Abstract
Legumes are well recognized for their nutritional and health benefits as well as for their impact in the sustainability of agricultural systems. Phosphate (Pi) deficiency is a major nutritional factor limiting legume production, particularly in acidic and calcareous soils. Pi deficiency limits N2 fixation, since it has been described to have a strong impact on the growth and survival of both rhizobia and host plant. Legumes have evolved complex mechanisms to cope with Pi limitation. The maintenance of symbiotic N2 fixation seems to be a key aspect to assure legume productivity in low Pi environments. During the last decades, physiological components and molecular players underlying Pi-deficiency adaptation responses have been elucidated. Molecular, biochemical, physiological, and morphological responses are triggered to stimulate soil Pi uptake or to optimize its intracellular use efficiency and allocation over all plant organs. Research conducted on model species such as Medicago truncatula or important pulses such as common bean, soybean, and white lupin have provided valuable clues about the different mechanisms ensuring Pi homeostasis in nodules under Pi-limited conditions. In this chapter, we provide a general overview on the recent achievements on the impact of Pi deficiency on symbiotic N2 fixation in a broad range of legume species. A critical discussion of the main results and open questions is provided, highlighting the different approaches used to address the current needs of agriculture under the climate change context.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
Legumes are well recognized for their nutritional and health benefits as well as for their impact in the sustainability of agricultural systems (Araújo et al. 2015). Legume cultivation can play a central role as they represent the second major crop of agricultural importance worldwide, covering about 14% of the total cultivated land (Aranjuelo et al. 2014). When considering the nutritional impact of leguminous plants, namely, grain legumes which are also known as pulses, the international Food and Agriculture Organization (FAO) from the United Nations recognized the importance of these products by declaring 2016 the International Year of Pulses (FAO 2016). The standard Codex Alimentarius (2007) defines pulses as the dry seeds of leguminous plants, distinguished from the leguminous oil seeds by their low fat content, and includes species like beans (Phaseolus spp.), lentils (Lens spp.), peas (Pisum sativum), chickpea (Cicer arietinum), faba beans (Vicia faba), and cowpeas (Vigna spp.).
Legumes are of particular interest in agriculture because of their high nutritional value and innate ability to form symbiotic relations with dinitrogen (N2)-fixing bacteria that often results in the formation of N2-fixing nodules . This symbiotic relationship with soil bacteria from the genus Rhizobium that use the solar energy captured by the plant to break the triple bonds in inert atmospheric N2 and form reactive nitrogen (N) species such as ammonium (NH4 +) enables a more efficient plant growth, development, and seed production (Goh et al. 2013). Hence, this attribute provides legumes with a considerable advantage over the nonleguminous crops, especially when grown on soils with low N content (Brockwell et al. 1995). Both the World Health Organization (WHO) and FAO recommended increasing legume production because of their nutritional value and ability to fix N2 in the soil (FAO 2013). This unique association comes from evidence suggesting that methods assigned to improve ecosystem sustainability can be closely related with goals of improving nutrition. A case study in Malawi revealed that the use of legumes to restore soil fertility also triggered an enhanced consumption of their products (Kerr et al. 2013). From a nutritional point of view, pulses had been even designated as “the poor man’s meat” mainly because of their high source of proteins and affordable costs, while containing also a vast array of micronutrients in large quantities. Legume seeds are rich in most B vitamins as well as in iron, calcium, and zinc. Some crops like groundnut (Arachis hypogaea L.) and soybean (Glycine max L. Merr.) also contain higher amounts of fat, thus contributing to increased energy rates (Messina 1999). Additionally, the leaves of common bean and cowpea have similar nutrient concentrations with the seeds and even have higher amounts of micronutrients, as it also contains provitamin A and vitamin C (Messina 1999).
Besides, the decrease in the addition of N fertilizers has an overall positive impact on both agricultural costs and ecological integrity, as leaching of nitrate (NO3 −) in the water supply can result in severe groundwater contamination (Crews and Peoples 2004). Moreover, a recent study conducted on a long-term N addition to the soil found that this treatment negatively impacted on the legume–rhizobium mutualism causing up to 30% reduction in plant biomass (Weese et al. 2015). From an agronomical point of view, when legumes are included in the intercropping systems, defined as the simultaneous cultivation of two or more crop species in the same field, it enhances the yield by improving resource-use efficiency (Li et al. 2003; Zhang et al. 2015; Xue et al. 2016).
When considering the essential nutrients needed for the development of crops with high yield potential, N and phosphorus (P) are the most limiting factors. N is an essential element required for successful plant growth as it is used by plants to synthesize amino acids, the building blocks of proteins, and other vital compounds, such as chlorophyll, nucleic acids, and enzymes. Among the signs of N deficiency in plants, stunt growth and chlorosis are the most encountered (Marschner and Marschner 2012). Although reactive N species such as NH4 +, NO3 −, and nitrite (NO2 −) account for less than 5% of the total N in soil, they represent the main forms absorbed by most plants (Liu et al. 2014b). Hence, inorganic and organic fertilizers are applied to maintain the nutritional condition of different cropping systems, but this intensive use of chemicals although it has a positive impact on crop yield it also negatively impacts on the environment. In a report released by the US Department of Agriculture (USDA), it was stated that besides the increased agricultural productivity, the N addition also causes ozone-induced injury to crops and forests, eutrophication of aquatic ecosystems, biodiversity losses, and global climate changes (Ribaudo et al. 2011). Thus, by using legumes in rotation and intercropping systems, the use of N fertilizers can be substantially reduced, and the soil natural balance of N can be sustainably restored.
P is an essential macronutrient for plant and crop yield (Rodrı́guez and Fraga 1999). Phosphate (Pi) is the main P form that plant roots can absorb but its concentration is often limited for plant uptake (Chiou and Lin 2011). After uptake, it either remains as Pi or is assimilated by forming an ester with a hydroxyl group of a carbon (C) chain (e.g., sugar phosphates) or attaches to another Pi by forming an energy-rich pyrophosphate bond (e.g., adenosine triphosphate, ATP) (Marschner and Marschner 2012). Numerous metabolites include Pi in their molecular composition, such as sugar phosphates, nucleotides and nucleic acids, phospholipids, phosphoproteins, and energy-rich compounds like ATP (Czarnecki et al. 2013). Moreover, Pi play a major role in energy transformation and regulation of various enzymatic activities involved in photosynthesis, respiration, energy generation, and nucleic acid biosynthesis (Sulieman and Tran 2015). Based on these assumptions, it becomes clear that the low availability of Pi in soil imposes serious limitations for legume growth and development.
The present chapter is designed to provide a general overview and critical discussions on the recent achievements regarding the study of Pi-deficiency responses in a broad range of legume species, with an emphasis on the symbiotic N2 fixation (SNF) while highlighting different approaches used to address the current needs of agriculture and industry under the climate change context.
3.2 P is an Essential Nutrient for Legumes
A huge challenge derives from the fact that Pi has a low availability in the soil as it forms insoluble complexes with several minerals, while it is also poorly recovered from fertilizer addition because it is absorbed mainly by the soil and is not available for plants lacking specific adaptations (Balemi and Negisho 2012). With more than 40% of the world soils being deficient in Pi (Vance et al. 2003), the chemical fertilizer application alone is not a cost-effective way for increasing crop production in many Pi-limiting soils (Tilman et al. 2002). In turn, the addition of Pi fertilizers also has a high impact on the soil pH as well as on several morphological plant traits such as length and surface area of the roots, root architecture, root clusters (Shane and Lambers 2005), and even the root association with soil bacteria and fungi (Smith et al. 2000). Hence, plant adaptation strategies to overcome Pi deficiency include improved uptake efficiency, acquired through modification of the root morphology, exudation of chemical compounds into the rhizosphere, and association of roots with mycorrhiza (Vance et al. 2003), as well as improved utilization efficiency to produce higher dry matter per unit of Pi absorbed (Richardson et al. 2011). Research performed during field studies revealed that some legumes, such as white lupin (Lupinus albus L.), are able to better fix Pi when compared with other crops, mainly because of cluster-root (CR) formation (Abdolzadeh et al. 2010; Thuynsma et al. 2014). However, Pi deficiency negatively impacts on the leguminous plants’ ability to fix N2 by limiting the growth and function of the nodules. This aspect is further addressed in Sect. 3.4.2. This was thought to be a consequence of lower photosynthesis rate that limits the supply of C to the nodules (Sa and Israel 1991). Another study showed that low Pi inhibits nodulation and N2 fixation more than it affects plant growth and that this is due to the presence of an N-feedback mechanism induced by low Pi (Almeida et al. 2000). In addition to the low C supply and N-feedback regulation, other studies have documented that also the oxygen (O2) supply and oxidative stress are factors which can negatively affect nodulation and symbiotic efficiency (Arthikala et al. 2014; van Noorden et al. 2016). Nevertheless, mounting evidences are showing that Pi homeostasis in N2-fixing plant results from a coordinated interplay between the host plant and symbiotic microorganisms (Sulieman and Tran 2015).
3.2.1 The Agricultural Scenario of Legumes and Pi Deficiency Under Climatic Changes
Nowadays, climate change represents one of the most stringent problems that the world is facing, with several ramifications over the global environmental impact, agriculture and food security, as well as overall humanity life quality. The assessment reports on the climate change provided by the Intergovernmental Panel on Climatic Change (IPCC) underline the vulnerability of both human and natural systems and advocate that agriculture holds great potential to aid in mitigating these adverse effects while further supporting food security goals (IPCC 2007). With the predicted increase in the atmospheric carbon dioxide (CO2) levels, rise in temperature, higher frequency of weather instability, water scarcity, soil salinisation, etc., the agricultural sector must be prepared to tackle all these multifactorial issues and, at the same time, boost food productivity for the increasing population (FAO 2015). In a global effort to reduce the effects of climatic change, the Paris Agreement was set in motion, and 186 countries had published their action plan for ways to reduce greenhouse emissions (COP21 2015). Among these milestones, the implementation of sustainable, climate smart agriculture is essential to adapt to climate change. This emphasizes issues like the enhancement of agricultural productivity to increase food security while decreasing greenhouse gas emissions and increasing C sinks, as well as raising the adaptive capacity at multiple levels (Campbell et al. 2014).
