bti showed a slight variation between 1.5- and 3.9-fold (Araujo et al. 2013; Kamgang et al. 2011; Liu et al. 2004; Loke et al. 2010; Marcombe et al. 2011, 2014; Pocquet et al. 2014). The susceptibility of Bti-treated populations, compared to laboratory colonies or untreated field samples used as references, was also similar to those observed in non-treated samples, confirming the lack of Bti resistance in those populations after exposure (Ferreira and Silva-Filha 2013). The only exception to these findings is the report of two C. pipiens populations in New York State (USA), which had a history of Bti spraying and displayed resistance ratios (RR) at LC95 of 14- and 41-fold (Paul et al. 2005). In this study, data from the pretreatment period was not available, and it was not possible to conclude whether the decreased susceptibility found was a consequence of Bti treatments.
Selection studies using whole Bti crystals performed under laboratory conditions also failed to show significant susceptibility alterations. Several attempts showed a maximum increase of around threefold in the lethal concentration of Bti for the selected colonies, which was the same as the natural variation found among untreated populations, as previously described (Ferreira and Silva-Filha 2013; Wirth 2010). In conclusion, resistance to Bti crystals, which are the active ingredient of commercially available biolarvicides, has not been reported to date. Although resistance to the whole Bti crystal has not been detected, larvae resistance to individual toxins from the crystal has been demonstrated by artificial selection assays using single Cry toxins (Cadavid-Restrepo et al. 2012, Georghiou and Wirth 1997, Paris et al. 2011). It was also demonstrated that an Ae. aegypti colony selected with Bti, but without decreased susceptibility to Bti, nevertheless displayed resistance ratios (RR) of 68-, 9-, and 9-fold for Cry4Aa, Cry4Ba, and Cry11Aa, respectively (Tetreau et al. 2012). This result shows that the action of single toxins can be affected, although the synergy provided by the set of toxins is able to prevent the selection of resistance to the whole Bti crystal. In some Bti-selected colonies, molecules that act as receptors for Cry toxins, such as ALPs and APNs, have been shown to be under-expressed (Stalinski et al. 2016). Alteration of these molecules may be responsible for the reduction of susceptibility to individual toxins found in this laboratory colony. However, as described, the synergy promoted by toxins, in particular the role of Cyt1Aa as a receptor for Cry toxins, is a key factor in overcoming failures related to alterations of Cry binding to midgut receptors. Data show that a decrease in susceptibility to individual Cry toxins does not evolve to Bti resistance but these can be used as markers to access the level of selection pressure imposed on a certain population (Tetreau et al. 2013b). The synergism of Bti toxins confers a great advantage provided by their interaction with midgut cells, but other mechanisms of resistance unrelated to this step in the mode of action could potentially occur, although these have not yet been specifically recorded for Bti. These may include failures in proteolytic processing and innate immune response, which are currently under study (Cancino-Rodezno et al. 2010; Tetreau et al. 2013a). To date, Bti is still the biolarvicide available for mosquito control that has the most selective spectrum of action and lack of recorded field resistance, after decades of use. These major advantages are the result of the multiple set of toxins found in Bti crystals, the synergy of toxins, and the strategic role of Cyt toxin in overcoming failures occurring at the level of larvae midgut receptors, which has been the most important mechanism behind refractoriness to bacterial insecticidal toxins.
15.2.2 Lysinibacillus Sphaericus Resistance
The insecticidal activity of L. sphaericus, unlike Bti, is based on the action of one toxin that targets a single class of receptors (Nielsen-Leroux and Charles 1992), and this is a critical factor for the selection of resistance. L. sphaericus displays a high larvicidal activity in combination with effective performance under field conditions, although the potential for selection of resistance to the Bin toxin remains its major disadvantage. This section will summarize the major resistance reports available in the literature and advances in its management. Bin toxin resistance has been reported in field populations of C. pipiens / C. quinquefasciatus exposed to this agent and also in colonies selected with L. sphaericus, under laboratory conditions. The first report was of a C. pipiens population from France exposed to L. sphaericus for about 5 years that displayed high resistance levels (RR>20,000) (Sinègre et al. 1994). Subsequently, resistance cases of C. quinquefasciatus or C. pipiens populations were recorded in India (Rao et al. 1995), China (Yuan et al. 2000), Tunisia (Nielsen-Leroux et al. 2002), and Thailand (Mulla et al. 2003), along with a second resistant population (BP) in France (Chevillon et al. 2001; Nielsen-Leroux et al. 2002). There are also examples of L. sphaericus utilization for C. quinquefasciatus control programs in two urban areas in Recife and São Paulo city, in Brazil, which did not lead to resistance (Silva-Filha et al. 2008). It is likely that factors such as the interruption of treatment and/or rotation with Bti recorded in these areas may have disrupted the selection pressure. Selection performed under laboratory conditions using L. sphaericus has also confirmed that larvae may achieve high levels of resistance to the Bin toxin (RR ≈ 100,000) (Amorim et al. 2007; Pei et al. 2002; Rodcharoen and Mulla 1994; Wirth et al. 2000). The resistance reports available indicated that prolonged and intensive utilization of L. sphaericus, as the sole agent for control, may result in selection of high resistance in the treated populations.
The mechanism of resistance identified in some laboratory-selected and field-derived C. pipiens colonies is caused by target site alteration. In such cases, previous studies have shown that protoxin from crystals can be correctly processed, but the activated Bin toxin fails to bind to the midgut epithelium, due to lack of functional receptors (Darboux et al. 2002; Guo et al. 2013; Nielsen-LeRoux et al., 1995, 2002; Oliveira et al. 2004). There are only two cases reported to date, resistant SPHAE (France) and TUNIS (Tunisia) field-derived colonies, in which there are functional binding receptors on the midgut and the resistance mechanisms remain unknown (Nielsen-Leroux et al. 1997, 2002). To date, the lack of receptors in the larval midgut is the major resistance mechanism for the Bin toxin (Silva Filha et al. 2014), and this occurs due to mutations in the cpm1/cqm1 genes that prevent the expression of these midgut-bound α-glucosidases. Resistance to L. sphaericus was found to be monofactorial and recessively inherited in all the cases studied to date (Amorim et al. 2007; Nielsen-Leroux et al. 1995, 2002; Oliveira et al. 2004).
