Abstract
Reproduction is a key step of a species life history and includes a suite of strategies and tactics enacted to allow the maximization of reproductively active offspring in relation to available energy and parental life expectancy. Gender system, oogenesis pattern, maturation schedule, shifts in habitat utilization, spawning seasonality, mating behavior, and fertilization pattern are among the traits involved in the process of species adaptive optimization of reproduction.
In this frame, here we review the reproductive traits of the Antarctic silverfish (Pleuragramma antarctica) based on the available macroscopic and histological data. Then, we will step forward by focusing on two aspects of this species’ reproduction: (i) skipped spawning; (ii) potential location of reproduction sites along the Antarctic coasts.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
It is widely accepted that reproduction encompasses a whole suite of strategies which enable the species to maximize its reproductive output in relation to the available energy and parental care (Murua and Saborido-Rey 2003). The optimization of these two components is achieved through an adaptive process fine tuning reproductive traits such as the gender system, oogenesis pattern, maturation schedule, shifts in habitat utilization, spawning seasonality, mating behaviour, and fertilization pattern. A thorough understanding of the reproductive characteristics of a species is paramount not only for understanding its biology and ecology, but also for management purposes. Here we focus on the reproductive features of the Antarctic notothenioid Pleuragramma antarctica (Antarctic silverfish), a dominant fish in high-Antarctic shelf and slope waters (Duhamel et al. 2014) where the species plays a similar key role in the food web as does krill in the Seasonal Pack-ice Zone (SPZ).
The evolution and adaptation of Antarctic notothenioids is closely linked to the opening of the Drake Passage and the subsequent cooling and other changes that occurred (Eastman 1993). The next 20 million years provided ample time to shape many aspects of their biology and ecology, including reproduction.
Antarctic notothenioids share a number of reproductive traits such as low fecundity, large eggs (size spanning between 2 and 5 mm), prolonged gametogenesis, and group-synchronous oocyte maturation (e.g. Kock and Kellermann 1991; Shandikov and Faleeva 1992; Calvo et al. 1999; Kock 2005; La Mesa et al. 2003, 2007, 2008; Parker and Grimes 2010). Despite the fact that they share common features of their reproduction on the one hand, notothenioids exhibit a high diversity in reproductive strategies. For example, most Antarctic notothenioids lay demersal eggs (e.g. Kock and Kellermann 1991; Kock and Everson 1997; van der Molen and Matallanas 2004; Kock 2005) that, in a number of species, are deposited in nests and guarded (e.g. Kock et al. 2006; Jones and Near 2012; Ferrando et al. 2014). Others lay pelagic eggs, which drift in the upper part of the water column up to several months (e.g. Yukhov 1982; Kock and Kellermann 1991).
Here, we review the reproductive traits of the Antarctic silverfish. We focus on two aspects of their reproductive strategy: (i) skipping annual spawning when unfavourable conditions prevail; (ii) identifying locations where the species are known to spawn and/or potentially spawn along the Antarctic shelves and slope.
2 Reproductive Traits of Antarctic Silverfish
In his monography of Antarctic fish, Andriashev (1965) compiled what was known on the Antarctic silverfish until the mid-1960s. Reproduction was one of the biological characteristics of which little beyond that spawning occurred apparently in austral winter and larvae were first recorded from October–December was known (Regan 1916; DeWitt and Tyler 1960).
The Soviet Union started commercial fishing in the Southern Ocean in the late 1960s. Soviet scouting and commercial fishing vessels were fishing close to the Antarctic continent from the second half of the 1970s. Aggregations of the Antarctic silverfish were discovered in the northern Weddell Sea, in the Prydz Bay and Ross Sea in the late 1970s by FRV Mys Yunony (Shust 1998). Comparatively little was published in the accessible Soviet scientific literature at that time. These exploratory fishing cruises confirmed that the species was apparently one of the most abundant fish species in the high-Antarctic. In particular, the abundance and biomass of this fish in the north-western part of the Weddell Sea was considered as very high. In 1985, the relative biomass of P. antarctica, even in the less productive central area of the Weddell Sea, was 300 kg/km2 (Shust 1998). Some data on catches of P. antarctica by Soviet trawlers during exploratory fishing reported in CCAMLR documents can be found in Koubbi et al. (2017).
The species is drawn to greater depths than other plankton-feeders, and adult P. antarctica are generally found at depths beyond 300 m (Sosinski and Skora 1979; Shust et al. 1984; Ekau 1990).
German Antarctic expeditions, conducted between 1979 and 1981 to the Weddell Sea down to the Filchner Shelf Ice, found that Antarctic silverfish larvae made up to 95% of the plankton biomass obtained from net catches. Of particular interest was the occurrence of larvae between 8 and 12.5 mm standard length in January–February, which reinforced Andriashev’s assumption of a winter spawning of the species (Hubold 1984). The presence of silverfish eggs in the stomach of the benthic nototheniid Trematomus pennellii (Ekau et al. 1987) first suggested that eggs may be deposited on the bottom (Kellermann 1986). However, the very thin membrane of eggs, and significant yolk deposits, made pelagic eggs more likely. The finding of early embryonated eggs under the fast ice in September (Ghigliotti et al. 2015), and their location in the upper part of the platelet ice, close to the solid ice, as found later in the season (Guidetti et al. 2015), confirmed that eggs were positively buoyant, and floating in their latest stage of development before hatching.
Unfavorable weather and ice conditions during the winter period have thus far limited year-round sampling of adult individuals which could provide an unambiguous determination of when and where Antarctic silverfish spawn. For the time being histological methods are considered to be the only techniques which could provide further insight into the process and cycle of gonad maturation.