From an agronomical point of view, grain legume production, although often used to promote environmental services, falls behind when compared with major grain cereals. A recent study estimated the variability and yield of grain non-legume in the EU and USA across a timeframe of more than 50 years (Cernay et al. 2015). The results of this study revealed that in EU the yield of legume crops vs. non-legume crops is highly variable, whereas in the USA, these differences are considerably lower, and EU imported more than 60% of its legume supply from the USA during this timeframe. The authors also suggest improving the cropping and intercropping system in EU in order to increase the local supply of legume grains. In fact, several studies pointed out the intercropping systems represent promising management practices that have an overall benefic impact on both crop yield and soil environment. In this sense, cereal/legume intercropping is an effective way to bring the legume production up to speed with the cereal crops while improving Pi uptake and N2 fixation in soils with low Pi and N content. Field studies on intercropped faba bean and maize reported a 20% increase in production without fertilizer addition and up to 38% enhancement when Pi was added to the soil (Li et al. 2003). Another recent study showed that wheat grown together with faba bean also presented a significant enhancement of Pi uptake in Pi-deficient soils; however, no increase in biomass was observed, and this might have been due to the slow growth rates of the two species (Li et al. 2015a). These two studies underline the importance of choosing the best partner crops for intercropping systems, encouraging further studies of this broad agricultural field. A big-scale experimental study was conducted in 16 different sites covering a broad range of climate environments, from Atlantic to continental and temperate to arctic, to assess the grass–legume mixture efficiency (Suter et al. 2015). This multifactorial study used five different grass species intercropped with white clover (Trifolium repens) and assessed plant productivity and N yield average together with the climatic influence. As concerned the total N yield, a positive effect of grass–legume mixture was observed that was however strongly affected by temperature and the proportion of legume present over the 3 years during which the study was conducted (Suter et al. 2015). But more importantly, this study, along with other studies summarized in the present review, underlines the importance of legumes in contributing to the development of recourse-efficient and environmentally-friendly agricultural practices to mitigate the challenges arouse by the global climatic changes.
3.2.2 Pi Deficiency and Legume Production Under Abiotic Stresses: Consequences and Adaptive Strategies
As legumes are an essential part of sustainable farming systems, an increasing body of research is continuously being carried out to better understand and mostly improve its uses in agriculture and food industry. The SNF which takes place in the root nodules represents a highly renewable and environmentally sustainable source of N. However, this symbiotic association between plant roots and bacteria is quite sensitive to environmental changes. Abiotic stresses, such as drought, extreme temperatures, salinity, and heavy metals, have a negative impact on legume production (Aranjuelo et al. 2014; Araújo et al. 2015). As abiotic stresses also influence soil conditions , both the plant and their symbiotic partners are affected, but the rhizobia display a higher degree of tolerance to stress manifested through more efficient maintenance of homeostasis (Priefer et al. 2001). In turn, this can have a positive impact also on the plant host (Xu et al. 2012). However, the symbiotic process is highly affected by abiotic stresses, and this was hypothesized to be related to a concerted accumulation of sucrose and reduction of malate during the bacteroid’s respiration process (Ramos et al. 1999). As a consequence, also the N status of the plant is affected. This has been proposed to work via an N-feedback inhibition of nitrogenase activity that evolved either from a direct N-feedback inhibition in nodules or by an indirect N-feedback process due to N signaling from the aerial parts of the plant (Serraj et al. 2001). Aside from abiotic stress, nutrient availability also represents a major constrain for legume yields, with limited Pi availability receiving the most attention (Tesfaye et al. 2007; Sulieman and Tran 2015).
As stated previously, P is implicated in many biological processes driving seed germination, root development, and fruit ripening. Pi is usually translocated into the actively growing meristems. However, during plant maturation, Pi is transported into the fruits, where high-energy requirements are needed for fruit ripening and seed formation (Goh et al. 2013). Hence, Pi deficiencies during this period can affect both seed development and normal crop maturity. Pi deficiency is quite difficult to diagnose from a morphological point of view, with crops usually displaying no particular symptoms except for a general stunting during early growth. Some evidences could include abnormal leaf coloration due to the accumulation of anthocyanins observed in some species (Sarker and Karmoker 2011) or root morphological changes (Wang et al. 2015). Conversely, biochemical diagnosis is more adequate as several studies reported enhanced carbohydrate concentrations (Hammond and White 2011), decreased soluble and insoluble protein content, as well as increased levels of proline and phenolic compounds in the root (Hernández et al. 2007; Sarker and Karmoker 2011). In white lupin, it was shown that sugars and Pi-stress signaling are closely interrelated (Uhde-Stone et al. 2003; Liu et al. 2005), while in common bean, it was evidenced that sugar is allocated to the roots in higher amount during Pi deprivation (Rychter and Randall 1994). The activity of sucrose synthase, a key enzyme of C metabolism, on legume nodules was affected by mild water deficit (Gonzalez et al. 1998), while reduced amounts of organic acids (oxalic, malic, and malonic acids) were also observed in common bean Pi-stressed roots when compared to Pi-sufficient roots (Hernández et al. 2007). From a physiological point of view, Pi deficiency has a direct negative effect on photosynthesis through a decrease in the photosynthetic ability per unit of leaf area also associated with a morphological reduction of the leaf area (Chaudhary et al. 2008). Altogether, these evidences support the assumption that a clear connection between C, N, and P metabolism exists.
The most studied abiotic stress factor interacting with the available soil Pi pools is water deficit or drought . Several studies performed under field conditions reported evidence of interactions between these two particular conditions. For instance, studies conducted in the Mediterranean region reported that lentil plants performed better under Pi fertilization during dry years (Matar et al. 1992). Another study showed that clover plants grown in dry soil and high Pi soil maintained better leaf water potential when compared with plants grown under low Pi, with only the low Pi plants actually showing clear symptoms of water stress (Singh et al. 1997). The effects of Pi deficiency and water deprivation are mostly encountered at the root level. In soybean, low Pi availability and water deprivation resulted in a gradual decrease of plant’s vegetative development as well as nodule number, while the root length density was increased with more than 20% as compared with control plants in order to avail a greater area for nutrition (Gutiérrez-Boem and Thomas 1999). In addition to root growth, the formation of mycorrhizae as well as other rhizospheric microbial communities can substantially help alleviate the symptoms of both Pi deficiency and water stress (Gupta et al. 2015). This is because generally the soil arbuscular mycorrhizae fungi have a benefic influence on plant growth mainly due to their ability to uptake nutrients, especially Pi (Lambers et al. 2008). As water deficit is known to reduce the Pi availability, mycorrhizal association was shown to aid plants survival at multiple levels, from nutritional (Pi an N uptake) to physiological (stomatal regulation by osmotic adjustments) and cellular (enhanced activity of antioxidant enzymes) effects (Ruiz-Lozano 2003). In a study on berseem clover (Trifolium alexandrinum) grown in the semiarid conditions of the Mediterranean region, it was shown that the formation of mycorrhizae leads to enhanced biomass and increased Pi and N uptake, thus playing an important role in plant growth and development (Saia et al. 2014).
Many genes and gene networks are responsible for governing the adaptation of plants to both Pi starvation and water scarcity. Aside from the obvious genes implicated in the response to stress, also genes involved in the regulation of root system architecture as well as genes coding for Pi transporters were studied in legumes. In soybean , the GmEXPB2 gene coding a β-expansin gene is mainly expressed in roots and greatly induced by Pi starvation (Guo et al. 2011). By using overexpression and silencing approaches, the authors demonstrated that the GmEXPB2 gene is involved in root hair elongation, having a high impact on Pi uptake during low Pi availability and water deficit. Another example is the PvTIFY transcription factor characterized in common bean as being involved in the regulation of genes involved in Pi deficiency and jasmonate pathway (Aparicio-Fabre et al. 2013). As the jasmonate hormone regulates a myriad of processes, it is essential for plant growth and development as well as defense responses and abiotic stresses such as drought (Browse 2009). In common bean, a global comparative gene expression analysis reported that several PvTIFY genes as well as genes involved in the jasmonate biosynthetic pathway were responsive to Pi deficiency, suggesting that PvTIFY is either directly or indirectly (via the jasmonate pathway) involved in Pi adaptation mechanisms (Aparicio-Fabre et al. 2013).
To gain better insight on how to efficiently use leguminous plants to tackle the climatic change scenario in agriculture, an overview on the knowledge of the legume SNF process, along with the physiological and molecular aspects related to Pi deficiency, is required. Hence, the current information regarding these aspects is presented in the next subchapters.
3.3 Legume SNF: Back to Basics
Legume–rhizobia symbiosis provides synergistic advantages to both partners, allowing the host plant to benefit from the N2-fixing ability of bacteria, which in turn are supplied with C compounds as energy sources for growth. Such a process, which has a tremendous importance in the agronomic context, still deserves to be investigated, particularly at the molecular level due to the extreme complexity of the regulatory pathways underlying plant–bacteria interactions, nodule development and metabolism, and adaptive strategies activated in response to adverse environments.
3.3.1 Free-Living Rhizobial Biology: Diversity, Growth, and Stress Tolerance
Life on planet Earth is dependent on essential elements among which N that is incorporated into proteins and nucleic acids. Although several living organisms are able to assimilate N in the form of NH4 +, NO2 −, NO3 −, or urea (CO(NH2)2), only a restricted number of microorganisms can perform the so-called process of biological N2 fixation (BNF) , namely, the conversion of atmospheric N2 molecule (containing a highly stable covalent triple bond) to NH4 +. A schematic overview of this process in the legume nodules is presented in Fig. 3.1. This conversion is catalyzed by nitrogenases, a family of complex metalloenzymes (see Hu and Ribbe 2015). Recent studies, including measurements in tropical forests, estimated the global BNF rate in terms of 58 Tg N per year (Vitousek et al. 2013; Sullivan et al. 2014). The gaseous N2 molecule, which accounts for approximately 78% of the Earth’s atmosphere, dissolves into water at the ocean surface allowing biological fixation in the marine ecosystems (Zehr and Ward 2002). Due to the relevance N2-fixing organisms for life, it is essential to assess their presence, diversity, and distribution on our planet. This will allow a more accurate quantitative and qualitative estimation of the process on a global scale (Reed et al. 2011). Novel N2-fixing organisms showing different degree of fixation rates have been recently discovered in various habitats, like the tropical forests (Batterman et al. 2013).
Schematic representation of symbiotic N2 fixation by nodulating legumes. N2 is fixed by the bacteria in the nodules through the activity of nitrogenases. This requires high amounts of energy (ATP) which it reserves from the plant. Nitrogenases function in anaerobic conditions, so the leghemoglobin, produced within the legume–bacteria cooperation, binds the O2 creating an anaerobic environment
Free-living N2-fixing bacteria (diazotrophic) include Azotobacter spp. (obligately aerobic, heterotrophic Gram-negative bacteria in the class of γ-Proteobacteria) widely distributed in natural and agricultural soils of temperate regions (Sahoo et al. 2014) and members of the genus Clostridium (endospore-forming obligate anaerobes) (Amon et al. 2010). Cyanobacteria represent another example of free-living N2-fixing bacteria located in terrestrial and marine environments (Bullerjahn and Post 2014; Singh et al. 2016). Among soil actinomycetes, the genus Frankia is well known for its N2-fixing ability. Frankia free-living strains are found in different ecological niches in soil as components of microbial consortia (Chaia et al. 2010). The well-known N2-fixing rhizobia are distributed within two different classes of Proteobacteria, namely, α-Proteobacteria and β-Proteobacteria. The α-proteobacterial genera Rhizobium, Sinorhizobium, Mesorhizobium, Azorhizobium, and Bradyrhizobium contain bacteria present under free-living and symbiotic conditions, the latter allowing symbiotic association with a compatible host plant. Differences between the two physiological conditions, particularly at the level of cell surface structure, have been highlighted based on comparative proteome analyses carried out in M. loti (Tatsukami et al. 2013). The complexity of rhizobia ecology and evolution is reflected by their different lifestyle since symbiotic rhizobia, including highly mutualistic N2 fixers as well as non-fixing parasites, coexist with nonsymbiotic strains lacking the ability to infect plants (Denison and Kiers 2004). Several studies have focused on the role played by rhizobia as natural endophytes of relevant cereal crops, able to promote plant growth and enhanced grain yield, independent on root nodulation and BNF (Yanni et al. 1997; Ji et al. 2010; Chen and Zhu 2013). For instance, in rice, it was demonstrated that rhizobia can colonize the plant roots without inducing nodulation. In this case, the bacteria reside mainly in the intercellular spaces, in contrast with the root nodule symbiosis where the bacteria have an intracellular colonization. Moreover, it was shown that common symbiosis genes (DMI3, CASTOR, CYCLOPS) are not required for the endophytic colonization of rice roots by rhizobial strains (Chen and Zhu 2013).