The identification of the genes coding for the Cpm1/Cqm1 receptors in C. pipiens/C. quinquefasciatus (Darboux et al. 2001) opened the way for investigations of the molecular basis of resistance. Eight alleles from cpm1/cqm1 genes associated with Bin resistance have been characterized in populations from the USA, Brazil, France, and China (Chalegre et al. 2012, 2015; Darboux et al. 2002, 2007; Guo et al. 2013; Menezes et al. 2016; Romão et al. 2006). Seven of these alleles (cpm1 GEO from the USA; cqm1 REC , cqm1 REC-2 , cqm1 REC-D16 , and cqm1 REC-D25 from Brazil; cpm1 BP from France; cqm1R from China) were characterized by mutations, as transitions or deletions that generate a premature stop codon in their open reading frames. As a consequence, their transcripts code for truncated proteins, without the GPI anchor which is located at the C-terminus of protein. The loss of the GPI anchor prevents the protein localizing to the midgut surface, and the Bin toxin can no longer bind to the epithelium in order to produce its toxic effect and larvae mortality. Only one allele was found in a resistant population from France; cpm1 BP -del has a mutation that produces a different effect. In this case the mutant protein retained the predicted GPI anchor, but a 198 bp internal deletion, provoked by the insertion of a retrotransposon, generates an alternative splicing event, and the resulting transcript codes for a protein with internal deletion of 66 amino acids. This protein is unable to bind to the Bin toxin, despite being correctly located on the epithelium (Darboux et al. 2007). This mechanism prevents Bin interaction with the midgut epithelium, and it is responsible for the high level of resistance exhibited by larvae that are homozygous for the allele. Similarly, Ae. aegypti larvae are naturally refractory due to the lack of functional receptors in the midgut (Nielsen-Leroux and Charles 1992). Larvae express the Aam1 α-glucosidase, which is a Cqm1 ortholog that, although located in the midgut, does not have the ability to bind to the Bin toxin and thus prevents the toxic action of the Bin toxin on Ae. aegypti (Ferreira et al. 2010). The characterization of these mutations indicates that cpm1/cqm1 is a highly polymorphic gene and six mutations, of the eight described, are located in the same region. These mutations can have a high impact because, unlike those observed in resistance genes of other insecticidal compounds (e.g., pyrethroids) which often cause only a reduction in their capacity to bind to the active ingredient (Du et al. 2013; Rinkevich et al. 2013), they generate full refractoriness, as seen in the case of Bin receptors, which become absent from the midgut.
Resistance to L. sphaericus needs be monitored, since the selection of homozygous individuals can lead to serious operational failures. L. sphaericus resistance is also likely to be associated with discrete biological costs rather than marked impact on the fitness of resistant individuals, as has often been reported in the literature (Anilkumar et al. 2008). Some L. sphaericus-resistant colonies, for instance, have been maintained for more than 200 hundreds generations, under laboratory conditions (Chalegre et al. 2015). One direct consequence of L. sphaericus resistance in these insects is the potential lack of the Cpm1/Cqm1 α-glucosidase. However, C. quinquefasciatus larvae display a set of other α-glucosidases (Gabrisko 2013; Romão et al. 2006), and hypothetically, the lack of Cqm1 may be compensated by other α-glucosidases expressed in the larvae midgut. The role played by Cqm1 and the other α-glucosidases in larvae physiology has not yet been elucidated, but the long-term maintenance of these resistant colonies suggests that these insects could be successfully established which increases concerns about L. sphaericus resistance.
Monitoring the susceptibility of populations exposed to L. sphaericus is thus crucial for the effectiveness of this biolarvicide. Bioassays to determine the lethal concentrations of Bin toxin to larvae are the main tool used to evaluate susceptibility. However, L. sphaericus resistance is recessively inherited, and heterozygous individuals carrying r alleles are susceptible and can thus barely be detected by this tool. On the other hand, the identification of mutations of the cqm1 gene that confer resistance has enabled the development of PCR screens which have enhanced the capacity to directly monitor these recessive genes in population samples. Screening of these genes in C. quinquefasciatus populations in the city of Recife (Brazil) has revealed four of these alleles: cqm1 REC , cqm1 REC-2 , cqm1 REC-D16 and cqm1 REC-D25 (Chalegre et al. 2009, 2012, 2015). cqm1 REC , which was primarily identified in a laboratory-selected colony, was found to occur in Recife city areas at a frequency in the order of 10−3 in samples of untreated populations, while a significantly higher frequency (≈0.05) was recorded in larvae samples from a L. sphaericus-treated area. Furthermore, although the four alleles were found in Recife city, cqm1 REC was detected in all populations at a higher frequency, compared to the other alleles (Menezes et al. 2016). The dataset reported the frequency of these alleles in Recife populations and indicated that cqm1 REC may be a marker for the surveillance of resistance in C. quinquefasciatus populations from those areas. The frequency of other L. sphaericus resistance alleles in the geographical areas in which they were originally detected, or abroad, has not been studied.