A time series of 10 year of data collected during austral summer (December–April) to East Antarctica (Cosmonauts, Commonwealth and Mawson Seas) allowed Faleeva and Gerasimchuk (1987, 1990) to refine information on length and age at sexual maturity, which had previously been overestimated due to the limitation of macroscopic observations. Furthermore, their investigations provided evidence that in early autumn the Antarctic silverfish gonads are still far from pre-spawning condition, supporting the notion of a winter or even winter/spring spawning (June to September).
The presence of sometimes large numbers of oocytes in regression state some 2–3 months prior to spawning suggests that a proportion of Antarctic silverfish females does not spawn annually and that the process of gonad maturation can be interrupted in case of unfavorable environmental conditions, thus saving energy for survival (Faleeva and Gerasimchuk 1990). Later studies extending the geographic range of such investigation by including the Antarctic Peninsula (La Mesa et al. 2015a) and Ross Sea (Ghigliotti et al. 2017) confirmed the presence of readsorbing follicles sporadically present or massively occurring in the ovaries.
2.1 Macroscopical Observations
The Antarctic silverfish is a gonochoristic dimorphic species. Males are characterized by on average smaller size, and pelvic fin which are longer than in females (Gerasimchuk 1987a) (please note that the spelling might change according to the Russian or Ukrainian transliteration as Gerasimchuk or Herasymchuk, respectively).
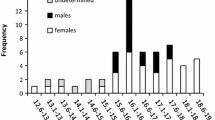
Differences between sexes have also been detected in the length at which specimens reach sexual maturity (Table 9.1) that is smaller in males and larger in females. [1] Gerasimchuk (1992); [2] Duhamel et al. 1993.
In the early austral summer, the gonadosomatic index (GSI) of sexually mature individuals, a widely used proxy of gonad development that expresses gonad weight as a percentage of total body weight, is low (ranging from 1 to 2% in both sexes). The GSI is slowly increasing towards the end of summer but in early autumn it is still well below 15–30%, usually found in females of many nototheniids and channichthyids immediately prior to spawning (Kock and Kellermann 1991; Duhamel et al. 1993) (Table 9.2 and references therein).
In low-Antarctic nototheniids and channichthyids, there appears to be a trend in the same species of increasing egg size and decreasing relative fecundity towards higher latitudes (Kock and Kellermann 1991), i.e. the same species generally produces more eggs at South Georgia than in the South Shetland Islands. Antarctic silverfish do not appear to follow that trend (Hubold 1992). Although not as high as in some Patagonian and low-Antarctic nototheniid species (e.g. Brickle et al. 2006), relative fecundity in Antarctic silverfish is fairly high (Table 9.3), and coupled with a relatively small egg diameter of about 2 mm.
2.2 Histological Data
Progress and timing of gonad maturation occurs in the three investigated areas, East Antarctica (Faleeva and Gerasimchuk 1990), Antarctic Peninsula (La Mesa et al. 2015b), and Ross Sea Region (Ghigliotti et al. 2017) at a similar time of the year. Within the 4 months of the austral summer, males pass through two stages: a resting stage is observed early in the season with inactive, sometimes dividing, spermatogonia at the periphery of the seminal gland, residual spermatozoids, and epithelium desquamation in proximal part of the testicular tubule. Active spermatogenesis then occurs the further summer progresses. It is characterized by intensive spermatogonial proliferation and emergence of primary spermatocytes. Limiting investigations to visual observation of testicles often resulted in over-estimation of maturity stage given the comparatively large size of the testis. Testis has often been found to occupy the rear 2/3 or the entire abdominal cavity and to be turbid white and yellowish. Nevertheless, spermatogenesis completion and emergence of spermatozoids had never been observed.
As notothenioids in general (see Shandikov and Faleeva 1992), females of Antarctic silverfish close to spawning are characterized by having group synchronous ovaries which consists of a batch of eggs maturing and ovulate in the current season, and a second batch of previtellogenic much smaller oocytes representing the next season’s spawn. Oocytes in the primary growth stage are homogeneous in size. A large nucleus occupies most of the cell surrounded by a thin strongly basophilic layer of cytoplasm (Fig. 9.1a). They are regularly occurring along with a cohort of oocytes in various stages of trophoplasmatic growth. Three distinct stages of vitellogenic cells have been observed: initial alveolus (IA), cortical alveolus (CA), and vitellogenic (Vtg). The cells are round with a thin acidophilic layer at the periphery of the cytoplasm (the future zona radiata) beneath the follicle. The nucleus is spherical in the IA cells (Fig. 9.1b), and becomes irregularly shaped, with niches along its wall, in the CA (Fig. 9.1c) and Vtg (Fig. 9.1d) cells. A large number of basophilic nucleoli are accumulated at the periphery of the nucleus; small grainy basophilic, not marginated, nucleoli are sometimes interspersed in the nucleoplasm or circularly displaced around the central area of the nucleus. Vesicles start to be accumulated at the periphery of the cytoplasm, in a few rows (IA), many rows (CA) or multiple rows occupying almost the entire cytoplasm (Vtg). In the vitellogenic oocytes, yolk granules start to be deposited from the periphery of the oocyte toward the centrally located nucleus.
Antarctic silverfish ovary. Cross sections of regular and reabsorbing follicles, hematoxylin-eosin stained: primary growth oocytes (a), initial alveolus oocyte (b), cortical alveolus stage oocyte (c), vitellogenic oocyte (d), post-ovulatory follicle (e), alpha atretic follicles (f). Scale bar = 100 μm
In January and February post ovulatory follicles (POFs), reaching 1600–1800 μm in size, were occasionally observed in the ovaries (Faleeva and Gerasimchuk 1990; Ghigliotti et al. 2017). They are clearly recognizable as having completely homogenized yolk, reduced lumen, convoluted shape and deep folded membrane (Fig. 9.1e).
