Abstract
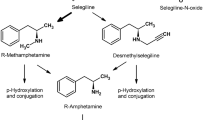
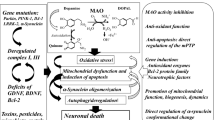
Monoamine oxidases (MAO)-A and MAO-B catalyze the oxidative deamination of monoamine neurotransmitters, such as dopamine (DA), noradrenaline, and serotonin, in the central and peripheral nervous system. Parkinson’s disease (PD) is an aging-related movement disorder, caused by a deficiency of the neurotransmitter DA in the striatum of the brain, caused by degeneration of the nigrostriatal DA neurons. During the1960s, L-DOPA, a direct precursor of DA, which is synthesized in vivo from tyrosine in DA neurons by tyrosine hydroxylase and is converted to DA by aromatic L-amino acid decarboxylase, was introduced to treat this DA deficiency in the striatum. In addition to L-DOPA as a treatment, MAO-B inhibitors (MAO-B-Is) have been used since the 1970s, first selegiline (L-(-)-deprenyl), then rasagiline, and more recently safinamide, as an effective therapy for PD by preventing the degradation of DA. Furthermore, monotherapy with MAO-B-I, selegiline, rasagiline, or safinamide has been proved to be effective in the case of early PD. Accumulating data suggest that MAO-B-Is may also have neuroprotective efficacy due to several mechanisms that may or may not be related to MAO inhibition. DA oxidation and formation of misfolded α-synuclein oligomers may be linked to dysfunctions of mitochondria, the autophagy-lysosomal system, and ubiquitin-proteasome system, resulting in DA neuron death in PD; and MAO-I may prevent these processes to afford neuroprotection. However, many clinical and basic studies have suggested, but not yet convincingly proved, neuroprotective effects of MAO-I in PD. It remains to be proved if the administration of MAO-B-I several decades before the onset of PD could prevent the occurrence of PD based on neuroprotection and, if so, to confirm the molecular mechanism involved.
Similar content being viewed by others
Abbreviations
- AADC:
-
Aromatic L-amino acid decarboxylase
- ALDH:
-
Aldehyde dehydrogenase
- DA:
-
Dopamine
- DOPAC:
-
3,4-Dihydroxyphenylacetic acid
- DOPAL:
-
3,4-Dihydroxyphenylacetaldehyde
- L-DOPA:
-
L-3,4-Dihydroxyphenylalanine/levodopa
- MAO:
-
Monoamine oxidase
- MAO-I:
-
MAO inhibitor
- MPP+:
-
1-Methyl-4-phenyl-pyridinium
- MPTP:
-
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- PD:
-
Parkinson’s disease
- ROS:
-
Reactive oxygen species
- TH:
-
Tyrosine hydroxylase
- VMAT2:
-
Vesicular monoamine transporter 2
References
Allain H, Pollak P, Neukirch HC. Members of the French Selegiline multicenter trial. Symptomatic effect of selegiline in de novo Parkinsonian patients. Mov Disord. 1993;8(Suppl 1):S36–40.
Ando T, Chock PB, Murphy DL, Chiueh CC. Role of the redox protein thioredoxin in cytoprotective mechanism evoked by (-)-deprenyl. Mol Pharmacol. 2005;68:1408–14.
Bar-Am O, Amit T, Kupershmidt L, Aluf Y, Mechlovich D, Kabha H, Danovitch L, Zurawski VR, Youdim MB, Weinreb O. Neuroprotective and neurorestorative activities of a novel iron chelator-brain selective monoamine oxidase-A/monoamine oxidase-B inhibitor in animal models of Parkinson’s disease and aging. Neurobiol Aging. 2015;36:1529–42.
Betarbet R, Sherer TB, Mackenzie G, Garcia-Osuna M, Panov AV, Greenmyre T. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–6.
Birkmayer W, Hornykiewicz O. The L-3,4-Dioxyphenylalanin (DOPA)-effect in Parkinson-akinesie. Wien Klin Wochenschr. 1961;73:787–8.