15.3 Management Strategies to Prevent L. sphaericus Resistance
The L. sphaericus resistance recorded in exposed populations from different countries highlights the need to design strategies to manage resistance to this agent. One of the most important approaches is the use of multiple strategies to reduce the density of mosquitoes. This is crucial for reducing insecticide use and hence the corresponding selection pressure that is caused by its use (Becker et al. 2003). It is highly recommended that environmental strategies be introduced to reduce the number of active breeding sites and to keep larvicide application at the minimum level possible, in order to prevent the onset of resistance. However, if resistance is detected in an exposed population, the interruption of L. sphaericus treatment is the primary measure to be taken. Mosquitoes are r-strategists, and populations can recover rapidly after interruption of the control interventions. The interruption of treatments, per se, allows the immigration of susceptible individuals from surrounding areas and leads to the dilution of resistance alleles. Reversal of L. sphaericus resistance is facilitated by the recessive inheritance of this phenotype (Amorim et al. 2007, 2010; Chevillon et al. 2001, Nielsen-Leroux et al. 1995, 1997, 2002; Oliveira et al. 2004). In a Chinese field population, a high resistance level (22,000-fold) was recorded, and, 6 months after stopping treatment, the resistance ratio decreased to sixfold (Yuan et al. 2000). The second strategy to be implemented is the replacement of L. sphaericus by other insecticides with different modes of action. Among the commercially available agents to be used in association with L. sphaericus, Bti-based biolarvicides are considered the most promising option because their toxins and mode of action are unrelated to the Bin toxin, as described previously in this chapter. Other dipteran-active Bacillus thuringiensis (Bt) strains also produce toxins that do not display cross-resistance to Bin toxin, such as those from Bacillus thuringiensis serovar. medellin (Btmed) and Bacillus thuringiensis serovar. jegathesan (Btjeg), although commercial products are not available to date. There are also other mosquitocidal toxins produced by L. sphaericus, that are effective on Bin-resistant mosquito strains, such as the Mtx and Cry48–49 toxins (Berry 2012). However, the expression of these toxins in native strains has limitations in terms of optimal amounts and stability. Further biotechnological development is needed for the production of biolarvicides based on these toxins. Recombinant L. sphaericus strains containing Bti toxins have been developed, and these have been shown to be active against larvae from Bin-resistant colonies. However, these modified strains showed low expression and/or instability of Bti proteins (Federici et al. 2010; Gammon et al. 2006). The integration of the Bin toxin into Bti strains has also been performed, and the recombinant constructs successfully produced Bti and Bin toxins with improved toxicity (Park et al. 2005). Products based on such recombinant bacteria have not been developed for field utilization but are a promising prospect (Federici et al. 2010).
Nowadays, it is strongly recommended that Bti be used in combination with L. sphaericus, since Bti commercial products are already available, are effective in overcoming Bin-resistance, and have a long history of successful field utilization. Bti can be used in rotation or mixed with L. sphaericus, and this strategy can be introduced for prevention or reversal of L. sphaericus resistance. Both rotation and mixtures may be effective, but mixtures may be more efficient in delaying the onset of resistance (Zahiri and Mulla 2003). Based on this successful association of the complementary features of L. sphaericus and Bti, commercial products containing a mixture of crystals produced by each agent in a single product have been developed (Anderson et al. 2011). These aim to target a wider range of mosquito species in a variety of settings. Successful trials have been carried out to control Culex and Aedes species that colonize typical breeding sites in urban areas and to control other mosquito species that occur in wetlands in environmentally sensitive areas (Anderson et al. 2011; Cetin et al. 2015; Dritz et al. 2011). These multi-toxin products have shown promising results and can be used in mosquito control programs as a safe tool with a low potential for resistance selection. In a broader view, other agents may also be considered for use in management of L. sphaericus resistance, and these may include biological control agents such as predators (fish, aquatic insects), entomopathogenic fungi, and nematodes (Hurst et al. 2006; Keiser et al. 2005; Lacey 2007; Lingenfelser et al. 2010). Spinosins are another group of larvicides that have been recently introduced for mosquito control, and field trials showed successful results (Hertlein et al. 2010). Synthetic insecticides, such as insect growth regulators, are another category to be considered, since these have a mode of action distinct from L. sphaericus and a relatively safe spectrum of action (Giraldo-Calderon et al. 2008, Guidi et al. 2013). In conclusion, resistance can be counteracted, and L. sphaericus is an effective component to be employed in association with other control measures in integrated programs in order to reduce mosquito populations.