The presence of developing follicles undergoing resorption (atretic follicles) is reported from the majority of females exhibiting maturing gonads from East Antarctica (Faleeva and Gerasimchuk 1990), a few females from the Antarctic Peninsula (La Mesa et al. 2015a), and a large number of maturing females from the Ross Sea Region (Ghigliotti et al. 2017). In most cases, the atretic process is reported to be in its initial phase (alpha atresia): oocytes shrinking in size, distorted follicles, disorganization of the cytoplasm in the oocyte, fragmentation of the chorion with cytoplasm flowing out, deformation and sometimes dissolution of the nucleus are among the most commonly described features (Fig. 9.1f).
3 Skipped Spawning: A Reproductive Strategy in the Antarctic Silverfish?
Field observations to be conducted year-round close to the Antarctic continent are still extremely difficult, dangerous, and cost-expensive. In particular, winter investigations suffer from these constraints. The limitations in mind, the available information nevertheless allows to develop a hypothesis with respect to the reproductive strategy which is probably followed by Antarctic silverfish.
Spawning is likely to occur in austral winter and early spring (July to end of August, beginning of September). This hypothesis is supported by the fact that both male and female gonads are developing but still far from spawning in late summer. The presence of newly hatched larvae in November (e.g. Hubold 1984; Vacchi et al. 2012) provides additional support for winter spawning of the Antarctic silverfish. The presence of large numbers of atretic follicles, also known as mass ovarian atresia, in the months leading up to spawning suggest that Antarctic silverfish are able to skip the process of gonad maturation when unfavorable living conditions are encountered. This may be interpreted as a tactic in order not to jeopardize survival of the fish.
A number of observations in recent years suggest that in other Antarctic fish species, such as mackerel icefish at South Georgia, 10–20% of the fish do not spawn each year. In years when feeding conditions were poor such as in those years when few krill was transported to South Georgia, up to 60% of mackerel icefish are able to interrupt the maturation process of the ovaries in order to save energy to sustain their living (Kock and Everson 2003). Skip spawning has been hypothesized for a number of Patagonian, Sub-Antarctic, and Antarctic species, Patagonian toothfish Dissostichus eleginoides (Arana 2009), the Antarctic toothfish D. mawsoni (Parker and Grimes 2010), the blackfin icefish Chaenocephalus aceratus (Vanella et al. 2005), the mackerel icefish Champsocephalus gunnari (Kock and Kellermann 1991; Kock and Everson 2003), and the pike icefish C. esox (Calvo et al. 1999).
Over evolutionary time, skipped spawning occasionally enacted when unfavorable environmental and physiological conditions prevail, can be interpreted as a manifestation of phenotypic plasticity in a species, in a wider adaptive sense. In other words, the capability to skip spawning, demonstrated at individual level, can be viewed as a facet of the overall specific reproductive strategy influencing the reproductive potential of fish populations and thus enhancing the species fitness (Jorgensen et al. 2006; Rideout and Tomkiewicz 2011; Shaw and Levin 2011).
The Antarctic silverfish is suggested to perform energy costly spawning migrations from open waters to ice-laden coastal waters where they were born or where environmental conditions are similar to those experienced at the larval stage following a homing-behaviour (Koubbi et al. 2011). In such a scenario, the capability to down-regulate the oocytes development through follicular atresia would reduce individual reproductive investment during breeding migration, at annual scale, but would result in a long-term adaptive advantage for the populations (Ghigliotti et al. 2017).
4 Reproduction Areas: Evidences and Hypotheses
There is sufficient evidence now to support the hypothesis that Antarctic silverfish reproduction occurs in coastal areas along major continental ice shelves (e.g. Faleeva and Gerasimchuk 1990; Eastman 1993; Hubold 1990; Kellermann 1987; La Mesa et al. 2010; Moline et al. 2008; Vacchi et al. 2012). However, running ripe females and males have never been sampled. Nevertheless, information is available on known and potential nursery areas of early larvae. They can serve as a proxy for the location of spawning grounds. In its wider sense, the term “nursery area” is used to define those regions or habitat where larvae, post-larvae and early juveniles of a given species occur and reside in significant amounts, with a substantial potential to contribute to the renewal of the adult fish population (Beck et al. 2001). Given the diversity in the life histories and developing times of the various species, a nursery area in its wide definition does not necessarily coincide with the place where reproduction occurs (Harden-Jones 1968). Being Antarctic fish characterized by a protracted larval development, in order to limit vagueness we will use the term “nursery area” in its strict sense, considering nursery grounds only areas where larvae up to 25 mm SL (0+ age class) have been recorded. Records of larger larvae, post-larvae, and juveniles, although important for reconstructing the life history of the Antarctic silverfish, have been excluded from our analysis because of the high spatial dispersal of these stages (La Mesa et al. 2010).
Early stages of silverfish, namely embryonated eggs or 0+ larvae (up to 25 mm SL), have been recorded in various locations around the Antarctic continent. In order to attempt a rough quantitative comparison among localities, the abundance of early life stages of Antarctic silverfish available in the scientific literature are ranked into four categories (see Table 9.4 and pertaining legend). By coupling the presence of the various Antarctic silverfish early stages and their abundance, each of the locations has been assigned to one of the following typology: (i) Hatching Area (HA, area where hatching eggs have been recorded), (ii) Likely Hatching Area (LHA, area in which eggs have never been found but just hatched larvae have been found abundant), (iii) Nursery Grounds (NG, area where early larval stages are occurring in large or very large numbers) and (iv) Likely Nursery Grounds (LNG, where silverfish larvae up to 25 mm SL were recorded in limited amount).