α-Proteobacteria include Methylobacterium, Blastobacter denitrificans, and Devosia neptuniae. Methylobacterium is a facultative methylotrophic bacterium associated with the plant phyllosphere (Minami et al. 2016), while the aquatic bacterium B. denitrificans is able to establish a symbiotic relationship with the flood-tolerant legume Aeschynomene indica (van Berkum et al. 1995). D. neptuniae, another non-rhizobia, Gram-negative, strictly aerobic short, and motile rod-shaped microorganism, is able to establish N2-fixing root nodule symbiosis with Neptunia natans, a unique aquatic legume from tropical/subtropical regions (Rivas et al. 2003). β-Proteobacteria include β-rhizobia such as Burkholderia, Cupriavidus (formerly Ralstonia) taiwanensis, and Herbaspirillum lusitanum (Gyaneshwar et al. 2011). Both Burkholderia and Cupriavidus have been found associated with species of the genus Mimosa, mainly native to the New World, while H. lusitanum has been characterized as an endophyte able to colonize agronomically relevant cereals (James et al. 1997).
The ability of rhizobia to withstand environmental stresses is directly correlated with their agronomical relevance as N2-fixing and growth-promoting bacteria since they can provide crop plants with enhanced tolerance to abiotic stresses like drought and salinity. It has been reported that M. truncatula plants nodulated with Sinorhizobium medicae or S. meliloti, having different degree of N2 fixation efficiency, showed a significant delay in drought-induced leaf senescence compared to non-nodulated plants (Staudinger et al. 2016). Free-living rhizobia growth is impaired under osmotic stress induced by excessive salts in soil, with negative consequences on nodulation and symbiotic capacity. The use of salt-tolerant rhizobia strains, able to perform N2 fixation in high-salinity soils, represents a promising strategy and sustainable alternatives to chemical fertilization (Rejili et al. 2012). In a recent study, Rhizobium leguminosarum bv. viceae strains were isolated from the salt-tolerant P. sativum cultivar “Resal” at sites with contrasting climatic conditions across Portugal (Cardoso et al. 2015). By screening its tolerance to salinity , several changes in the bacterial protein profiles were determined. While rhizobia from southeast Portugal were able to withstand water shortage in soil, the bacteria from northwest Portugal were more susceptible to water stress (Cardoso et al. 2015). These findings highlight the need for extensive screening of stress-tolerant rhizobial populations in order to enhance the strain collections useful for agronomical applications.
Increased desiccation tolerance is another desired trait that would support survival of rhizobia on legume seeds during drying and storage. The response to desiccation stress in prokaryotes involves complex physiological responses and activation of protection mechanisms against cellular damage, e.g., the production of exopolysaccharides, induction of stress-responsive proteins, repair of membrane/DNA damage, and accumulation of intracellular sugars (Vriezen et al. 2007; Cytryn et al. 2007; Vanderlinde et al. 2010). Osmoprotectants are accumulated within rhizobia cells in response to osmotic and desiccation stress, either by de novo synthesis or by active uptake systems. The presence of the nonreducing disaccharide trehalose, involved in the protection of macromolecules against denaturation during desiccation stress, significantly improved desiccation tolerance in Bradyrhizobium japonicum (Streeter 2003) and R. leguminosarum bv. trifolii (McIntyre et al. 2007). More recently, an investigation performed on peat-cultured R. leguminosarum bv. trifolii TA1 and B. japonicum CB1809 strains revealed multifactorial features associated with increased desiccation tolerance which requires intracellular trehalose accumulation together with enhanced expression of proteins involved in cell envelope protection, DNA damage repair, and oxidative stress response (Casteriano et al. 2013). Stress-specific molecular responses have been analyzed by Mhamdi et al. (2015) in strains belonging to Mesorhizobium, Sinorhizobium, and Rhizobium genera challenged with sodium chloride (NaCl) and polyethylene glycol (PEG). A negative correlation between cell viability and lipid peroxidation was observed, while increased levels of C19:0 cyclo-fatty acid were detected in Sinorhizobium- and Mesorhizobium-tolerant strains, as a protective mechanism for preserving membrane integrity. Superoxide dismutase (SOD) and catalase (CAT) activity were enhanced in response to both stress agents to provide protection against free radical species (Mhamdi et al. 2015).
3.3.2 Nodulation
Root nodule symbiosis observed in legumes is a fascinating example of successful symbiosis between plants and bacteria, characterized by a perfect balance between the host and the microsymbiont. Legumes facing N shortage secrete secondary metabolites, such as flavonoids, as signaling molecules that attract compatible symbiotic rhizobia by triggering the expression of the bacteria nodulation genes (Abdel-Lateif et al. 2012). The latter encode enzymes required for the synthesis of the nodulation factor (NodF), a lipo-chitooligosaccharide which induces the plant molecular responses leading to root nodule symbiosis (Oldroyd et al. 2011). NodFs bind plant receptors which belong to the receptor-like kinases (RLKs) class, key components of the plant innate immunosystem, thus posing the question about the possible common features between the rhizobial–plant and pathogen–plant interactions and the possibility to exploit the plant immune response to modulate rhizobial infection and host range (Toth and Stacey 2015).
In many legumes, rhizobia enter the root epidermis using infection threads (ITs ) or tubular plant-derived structures surrounded by a membrane which is contiguous with the plant cell plasmalemma and a layer of cell wall material. Rhizobia are trapped within the IT, and they remain isolated from the host cell cytoplasm. Fournier et al. (2015) reported on the use of live tissue imaging to monitor the early steps of the rhizobia–plant interaction, focusing on the transition from the entrapment of bacteria within the root hair cell to the formation of the IT. The authors suggest a new model in which the so-called infection chamber first gives rise to a globular apoplastic compartment that contains the bacteria resembling the structure of the future IT. Subsequently, the infection chamber is remodeled with a transition from radial morphology to the tubular structure typical of ITs (Fournier et al. 2015). ITs deliver rhizobia into the actively dividing cortical cells that will give rise to the nodule primordium, subsequently converted into the nodule, a new root organ. Within the nodule, bacteria differentiate into bacteroids, the SNF sites. Ammonia (NH3) is supplied to the host plant which, in turn, provides rhizobia with carbohydrates. Terminal bacteroid differentiation is accomplished through cell enlargement (up to ten times compared to the size of the free-living bacteria), genome endoreduplication, and membrane permeabilization (Mergaert et al. 2006), and the entire process is ruled by plant antimicrobial peptides, named nodule-specific cysteine-rich (NCR) peptides , showing similarities to defensin-type factors (Haag et al. 2013; Alunni and Gourion 2016).
Legumes produce indeterminate or determinate nodules, differing in morphology and developmental program. Legumes from the inverted repeat-lacking clade (IRLC) (e.g., P. sativum L., V. faba L., and M. sativa L.), which develop indeterminate nodules, are able to secrete antimicrobial peptides that trigger endoreduplication of the bacterial genome and transition to a quiescent state. The rhizobial BacA (bacteroid development factor) protein is required for the uptake of plant-derived NCR peptides which rule bacteroid differentiation (Marlow et al. 2009). Bacteroids are surrounded by peribacteroid solution and peribacteroid membranes derived from the plasma membrane of the host plant cell. The peribacteroid solution contains high sugar levels, particularly inositols (Teijma et al. 2003), and molecules able to induce differentiation of rhizobia into bacteroids (Ohkama-Ohtsu et al. 2015).
Due to the high metabolic costs of nodulation, nodule number is tightly regulated by mechanisms that still need to be fully understood. As described later on in this chapter, Pi plays a major role on this process. The role of signaling peptides in the local and systemic control of nodule and lateral root formation, particularly at the early stage of nodule development, has been reviewed by Djordjevic et al. (2015). Reactive oxygen species (ROS) accumulated through the activity of respiratory burst oxidative homologues (RBOHs) , namely, NADPH oxidases, play key roles in several plant signal transduction pathways, among which are those that regulate the symbiosis between legumes and N2-fixing bacteria (Arthikala et al. 2014). It has been previously demonstrated that ROS levels are transiently enhanced in tips of actively growing Phaseolus vulgaris root hair cells following exposure to NodFs (Cardenas et al. 2008), while ROS production is reduced in M. truncatula roots following the first hour of treatment with NodFs. Such a decrease is associated with the downregulation of the MtRBOH2 and MtRBOH3 genes (Lohar et al. 2007), whereas the use of inhibitors of NADPH oxidases in M. truncatula impairs both ROS production and the early rhizobial interaction in root hairs (Peleg-Grossman et al. 2007).
The complex molecular events underlying rhizobial infection and nodule organogenesis are under phytohormone control. Ethylene is a negative regulator of the legume–rhizobia symbiosis , acting at different stages during nodule formation by suppressing the signaling pathways triggered by NodFs (Guinel 2015). Larrainzar et al. (2015) investigated these issues using “omics” approaches to monitor the transcriptional changes occurring in roots of M. truncatula inoculated with Sinorhizobium medicae. The use of M. truncatula mutants showing reduced sensitivity to NodFs as well as the ethylene insensitive/Nod factor-hypersensitive mutant sickle revealed thousands of candidate genes modulated by NodFs and ethylene , allowing the prediction of key nodes controlling perception/transduction of signals brought to plants by NodFs (Larrainzar et al. 2015). The role of auxin signaling in rhizobial infection is not completely clarified, although it is possible that auxin regulates induction of cell division associated with infection (Breakspear et al. 2014; Schaller et al. 2015). Cytokinins are required for nodule development with cytokinin signaling responses occurring in both nodule primordia and developed nodules as recently showed by Reid et al. (2016) who found that the Lotus japonicus Ckx3 (cytokinin oxidase/dehydrogenase) gene was induced by NodF during the early phase of nodule initiation. Jasmonate is also an emerging player in the control of symbiotic nodulation. A jasmonate ZIM-domain (JAZ) protein interacting with leghemoglobin in Astragalus sinicus was identified by Li et al. (2015b) who demonstrated its requirement for maintenance of proper nodule number, bacteroid development, and nitrogenase activity and highlighted a novel role for jasmonate during legume–rhizobia symbiosis.