References
Amorim LB, Oliveira CMF, Rios EM, Regis L, Silva-Filha MHNL (2007) Development of Culex quinquefasciatus resistance to Bacillus sphaericus strain IAB59 needs long term selection pressure. Biol Control 42:155–160
Amorim LB, De Barros RA, Chalegre KD, De Oliveira CM, Regis LN, Silva-Filha MH (2010) Stability of Culex quinquefasciatus resistance to Bacillus sphaericus evaluated by molecular tools. Insect Biochem Mol Biol 40:311–316
Anderson JF, Ferrandino FJ, Dingman DW, Main AJ, Andreadis TG, Becnel JJ (2011) Control of mosquitoes in catch basins in Connecticut with Bacillus thuringiensis israelensis, Bacillus sphaericus, [corrected] and spinosad. J Am Mosq Control Assoc 27:45–55
Anilkumar KJ, Pusztai-Carey M, Moar WJ (2008) Fitness costs associated with Cry1Ac-resistant Helicoverpa zea (Lepidoptera: Noctuidae): a factor countering selection for resistance to Bt cotton? J Econ Entomol 101:1421–1431
Araujo AP, Araujo Diniz DF, Helvecio E, De Barros RA, De Oliveira CM, Ayres CF, De Melo-Santos MA, Regis LN, Silva-Filha MH (2013) The susceptibility of Aedes aegypti populations displaying temephos resistance to Bacillus thuringiensis israelensis: a basis for management. Parasit Vectors 6:297
Arredondo-Jimenez JI, Lopez T, Rodriguez MH, Bown DN (1990) Small scale field trials of Bacillus sphaericus (strain 2362) against anopheline and culicine mosquito larvae in southern Mexico. J Am Mosq Control Assoc 6:300–305
Bayyareddy K, Andacht TM, Abdullah MA, Adang MJ (2009) Proteomic identification of Bacillus thuringiensis subsp. israelensis toxin Cry4Ba binding proteins in midgut membranes from Aedes (Stegomyia) aegypti Linnaeus (Diptera, Culicidae) larvae. Insect Biochem Mol Biol 39:279–286
Becker N, Petric D, Dahl C, Lane J, Kaiser A (2003) Integrated pest management. In: Becker N (ed) Mosquitos and their control. Kluwer Academic/Plenum Publishers, New York, pp 417–424
Beltrão BM, Silva-Filha MH (2007) Interaction of Bacillus thuringiensis svar. israelensis Cry toxins with binding sites from Aedes aegypti (Diptera: Culicidae) larvae midgut. FEMS Microbiol Lett 266:163–169
Berry C (2012) The bacterium, Lysinibacillus sphaericus, as an insect pathogen. J Invertebr Pathol 109:1–10
Berry C, Hindley J, Ehrhardt AF, Grounds T, De Souza I, Davidson EW (1993) Genetic determinants of host ranges of Bacillus sphaericus mosquito larvicidal toxins. J Bacteriol 175:510–518
Berry C, O'neil S, Ben-Dov E, Jones AF, Murphy L, Quail MA, Holden MT, Harris D, Zaritsky A, Parkhill J (2002) Complete sequence and organization of pBtoxis, the toxin-coding plasmid of Bacillus thuringiensis subsp. israelensis. Appl Environ Microbiol 68:5082–5095
Boonserm P, Davis P, Ellar DJ, Li J (2005) Crystal structure of the mosquito-larvicidal toxin Cry4Ba and its biological implications. J Mol Biol 348:363–382
Bravo A, Gómez I, Conde J, Muñoz-Garay C, Sánchez J, Miranda R, Zhuang M, Gill SS, Soberón M (2004) Oligomerization triggers binding of a Bacillus thuringiensis Cry1Ab pore-forming toxin to aminopeptidase N receptor leading to insertion into membrane microdomains. Biochim Biophys Acta 1667:38–46
Bravo A, Gill SS, Soberón M (2007) Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 49:423–435
Bravo A, Likitvivatanavong S, Gill SS, Soberón M (2011) Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem Mol Biol 41:423–431
Broadwell AH, Baumann L, Baumann P (1990) Larvicidal properties of the 42 and 51 kilodalton Bacillus sphaericus proteins expressed in different bacterial hosts: evidence for a binary toxin. Curr Microbiol 21:361–366
Cadavid-Restrepo G, Sahaza J, Orduz S (2012) Treatment of an Aedes aegypti colony with the Cry11Aa toxin for 54 generations results in the development of resistance. Mem Inst Oswaldo Cruz 107:74–79
Cancino-Rodezno A, Alexander C, Villasenor R, Pacheco S, Porta H, Pauchet Y, Soberon M, Gill SS, Bravo A (2010) The mitogen-activated protein kinase p38 is involved in insect defense against Cry toxins from Bacillus thuringiensis. Insect Biochem Mol Biol 40:58–63
Cancino-Rodezno A, Lozano L, Oppert C, Castro JI, Lanz-Mendoza H, Encarnacion S, Evans AE, Gill SS, Soberon M, Jurat-Fuentes JL, Bravo A (2012) Comparative proteomic analysis of Aedes aegypti larval midgut after intoxication with Cry11Aa toxin from Bacillus thuringiensis. PLoS One 7:e37034
Cantón PE, Zanicthe Reyes EZ, Ruiz De Escudero I, Bravo A, Soberón M (2011) Binding of Bacillus thuringiensis subsp. israelensis Cry4Ba to Cyt1Aa has an important role in synergism. Peptides 32:595–600
Cetin H, Oz E, Yanikoglu A, Cilek JE (2015) Operational evaluation of Vectomax(R) WSP (Bacillus thuringiensis subsp. israelensis+Bacillus sphaericus) against larval Culex pipiens in septic tanks. J Am Mosq Control Assoc 31:193–195
Chalegre KD, Romão TP, Amorim LB, Anastacio DB, De Barros RA, De Oliveira CM, Regis L, De-Melo-Neto OP, Silva-Filha MH (2009) Detection of an allele conferring resistance to Bacillus sphaericus binary toxin in Culex quinquefasciatus populations by molecular screening. Appl Environ Microbiol 75:1044–1049
Chalegre KD, Romão TP, Tavares DA, Santos EM, Ferreira LM, Oliveira CMF, De-Melo-Neto OP, Silva-Filha MHNL (2012) Novel mutations associated to Bacillus sphaericus resistance are identified in a polymorphic region of the Culex quinquefasciatus cqm1 gene. Appl Environ Microbiol 78:6321–6326