Terra Nova Bay (TNB), in the Ross Sea, is the only area from where embryonated eggs and newly hatched larvae have been recorded. In TNB, Antarctic silverfish embryonated eggs have been found floating in large quantities among platelet ice under the sea-ice in September (Ghigliotti et al. 2015). Mass hatching has directly been observed in November (Vacchi et al. 2004, 2012; Guidetti et al. 2015). In the same areas, early larvae (7–9 mm SL) have been recorded in abundance in the following summer months (Guglielmo et al. 1998; Granata et al. 2000). This makes TNB both a hatching area (HA) and a nursery ground (NG) and strongly supports the notion that mass spawning occurs in the northern part of the Ross Sea. The finding, during several annual surveys, of adult silverfish remains trapped in the sea-ice in the TNB nursery area is a further strong indication for spawning in the northern Ross Sea (Vacchi et al. 2012). Alternative hypotheses are that eggs are spawned close to the sea-ice and remain in a challenging environment for all the embryonic development period, or dispatched in deeper ice-free water and then rise in the water column later on owing to their buoyancy and local hydrodynamic flows (Vacchi et al. 2012).
Although lower in numbers, larvae had been collected on several occasions in southern parts of the Ross Sea. P. antarctica early larvae were collected at Ross Island during the “Discovery” and “Terra Nova” Expeditions (Regan 1916). Early larvae were collected in McMurdo Sound during spring and summer months (DeWitt and Tyler 1960; Hopkins 1987; Outram 2000). Recently, several newly-hatched larvae, which were genetically assigned to Pleuragramma, were sampled in mid-March in the Bay of Whales, eastern Ross Sea (Brooks and Goetz 2014; Caccavo et al. 2015). This finding, together with the occurrence of a number of 10 mm long larvae, previously reported by Biggs (1982) in the same area, strongly suggests the potential role of the Bay of Whales in the eastern Ross Sea as a nursery ground for this species.
In the D’Urville Sea region, annual surveys carried out from 1996 to 2010 (Koubbi et al. 1997, 2009, 2011) provided data to identify a second nursery ground for Pleuragramma. The Terre Adélie coastal area (Pierre Lejay Bay) and seabed depression adjacent to the Mertz Glacier Tongue and Commonwealth Bay hosted P. antarctica larvae in large numbers with mean size of 17.3 and 13.8 mm SL, respectively. At Pierre Lejay Bay a dense aggregation of Antarctic silverfish larvae, extending about 1.8 by 1.5 nautical miles, was noticed. Such an aggregation was made of at least 17/m3 individuals, while densities in the remainder of the study area were on average lower than one individual/m3 (Koubbi et al. 1997).
In other regions of the Indian Sector, an interesting record on the mass occurrence of early-stage Pleuragramma in Wilhelm II coast (Davis Sea) came from the Heroic Age of the Antarctic Expeditions around the turn to the Twentieth century. From 22 November to 1 December 1902 more than one thousand Pleuragramma larvae of about 20 mm TL were taken by fine-meshed tow-nets at the German Gauss Station (Pappenheim 1912). These large density suggest the presence of another nursery ground in the Davis Sea.
A survey carried 1991 in the Prydz Bay region (Cooperation Sea) identified another nursery ground. In 93 near-surface tows (30–50 m depth) of 30 min duration with the International Young Gadoid Pelagic Trawl (IYGPT, P. antarctica constituted the most important part of the catch. Up to 9100 individuals/30 min haul occurred in the central part of the bay. Most of the fishes were age class I juveniles of 45 mm SL but a noticeable part of the catch was also represented by Pleuragramma larvae of 15 mm SL (Williams 1991). A recent extensive survey in the pelagic zone of the Cosmonauts Sea and Prydz Bay (Van de Putte et al. 2010) confirmed the occurrence of Antarctic silverfish fingerlings of age class 2+ (54–95 mm SL) and larvae of age class 0+ in coastal waters of Prydz Bay, although in low numbers.
The Weddell Sea region has been studied in some detail for the presence and abundance of ichthyonekton since 1979/1980 (Hempel et al. 1983; Kellermann 1986; Hubold and Ekau 1987; Ekau et al. 1987; Piatkowski 1987; Boysen-Ennen and Piatkowski 1988; Hubold 1990; Piatkowski et al. 1990; White and Piatkowski 1993). Larval P. antarctica typically predominated shelf waters of the southern and southeastern Weddell Sea (Keller 1983; Hubold 1985, 1990) representing some 90% of the fish larvae in summer (Hubold 1990; Piatkowski et al. 1990). P.antarctica larvae ranging 8–16 mm SL in size, were aggregating in the upper 25–50 m of the water column over the continental slope and innershelf depressions located in the southern Weddell Sea off the Filchner Ice Shelf and in the south eastern part of Weddell Sea in January–February (Hubold 1984, 1985). The density of larvae peaked at 3000–4000 individuals/1000 m3 (Keller 1983) and supports the hypothesis that P. antarctica hatched near Vestkapp by mid-November (Hubold 1990), making this zone a likely hatching area (LHA) for the species.
A large number of ichthyoplankton surveys were performed in the western Antarctic Peninsula (wAP) since 1975/1976 (Kellermann 1986; Kellermann and Schadwinkel 1991, Kellermann and Kock 1988; Loeb 1991; Morales-Nin et al. 1995; Donnelly and Torres 2008; Jones et al. 2014; Ross et al. 2014; Parker et al. 2015; Mintenbeck and Torres 2017). Typically, the number of fish larvae up to 25 mm SL was low, ranging from zero to about one hundred individuals in the catches (higher numbers of more developed postlarvae and juveniles were routinely collected).