Birkmayer W, Riederer P, Youdim MB, Linauer W. The potential of the anti akinetic effect after L-dopa treatment by an inhibitor of MAO-B, Deprenil. J Neural Transm. 1975;36:303–26.
Birkmayer W, Knoll J, Riederer P, Youdim M, Hars V, Marton A. Increased life expectancy resulting from addition of L-deprenyl to madopar® treatment in Parkinson’s disease: a long term study. J Neural Transm. 1985;64:113–27.
Bonifati V, Rizzu P, van Baren MJ, Schaap OJ, Breedveld GJ, Krieger E, Dekker MCJ, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Sweten JC, Brice A, Meco G, van Duijin CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–9.
Braga CA, Follmer C, Palhano FL, Khattar E, Freitas MS, Romano FL, Khattar E, Freitas MS, Romão L, Giovanni SD, Lashuel HA, Silva JL, Foguel D. The anti-Parkinsonian drug selegiline delays the nucleation phase of alpha-synuclein aggregation leading to the formation of nontoxic species. J Mol Biol. 2011;405:254–73.
Burbulla LF, Song P, Mazzulli R, Zampese E, Wang YC, Jeon S, Santos DP, Blanz J, Obermair CD, Strojny C, Savas JN, Kiskinis E, Zhuang X, Krüger R, Sumeier DJ, Krainc D. Dopamine oxidation mediates mitochondrial and lysosomal dysfunction in Parkinson’s disease. Science. 2017; https://doi.org/10.1126/science.aam9080.
Casida JE, Ford B, Jinsinama Y, Sullivan P, Cooney A, Goldstein DS. Benomyl, aldehyde dehydrogenase, DOPAL, and catecholaldehyde hypothesis for the pathogenesis of Parkinson’s disease. Chem Res Toxicol. 2014;27:1359–61.
Cereda E, Cilia R, Canesi M, Tesei S, Mariani CB, Zecchinelli AL, Pezzoli G. Efficacy of rasagiline and selegiline in Parkinson’s disease: a head-to-head 3-year retrospective case-control study. J Neurol. 2017;264:1254–63.
Chen K, Shih JC. Monoamine oxidase A and B: structure, function, and behavior. Adv Pharmacol. 1998;42:292–6.
Chen L, Xie Z, Turkson S, Zhuang X. A57T human α-synuclein overexpression in transgenic mice induces pervasive mitochondria macroautophagy defects producing dopamine neuron degeneration. J Neuirosci. 2015;35:890–905.
Chung JY, Lee JW, Ryu CH, Min HK, Yoon YJ, Lim MJ, Park CH. 1-[2-(4-Benzyloxyphenoxy)ethyl]imidazole inhibits monoamine oxidase B and protects against neuronal loss and behavioral impairment in rodent model of Parkinson’s disease. J Neurosci Res. 2015;93:1267–78.
Collins MA, Neafsey EJ. Beta-carboline analogues of N-methyl-4-phenyl-1,2,5,6-tetrahydropyridine (MPTP): endogenous factors underlying idiopathic parkinsonism? Neurosci Lett. 1985;55:179–84.
Collins MA, Neafsey EJ. Beta-carboline analogues of MPP+ as environmental neurotoxins. In: Storch A, Collins MA, editors. Neurotoxic factors in Parkinson’s disease and related disorders. New York: Kluwer Academic Publishing/Plenum; 2000. p. 115–30.
Cotzias GC, Papavasiliou PS, Gellene R. Modification of parkinsonism – chronic treatment with L-DOPA. N Engl J Med. 1969;280:337–45.
Danielson SR, Held JM, Schilling B, Oo M, Gibson BW, Anderson JK. Preferentially increased nitration of alpha-synuclein at tyrosine-39 in a cellular oxidative model of Parkinson’ disease. Anal Chem. 2009;81:7823–8.