Chalegre KD, Tavares DA, Romao TP, Menezes HSG, Nascimento AL, Oliveira CMF, De-Melo-Neto OP, Silva-Filha MHNL (2015) Co-selection and replacement of resistance alleles to Lysinibacillus sphaericus in a Culex quinquefasciatus colony. FEBS J 282:3592–3602
Charles JF (1987) Ultrastructural midgut events in Culicidae larvae fed with Bacillus sphaericus 2297 spore/crystal complex. Ann Inst Pasteur Microbiol 138:471–484
Charles JF, Nielsen-Leroux C, Delecluse A (1996) Bacillus sphaericus toxins: molecular biology and mode of action. Annu Rev Entomol 41:451–472
Charles JF, Silva-Filha MH, Nielsen-Leroux C, Humphreys MJ, Berry C (1997) Binding of the 51- and 42-kDa individual components from the Bacillus sphaericus crystal toxin to mosquito larval midgut membranes from Culex and Anopheles sp. (Diptera: Culicidae). FEMS Microbiol Lett 156:153–159
Chevillon C, Bernard C, Marquine M, Pasteur N (2001) Resistance to Bacillus sphaericus in Culex pipiens (Diptera: Culicidae): interaction between recessive mutants and evolution in southern France. J Med Entomol 38:657–664
Colletier JP, Sawaya MR, Gingery M, Rodriguez JA, Cascio D, Brewster AS, Michels-Clark T, Hice RH, Coquelle N, Boutet S, Williams GJ, Messerschmidt M, Deponte DP, Sierra RG, Laksmono H et al (2016) De novo phasing with X-ray laser reveals mosquito larvicide BinAB structure. Nature 539:43–47
Crickmore N, Bone EJ, Wiliams JA, Ellar DJ (1995) Contribution of the individual components of the delta-endotoxin crystal to the mosquitocidal activity of Bacillus thuringiensis subs. israelensis. FEMS Microbiol Lett 131:249–254
Darboux I, Nielsen-Leroux C, Charles JF, Pauron D (2001) The receptor of Bacillus sphaericus binary toxin in Culex pipiens (Diptera: Culicidae) midgut: molecular cloning and expression. Insect Biochem Mol Biol 31:981–990
Darboux I, Pauchet Y, Castella C, Silva-Filha MH, Nielsen-Leroux C, Charles JF, Pauron D (2002) Loss of the membrane anchor of the target receptor is a mechanism of bioinsecticide resistance. Proc Natl Acad Sci U S A 99:5830–5835
Darboux I, Charles JF, Pauchet Y, Warot S, Pauron D (2007) Transposon-mediated resistance to Bacillus sphaericus in a field-evolved population of Culex pipiens (Diptera: Culicidae). Cell Microbiol 9:2022–2029
Davidson EW (1988) Binding of the Bacillus sphaericus (Eubacteriales: Bacillaceae) toxin to midgut cells of mosquito (Diptera: Culicidae) larvae: relationship to host range. J Med Entomol 25:151–157
Davidson EW (1989) Variation in binding of Bacillus sphaericus toxin and wheat germ agglutinin to larval midgut cells of six species of mosquitoes. J Invertebr Pathol 53:251–259
De Barjac H (1978) A new variety of Bacillus thuringiensis very toxic to mosquitoes: B. thuringiensis var. israelensis serotype 14. C R Seances Hebdomadaires Acad Sci Ser D 286:797–800
De Maagd RA, Bravo A, Berry C, Crickmore N, Schnepf HE (2003) Structure, diversity, and evolution of protein toxins from spore-forming entomopathogenic bacteria. Ann Rev Genet 37:409–433
De Melo JV, Vasconcelos RH, Furtado AF, Peixoto CA, Silva-Filha MH (2008) Ultrastructural analysis of midgut cells from Culex quinquefasciatus (Diptera: Culicidae) larvae resistant to Bacillus sphaericus. Micron 39:1342–1350
Dritz DA, Lawler SP, Evkhanian C, Graham P, Baracosa V, Dula G (2011) Control of mosquito larvae in seasonal wetlands on a wildlife refuge using VectoMax CG. J Am Mosq Control Assoc 27:398–403
Du Y, Nomura Y, Satar G, Hu Z, Nauen R, He SY, Zhorov BS, Dong K (2013) Molecular evidence for dual pyrethroid-receptor sites on a mosquito sodium channel. Proc Natl Acad Sci U S A 110:11785–11790
Federici BA, Park HD, Bideshi DK (2010) Overview of the basic biology of Bacillus thuringiensis with emphasis on genetic engineering of bacterial larvicides for mosquito control. Open J Toxicol 3:83–100
Ferreira LM, Silva-Filha MHNL (2013) Bacterial larvicides for vector control: mode of action of toxins and implications for resistance. Biocontrol Sci Tech 23:1137–1168
Ferreira LM, Romão TP, De-Melo-Neto OP, Silva-Filha MH (2010) The orthologue to the Cpm1/Cqm1 receptor in Aedes aegypti is expressed as a midgut GPI-anchored alpha-glucosidase, which does not bind to the insecticidal binary toxin. Insect Biochem Mol Biol 40:604–610
Ferreira LM, Romão TP, Nascimento NA, Costa MD, Rezende AM, De-Melo-Neto OP, Silva-Filha MH (2014) Non conserved residues between Cqm1 and Aam1 mosquito alpha-glucosidases are critical for the capacity of Cqm1 to bind the Binary (Bin) toxin from Lysinibacillus sphaericus. Insect Biochem Mol Biol 50:34–42
Gabrisko M (2013) Evolutionary history of eukaryotic alpha-glucosidases from the alpha-amylase family. J Mol Evol 76:129–145
Gahan LJ, Pauchet Y, Vogel H, Heckel DG (2010) An ABC transporter mutation is correlated with insect resistance to Bacillus thuringiensis Cry1Ac toxin. PLoS Genet 6:e1001248
Gammon K, Jones GW, Hope SJ, De Oliveira CM, Regis L, Silva Filha MH, Dancer BN, Berry C (2006) Conjugal transfer of a toxin-coding megaplasmid from Bacillus thuringiensis subsp. israelensis to mosquitocidal strains of Bacillus sphaericus. Appl Environ Microbiol 72:1766–1770
Georghiou GP, Wirth MC (1997) Influence of exposure to single versus multiple toxins of Bacillus thuringiensis subsp. israelensis on development of resistance in the mosquito Culex quinquefasciatus (Diptera: Culicidae). Appl Environ Microbiol 63:1095–1101