Autoregulation of nodulation (AON) is a systemic signaling pathway which limits the number of nodules formed by the host legume plant when symbiosis with rhizobia takes place (Mortier et al. 2012). According to the most recent working hypothesis, developed in the model legume M. truncatula, root signaling peptides are translocated to the shoot where they bind to the shoot receptor complex SUNN (supernumerary nodules, belonging to the class of leucine-rich repeat receptor-like kinases or LRR-RLK), inducing a signal transduction pathway which impairs nodule formation. This mechanism requires the interaction of SUNN receptor with CRN (CORYNE) and CLV2 (CLAVATA ) proteins which are essential players in the control of root meristem activity (Crook et al. 2016).
3.3.3 Nodule Function: Nitrogenase Activity
Atmospheric N2 is made available to the biosphere through BNF catalyzed by nitrogenase , a metalloprotein that consists of two components, the Fe protein (dinitrogenase reductase) and the MoFe protein (dinitrogenase) (Seefeldt et al. 2009). Electrons are delivered from Fe protein to MoFe protein in a reaction which requires the hydrolysis of at least two ATP molecules for each transferred electron. The Fe protein is extremely sensitive to O2, and all diazotrophs maintain an anaerobic environment around nitrogenase.
Several methods have been developed to quantify the rates of N2 fixation in terrestrial ecosystems , among which are 15N isotope dilution (McAuliffe et al. 1958), acetylene reduction assay (ARA) (Hardy et al. 1968), N accretion (Knowles 1980), 15N natural abundance (Shearer and Kohl 1986), and N difference (Weaver and Danso 1994). Stable isotope methods are generally considered the most accurate for measuring of SNF (Danso 1995). The 15N isotope-based tracer techniques , which have significantly contributed to expand the knowledge of the dynamics occurring within the soil/plant system, rely on the Ndff equation: N = N 1 + Ns + No (where N is the total amount of N in the plant, N 1 is the N recovered from the labeled pool, N s is the N recovered from the unlabeled soil pool, and No is the N found in the plant at the beginning of the experiment) (Barraclough 1995). The discovery that the nitrogenase enzyme responsible for N2 fixation also reduces acetylene (C2H2) to ethylene (C2H4) (Dilworth 1966) provided a useful assay for the quantification of the N2 fixation process. ARA is still widely used because it provides a highly sensitive and inexpensive way to quantify relative nitrogenase enzyme activity in N2-fixing tissues. Both acetylene and C2H4 are easy to measure by flame ionization gas chromatography . Thus, ARA can provide a simple, inexpensive measure of nitrogenase activity. There are two significant complications that can limit the use of the ARA for quantifying N2 fixation: (1) the assay measures total electron flow through nitrogenase but only a proportion of electron flow is actually directed toward N2 reduction (2) and this proportion can change depending on genetic and environmental factors. It has been reported that acetylene itself causes partial suppression of nitrogenase activity by limiting O2 diffusion (Minchin et al. 1986). Recommendations and refinements to the original protocol have been presented by Vessey (1994) who underlined the importance of several key parameters, e. g., tissue preparation, time, and gas sample storage.
Hydrogen (H2) evolution under normal atmospheric N2 and O2 levels (80:20 vol/vol) can be used to measure the apparent nitrogenase activity (ANA), namely, the situation where electrons are used to reduce both H+ and N2 (Hunt et al. 1987). H2 evolving from the nodules provides an indirect, nondestructive measure of the N2 fixation activity of nitrogenase. When N2 in the air around nodules is replaced by argon (Ar), the electron flow through nitrogenase is directed onto H+. The resulting H2 evolution can be measured as a simple and nondestructive way to estimate total nitrogenase activity (TNA). The relative efficiency of nitrogenase in terms of electron allocation can be calculated as 1-ANA/TNA (electron allocation coefficient, EAC) (Fischinger and Schulze 2010). A mathematical model can be used to estimate the rates of O2, CO2, N2, and H2 exchange from legume nodules under steady-state conditions of N2 fixation. Based on this model, the rates of gas exchange, relative growth rate (RGR), TNA, EAC, uptake hydrogenase activity (HUP), and chemical features of the resulting N-containing molecules have been calculated with results that were in agreement with those obtained through experimental activity (Layzell et al. 1988). When considering the effects of nodule features on the rates of gas exchange, apparent respiratory cost (CO2/NH3), and sucrose cost (sucrose consumed/NH3), ureide-producing nodules were estimated to consume 8% less sucrose per N fixed when compared to amide (asparagine)-producing nodules. However, ureide-producing nodules would show an apparent respiratory cost of 5% higher than that in amide-producing nodules. In both ureide-producing and amide-producing nodules, the apparent respiratory cost of N2 fixation (CO2/NH3) was mainly dependent on EAC, followed by TNA, nodule RGR, and nodule size. EAC is modulated by the competitive inhibition of H2, while the degree of inhibition is affected by the nodule’s permeability to gas diffusion. Moloney et al. (1994) tested this hypothesis by measuring EAC in soybean nodules exposed to different partial pressures of H2 and N2, with or without changes in TNA or nodule permeability to gas diffusion. Results were compared with predictions from a mathematical model that combined equations for gas diffusion and competitive inhibition of N2 fixation (Moloney and Layzell 1993). Both empirical and theoretical data revealed the same trend, namely, that decreases in EAC values were associated with increases in external pH2, decreases in external pN2, and decreases in nodule permeability to O2 diffusion. The ability of the model to predict EAC provided strong support for the hypothesis that H2-mediated inhibition of N2 fixation plays a major role in the in vivo control of EAC. The model also hypothesized that the presence of a variable barrier to gas diffusion affects the H2 and N2 concentration in infected cells and consequently the degree of H2 inhibition. Continuous measurement of H2 evolution has been reported only for periods shorter than 48 h since H2 analyzers are highly sensitive to humidity, and the analyzers react to O2 pressure in the gas flow and temperature in their surroundings (Gordon et al. 2002). A new method for the noninvasive measure of nodule activity during prolonged time periods has been described by Cabeza et al. (2014, 2015) who provided novel insights into the regulation of N2 fixation, such as the occurrence of daily rhythms in nodule activity.
The combination of chromatographic techniques with the use of isotopes as tracers has become an efficient tool for the study of metabolic fluxes in plants (Freund and Hegeman 2016). Nuclear magnetic resonance (NMR) in combination with isotope labeling is used in plant metabolomics to decipher metabolic fluxes, as a rapid, selective, highly reproducible, and site-specific tool. Isotope ratio mass spectrometry (IRMS) is also considered as one of the most suitable techniques for measuring isotopic ratios and isotopic enrichments due to high precision, sensitivity, and accuracy (Freund and Hegeman 2016). An IRMS-based method, allowing the measurement of δ13C and δ15N values of amino acids within the plant and symbiotic bacteria, has been described by Molero et al. (2011). The method revealed the pattern of C and N exchange between leaves and nodules, highlighting that γ-aminobutanoic acid (GABA), and glycine may represent an important form of C transport from leaves to the nodules.
3.3.4 Nodule Energy Status
Legume nodules are sites of intensive C and energy turnover, particularly in P-rich plant organs (Schulze et al. 2006). A correlation of SNF rates with the adenylate ratios, namely, ATP–ADP or adenylate energy charge AEC = ([ATP] + 0.5[ADP])/([ATP] + [ADP] + [AMP]), has been highlighted (Wei et al. 2004). Early studies by de Lima et al. (1994) showed that exposure of soybean nodules to 10% O2, stem girdling, or NO3 − fertilization resulted in decreased AEC values in nodules. Wei et al. (2004) used nonaqueous centrifugal density gradient fractionation of the central infected zone tissue of soybean nodules to recover adenylate pools from subcellular compartments. When nodules were switched from air to Ar/O2, AEC in the plant cytosol significantly increased, whereas AEC of the mitochondrial compartment in this central zone tissue remained stable. AEC values in the bacteroid compartment did not change in the short term but a decline was then observed after 60 min. Wei et al. (2004) also provided a simulation model in which ATP hydrolysis was linked to glutamine synthetase and asparagine synthetase activity required for the assimilation of fixed N, as well as to the translocation of C4 acids across the symbiosome membrane. The large distances between sites of ATP production and hydrolysis were predicted to generate gradients in ATP, ADP, and AMP within the cytosol of the infected cell (Wei et al. 2004).
3.4 Physiological and Molecular Aspects of Pi Deficiency in Legumes
The Pi starvation response is a multifaceted adaptation that aims to improve Pi acquisition, and the internal recycling of Pi is composed of metabolic, physiological, and morphological components (Salazar-Henao et al. 2016). Pi acquisition by plants from the external environment is influenced by N metabolism. Early studies reported that pretreatments with NH4 + and NO3 − can enhance Pi uptake in Zea mays roots (Smith and Jackson 1987). The uptake of NH4 + is associated with proton (H+) release and decrease in the rhizosphere pH, which in turn facilitates Pi solubility and uptake (Zhao et al. 2009), and this might be possibly due to enhanced activity of the plasma membrane H+-ATPase (Zeng et al. 2012). On the contrary, when NO3 − is provided, Pi uptake is affected (Watanabe et al. 1998). More recently, Zhu et al. (2016) investigated these issues in two rice (Oryza sativa L.) cultivars, “Nipponbare” and “Kasalath,” which differ in the ability of reutilizing Pi from their cell wall. This study demonstrated that NH4 + positively modulates the pectin content and pectin methylesterase activity in root cell walls under Pi deficiency, resulting in Pi remobilization from the cell wall and increased availability of soluble Pi in roots and shoots. Moreover, increased nitric oxide (NO) levels were detected in rice roots supplied with NH4 + as unique N source (Zhu et al. 2016).
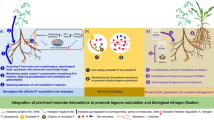
A striking and particular fact of P nutrition in legumes is that N2 fixation actually requires higher amounts of Pi for optimal functioning when compared with non-leguminous plants (Sulieman et al. 2013a; Tesfaye et al. 2007; Chiou and Lin 2011; Sulieman and Tran 2015), suggesting that legumes are more prone to suffer from Pi deficiency. This has been linked to the nodule development and energetic transformation, especially for the synthesis of mitochondria and symbiosome membranes (Mus et al. 2016). Several reports from the literature have unveiled the effects of Pi deficiency in several species from models like M. truncatula (Cabeza et al. 2014) to crops such as common bean (Araújo et al. 2008; Olivera et al. 2004) and white lupin (Thuynsma et al. 2014) or the legume pastures white clover (Almeida et al. 2000; Høgh-Jensen et al. 2002), alfalfa (Schulze and Drevon 2005), and stylo (Stylosanthes spp.) (Liu et al. 2016). All these legumes show different strategies to enhance Pi acquisition or improve its remobilization under Pi limitation (Fig. 3.2).