Dexter DT, Wells FR, Agid F, Agid Y, Lees AJ, Jenner P, Marsden CD. Increased nigral iron content in postmortem parkinsonian brain. Lancet. 1987;330:1219–20.
Ehringer H, Hornykiewicz O. Verteilung von Noradrenalin und Dopamin (3-Hydroxytyramin) im Gehirn des Menschen und ihr Verhalten bei Erkrankungen des Extrapyramidalen Systems. Klin Wochenschr. 1960;38:1236–9.
Fahn S. The medical treatment of Parkinson disease from James Parkinson to George Cotzias. Mov Disord. 2015;30:331–49.
Fitzmaurice AG, Rhodes SL, Lulla A, Murphy NP, Lam HA, O’Donnel KC, Bamhill L, Casida JE, Cockbaurn M, Segasti A, Stahl MC, Maidment NT, Ritz B. Aldehyde dehydrogenase inhibition as a pathogenic mechanism in Parkinson disease. Prod Natl Acad Sci USA. 2013;110:636–41.
Fowler JS, Volkow ND, Wang GJ, Pappas N, Shea C, MacGregor RR. Visualization of monoamine oxidase in human brain. Adv Pharmacol. 1998;42:304–7.
Goldstein DS, Holmes C, Kopin IJ, Sharabi Y. Intraneuronal vesicular uptake of catecholamines is decreased in patients with Lewy body diseases. J Clin Invest. 2011;121:3320–30.
Goldstein DS, Sullivan P, Holmes C, Miller GW, Alter S, Strong R, Mash DC, Kopin IJ, Sharabi Y. Determination of the toxic dopamine metabolite DOPAL in Parkinson’s disease. J Neurochem. 2013;126:591–603.
Goldstein DS, Jinsmaa Y, Sullivan P, Holmes C, Kopin IJ, Sharabi Y. Comparison of monoamine oxidase inhibitors in decreasing production of the autotoxic dopamine metabolite 3,4-dihydorxyphenylactaldehyde in PC 12 cells. J Pharmacol Exp Therap. 2016;356:483–92.
Grünblatt E, Riederer P. Aldehyde dehydrogenase (ALDH) in Alzheimer’s and Parkinson’s disease. J Neutral Transm. 2016;123:83–90.
Hara MR, Thomas B, Cascio MB, Bae B, Hester LD, Dawson VL, Dawson TM, Sawa A, Snyder S. Neuroprotection by pharmacologic blockade of the GAPDH death signal. Proc Natl Acad Sci USA. 2006;103:3887–9.
Hattori N, Mizuno Y. Twenty years since the discovery of the parkin gene. J Neural Transm. 2017;124:1037–54.
Hauser RA, Li R, Pérez A, Ren X, Weintraub D, Elm J, Goudreau JL, Morgan JC, Fang JY, Aminoff MJ, Christine CW, Dhall R, Umeh CC, Boyd JT, Stover N, Leehey M, Zweig RM, Nicholas AP, Bodis-Wollner I, Willis A, Kieburz K, Tilley BC. Longer duration of MAO B inhibitor exposure is associated with clinical decline in Parkinson’s disease. J Park Dis. 2017;7:117–27.
Hirsch EC, Brandel JP, Galle P, Javoy-Agid F, Agid Y. Iron and aluminum increase in the substantia nigra of patients with Parkinson’s disease: an X-ray microanalysis. J Neurochem. 1991;56:446–51.
Hirsch EC, Vyas S, Hunot S. Neuroinflammation in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:S210–2.
Imamura K, Nishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol. 2003;106:518–26.
Imamura K, Nishikawa N, Ono K, Suzuki H, Sawada M, Nagatsu T, Yoshida M, Hashizume Y. Cytokine production of activated microglia and decrease in neurotrophic factors of neurons in the hippocampus of Lewy body disease brains. Acta Neuropathol. 2005;109:141–50.