At least, three sites of wAP (Bransfield Strait, Gerlache Strait, and Marguerite Bay) can be considered as LNGs. In these wAP areas, the larval density is generally 10 times less than in the Weddell Sea. A substantial decline has occurred in the northernmost location of larvae after 1999–2000 (Ross et al. 2014). Despite the occurrence of substantial numbers of larvae, it remains unclear so far if Antarctic silverfish spawn successfully in the wAP region. Recruitment of Pleuragramma to the waters of wAP shelf has been attributed to larval dispersal from spawning occurring in the western Weddell Sea (Kellermann 1986; La Mesa et al. 2015b) and the Bellinghausen Sea, southwest of the wAP (Kellermann and Schadwinkel 1991). A LHA could be located in the northwestern Weddell Sea. Larvae originating from this area could be transported from the Weddell Sea via the Weddell gyre/Antarctic Coastal Current as it flows through the Antarctic Sound and around the tip of the Antarctic Peninsula into the eastern Bransfield Strait (La Mesa et al. 2015b). Larvae originating from the other LHAs in the Bellinghausen Sea south of the Peninsula could be drifted northeast by the Antarctic Circumpolar Current merging with the general northeasterly flow at the shelf break along the Peninsula (Mintenbeck and Torres 2017).
The resulting distribution of hatching areas, likely hatching areas, nursery areas and likely nursery areas along the coasts of Antarctica is shown in Fig. 9.2.
Hubold (1984) related the retention of developing eggs and young stages in the Weddell Sea to the presence of polynyas that are known to act as hydrographic engines, and that are widespread along the Antarctic coast (e.g. Kern 2009). However, no evidences are available to date that might confirm or reject this interesting hypothesis.
5 Conclusive Considerations
Scientific knowledge on the reproduction cycle of the Antarctic silverfish has improved in the past 30 years, with most of the information originating from the Ross Sea. In Fig. 9.3 the year-round events characterizing the Antarctic silverfish reproduction in the Ross Sea are shown along a timeline.
Besides illustrating actual information, Fig. 9.3 also highlights areas where knowledge are still lacking. Indeed, the time of spawning is still approximate, but it is likely to occur from July to September. Moreover, the occurrence of small larvae as late as February–March may suggest that spawning is extending over a comparatively long period of several months. Eggs are pelagic but if they are released in surface waters or close to the sea-floor from where they may rise later is still unknown. Logistic constraints have historically limited year-round field activity, however future scenarios in this regard are encouraging.
For instance, newly established permanent Antarctic Stations are expected to provide new opportunities for Antarctic silverfish winter sampling, such as the new Korean Jang Bogo Station in the Ross Sea, in close vicinity of the Silverfish Bay – Cape Washington hatching area, that already offered the possibility to extend backwards to September the sampling of embryonated eggs (Ghigliotti et al. 2015).
In addition, advances in the logistic capabilities of research vessels, by allowing to operate year-round in ice-covered waters, could further improve both a geographical and temporal extension of researches focusing on the Antarctic silverfish life cycle and reproduction.
New tools for non-invasive, spatial and temporal extensive surveys have been developed in recent years improving researches by visual and acoustic methods (Azzali et al. 2010; O’Driscoll et al. 2011; Fox 2015; Guidetti et al. 2015). The definition of Antarctic silverfish adults acoustic target strength (Azzali et al. 2010), and the improvement of acoustic technology in general (reviewed in O’Driscoll et al. 2017), provides new possibilities for recording silverfish presence, abundance and movements over a wide time frame.
On the whole, the availability of new infrastructure for all-year-round research in the Southern Ocean, progress in remote sensing and image processing technology offer the possibility to collect data in areas and periods previously unattainable, laying the bases to step forward in the knowledge of the Antarctic silverfish reproductive strategies.
References
Andriashev AP (1965) A general review of the Antarctic fish fauna. In: van Mieghem J, van Oye P (eds) Biogeography and ecology in Antarctica. Springer, Dordrecht, pp 491–550. doi:10.1007/978-94-015-7204-0_15
Arana P (2009) Reproductive aspects of the Patagonian toothfish (Dissostichus eleginoides) off southern Chile. Lat Am J Aquat Res 37:381–394. doi:10.3856/vol37-issue3-fulltext-9
Azzali M, Leonori I, Biagiotti I et al (2010) Target strength studies on Antarctic silverfish Pleuragramma antarcticum in the Ross Sea. CCAMLR Sci 17:75–104
Beck MW, Heck KL, Able KW et al (2001) The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. Bioscience 51:633–641
Biggs DC (1982) Zooplankton excretion and NH 4 + cycling in near-surface waters of the Southern Ocean. I. Ross Sea, austral summer 1977–78. Polar Biol 1:55–67
Boysen-Ennen E, Piatkowski U (1988) Meso- and macrozooplankton communities in the Weddell Sea, Antarctica. Polar Biol 9:17–55