Giraldo-Calderon GI, Perez M, Morales CA, Ocampo CB (2008) Evaluation of the triflumuron and the mixture of Bacillus thuringiensis plus Bacillus sphaericus for control of the immature stages of Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) in catch basins. Biomedica 28:224–233
Goldberg LH, Margalit J (1978) A bacterial spore demonstrating rapid larvicidal activity against Anopheles segentii, Uranotaenia unguiculata, Culex univitatus, Aedes aegypti and Culex pipiens. Mosq News 37:355–358
Guidi V, Luthy P, Tonolla M (2013) Comparison between diflubenzuron and a Bacillus thuringiensis israelensis- and Lysinibacillus sphaericus-based formulation for the control of mosquito larvae in urban catch basins in Switzerland. J Am Mosq Control Assoc 29:138–145
Guillet P, Kurtak DC, Phillipon B, Meyer R (1990) Use of Bacillus thuringiensis for onchocerciasis control in West Africa. In: De Barjac H, Sutherland D (eds) Bacterial control of mosquitoes and black-flies, 1st edn. Rutgers University Press, New Brunswick, pp 187–201
Guo QY, Cai QX, Yan JP, Hu XM, Zheng DS, Yuan ZM (2013) Single nucleotide deletion of cqm1 gene results in the development of resistance to Bacillus sphaericus in Culex quinquefasciatus. J Insect Physiol 59:967–973
Hertlein MB, Mavrotas C, Jousseaume C, Lysandrou M, Thompson GD, Jany W, Ritchie SA (2010) A review of spinosad as a natural product for larval mosquito control. J Am Mosq Control Assoc 26:67–87
Hofte H, Whiteley HR (1989) Insecticidal crystal proteins of Bacillus thuringiensis. Microbiol Rev 53:242–255
Hurst TP, Brown MD, Kay BH, Ryan PA (2006) Evaluation of Melanotaenia duboulayi (Atheriniformes: Melanotaeniidae), Hypseleotris galli (Perciformes: Eleotridae), and larvicide VectoLex WG (Bacillus sphaericus) for integrated control of Culex annulirostris. J Am Mosq Control Assoc 22:418–425
Kale A, Hire RS, Hadapad AB, D'souza SF, Kumar V (2013) Interaction between mosquito-larvicidal Lysinibacillus sphaericus binary toxin components: analysis of complex formation. Insect Biochem Mol Biol 43:1045–1054
Kalfon A, Charles JF, Bourgouin C, De Barjac H (1984) Sporulation of Bacillus sphaericus 2297: an electron microscope study of crystal-like inclusion biogenesis and toxicity to mosquito larvae. J Gen Microbiol 130:893–900
Kamgang B, Marcombe S, Chandre F, Nchoutpouen E, Nwane P, Etang J, Corbel V, Paupy C (2011) Insecticide susceptibility of Aedes aegypti and Aedes albopictus in Central Africa. Parasit Vectors 4:79
Keiser J, Maltese MF, Erlanger TE, Bos R, Tanner M, Singer BH, Utzinger J (2005) Effect of irrigated rice agriculture on Japanese encephalitis, including challenges and opportunities for integrated vector management. Acta Trop 95:40–57
Knowles BH, Ellar DJ (1987) Colloid-osmotic lysis is a general feature of the mechanism of action of Bacillus thuringiensis δ-endotoxins with different insect specificity. Biochim Biophys Acta 924:507–518
Krasikov VV, Karelov DV, Firsov LM (2001) Alpha-glucosidases. Biochemistry (Mosc) 66:267–281
Lacey L (2007) Bacillus thuringiensis serovariety israelensis and Bacillus sphaericus for mosquito control. J Am Mosq Control Assoc 23:133–163
Likitvivatanavong S, Chen J, Evans AM, Bravo A, Soberón M, Gill SS (2011) Multiple receptors as targets of Cry toxins in mosquitoes. J Agric Food Chem 59:2829–2838
Lingenfelser A, Rydzanicz K, Kaiser A, Becker N (2010) Mosquito fauna and perspectives for integrated control of urban vector-mosquito populations in southern Benin (West Africa). Ann Agric Environ Med 17:49–57
Liu H, Cupp EW, Guo A, Liu N (2004) Insecticide resistance in Alabama and Florida mosquito strains of Aedes albopictus. J Med Entomol 41:946–952
Loke SR, Andy-Tan WA, Benjamin S, Lee HL, Sofian-Azirun M (2010) Susceptibility of field-collected Aedes aegypti (L.) (Diptera: Culicidae) to Bacillus thuringiensis israelensis and temephos. Trop Biomed 27:493–503
Marcombe S, Darriet F, Agnew P, Etienne M, Yp-Tcha MM, Yebakima A, Corbel V (2011) Field efficacy of new larvicide products for control of multi-resistant Aedes aegypti populations in Martinique (French West Indies). AmJTrop Med Hyg 84:118–126
Marcombe S, Farajollahi A, Healy SP, Clark GG, Fonseca DM (2014) Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PLoS One 9:e101992
Margalit J, Dean D (1985) The story of Bacillus thuringiensis var. israelensis (B.t.i.) J Am Mosq Control Assoc 1:1–7
Menezes HSG, Chalegre KD, Romao TP, Oliveira CMF, De-Melo-Neto OP, Silva-Filha MHNL (2016) A new allele conferring resistance to Lysinibacillus sphaericus is detected in low frequency in Culex quinquefasciatus field populations. Parasit Vectors 9:1–7
Mulla MS, Thavara U, Tawatsin A, Chomposri J, Su T (2003) Emergence of resistance and resistance management in field populations of tropical Culex quinquefasciatus to the microbial control agent Bacillus sphaericus. J Am Mosq Control Assoc 19:39–46
Nicolas L, Nielsen-Leroux C, Charles JF, Delécluse A (1993) Respective role of the 42- and 51-kDa components of the Bacillus sphaericus toxin overexpressed in Bacillus thuringiensis. FEMS Microbiol Lett 106:275–280
Nielsen-Leroux C, Charles JF (1992) Binding of Bacillus sphaericus binary toxin to a specific receptor on midgut brush-border membranes from mosquito larvae. Eur J Biochem 210:585–590
Nielsen-Leroux C, Charles JF, Thiery I, Georghiou GP (1995) Resistance in a laboratory population of Culex quinquefasciatus (Diptera: Culicidae) to Bacillus sphaericus binary toxin is due to a change in the receptor on midgut brush-border membranes. Eur J Biochem 228:206–210