Ives NJ, Stowe RL, Marro J, Counsell C, Macleod A, Clarke CE, Gray R, Wheatley K. Monoamine oxidase type B inhibitors in early Parkinson’s disease: meta-analysis of 17 randomized trials involving 3525 patients. Brit Med J. 2004;329:593–59.
Jellinger K, Kienzl E, Rumpelmair G, Riederer P, Stachelberger H, Ben-Shachar D, Youdim MBH. Iron-melanin complex in substantia nigra of parkinsonian brains: an X-ray microanalysis. J Neurochem. 1992;59:1168–71.
Jiang H, Wang J, Rogers J, Xie J. Brain iron metabolism dysfunction in Parkinson’s disease. Mol Neurobiol. 2017;54:3078–101.
Kim T-I, Mao X, Park H, Chou S-C, Karuppagounder SS, Umanah GE, Yun SP, Brahmachari S, Panicker N, Chen R, Andrabi SA, Qi C, Poirier GG, Pletnikova O, Troncoso JC, Bekris LM, Leverenz JB, Pantelyat A, Ko HS, Rosenthal LS, Dawson TM, Dawson VL. Poly(ADP-ribose) drives pathologic α-synuclein neurodegeneration in Parkinson’s disease. Science. 2018;362:557.
Kitada T, Asakawa S, Hattori N, Matsumine H, Yamamura Y, Minoshima S, Yokochi M, Mizuno Y, Shimizu N. Mutation in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–8.
Kotake Y, Tasaki Y, Hirobe M, Ohta S. Deprenyl decreases an endogenous parkinsonism-inducing compound, 1-benzyl-1,2,3,4-tetrahydroisoquinoline, in mice: in vivo and in vitro studies. Brain Res. 1998;787:341–3.
Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–80.
Larsen JP, Boas J, Erdal JK. The Norwegian-Danish study group. Does selegiline modify the progression of early Parkinson’s disease? Results from a five-year study. Eur J Neurol. 1999;6:539–47.
Le W. Role of iron in UPS impairment model of Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(Suppl 1):S158–61.
Liu G, Yu J, Ding J, Xie C, Sun L, Rudenko I, Zheng W, Sastry N, Luo J, Rudow G, Troncoso JC, Cai H. Aldehyde dehydrogenase 1 defines and protects a nigrostriatal dopaminergic neuron subpopulation. J Clin Invest. 2014;124:3032–46.
Lloyd KG, Davidson L, Hornykiewicz O. The neurochemistry of Parkinson’s disease: effect of L-DOPA therapy. J Pharmacol Exp Therap. 1975;153:453–64.
Mallajosyula JK, Kaur D, Chinta SJ, Rajagopalan S, Rane A, Nicholls DG, Di Monte DA, Macarthur H, Andersen JK. MAO B elevation in mouse brain astrocytes results in Parkinson’s pathology. PLoS One. 2008;3:e1616.
Maruyama W, Takahashi T, Naoi M. (-)-Deprenyl protects human dopaminergic neuroblastoma SH-SY5Y cells from apoptosis induced by peroxynitrate. J Neurochem. 1998;70:2510–5.
Matsubara K. N-methyl-beta-carbonium neurotoxins in Parkinson’s disease. In: Storch A, Collins MA, editors. Neurotoxic factors in Parkinson’s disease and related disorders. New York: Kluwer Academic Publishing/Plenum; 2000. p. 115–30.
McGeer EG, McGeer PL. The role of anti-inflammatory agents in Parkinson’s disease. CNS Drugs. 2007;20:351–7.
McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s disease and Alzheimer’s disease brain. Neurology. 1988;38:1285–91.
Michel PP, Hirsch EC, Hunot S. Understanding dopaminergic cell death pathways in Parkinson disease. Neuron. 2016;90:678–91.
Mizuno Y, Ohta S, Tanaka M, Takamiya S, Suzuki K, Sato T, Oya H, Ozawa T, Kagawa Y. Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem Biophys Res Commun. 1989;163:1450–5.