Brickle P, Laptikhovsky V, Arkhipkin A et al (2006) Reproductive biology of Patagonotothen ramsayi (Regan, 1913) (Pisces: Nototheniidae) around the Falkland Islands. Polar Biol 29:570–580. doi:10.1007/s00300-005-0090-5
Brooks C, Goetz K (2014) Pleuragramma antarcticum distribution in the Ross Sea during late austral summer 2013. In: CCAMLR Working Group on Ecosystem Monitoring and Management WG-EMM 14/61
Caccavo JA, Brooks C, Zane L et al (2015) Identification of Pleuragramma antarctica larvae in the Ross Sea via mitochondrial DNA. In: CCAMLR Working Group on Fish Stock Assessment WG-FSA 15/38
Calvo J, Morriconi E, Rae GA (1999) Reproductive biology of the icefish Champsocephalus esox (Günther, 1861) (Channichthyidae). Antarct Sci 11:140–149. doi:10.1017/S0954102099000206
DeWitt HH, Tyler JC (1960) Fishes of the Stanford Antarctic biological research program, 1958–1959. Stanf Ichthyol Bull 7:162–199
Donnelly J, Torres JJ (2008) Pelagic fishes in the Marguerite Bay region of the West Antarctic Peninsula continental shelf. Deep-Sea Res II 55:523–539
Duhamel G, Kock K-H, Balguerias E et al (1993) Reproduction in fish of the Weddell Sea. Polar Biol 13:193–200
Duhamel G, Hulley P-A, Causse R et al (2014) Biogeographic patterns of fish. In: De Broyer C, Koubbi P, Griffiths HJ et al (eds) Biogeographic atlas of the Southern Ocean. Scientific Committee on Antarctic Research, Cambridge, pp 328–362
Eastman JT (1993) Antarctic fish biology: evolution in a unique environment. Academic, San Diego
Ekau W (1990) Demersal fish fauna of the Weddell Sea, Antarctica. Antarct Sci 2:129–137
Ekau W, Hubold G, Wöhrmann A (1987) Fish and fishlarvae. Berichte zur Polarforschung 39:210–218
Faleeva TI, Gerasimchuk VV (1987) Issues on the reproductive biology of Antarctic silverfish (in Russian). Abstracts of II All-Union Conference “Natural resources of the Southern Ocean and the problems of their rational exploitation.”, Kerch, pp 131–132
Faleeva TI, Gerasimchuk VV (1990) Reproduction biology peculiarities of Antarctic silverfish, Pleuragramma antarcticum (Nototheniidae). Quest Ichthyology 30:67–79 (in Russian)
Ferrando S, Castellano L, Gallus L et al (2014) A demonstration of nesting in two Antarctic icefish (genus Chionodraco) using a fin dimorphism analysis and ex situ videos. PLoS One 9(3):e90512. doi:10.1371/journal.pone.0090512
Fox D (2015) Fish live beneath Antarctica. Nat Sci Am. doi:10.1038/nature.2015.16772
Gerasimchuk VV (1987a) On the fecundity of the Antarctic sidestripe, Pleurogramma antarcticum. Voprosy Ikhtiologii 5:858–860
Gerasimchuk VV (1987b) Biology and fishing prospects of Antarctic silverfish Pleuragramma antarcticum (Pisces: Nototheniidae) in adjacent seas of the Indian sector of the Southern Ocean. Dissertation, All Union Research Institute of Marine Fisheries and Oceanography
Gerasimchuk VV (1992) A brief outline of the biology of the Antarctic silverfish, Pleuragramma antarcticum Boulenger, 1902 (Nototheniidae) from the Antarctic Indian Ocean. In: Working document CCAMLR WG-FSA-92/11, 47
Ghigliotti L, Pisano E, Carlig E et al (2015) Towards an all year round monitoring the Antarctic silverfish nursery area in the Ross sea. CCAMLR WG-FSA-15/58
Ghigliotti L, Ferrando S, Carlig E et al (2017) Reproductive features of the Antarctic silverfish (Pleuragramma antarctica) from the Western Ross Sea. Polar Biol 40:199–211. doi:10.1007/s00300-016-1945-7
Granata A, Guglielmo L, Greco S et al (2000) Spatial distribution and feeding habits of larval and juvenile Pleuragramma antarcticum in the western Ross Sea (Antarctica). In: Faranda F, Guglielmo L, Ianora A (eds) Ross Sea ecology. Springer, Berlin, pp 369–393
Guglielmo L, Granata A, Greco S (1998) Distribution and abundance of postlarval and juvenile Pleuragramma antarcticum (Pisces, Nototheniidae) off Terra Nova Bay (Ross Sea, Antarctica). Polar Biol 19:37–51. doi:10.1007/s003000050214
Guidetti P, Ghigliotti L, Vacchi M (2015) Insights into spatial distribution patterns of early stages of the Antarctic silverfish, Pleuragramma antarctica, in the platelet ice of Terra Nova Bay, Antarctica. Polar Biol 38:333–342. doi:10.1007/s00300-014-1589-4
Harden-Jones FR (1968) Fish migration. Edward Arnold, London
Hempel G, Hubold G, Kaczmaruk B et al (1983) Distribution of some groups of zooplankton in the inner Weddell Sea in summer 1979/80. Ber Polarforsch 9:1–35
Hopkins TL (1987) Midwater food web in McMurdo Sound, Ross Sea, Antarctica. Mar Biol 96:93–106
Hubold G (1984) Spatial distribution of Pleuragramma antarcticum (Pisces: Nototheniidae) near the Filchner- and Larsen Ice Shelves (Weddell Sea Antarctica). Polar Biol 3:231–236
Hubold G (1985) The early life history of the high Antarctic Silverfish, Pleuragramma antarcticum. In: Siegfried WR, Condy PR, Laws RM (eds) Antarctic nutrient cycles and food webs. Springer-Verlag, Berlin, pp 445–451
Hubold G (1990) Seasonal patterns of ichthyoplankton distribution and abundance in the southern Weddell Sea. In: Kerry KR, Hempel G (eds) Antarctic ecosystems ecological change and conservation. Springer-Verlag, Heidelberg, pp 149–158
Hubold G (1992) Zur Ökologie der Fische im Weddellmeer. Rep Polar Res 103:1–157
Hubold G, Ekau W (1987) Midwater fish fauna of the Weddell Sea, Antarctica. In: Proceedings of the 5th Congress of European Ichthyologists, Stockholm 1985, pp 391–396