Schematic representation of morphological, physiological, and biochemical responses to phosphate (Pi) deficiency in plants. Aside from the morphological changes in root architecture, Pi uptake is also facilitated by the expression of high-affinity Pi transporters. In addition to this, the exudation of organic acids, phosphatases, and phytases also mobilizes additional Pi resources. At the aerial parts level, high Pi deficiency can be associated with reduced shoot branching and early leaf senescence
3.4.1 Impact of Pi Deficiency in Root and Shoot Traits
Alterations in morphological , anatomical, and architectural root traits are among the best described responses to Pi deprivation in an attempt to maximize Pi uptake from the soil (Negi et al. 2016; Mori et al. 2016; Yuan et al. 2016). The mechanisms by which roots are able to perceive Pi starvation remain not totally understood. In this context, root caps in the root–soil interface are expected to have an important role in sensing nutrient deficiency and respond to it (Svistoonoff et al. 2007). Coupled to the typical growth arrest of primary root in response to Pi deprivation, another well characterized response in legumes is root branching. Root branching contributes to increase topsoil foraging, maximizing Pi acquisition in the sense that the roots are searching for Pi-enriched patches. Common bean genotypes with increased basal root whorl number showed improved performance under low Pi availability in soils (Miguel et al. 2013).
Cluster root (CR) formation is a desirable trait to improve Pi acquisition when global Pi resources became scarce, representing a common adaptive strategy of legumes. Coupled to this, the colonization of mycorrhizae leads to higher Pi use efficiency as well as increased total N content in plants (Schulze et al. 2006; Sprent and James 2007). In a study conducted on L. albus and L. pilosus, Wang et al. (2015) showed that the CR percentage was strongly and negatively correlated with plant Pi status in species-specific manner. In L. albus, low Pi status at the shoot level is reflected in the amount of sucrose translocated to the roots (Wang et al. 2015). The main results of this study indicated that sucrose appears to have an important role in CR formation simultaneously acting both as a C source and a long-distance signal reporting the shoot Pi status to the root system. Interestingly, L. angustifolius does not form CR under Pi starvation, but it developed a strategy for achieving critical Pi uptake in low Pi environments by altering shoot growth and root architecture and secreting carboxylates from roots (Chen et al. 2013). On the other side, Pi supplementation seems to inhibit lupin’s ability to form CRs, and also this response is dependent on the studied species (Abdolzadeh et al. 2010). Such studies bring evidence of genotype-dependent performances under low Pi availability suggesting that a determinant genetic control of this trait exists.
The response of two legume tree species, namely, Virgilia divaricata and V. oroboides, was investigated during low Pi conditions (Magadlela et al. 2014). While the growth of V. divaricata was not affected by Pi deficiency, the same was not observed in V. oroboides, which showed lower performance under such conditions. The authors were able to link the poor performance of V. oroboides to a lower Pi uptake, as well as a differential allocation of N and C nutrients, which were directed more to the aerial parts in the detriment of the roots and nodules, resulting in a decline of N nutrition, growth respiration, and overall photosynthetic costs. On the other hand, V. divaricata increased the Pi allocation to nodules and benefited N nutrition, while maintaining its photosynthetic costs. This is an example of different adaptation strategies to Pi starvation through the alteration of biomass allocation (Magadlela et al. 2014). Consequently, the selection of genotypes with better performance in low Pi soils could lead to sustainable crop production under Pi-limited environments.
The coordination of shoot and root responses to Pi deprivation involves trafficking of systemic signals, such as sugar, microRNAs (miRNAs), and hormones, through the vasculature (Lin et al. 2014). Nevertheless, the morphological responses of roots are thought to be chiefly controlled by the local concentration of Pi (Ticconi et al. 2004). However, it may be assumed that local and systemic signals are integrated and that long-distance signals might also influence some, if not all, root morphological responses (Salazar-Henao et al. 2016).
3.4.2 Impact of Pi Deficiency in SNF
In low Pi soils , legumes depending on N2 fixation respond positively to Pi fertilization and show increased N content in shoots and roots (Richardson and Simpson 2011). Pi-limited grain legumes can provide normal SNF rates for as long as 3 weeks, taking advantage only of seed P reserves (Schulze et al. 2006). Due to the high Pi requirement of N2-fixing nodules, shortage of Pi has a particularly large impact on legumes relying on SNF. Pi deficiency seems to affect nodulation and N2 fixation to a greater extent than the aboveground plant growth (Almeida et al. 2000; Olivera et al. 2004). Low Pi availability resulted in a decline of N2 fixation, while a sudden removal of Pi from the medium totally blocked the nodule growth and changed the relative growth rate of both shoots and roots in white clover (Høgh-Jensen et al. 2002, Almeida et al. 2000). On the other side, higher Pi applications significantly inhibited nodule function also in soybean and M. truncatula (Qin et al. 2012; Sulieman et al. 2013a), suggesting that a tight regulation of the Pi pools is needed to ensure efficient legume growth and development. Hence, the abovementioned studies provided strong evidence that elevated Pi concentrations in nodules are essential for N2 fixation during Pi deficiency . Almeida et al. (2000) proposed that in Pi-deficient plants, the impairment of SNF could be a result of several factors among which (1) the impairment of the host plant growth, (2) the growth and functioning of the nodule, and (3) the interaction among these factors.
Pi allocation to nodules plays a key role to assure essential N nutrition (Magadlela et al. 2014). Other species that increase the allocation of Pi in the nodule upon Pi starvation include M. truncatula, M. sativa, and L. luteus (Sulieman et al. 2013b; Kleinert et al. 2014). Increased Pi uptake is mediated by the high affinity of Pi transporters in the nodules (Qin et al. 2012). Research has shown that, under normal condition, around 20% of plant total P is allocated to the nodule (Kouas et al. 2005), while considerably higher levels of Pi are required under low Pi conditions (Thuynsma et al. 2014). An upgrading of N2 fixation ability in nodules can compensate for the reduction in the number of nodules observed under low Pi conditions (Almeida et al. 2000; Qin et al. 2012; Schulze et al. 2006). Alfalfa nodules grown under the Pi-deficient conditions are smaller but have had a higher O2 uptake per N2 reduced, coinciding with increased nodule permeability and conductance (Schulze and Drevon 2005). Nodule permeability to O2 through the regulation of O2 diffusion represents a key factor for the appropriate functioning of the nitrogenase enzyme (Schulze 2004). Thus, increased O2 uptake appears to be a mechanism to adjust nodule metabolism to Pi deficiency in indeterminate N2-fixing nodules such as in alfalfa, as previously shown for determinate (common bean and soybean) nodule forms.
The effects of long-term Pi deficiency and subsequent recovery on bacteroid mass/number per unit nodule mass and the energy status of soybean nodules were investigated by Sa and Israel (1991). The continuous Pi deficiency significantly decreased the whole-plant dry mass, Pi and N content, and specific nitrogenase activity, as compared to the Pi-sufficient control. The whole nodules of Pi-deficient controls contained 70–75% lower ATP concentrations than nodules of Pi-sufficient controls . The energy charge and ATP concentrations in the bacteroid fraction of nodules were not significantly affected by Pi treatment. However, ATP and total adenylate concentrations and AEC values in the plant cell fraction of nodules were significantly decreased to 91%, 62%, and 50%, respectively, by the Pi deficiency. The specific activity of nodules (Nfixed per unit nodule biomass), AEC, and ATP concentration in the plant cell fraction increased to the levels of non-stressed controls after alleviation of external Pi limitation. The bacteroid mass per unit nodule mass and bacteroid N concentration did not increase to the level of non-stressed controls until 7 days after alleviation of external Pi limitation.
3.4.3 Pi Deficiency Induces the Excretion of Organic Acids and Pi-Releasing Enzymes
Legume genotypes with contrasting utilization of P for SNF are interesting systems to study the molecular mechanisms underlying N2 fixation impairment under Pi deficiency. Many studies have focused on the role that acidic phosphatases play in intra- and/or extracellular Pi scavenging and recycling during Pi starvation (for review, see Plaxton and Tran 2011). Plants grown under limited Pi supply can increase the activity of phosphatases in roots to hydrolyze organic P compounds in the soil, thus improving plant Pi acquisition (Araújo et al. 2008; Plaxton and Tran 2011). However, little information is available about the role of these enzymes for internal plant metabolism at limited Pi conditions.
Phytate is one of the major organic forms of P in the soil but this P form is unavailable to plants unless mineralization takes place. Phytases are capable of hydrolyzing phytate to a series of lower phosphate esters of myoinositol and phosphate contributing for increasing the rhizospheric Pi contents for root uptake. It is argued that phytase activity in nodules would contribute to the adaptation of the rhizobia–legume symbiosis to low Pi environments as seen in P. vulgaris nodules (Araújo et al. 2008). In this context, Lazali et al. (2013) investigated the expression profiles of phytase genes in two recombinant inbred lines of P. vulgaris characterized by contrasting N2 fixation ability under Pi deficiency, inoculated with R. tropici CIAT 899 strain and grown under low or high Pi supply. They detected accumulation of phytase transcripts in the nodule cortex and infected zone of both lines, but phytase gene expression was significantly enhanced in the P. vulgaris line with high N2 fixation ability in the absence of Pi. This finding was well correlated with an increase in phytase (33%) and phosphatase (49%) activities and enhanced SNF efficiency (34%). The authors underlined a possible role of phytase activity within nodules in helping adaptation of the rhizobia–legume symbiosis to low Pi environments.
Plant acid phosphatases (APases) catalyze the hydrolysis of Pi from a group of phosphomonoesters and anhydrides with optimal activity pH below 7.0 (Duff et al. 1994). Increased APase activities and exudation is considered an efficient strategy for plants to mobilize and utilize organic P . This strategy was recently associated with the performance of stylo, a dominant pasture legume widely grown in tropical and subtropical areas where acid soils cause Pi deficiency (Chandra 2009). The utilization of extracellular deoxyribonucleotide triphosphate (dNTP) and the underlying physiological and molecular mechanisms were examined for two stylo genotypes with contrasting Pi efficiency (Liu et al. 2016). The results showed that the Pi-efficient genotype (TPRC2001-1) was superior to the Pi-inefficient genotype (Fine-stem) when using dNTP as the sole Pi source. Moreover, Pi starvation can increase root-associated APase activities in stylo, which might be caused by enhanced expression levels of the purple acid phosphatases (PAP) in roots of both stylo genotypes. Furthermore, the higher expression levels of SgPAP7 and SgPAP10 in TPRC2001-1 roots contribute to its higher root-associated APase activities and thus facilitate greater utilization of extracellular dNTP by TPRC2001-1 than by Fine-stem. Other studies have also highlighted that some members of PAP gene family have a potential role in plants’ response to symbiosis with rhizobia or arbuscular mycorrhizal fungi under Pi-limited conditions (Li et al. 2012).