Mizuno Y, Kondo T, Kuno S, Nomoto M, Yanagisawa N. Early addition of selegiline to L-DOPA treatment is beneficial for patients with Parkinson disease. Clin Neuropharmacol. 2010;33:1–3.
Mizuta I, Ohta M, Ohta K, Nishimura M, Mizuta E, Hayashi K, Kuno S. Selegiline and desmethylselegiline stimulate NGF, BDNF, and GDNF synthesis in cultured mouse astrocytes. Biochem Biophys Res Commun. 2000;279:751–5.
Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-alpha (TNF-alpha) increases in the brain and in the cerebrospinal fluid from parkinsonian patients. Neurosci Lett. 1994a;165:208–10.
Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994b;180:147–50.
Mogi M, Togari A, Kondo T, Mizuno Y, Komure O, Kuno S, Ichinose H, Nagatsu T. Brain-derived growth factor and nerve growth factor concentrations are decreased in the substantia nigra in Parkinson’s disease. Neurosci Lett. 1999;270:45–8.
Müller T, Riederer P, Grünblatt E. Simultaneous determination of MAO-A and -B activity following first time intake of an irreversible MAO-B inhibitor in patients with Parkinson’s disease. J Neural Transm. 2017;124:745–8.
Myllylä VV, Sotaniemi KA, Hakulinen P, Mäki-Ikola O, Heinonen EH. Selegiline as the primary treatment of Parkinson’s disease – a long-term double blind study. Acta Neurol Scand. 1997;95:211–8.
Mytilineou C, Leonardi EK, Radcliffe P, Heinonen EH, Han S, Werner P, Cohen G, Olanow W. Deprenyl and desmethylselegiline protect mesencephalic neurons from toxicity induced by glutathione depletion. J Pharmacol Exp Therap. 1998;284:700–6.
Nagatsu T. Amine-related neurotoxins in Parkinson’s disease: past, present, and future. Neurotoxicol Teratol. 2002;24:565–9.
Nagatsu T, Nagatsu I. Tyrosine hydroxylase (TH), its cofactor tetrahydrobiopterin (BH4), other catecholamine-related enzymes, and their human genes in relation to the drug and gene therapies of Parkinson’s disease (PD): historical overview and future prospects. J Neural Transm. 2016;123:1255–78.
Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm Des. 2005;11:999–1016.
Nagatsu T, Sawada M. Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: possible implication of glial cells. J Neural Transm. 2006a;Suppl 71:53–65.
Nagatsu T, Sawada M. Cellular and molecular mechanisms of Parkinson’s disease: neurotoxins, causative genes, and inflammatory cytokines. Cell Mol Neurobiol. 2006b;26:781–802.
Nagatsu T, Kato T, Numata Y, Ikuta K, Sano M, Nagatsu I, Kondo Y, Inagaki S, Iizuka R, Hori A, Narabayashi H. Phenylethanolamine N-methyltransferase activity and other enzymes of catecholamine metabolism in human brain. Clin Chim Acta. 1977;75:221–31.
Nagatsu T, Mogi M, Ichinose H, Togari A. Cytokines in Parkinson’s disease. J Neural Trnasm. 2000;Suppl 58:143–51.
Nakao K, Nakamura C, Sato H, Imamura K, Takeshima T, Nakashima K. Novel cytoprotective mechanism of anti-parkinsonian drug deprenyl: PI3K and Nrf2-derived induction of antioxidative proteins. Biochem Biophys Res Commun. 2006;339:915–22.
Naoi M, Maruyama Y, Dostert P, Hashizume Y, Nakahara D, Takahashi T, Ota M. Dopamine-derived endogenous 1(R), 2 (N)-dimethyl-1,2,3,4-tetrahydroisoquinoline, N-methyl-(R)-salsolinol, induced parkinsonism in rats: biochemical, pathological and behavioral studies. Brain Res. 1996;709:285–95.