Jones CD, Near TJ (2012) The reproductive behaviour of Pogonophryne scotti confirms widespread egg-guarding parental care among Antarctic notothenioids. J Fish Biol 80:2629–2635. doi:10.1111/j.1095-8649.2012.03282.x
Jones CD, Koubbi P, Catalano B et al (2014) Mesopelagic and larval fish survey. NOAA Techn Mem NMFS SWFSC 524:28–40
Jorgensen C, Ernande B, Fiksen Ø et al (2006) The logic of skipped spawning in fish. Can J Fish Aquat Sci 63:200–211
Keller R (1983) Contributions to the early life history of Pleuragramma antarcticum Boul. 1902 (Pisces, Notothenioidei) in the Weddell Sea. Meeresforschung 30:10–24
Kellermann A (1986) On the biology of early life stages of notothenioid fishes (Pisces) off the Antarctic Peninsula. Ber Polarforsch 31:1–149
Kellermann A (1987) Food and feeding ecology of postlarval and juvenile Pleuragramma antarcticum (Pisces; Notothenioidei) in the seasonal pack ice zone off the Antarctic Peninsula. Polar Biol 7:307–315
Kellermann A, Kock KH (1988) Patterns of spatial and temporal distribution and their variation in early life stages of Antarctic fish in the Antarctic Peninsula region. In: Sahrhage D (ed) Antarctic Ocean and resources variability. Springer, Berlin, pp 147–159
Kellermann A, Schadwinkel S (1991) Winter aspects of the ichthyoplankton community in Antarctic Peninsula waters. Polar Biol 11:117–127
Kern S (2009) Wintertime Antarctic coastal polynya area: 1992–2008. Geophys Res Lett 36:2009. doi:10.1029/2009GL038062
Kock KH (2005) Antarctic icefishes (Channichthyidae): a unique family of fishes. A review. Polar Biol 28:862–895. doi:10.1007/s00300-005-0019-z
Kock KH, Everson I (1997) Biology and ecology of mackerel icefish, Champsocephaeus gunnari: an Antarctic fish lacking hemoglobin. Comp Biochem Physiol 118:1067–1077
Kock KH, Everson I (2003) Shedding new light on the life cycle of mackerel icefish in the Southern Ocean. J Fish Biol 63:1–21
Kock KH, Kellermann A (1991) Reproduction in Antarctic notothenioid fish – a review. Antarct Sci 3:125–150. doi:10.1017/S0954102091000172
Kock KH, Pshenichnov LK, DeVries AL (2006) Evidence for egg brooding and parental care in icefish and other notothenioids in the Southern Ocean. Antarct Sci 18:223–227. doi:10.1017/S0954102006000265
Koubbi P, Hureau JC, Vacchi M et al (1997) Results of the preliminary survey on the coastal distribution of fish larvae in Adelie land (Southern Ocean) during January–February 1996. Cybium 21:381–392
Koubbi P, Duhamel G, Hecq JH et al (2009) Ichthyo-plankton in the neritic and coastal zone of Antarctica and Subantarctic islands: a review. J Mar Syst 78:547–556
Koubbi P, O’Brien C, Loots C et al (2011) Spatial distribution and inter-annual variations in the size frequency distribution and abundances of Pleuragramma antarcticum larvae in the Dumont d’Urville Sea from 2004 to 2010. Polar Sci 5:225–238
Koubbi P, Grant S, Ramm D et al (2017) Conservation and management of Antarctic silverfish Pleuragramma antarctica populations and habitats. In: Vacchi M, Pisano E, Ghigliotti L (eds) The Antarctic silverfish, a keystone species in a changing ecosystem. Advances in Polar Ecology 3, 10.1007/978-3-319-55893-6_13
La Mesa M, Caputo V, Rampa R et al (2003) Macroscopic and histological analyses of gonads during the spawning season of Chionodraco hamatus (Pisces, Channichthyidae) off Terra Nova Bay, Ross Sea, Southern Ocean. Polar Biol 26:621–628. doi:10.1007/s00300-003-0519-7
La Mesa M, Caputo V, Eastman JT (2007) Gametogenesis in the dragonfishes Akarotaxis nudiceps and Bathydraco marri (Pisces, Notothenioidei: Bathydraconidae) from the Ross Sea. Antarct Sci 19:64–70. doi:10.1017/S0954102007000090
La Mesa M, Caputo V, Eastman JT (2008) The reproductive biology of two epibenthic species of Antarctic nototheniid fish of the genus Trematomus. Antarct Sci 20:355–364
La Mesa M, Catalano B, Russo A et al (2010) Influence of environmental conditions on spatial distribution and abundance of early life stages of Antarctic silverfish, Pleuragramma antarcticum (Nototheniidae), in the Ross Sea. Antarct Sci 22:243–254
La Mesa M, Riginella E, Mazzoldi C et al (2015a) Reproductive resilience of ice-dependent Antarctic silverfish in a rapidly changing system along the Western Antarctic Peninsula. Mar Ecol 36:235–245. doi:10.1111/maec.12140
La Mesa M, Piñones A, Catalano B et al (2015b) Predicting early life connectivity of Antarctic silverfish, an important forage species along the Antarctic Peninsula. Fish Oceanogr 24:150–161
Loeb V (1991) Distribution and abundance of larval fishes collected in the western Bransfield Strait region, 1986–87. Deep Sea Res Part A 38:1251–1260
Mintenbeck K, Torres JJ (2017) Impact of climate change on the Antarctic silverfish and its consequences for the Antarctic ecosystem. In: Vacchi M, Pisano E, Ghigliotti L (eds) The Antarctic silverfish, a keystone species in a changing ecosystem. Advances in Polar Ecology 3, doi:10.1007/978-3-319-55893-6_12
Moline MA, Karnovsky NJ, Brown Z et al (2008) High latitude changes in ice dynamics and their impact on polar marine ecosystems. Ann N Y Acad Sci 1134:267–319
Morales-Nin B, Palomera I, Schadwinkel S (1995) Larval fish distribution and abundance in the Antarctic Peninsula region and adjacent waters. Polar Biol 15:143–154