Nielsen-Leroux C, Pasquier F, Charles JF, Sinegre G, Gaven B, Pasteur N (1997) Resistance to Bacillus sphaericus involves different mechanisms in Culex pipiens (Diptera: Culicidae) larvae. J Med Entomol 34:321–327
Nielsen-Leroux C, Pasteur N, Pretre J, Charles JF, Sheikh HB, Chevillon C (2002) High resistance to Bacillus sphaericus binary toxin in Culex pipiens (Diptera: Culicidae): the complex situation of West Mediterranean countries. J Med Entomol 39:729–735
Oei C, Hindley J, Berry C (1992) Binding of purified Bacillus sphaericus binary toxin and its deletion derivatives to Culex quinquefasciatus gut: elucidation of functional binding domains. J Gen Microbiol 138:1515–1526
Oliveira CMF, Silva-Filha MH, Nielsen-Leroux C, Pei G, Yuan Z, Regis L (2004) Inheritance and mechanism of resistance to Bacillus sphaericus in Culex quinquefasciatus (Diptera: Culicidae) from China and Brazil. J Med Entomol 41:58–64
Opota O, Charles JF, Warot S, Pauron D, Darboux I (2008) Identification and characterization of the receptor for the Bacillus sphaericus binary toxin in the malaria vector mosquito, Anopheles gambiae. Comp Biochem Physiol -Part B Biochem Mol Biol 149:419–427
Opota O, Gauthier NC, Doye A, Berry C, Gounon P, Lemichez E, Pauron D (2011) Bacillus sphaericus binary toxin elicits host cell autophagy as a response to intoxication. PLoS One 6:e14682
Paris M, Tetreau G, Laurent F, Lelu M, Després L, David JP (2011) Persistence of Bacillus thuringiensis israelensis (Bti) in the environment induces resistance to multiple Bti toxins in mosquitoes. Pest Manag Sci 67:122–128
Park HW, Bideshi DK, Wirth MC, Johnson JJ, Walton WE, Federici BA (2005) Recombinant larvicidal bacteria with markedly improved efficacy against Culex vectors of West Nile virus. Am J Trop Med Hyg 72:732–738
Pauchet Y, Luton F, Castella C, Charles JF, Romey G, Pauron D (2005) Effects of a mosquitocidal toxin on a mammalian epithelial cell line expressing its target receptor. Cell Microbiol 7:1335–1344
Paul A, Harrington LC, Zhang L, Scott JG (2005) Insecticide resistance in Culex pipiens from New York. J Am Mosq Control Assoc 21:305–309
Pei G, Oliveira CM, Yuan Z, Nielsen-Leroux C, Silva-Filha MH, Yan J, Regis L (2002) A strain of Bacillus sphaericus causes slower development of resistance in Culex quinquefasciatus. Appl Environ Microbiol 68:3003–3009
Pérez C, Fernandez LE, Sun J, Folch JL, Gill SS, Soberón M, Bravo A (2005) Bacillus thuringiensis subsp. israelensis Cyt1Aa synergizes Cry11Aa toxin by functioning as a membrane-bound receptor. Proc Natl Acad Sci U S A 102:18303–18308
Pérez C, Muñoz-Garay C, Portugal LC, Sánchez J, Gill SS, Soberón M, Bravo A (2007) Bacillus thuringiensis ssp. israelensis Cyt1Aa enhances activity of Cry11Aa toxin by facilitating the formation of a pre-pore oligomeric structure. Cell Microbiol 9:2931–2937
Pigott CR, Ellar DJ (2007) Role of receptors in Bacillus thuringiensis crystal toxin activity. Microbiol Mol Biol Rev 71:255–281
Pocquet N, Darriet F, Zumbo B, Milesi P, Thiria J, Bernard V, Toty C, Labbe P, Chandre F (2014) Insecticide resistance in disease vectors from Mayotte: an opportunity for integrated vector management. Parasit Vectors 7:299
Rao DR, Mani TR, Rajendran R, Joseph AS, Gajanana A, Reuben R (1995) Development of a high level of resistance to Bacillus sphaericus in a field population of Culex quinquefasciatus from Kochi. India J Am Mosq Control Assoc 11:1–5
Regis L, Silva-Filha MH, Nielsen-Leroux C, Charles JF (2001) Bacteriological larvicides of dipteran disease vectors. Trends Parasitol 17:377–380
Rinkevich FD, Du Y, Dong K (2013) Diversity and convergence of sodium channel mutations involved in resistance to pyrethroids. Pestic Biochem Physiol 106:93–100
Rodcharoen J, Mulla MS (1994) Resistance development in Culex quinquefasciatus to the microbial agent Bacillus sphaericus. J Econ Entomol 87:1133–1140
Rodcharoen J, Mulla MS, Chaney JD (1991) Microbial larvicides for the control of nuisance aquatic midges (Diptera: Chironomidae) inhabiting mesocosms and man-made lakes in California. J Am Mosq Control Assoc 7:56–62
Rodrigues IB, Tadei WP, Dias JM (1999) Larvicidal activity of Bacillus sphaericus 2362 against Anopheles nuneztovari, Anopheles darlingi and Anopheles braziliensis (Diptera, Culicidae). Rev Inst Med Trop Sao Paulo 41:101–105
Romão TP, De Melo Chalegre KD, Key S, Ayres CF, Fontes De Oliveira CM, De-Melo-Neto OP, Silva-Filha MH (2006) A second independent resistance mechanism to Bacillus sphaericus binary toxin targets its alpha-glucosidase receptor in Culex quinquefasciatus. FEBS J 273:1556–1568
Romão TP, De-Melo-Neto OP, Silva-Filha MH (2011) The N-terminal third of the BinB subunit from the Bacillus sphaericus binary toxin is sufficient for its interaction with midgut receptors in Culex quinquefasciatus. FEMS Microbiol Lett 321:167–174
Schwartz JL, Potvin L, Coux F, Charles JF, Berry C, Humphreys MJ, Jones AF, Bernhart I, Dalla Serra M, Menestrina G (2001) Permeabilization of model lipid membranes by Bacillus sphaericus mosquitocidal binary toxin and its individual components. J Membr Biol 184:171–183
Silva Filha MHNL, Peixoto CA (2003) Immunocytochemical localization of the Bacillus sphaericus toxin components in Culex quinquefasciatus (Diptera: Culicidae) larvae midgut. Pestic Biochem Physiol 77:138–146
Silva Filha MHNL, Berry C, Regis LN (2014) Lysinibacillus sphaericus: toxins and mode of action, applications for mosquito control and resistance management. In: Dhadialla TS, Gill SS (eds) Insect midgut and insecticidal proteins. Academic Press, Oxford, pp 89–176