Ono K, Hirohata M, Yamada M. Anti-fibrillogenic and fibril-destabilizing activities of anti-parkinsonian agents for alpha-synuclein fibrils in vitro. J Neurosci Res. 2007;85:1547–57.
Ozaki N, Nakahara D, Mogi M, Harada M, Kiuchi K, Kaneda N, Miura Y, Kasahara Y, Nagatsu T. Inactivation of tyrosine hydroxylase in rat striatum by 1-methyl-4-phenylpyridinium iron (MPP+). Neurosci Lett. 1988;85:228–32.
Paisan-Ruiz C, Jain S, Evans EW, Gilks WP, Simon J, van der Brug M, de Munain AL, Aparicio S, Gil AM, Khan N, Johnson J, Martinez JR, Nicholl D, Carrera IM, Pena AS, de Silva R, Lees A, Marti-Masso JF, Perez-Tur J, Wood NW, Singleton AB. Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron. 2004;44:595–600.
Pålhagen S, Heinonen E, Hägglund J, Kaugesaar T, Mäki-Ikola O, Palm R. The Swedish Parkinson study group. Selegiline slows the progression of the symptoms of Parkinson disease. Neurology. 2006;66:1200–6.
Parkinson Study Group. Effect of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med. 1993;328:176–83.
PD Med Collaborative Group, Gray R, Ives N, Rick C, Patel S, Gray A, Jenkinson C, McIntosh E, Wheatley K, Williams A, Clarke CE. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson’s disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. 2014;384: 1196–205.
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Deheijia A, Dutra A, Pike B, Root H, Robenstein J, Boyer R, Stenrous ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoiosin RC, DiIorio G, Golbe LI, Nussbaum RL. Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–7.
Przuntek H, Conrad B, Dichgans J, Kraus PH, Krauseneck P, Pergande G, Rinne U, Schimrigk K, Schnitker J, Vogel HP. SELEDO: a 5-year long-term trial on the effect of selegiline in early parkinsonian patients treated with levodopa. Eur J Neurol. 1999;6:141–50.
Riederer P. MAO inhibitors and selegiline. In: Nagatsu T, Takahashi A, Yanagisawa N, Mizuno Y, Kondo T, Takahashi R, Mezaki T, Riederer P, Riederer C, editors. From east to west: pioneers in Parkinson’s disease in Japan. Tokyo: QOL Laboratory Corp; 2014. p. 36–7.
Riederer P, Berg D, Casadei N, Cheng F, Classen J, Dresel C, Jost W, Krüger R, Müller T, Reichmann H, Rieß O, Storch A, Strobel S, van Eimeren T, Völker HU, Winkler J, Winklhofer KF, Wüllner U, Zunke F, Monoranu CM. alpha-Synuclein in Parkinson's disease: causal or bystander? J Neural Transm. 2019;126:815–840.
Robakis D, Fahn S. Defining the role of the monoamine oxidase-B inhibitors for Parkinson’s disease. CNS Drugs. 2015;29:433–41.
Sánchez-Danés A, Richaud-Patin Y, Carballo-Carbajal I, Jiménez-Delgado S, Caig C, Mora S, Di Guglielmo C, Ezglielmo C, Ezguerra M, Patel B, Gilalt A, Canals JM, Memo M, Auberch J, López-Barneo J, Vila M, Cuervo AM, Tolsa E, Consiglic A, Raya A. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med. 2012;4:380–95.
Schapira AHV, Cooper JM, Dexter D, Jenner P, Clark JB, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. Lancet. 1989;1:1269.
Shimura H, Hattori N, Kubo S, Mizuno Y, Asakawa S, Minoshima S, Shimizu N, Imai K, Chiba T, Tanaka K, Suzuki T. Familial Parkinson gene product, parkin, is a ubiquitin-protein ligase. Nat Genet. 2000;25:302–5.