Murua H, Saborido-Rey F (2003) Female reproductive strategies of marine fish and their classification in the North Atlantic. J Northwest Atl Fish Sci 33:23–31
O’Driscoll RL, Macaulay GJ, Gauthier S et al (2011) Distribution, abundance and acoustic properties of Antarctic silverfish (Pleuragramma antarcticum) in the Ross Sea. Deep-Sea Res II 58:181–195
O’Driscoll RL, Leonori I, De Felice A et al (2017) Acoustic methods of monitoring Antarctic silverfish distribution and abundance. In: Vacchi M, Pisano E, Ghigliotti L (eds) The Antarctic silverfish, a keystone species in a changing ecosystem. Advances in Polar Ecology 3, 10.1007/978-3-319-55893-6_11
Outram D (2000) Spatial and temporal variability in early growth rates of the Antarctic silverfish, Pleuragramma antarcticum, around the Antarctic continent. MSc thesis, Moss Landing Marine Laboratories California State University, Stanislaus, USA
Pappenheim P (1912) Die Fische tier Deutschen Südpolar-Expedition 1901–1903. 1. Die Fische der Antarktis und Subantarktis. Dtsch Südpol Exp 1901–1903, Zool:162–184
Parker SJ, Grimes PJ (2010) Length and age at spawning of Antarctic toothfish (Dissostichus mawsoni) in the Ross Sea. CCAMLR Sci 17:53–73
Parker ML, Fraser WF, Ashford JR et al (2015) Assemblages of micronektonic fishes and invertebrates in a gradient of regional warming along the Western Antarctic Peninsula. J Mar Syst 152:18–41
Piatkowski U (1987) Zoogeographical investigations and community analysis on Antarctic macroplankton. Ber Polarforsch 34:73–81
Piatkowski U, White MG, Dimmler W (1990) Micronekton of the Weddell Sea: distribution and abundance. Ber Polarforsch 68:73–81
Regan CT (1916) Larval and postlarval fishes. 1. Antarctic and subantarctic fishes. Nat Hist Rep Br Antarct “Terra Nova” Exp 1910 Zool 1:125–156
Rideout RM, Tomkiewicz J (2011) Skipped spawning in fishes: more common than you might think. Mar Coast Fish 3:176–189
Ross RM, Quetin LB, Newberger T et al (2014) Trends, cycles interannual variability for three pelagic species west of the Antarctic Peninsula 1993–2008. Mar Ecol Prog Ser 515:11–32
Shandikov GA, Faleeva TI (1992) Features of gametogenesis and sexual cycles of six notothenioid fishes from East Antarctica. Polar Biol 11:615–621. doi:10.1007/BF00237956
Shaw AK, Levin SA (2011) To breed or not to breed: a model of partial migration. Oikos 120:1971–1879
Shust KV (1998) Fish and fishery resources of Antarctica. Ph.D. thesis, Moscow, Russia, 163 pp (in Russian)
Shust KV, Parfenovich SS, Gerasimchuk VV (1984) Distribution of the Antarctic silverfish (Pleuragramma antarcticum Boulenger) in relation to oceanographical conditions within its area of habitat and in relation to biographical characteristics of the species. Mhysl, Moscow (in Russian)
Sinque C, Koblitz S, Costa LM (1986) Ichthyoplankton of Bransfield Strait-Antarctica. Neritica, Pontal do Sul, PR 1:91–102
Sosinski J, Skora KE (1979) Nowe gatunki ryb przemyslowych rejonu antarktyki. Biul MorskInst Ryb 54(4):12–15
Vacchi M, La Mesa M, Dalù M et al (2004) Early life stages in the life cycle of Antarctic silverfish, Pleuragramma antarcticum in Terra Nova Bay, Ross Sea. Antarct Sci 16:299–305
Vacchi M, DeVries AL, Evans CW et al (2012) A nursery area for the Antarctic silverfish Pleuragramma antarcticum at Terra Nova Bay (Ross Sea): first estimate of distribution and abundance of eggs and larvae under the seasonal sea-ice. Polar Biol 35:1573–1585. doi:10.1007/s00300-012-1199-y
Van de Putte AP, Jackson GD, Pakhomov E et al (2010) Distribution of squid and fish in the pelagic zone of the Cosmonaut Sea and Prydz Bay region during the BROKE-West campaign. Deep-Sea Res II 57:956–967
Van der Molen S, Matallanas J (2004) Reproductive biology of female Antarctic spiny plunderfish Harpagifer spinosus (Notothenioidei: Harpagiferidae), from Îles Crozet. Antarct Sci 16:99–105. doi:10.1017/S0954102004001865
Vanella FA, Calvo J, Morriconi ER et al (2005) Somatic energy content and histological analysis of the gonads in Antarctic fish from the Scotia Arc. Sci Mar 69:305–316. doi:10.3989/scimar.2005.69s2305
White MG, Piatkowski U (1993) Abundance, horizontal and vertical distribution of fish in eastern Weddell Sea micronekton. Polar Biol 3:41–53
Williams R (1991) Potential nursery areas for fish in the Prydz Bay region. In: CCAMLR Working Group on Fish Stock Assessment WG-FSA 91/35
Yukhov VL (1982) Antarkticheskii klykach [the Antarctic toothfish]. Moscow: Nauka, 114 pp (in Russian)
Acknowledgments
This work is also intended as a tribute to the memory of Tatiana I. Faleeva who greatly contributed to the knowledge on the reproductive biology of the Antarctic silverfish.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Ghigliotti, L., Herasymchuk, V.V., Kock, KH., Vacchi, M. (2017). Reproductive Strategies of the Antarctic Silverfish: Known Knowns, Known Unknowns and Unknown Unknowns. In: Vacchi, M., Pisano, E., Ghigliotti, L. (eds) The Antarctic Silverfish: a Keystone Species in a Changing Ecosystem. Advances in Polar Ecology, vol 3. Springer, Cham. https://doi.org/10.1007/978-3-319-55893-6_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-55893-6_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55891-2
Online ISBN: 978-3-319-55893-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)