3.4.4 Novel Clues on the Impact of Pi Deficiency in Legumes
Recently, Sulieman and Tran (2015) provided a comprehensive review of the complex mechanisms used by legumes to control Pi homeostasis in nodules, when Pi levels decrease. One of these strategies allows maintaining higher Pi concentration in nodules compared to other organs. Indeed, up to 20% of total plant P is found in the nodules, and under limiting Pi conditions, the Pi levels in nodules are even enhanced. A significant example is provided by Thuynsma et al. (2014) who showed that L. albus responds to Pi shortage by increasing CR production , a highly expensive metabolic process, to improve Pi supply to nodules. Expansion of CRs results in larger root surface area and exudation of organic acids which facilitate Pi absorption (Thuynsma et al. 2014). Increasing Pi acquisition also involves uptake mediated by high-affinity Pi transporters (Jia et al. 2011; Qin et al. 2012; Liu et al. 2014a), while root colonization by mycorrhizae results in the efficient translocation of high Pi amounts into the host plant (Wang et al. 2011). In addition, induction of APase is triggered by Pi starvation as a universal strategy in higher plants (Araújo et al. 2008), while addition of N to soil with low Pi content increases as well the activity of extracellular phosphatases (Tredeser and Vitousek 2001).
In most cases, the improved response toward Pi starvation results from a concerted action of multiple strategies, as seen in white lupin. The efficient C use and N assimilation process together with enhanced nodulation in CR zones and elevated organic acid production in nodules makes this species highly adaptable to Pi deficiency (Schulze et al. 2006). A complex cross talk of regulatory processes and molecular player, acting both locally or at the whole-plant level, assures Pi homeostasis in nodules under Pi starvation. Many genes responsible for the regulation of Pi homeostasis have been identified in Arabidopsis (Lin et al. 2009), while in legume species, such studies are relatively fewer, though a number of genes associated with this process were also identified in several legumes (Table 3.1). On the other hand, the development of post-genome methodologies, such as global analysis of coding and noncoding transcriptomes, proteomes, and metabolomes integrated in solid bioinformatics platforms, has noticeably improved our knowledge and holistic understanding of various plant functions, including the response to environmental stresses (Mochida and Shinozaki 2011). In this subsection, we describe some studies in which the use of omics considerably increased our knowledge on the response of legume N2 fixation under Pi deficiency.
BNF and Pi concentration in different organs of M. truncatula during a whole-plant Pi depletion experiment was investigated by Cabeza et al. (2014). N2 fixation activity per plant diverged from that of fully nourished plants beginning at day 5 of the Pi depletion process , since fewer nodules were formed, while the activity of the existing nodules was maintained for as long as 2 weeks into Pi depletion. RNA-seq analysis revealed several mechanisms underlying nodule adaptation to Pi deprivation. Among the 1140 differentially expressed genes, several genes for Pi remobilization from organic structures and nodule malate formation were up-regulated, while genes involved in fermentation were downregulated. During Pi depletion , nodule metabolism is shifted for acclimating nodules to low Pi availability before the tissue itself is depleted. Among those, reduced activity of fermentation pathways, increased CO2 fixation, and upregulation of phosphatases contribute to mobilize Pi from organic structures. This enhanced turnover of the limited Pi quantities available allowed plants to uphold the high N2 fixation rates of existing nodules well into the Pi depletion process.
The adaptation of Mesorhizobium –chickpea to Pi limitation was deeply investigated by Nasr Esfahani et al. (2016). Chickpea (C. arietinum L. cultivar ILC482) was inoculated with two symbionts (McCP-31 and MmSWRI9) of the genus Mesorhizobium. ILC482 is a high-yielding elite Kabuli variety with relatively high adaptability to water scarcity (Rozrokh et al. 2012). McCP-31-inoculated plants showed bigger nodule dry mass and enhanced SNF than the MmSWRI9 ones. Metabolic profiling revealed that differential responses in C and N metabolism-related metabolites were observed between MmSWRI9- and McCP-31-inoculated plants in response to Pi deficiency. Pi deficiency significantly increased the levels of amino acid as asparagine, homoserine, isoleucine, 3-cyano-L-alanine, methionine, lysine, tyrosine, and phenylalanine in the MmSWRI9-induced nodules. On the other side, the McCP-31-induced nodules showed significantly increased levels of glutamine, GABA, L-alanine, 3-cyano-L-alanine, aspartate, glutamate, threonine, lysine, and 5-oxoproline. The total level of identified sugars was increased by 68.8% in McCP-31-induced nodules, whereas the level of total detected sugars in MmSWRI9-induced nodules remained unchanged under Pi starvation. When analyzing the differences in terms of organic acids, the levels of malate, glycolate, malonate, and isocitrate were decreased in the MmSWRI9-nodulated roots, whereas the levels of α-ketoglutarate, malate, succinate, citramalate, galactonate, threonate, itaconate, and threonic acid-1,4-lactone displayed significant accumulation in the McCP-31-nodulated roots under Pi-deficient conditions. These metabolomic data results unshaded the complex cross talk among numerous signaling pathways in the regulation of Mesorhizobium–chickpea adaptation to Pi limitation . Nevertheless, it cannot be disregarded that other master players, such as miRNAs, might also contribute to regulation of SNF capacity in chickpea under low Pi availability (Nasr Esfahani et al. 2016).
MiRNA-mediated regulation of gene expression plays essential roles in almost all biological processes in plants including the modulation of legume response to Pi starvation (Jones-Rhoades et al. 2006; Zeng et al. 2010; Branscheid et al. 2010; Peláez et al. 2012; Ramírez et al. 2013). MiRNAs, as a class of small noncoding (20–24 nucleotides) RNAs, act at the posttranscriptional level leading to gene silencing through mRNA cleavage and translation repression based on sequence complementation (Bartel 2004). An miRNA macroarray was used to detect miRNAs in the leaves, roots, and nodules of control and Pi-deprived common bean plants (Valdés-López et al. 2010). In this study, several miRNAs that have never been reported as Pi stress responsive in other plant species (e.g., miR157, miR160, miR165/miR166, miR169, miR393, pvu-miR2118) were differentially regulated under Pi deficiency in one or more common bean organs. MiR172, which targets the transcription factor APETALA2-related (AP2), was the only miRNA detected exclusively in common bean nodules, and its expression was slightly increased under Pi deprivation. MiR172 and AP2 have been previously associated with Pi starvation in roots and nodules of common bean (Hernández et al. 2009), as well as M. truncatula (Lelandais-Briere et al. 2009). Although miR172-mediated improvement of SNF in common bean R. etli was functionally validated (Nova-Franco et al. 2015), its role in Pi-mediated responses needs to be further elucidated.
The identification of genotypes with improved performances under Pi-limited environments opens new possibilities to understand which are the molecular players underlying such response. In an attempt to identify proteins responsive to Pi deficiency in the Pi-efficient soybean cultivar BX10, Sha et al. (2016) conducted a proteomic study, in which the protein accumulation profiles of shoots and roots were studied under Pi-deficient and Pi-sufficient conditions. Among the 88 proteins identified, 61 were responsive to Pi deficiency, most of them being described for the first in response to Pi starvation. Interestingly, several proteins associated with energy metabolism (e.g., vacuolar ATPase subunit B, ATP synthase CF1 α subunit) and cell cycle and division (e.g., patellin, actin-depolymerizing factor 1) were accumulated under Pi deficiency, indicating possible mechanisms activated to ensure of Pi homeostasis.
3.5 Conclusions
Legumes are well recognized for their nutritional and health benefits as well as for their impact in the sustainability of agricultural systems, due to their ability to form symbiosis with N2-fixing microorganisms. Nevertheless, legume N2 fixation has a high-energy cost, and Pi deficiency strongly hampers legume production, especially in low Pi soils typical of most of the tropical regions. The identification of legume genotypes with contrasting utilization of Pi for SNF, resulting from running breeding programs, has provided excellent models to study the molecular mechanisms underlying N2 fixation impairment under Pi deficiency.
Legumes have evolved a complexity of mechanisms to cope with Pi limitation, which relies on strategies that aim to enhance Pi acquisition or improve its remobilization under Pi limitation. Roots have been the privileged studied organ, not only because they are the first organ to sense Pi shortage but also because of their role in SNF. The maintenance of SNF seems to be a key aspect to assure legume productivity in low Pi environments. To ensure proper SNF under Pi shortage, several strategies to maintain higher Pi concentrations in nodules were described. They include morphological and architectural changes in root and shoot traits, alteration in overall C and N whole-plant metabolism, and exudation of organic acids or Pi-releasing enzymes coupled to an enhanced Pi uptake system, mediated by specific Pi transporters. Many genes, enzymes, and miRNAs are involved in enhanced SNF under Pi-limited environments through the use of simple molecular biology and functional biology approaches. Nevertheless, the recent emergence of “holistic” omics-based studies will certainly make significant advances beyond the current state of the art.
Although considerable research efforts have been carried out to understand the molecular and regulatory mechanisms responsible for SNF under Pi limitation, the knowledge on this topic is still scarce. This also includes studies looking for alternative and optimized legume–rhizobium associations or the development of improved strains. In addition to this, in natural ecosystems, the interaction with other environmental factors that affect soil properties, such as water deficit or soil acidity, has to be considered. Previous studies have shown that all these factors impact on O2, C, and N availability, crucial for N2 fixation homeostasis. More studies exploring the combination of abiotic stresses, focused on enhanced SNF, should be integral part of legume improvement breeding programs. They are expected to address efficiently the current and future demands of modern agriculture and food production presently exacerbated by the variability in global climate change.