Silva-Filha MH, Nielsen-Leroux C, Charles JF (1997) Binding kinetics of Bacillus sphaericus binary toxin to midgut brush-border membranes of Anopheles and Culex sp. mosquito larvae. Eur J Biochem 247:754–761
Silva-Filha MH, Nielsen-Leroux C, Charles JF (1999) Identification of the receptor for Bacillus sphaericus crystal toxin in the brush border membrane of the mosquito Culex pipiens (Diptera: Culicidae). Insect Biochem Mol Biol 29:711–721
Silva-Filha MHNL, Chalegre KD, Anastacio DB, Oliveira CMF, Silva SB, Acioli RV, Hibi S, Oliveira DC, Parodi ESM, Marques Filho CAM, Furtado AF, Regis L (2008) Culex quinquefasciatus field populations subjected to treatment with Bacillus sphaericus did not display high resistance levels. Biol Control 44:227–234
Sinègre G, Babinot M, Vigo G, Jullien JL (1994) First occurrence of Culex pipiens resistance to Bacillus sphaericus in Southern France. VIII European Meeting of Society of Vector Ecology 5–8 September 1994. Faculty of Biologia. University of Barcelona Spain, Barcelona
Singh GJ, Gill SS (1988) An electron microscope study of the toxic action of Bacillus sphaericus in Culex quinquefasciatus larvae. J Invertebr Pathol 52:237–247
Singkhamanan K, Promdonkoy B, Srikhirin T, Boonserm P (2013) Amino acid residues in the N-terminal region of the BinB subunit of Lysinibacillus sphaericus binary toxin play a critical role during receptor binding and membrane insertion. J Invertebr Pathol 114:65–70
Soberón M, Fernández LE, Pérez C, Gill SS, Bravo A (2007) Mode of action of mosquitocidal Bacillus thuringiensis toxins. Toxicon 49:597–600
Srisucharitpanit K, Yao M, Promdonkoy B, Chimnaronk S, Tanaka I, Boonserm P (2014) Crystal structure of BinB: a receptor binding component of the binary toxin from Lysinibacillus sphaericus. Proteins 82:2703–2712
Stalinski R, Laporte F, Tetreau G, Despres L (2016) Receptors are affected by selection with each Bacillus thuringiensis israelensis Cry toxin but not with the full Bti mixture in Aedes aegypti. Infect Genet Evol 44:218–227
Tangsongcharoen C, Chomanee N, Promdonkoy B, Boonserm P (2015) Lysinibacillus sphaericus binary toxin induces apoptosis in susceptible Culex quinquefasciatus larvae. J Invertebr Pathol 128:57–63
Tetreau G, Bayyareddy K, Jones CM, Stalinski R, Riaz MA, Paris M, David JP, Adang MJ, Despres L (2012) Larval midgut modifications associated with Bti resistance in the yellow fever mosquito using proteomic and transcriptomic approaches. BMC Genomics 13:248
Tetreau G, Stalinski R, David JP, Despres L (2013a) Increase in larval gut proteolytic activities and Bti resistance in the dengue fever mosquito. Arch Insect Biochem Physiol 82:71–83
Tetreau G, Stalinski R, David JP, Despres L (2013b) Monitoring resistance to Bacillus thuringiensis subsp. israelensis in the field by performing bioassays with each Cry toxin separately. Mem Inst Oswaldo Cruz 108:894–900
Torres-Martinez M, Rubio-Infante N, Garcia-Hernandez AL, Nava-Acosta R, Ilhuicatzi-Alvarado D, Moreno-Fierros L (2016) Cry1Ac toxin induces macrophage activation via ERK1/2, JNK and p38 mitogen-activated protein kinases. Int J Biochem Cell Biol 78:106–115
Vachon V, Laprade R, Schwartz JL (2012) Current models of the mode of action of Bacillus thuringiensis insecticidal crystal proteins: a critical review. J Invert Pathol 111:1–12
Vasquez MI, Violaris M, Hadjivassilis A, Wirth MC (2009) Susceptibility of Culex pipiens (Diptera: Culicidae) field populations in Cyprus to conventional organic insecticides, Bacillus thuringiensis subsp. israelensis, and methoprene. J Med Entomol 46:881–887
Wirth MC (2010) Mosquito resistance to bacterial larvicidal proteins. Open J Toxicol 3:101–115
Wirth MC, Georghiou GP, Malik JI, Abro GH (2000) Laboratory selection for resistance to Bacillus sphaericus in Culex quinquefasciatus (Diptera: Culicidae) from California. USA J Med Entomol 37:534–540
Wirth MC, Ferrari JA, Georghiou GP (2001) Baseline susceptibility to bacterial insecticides in populations of Culex pipiens complex (Diptera: Culicidae) from California and from the Mediterranean Island of Cyprus. J Econ Entomol 94:920–928
Yuan ZM, Zhang YM, Liu EY (2000) High-level field resistance to Bacillus sphaericus C3-41 in Culex quinquefasciatus from southern China. Biocontrol Sci Tech 10:43–51
Zahiri NS, Mulla MS (2003) Susceptibility profile of Culex quinquefasciatus (Diptera: Culicidae) to Bacillus sphaericus on selection with rotation and mixture of B. sphaericus and B. thuringiensis israelensis. J Med Entomol 40:672–677
Zhang X, Candas M, Griko NB, Taussig R, Bulla LA Jr (2006) A mechanism of cell death involving an adenylyl cyclase/PKA signaling pathway is induced by the Cry1Ab toxin of Bacillus thuringiensis. Proc Natl Acad Sci U S A 103:9897–9902
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Silva-Filha, M.H.N.L. (2017). Resistance of Mosquitoes to Entomopathogenic Bacterial-Based Larvicides: Current Status and Strategies for Management. In: Fiuza, L., Polanczyk, R., Crickmore, N. (eds) Bacillus thuringiensis and Lysinibacillus sphaericus. Springer, Cham. https://doi.org/10.1007/978-3-319-56678-8_15
Download citation
DOI: https://doi.org/10.1007/978-3-319-56678-8_15
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56677-1
Online ISBN: 978-3-319-56678-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)