Shoulson I, Oakes D, Fahn S, Lang A, Langston JW, LeWitt P, Olanow CW, Penney JB, Tanner C, Kieburtz K, Rudolph A, the Parkinson Study Group. Impact of sustained deprenyl (selegiline) in levodopa-treated Parkinson’s disease: a randomized placebo-controlled extension of the deprenyl and tocopherol antioxidative therapy of Parkinsonism trial. Ann Neurol. 2002;51:604–12.
Singh N, Haldar S, Tripathi AK, McElwee KK, Horback K, Beserra A. Iron in neurodegenerative disorders of protein misfolding: a case of prion disorders and Parkinson’s. Antioxid Redox Signal. 2014;21:471–84.
Sofic E, Riederer P, Heisen H, Bechmann H, Reynolds GP, Hebenstreit G, Youdim MBH. Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm. 1988;74:199–205.
Tang YP, Ma YL, Chao CC, Chen KY, Lee EHI. Enhanced glial cell line-derived neurotrophic factor mRNA expression upon (-)-deprenyl and melatonin treatments. J Neurosci Res. 1998;53:593–604.
Tatton WG, Wadia JS, Ju WYH, Chalmers-Redman RMA, Tatton NA. (-)-Deprenyl reduces neuronal apoptosis and facilitates neuronal outgrowth by altering protein synthesis without inhibiting monoamine oxidase. J Neural Trnsm. 1996;48:45–59.
Tetrud JW, Langston JW. The effect of deprenyl (selegiline) on the natural history of Parkinson’s disease. Science. 1989;245:519–22.
Valente EM, Abou-Sleiman PM, Caputo V, Muqit MMK, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science. 2004;304:1158–60.
Woodard CM, Campos BA, Kuo S-H, Nirenberg MJ, Nestor MW, Zimmer M, Mosharov E, Sulzer D, Zhou H, Paul D, Clark L, Schadt EE, Sardi SP, Rubin L, Eggan K, Brock M, Lipnick S, Rao M, Chang S, Li S, Noggle S. iPS cell derived dopamine neurons reveal differences between monozygotic twins discordant for Parkinson’s disease. Cell Rep. 2014;9:1173–82.
Youdim MB, Fridkin M, Zheng H. Bifunctional drug derivatives of MAO B inhibitor rasagiline and iron chelator VK-28 rasagiline as a more effective approach to treatment of brain aging and aging neurodegenerative diseases. Mech Aging Dev. 2005;126:317–26.
Zhao YJ, Wee HL, Au WL, Seah SH, Luo N, Li SC, Tan LCS. Selegiline use is associated with a slower progression in early Parkinson’s disease as evaluated by Hoehn and Yahr stage transition times. Parkinsonism Relat Disord. 2011;17:194–7.
Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessel AJ, Pfeiffer RF, Patenge N, Carbajal IC, Viergge P, Asmus F, Mueller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44:601–7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
Nagatsu, T., Nakashima, A. (2020). Monoamine Oxidase Inhibitor (MAO-I)-Mediated Neuroprotection for Treating Parkinson’s Disease. In: Riederer, P., Laux, G., Mulsant, B., Le, W., Nagatsu, T. (eds) NeuroPsychopharmacotherapy. Springer, Cham. https://doi.org/10.1007/978-3-319-56015-1_238-2
Download citation
DOI: https://doi.org/10.1007/978-3-319-56015-1_238-2
Received:
Accepted:
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-56015-1
Online ISBN: 978-3-319-56015-1
eBook Packages: Springer Reference MedicineReference Module Medicine
Publish with us
Chapter history
-
Latest
Monoamine Oxidase Inhibitor (MAO-I)-Mediated Neuroprotection for Treating Parkinson’s Disease- Published:
- 10 April 2020
DOI: https://doi.org/10.1007/978-3-319-56015-1_238-2
-
Original
Monoamine Oxidase Inhibitor (MAO-I)-Mediated Neuroprotection for Treating Parkinson’s Disease- Published:
- 19 November 2019
DOI: https://doi.org/10.1007/978-3-319-56015-1_238-1