References
Abdel-Latief K, Bogusz D, Hocher V (2012) The role of flavonoids in the establishment of plant roots endosymbioses with arbuscular mycorrhiza fungi, rhizobia and Frankia bacteria. Plant Signal Behav 7:636–641. doi:10.4161/psb.20039
Abdolzadeh A, Wang X, Veneklaas EJ, Lambers H (2010) Effects of phosphorus supply on growth, phosphate concentration and cluster-root formation in three Lupinus species. Ann Bot 105:365–374. doi:10.1093/aob/mcp297
Almeida JPF, Hartwig UA, Frehner M, Josef N, Lüscher A (2000) Evidence that P deficiency induces N feedback regulation of symbiotic N2 fixation in white clover (Trifolium repens L.) J Exp Bot 51:1289–1297. doi:10.1093/jexbot/51.348.1289
Alunni B, Gourion B (2016) Terminal bacteroid differentiation in the legume-rhizobium symbiosis: nodule-specific cysteine-rich peptides and beyond. New Phytol 211:411–417. doi:10.1111/nph.14025
Amon J, Titgemeyer F, Burkovski A (2010) Common patterns – unique features: nitrogen metabolism and regulation in Gram-positive bacteria. FEMS Microbiol Rev 34:588–605. doi:10.1111/j.1574-6976.2010.00216.x
Aparicio-Fabre R, Guillén G, Loredo M, Arellano J, Valdés-López O, Ramírez M, Iñiguez LP, Panzeri B, Castiglioni P, Cremonesi P, Stronzzi F, Stella A, Girard L, Sparvoli F, Hernández G (2013) Common bean (Phaseolus vulgaris L.) PvTIFY orchestrates global changes in transcript profile response to jasmonate and phosphorus deficiency. BMC Plant Biol 13:26. doi:10.1186/1471-2229-13-26
Aranjuelo I, Arrese-Igor C, Molero G (2014) Nodule performance within a changing environmental context. J Plant Physiol 171:1076–1090. doi:10.1016/j.jplph.2014.04.002
Araújo AP, Plassard C, Drevon JJ (2008) Phosphatase and phytase activities in nodules of common bean genotypes at different levels of phosphorus supply. Plant Soil 312:129–138. doi:10.1007/s11104-008-9595-3
Araújo SS, Beebe S, Crespi M, Delbreil B, González EM, Gruber V, Lejeune-Henaut I, Link W, Monteros MJ, Prats E, Rao I, Vadez V, Vaz Patto MC (2015) Abiotic stress responses in legumes: strategies used to cope with environmental challenges. Crit Rev Plant Sci 34:237–280. doi:10.1080/07352689.2014.898450
Arthikala M-K, Sanchez-Lopez R, Nava N, Santana O, Cárdenas L, Quinto C (2014) RbohB, a Phaseolus vulgaris NADPH oxidase gene, enhances symbiosome number, bacteroid size, and nitrogen fixation in nodules and impairs mycorrhizal colonization. New Phytol 202:886–900. doi:10.1111/nph.12714
Balemi T, Negisho K (2012) Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: a review. J Soil Sci Plant Nutr 12:547–561
Barraclough D (1995) 15N isotope dilution techniques to study soil nitrogen transformations and plant uptake. Fertil Res 42:185–192. doi:10.1007/BF00750513
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297. doi:10.1016/S0092-8674(04)00045-5
Batterman SA, Hedin LO, van Breugel M, Ransijn J, Craven DJ, Hall JS (2013) Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature 502:224–227. doi:10.1038/nature12525
Branscheid A, Sieh D, Pant BD, May P, Devers EA, Elkorg A, Schauser L, Scheible WR, Krajinski F (2010) Expression pattern suggests a role of MiR399 in the regulation of the cellular response to local Pi increase during arbuscular mycorrhizal symbiosis. Mol Plant-Microbe Interact 23:915–926. doi:10.1094/MPMI-23-7-0915
Breakspear A, Liu C, Roy S, Stacey N, Rogers C, Trick M, Morieri G, Mysore KS, Wen J, Oldroyd GE, Downie GA, Murray JD (2014) The root hair ‘infectome’ of Medicago truncatula uncovers changes in cell cycle genes and reveals a requirement for auxin signaling in rhizobial infection. Plant Cell 26:4680–4701. doi:10.1105/tpc.114.133496
Brockwell J, Bottomley PJ, Thies JE (1995) Manipulation of rhizobia microflora for improving legume productivity and soil fertility: a critical assessment. Plant Soil 174:143–180. doi:10.1007/BF00032245
Browse J (2009) Jasmonate passes muster: a receptor and targets for the defense hormone. Annu Rev Plant Biol 60:183–205. doi:10.1146/annurev.arplant.043008.092007
Bullerjahn GS, Post AF (2014) Physiology and molecular biology of acquatic cyanobacteria. Front Microbiol 5:359. doi:10.3389/fmicb.2014.00359
Cabeza RA, Liese R, Lingner A, Stieglitz I, Neumann J, Salinas-Riester G, Pommerenke C, Dittert K, Schulze J (2014) RNA-seq transcriptome profiling reveals that Medicago truncatula nodules acclimate N2 fixation before emerging P deficiency reaches the nodules. J Exp Bot 65:6035–6048. doi:10.1093/jxb/eru341
Cabeza RA, Liese R, Fischinger SA, Sulieman S, Avenhaus U, Lingner A, Hein H, Koester B, Baumgarten V, Dittert K, Schulze J (2015) Long-term non-invasive and continuous measurements of legume nodule activity. Plant J 81:637–648. doi:10.1111/tpj.12751
Campbell BM, Thornton P, Zougmoré R, van Asten P, Lipper L (2014) Sustainable intensification: what is its role in climate smart agriculture? Curr Opin Environ Sustain 8:39–43. doi:10.1016/j.cosust.2014.07.002
Cardenas L, Martınez A, Sanchez F, Quinto C (2008) Fast, transient and specific intracellular ROS changes in living root hair cells responding to Nod factors (NFs). Plant J 56:802–813. doi:10.1111/j.1365-313X.2008.03644.x
Cardoso P, Freitas R, Figueira E (2015) Salt tolerance of rhizobial populations from contrasting environmental conditions: understanding the implications of climate change. Ecotoxicology 24:143–152. doi:10.1007/s10646-014-1366-8
Casteriano A, Wilkes MA, Deaker R (2013) Physiological changes in rhizobia after growth in peat extract may be related to improved desiccation tolerance. Appl Environ Microbiol 79:3998–4007. doi:10.1128/AEM.00082-13
Cernay C, Ben-Ari T, Pelzer E, Meynard J-M, Makowski D (2015) Estimating variability in grain legume yields across Europe and the Americas. Sci Rep 5:11171. doi:10.1038/srep11171
Chaia EE, Wall LG, Huss-Danell K (2010) Life in soil by the actinorhizal root nodule endophyte Frankia. A review. Symbiosis 5:201–226. doi:10.4161/psb.25453
Chandra A (2009) Diversity among Stylosanthes species: habitat, edaphic and agro-climatic affinities leading to cultivar development. J Environ Biol 30:471–478
Chaudhary MI, Adu-Gyamfi JJ, Saneoka H, Nguyen NT, Suwa R, Kanai S, El-Shemy HA, Lightfoot DA, Fujita K (2008) The effect of phosphorus deficiency on nutrient uptake, nitrogen fixation and photosynthetic rate in mashbean, mungbean and soybean. Acta Physiol Plant 30:537–544. doi:10.1007/s11738-008-0152-8
Chen C, Zhu H (2013) Are common symbiosis genes required for endophytic rice-rhizobial interactions? Plant Signal Behav 8:e25453. doi:10.4161/psb.25453
Chen YL, Dunbabin VM, Diggle AJ, Siddique KHM, Rengel Z (2013) Phosphorus starvation boosts carboxylate secretion in P-deficient genotypes of Lupinus angustifolius with contrasting root structure. Crop Pasture Sci 64:588–599. doi:10.1071/CP13012
Chiou T-J, Lin S-I (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62:185–206. doi:10.1146/annurev-arplant-042110-103849
Codex Alimentarius (2007) Cereals, pulses, legumes and vegetable proteins, 1st edn. WHO & FAO, Rome
COP21 (2015) http://www.cop21.gouv.fr/en/more-details-about-the-agreement/
Crews TE, Peoples MB (2004) Legume versus fertilizer sources of nitrogen. Agric Ecosyst Environ 102:279–297
Crook AD, Schnabel EL, Frugoli JA (2016) The systemic nodule number regulation kinase SUNN in Medicago truncatula interacts with MtCLV2 and MtCRN. Plant J. doi:10.1111/tpj.13234
Cytryn EJ, Sangurdekar DP, Streeter JG, Franck WL, Chang WS, Stacey G, Emerich DW, Joshi T, Xu D, Sadowsky MJ (2007) Transcriptional and physiological responses of Bradyrhizobium japonicum to desiccation induced stress. J Bacteriol 189:6751–6762. doi:10.1128/JB.01543-07
Czarnecki O, Yang J, Weston DJ, Tuskan GA, Chen J-G (2013) A dual role of strigolactones in phosphate acquisition and utilization in plants. Int J Mol Sci 14:7681–7701. doi:10.3390/ijms14047681
Danso SKA (1995) Assessment of biological nitrogen fixation. Fertil Res 42:33–41. doi:10.1007/BF00750498
de Lima M, Oresnik IJ, Fernando SM, Hunt S, Smith R, Turpin DH, Layzell DB (1994) The relationship between nodule adenylates and the regulation of nitrogenase activity by oxygen in soybean. Physiol Plant 91:687–695. doi:10.1111/j.1399-3054.1994.tb03006.x
Denison RF, Kiers ET (2004) Lifestyle alternatives for rhizobia: mutualism, parasitism, and forgoing symbiosis. FEMS Microbiol Lett 237:187–193. doi:10.1016/j.femsle.2004.07.013
Dilworth MJ (1966) Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim Biophys Acta 127:285–294. doi:10.1016/0304-4165(66)90383-7
Djordjevic MA, Mohd-Radzman NA, Imin N (2015) Small peptide signals that control root nodule number, development, and symbiosis. J Exp Bot 66:5171–5181. doi:10.1093/jxb/erv357
Duff SMG, Sarath G, Plaxton WC (1994) The role of acid phosphatases in plant phosphorus metabolism. Physiol Plant 90:791–800. doi:10.1111/j.1399-3054.1994.tb02539.x
FAO (2013) Synthesis of guided principles on agriculture programming for nutrition. FAO, Rome
FAO (2015) Coping with climatic changes. The roles of genetic resources for food and agriculture. FAO, Rome
FAO (2016) http://www.fao.org/pulses-2016/en/
Fischinger SA, Schulze J (2010) The argon-induced decline in nitrogenase activity commences before the beginning of a decline in nodule oxygen uptake. J Plant Physiol 167:1112–1115. doi:10.1016/j.jplph.2010.03.014
Fournier J, Teillet A, Chabaud M, Ivanov S, Genre A, Limpens E, de Carvalho-Niebel F, Barker DG (2015) Remodeling of the infection chamber before infection thread formation reveals a two-step mechanism for rhizobial entry into the host legume root hair. Plant Physiol 167:1233–1242. doi:10.1104/pp.114.253302
Freund DM, Hegeman AD (2016) Recent advances in stable isotope-enabled mass spectrometry-based plant metabolites. Curr Opin Biotechnol 43:41–48. http://dx.doi.org/10.1016/j.copbio.2016.08.002
Goh C-H, Veliz Vallejos DF, Nicotra AB, Mathesius U (2013) The impact of beneficial plant-associated microbes on plant phenotypic plasticity. J Chem Ecol 39:826–839. doi:10.1007/s10886-013-0326-8
Gonzalez EM, Aparicio-Tejo PM, Gordon AJ, Minchin JR, Royuela M, Arrese-Igor C (1998) Water-deficit effects on carbon and nitrogen metabolism of pea nodules. J Exp Bot 49:1705–1714. doi:10.1093/jxb/49.327.1705
Gordon AJ, Skot L, James CL, Minchin FR (2002) Short-term metabolic responses of soybean root nodules to nitrate. J Exp Bot 53:423–428. doi:10.1093/jexbot/53.368.423
Guinel FC (2015) Ethylene a hormone at the center-stage of nodulation. Front Plant Sci 6:1121. doi:10.3389/fpls.2015.01121
Guo WB, Zhao J, Li XX, Qin L, Yan XL, Liao H (2011) A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J 66:541–552. doi:10.1111/j.1365-313X.2011.04511.x
Gupta R, Bisaria VS, Sharma S (2015) Effect of agricultural amendments on Cajanus cajan (pigeon pea) and its rhizospheric microbial communities – a comparison between chemical fertilizers and bioinoculants. PLoS One 10:e0132770. doi:10.1371/journal.pone.0132770
Gutiérrez-Boem FH, Thomas GW (1999) Phosphorus nutrition and water deficit in field-grown soybeans. Plant Soil 207:87–96. doi:10.1023/A:1004469403667
Gyaneshwar P, Hirsch AM, Moulin L, Chen WM, Elliott GN, Bontemps C, Estrada-de Los Santos P, Gross E, Dos Reis FB, Sprent JI, Young JP, James EK (2011) Legume-nodulating betaproteobacteria: diversity, host range, and future prospects. Mol Plant Microbe Interact 24:1276–1288. doi:10.1094/MPMI-06-11-0172
Haag AF, Arnold MF, Myka KK, Kerscher B, Dall’Angelo S, Zanda M, Mergaert P, Ferguson GP (2013) Molecular insights into bacteroid development during Rhizobium-legume symbiosis. FEMS Microbiol Rev 37:364–383. doi:10.1111/1574-6976.12003
Hammond JP, White PJ (2011) Sugar signaling in root responses to low phosphorus availability. Plant Physiol 156:1033–1040. doi:10.1104/pp.111.175380
Hardy RWF, Holsten RD, Jackson EK, Burns RC (1968) The acetylene-ethylene assay for N2-fixation: laboratory and field evaluation. Plant Physiol 43:1185–1207. doi:10.1104/pp.43.8.1185
Hernández G, Ramírez M, Valdés-López O, Tesfaye M, Graham MA, Czechowski T, Schlereth A, Wandrey M, Erban A, Cheung F, Wu HC, Lara M, Town CD, Kopka J, Udvardi MK, Vance CP (2007) Phosphorus stress in common bean: root transcript and metabolic responses. Plant Physiol 144:752–767. doi:10.1104/pp.107.096958
Hernández G, Valdés-López O, Ramírez M, Goffard N, Weiller G, Aparicio-Fabre R, Fuentes SI, Erban A, Kopka J, Udvardi MK, Vance CP (2009) Global changes in the transcript and metabolic profiles during symbiotic nitrogen fixation in phosphorus-stressed common bean plants. Plant Physiol 151:1221–1238. doi:10.1104/pp.109.143842
Høgh-Jensen H, Schjoerring JK, Soussana J-F (2002) The influence of phosphorus deficiency on growth and nitrogen fixation of white clover plants. Ann Bot 90:745–753. doi:10.1093/aob/mcf260
Hu Y, Ribbe MW (2015) Nitrogenase and homologs. J Biol Inorg Chem 20:435–445. doi:10.1007/s00775-014-1225-3
Hunt S, King BJ, Canvin DT, Layzell DB (1987) Steady and non steady state gas exchange characteristics of soybean nodules in relation to the oxygen diffusion barrier. Plant Physiol 84:164–172
IPCC (2007) Climate change impacts, adaptation and vulnerability working group II. Contribution to the intergovernmental panel on climate change. In Fourth Assessment Report. Summary for policymakers
James EK, Olivares FL, Baldani JI, Döbereiner J (1997) Herbaspirillum, an endophytic diazotroph colonizing vascular tissue in the leaves of Sorghum bicolor. J Exp Bot 48:785–798. doi:10.1093/jxb/48.3.785
Ji KX, Chi F, Yang MF, Shen SH, Jing YX, Dazzo FB, Cheng HP (2010) Movement of rhizobia inside tobacco and lifestyle alternation from endophytes to free-living rhizobia on leaves. J Microbiol Biotechnol 20:238–244. doi:10.4014/jmb.0906.06042
Jia H, Ren H, Gu M, Zhao J, Sun S, Zhang X, Chen J, Wu P, Xu G (2011) The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol 156:1164–1175. doi:10.1104/pp.111.175240
Jones-Rhoades MW, Bartel DP, Bartel B (2006) MicroRNAS and their regulatory roles in plants. Annu Rev Plant Biol 57:19–53. doi:10.1146/annurev.arplant.57.032905.105218
Kerr RB, Shumba L, Dakishoni L, Lupafya E, Berti PR, Classen L, Snapp SS, Katundu M (2013) Participatory, agroecological and gender-sensitive approaches to improved nutrition: a case study in Malawi. FAO Expert Meeting ‘Nutrition-Sensitive Food and Agriculture Systems’
Kleinert A, Venter M, Kossmann J, Valentine A (2014) The reallocation of carbon in P deficient lupins affects biological nitrogen fixation. J Plant Physiol 171:1619–1624. doi:10.1016/j.jplph.2014.07.017
Knowles R (1980) Nitrogen fixation in natural plant communities and soils. In: Bergersen FJ (ed) Methods for evaluating biological nitrogen fixation. Wiley, New York, pp 557–582
Kouas S, Labidi N, Debez A, Abdelly C (2005) Effect of P on nodule formation and N fixation in bean. Agron Sustain Dev 25:389–393. doi:10.1051/agro:2005034
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103. doi:10.1016/j.tree.2007.10.008
Larrainzar E, Riely BK, Kim SC, Carrasquilla-Garcia N, Yu HJ, Hwang HJ, Oh M, Kim GB, Surendrarao AK, Chasman D, Siahpirani AF, Penmetsa RV, Lee GS, Kim N, Roy S, Mun JH, Cook DR (2015) Deep sequencing of the Medicago truncatula root transcriptome reveals a massive and early interaction between nodulation factor and ethylene signals. Plant Physiol 169:233–265. doi:10.1104/pp.15.00350
Layzell DB, Gaito ST, Hunt S (1988) Model of gas exchange and diffusion in legume nodules (I. Calculation of gas exchange rates and the energy cost of N2 fixation). Planta 173:117–127. doi:10.1007/BF00394496
Lazali M, Zaman-Allah M, Amenc L, Ounane G, Abadie J, Drevon JJ (2013) A phytase gene is overexpressed in root nodules cortex of Phaseolus vulgaris–rhizobia symbiosis under phosphorus deficiency. Planta 238:317–324. doi:10.1007/s00425-013-1893-1
Lelandais-Briere C, Naya L, Sallet E, Calenge F, Frugier F, Hartmann C, Gouzy J, Crespi M (2009) Genome-wide Medicago truncatula small RNA analysis revealed novel microRNAs and isoforms differentially regulated in roots and nodules. Plant Cell 21:2780–2796. doi:10.1105/tpc.109.068130
Li L, Zhang F, Li X, Christie P, Sun J, Yang S, Tang C (2003) Interspecific facilitation of nutrient uptake by intercropped maize and faba bean. Nutr Cycl Agroecosyst 65:61–71. doi:10.1023/A:1021885032241
Li C, Gui S, Yang T, Walk T, Wang X, Liao H (2012) Identification of soybean purple acid phosphatase genes and their expression responses to phosphorus availability and symbiosis. Ann Bot 109:275–285. doi:10.1093/aob/mcr246
Li C, Dong Y, Li H, Shen J, Zhang F (2015a) Shift from complementarily to facilitate on P uptake by intercropped wheat neighboring with faba bean when available soil P is depleted. Sci Rep 6:18663. doi:10.1038/srep18663
Li Y, Xu M, Wang N, Li Y (2015b) A JAZ protein in Astragalus sinicus interacts with a leghemoglobin through the TIFY domain and is involved in nodule development and nitrogen fixation. PLoS One 10:1–18. doi:10.1371/journal.pone.0139964
Lin W-Y, Lin S-I, Chiou T-J (2009) Molecular regulators of phosphate homeostasis in plants. J Exp Bot 60:1427–1438. doi:10.1093/jxb/ern303
Lin W-Y, Huang T-K, Leong SJ, Chiou T-J (2014) Long-distance call from phosphate: systemic regulation of phosphate starvation responses. J Exp Bot 65:1817–1827. doi:10.1093/jxb/ert431
Liu J, Samac DA, Bucciarelli B, Allan DL, Vance CP (2005) Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. Plant J 41:257–268. doi:10.1111/j.1365-313X.2004.02289.x
Liu P, Chen S, Song A, Zhao S, Fang W, Guan Z, Liao Y, Jiang J, Chen F (2014a) A putative high affinity phosphate transporter, CmPT1, enhances tolerance to Pi deficiency of chrysanthemum. BMC Plant Biol 14:18. doi:10.1186/1471-2229-14-18
Liu C-W, Sung Y, Chen B-C, Lai H-Y (2014b) Effects of nitrogen fertilizers on the growth and nitrate content of lettuce (Lactuca sativa L.) Int J Environ Res Public Health 11:4427–4440. doi:10.3390/ijerph110404427
Liu P, Xue Y-B, Chen Z-J, Liu G-D, Tian J (2016) Characterization of purple acid phosphatases involved in extracellular dNTP utilization in Stylosanthes. J Exp Bot 67:4141–4154. doi:10.1093/jxb/erw190
Lohar DP, Haridas S, Gantt JS, VandenBosch KA (2007) A transient decrease in reactive oxygen species in roots leads to root hair deformation in the legume-rhizobia symbiosis. New Phytol 173:39–49. doi:10.1111/j.1469-8137.2006.01901.x
Magadlela A, Kleinert A, Dreyer L, Valentine AJ (2014) Low-phosphorus conditions affect the nitrogen nutrition and associated carbon costs of two legume tree species from a Mediterranean-type ecosystem. Aust J Bot 62:1–9. doi:10.1071/BT13264
Marlow VL, Haah AF, Kobayashi H, Fletcher V, Scocchi M, Walker GC, Ferguson GP (2009) Essential role for the BacA protein in the uptake of a truncated eukaryotic peptide in Sinorhizobium meliloti. J Bacteriol 191:1519–1527. doi:10.1128/JB.01661-08
Marschner H, Marschner P (2012) Marschner’s mineral nutrition of higher plants. Academic Press, Amsterdam
Matar A, Torrent J, Ryan J (1992) Soil and fertilizer phosphorus and crop responses in the dryland mediterranean zone. Adv Soil Sci 18:81–146. doi:10.1007/978-1-4612-2844-8_3
McAuliffe CC, Chamblee DS, Uribe-Arango H, Woodhouse WW Jr (1958) Influence of inorganic nitrogen on nitrogen fixation by legumes as revealed by 15N dilution method. Agron J 50:334–337. doi:10.2134/agronj1958.00021962005000060014x
McIntyre HJ, Davies H, Hore TA, Miller SH, Dufour JP, Ronson CW (2007) Trehalose biosynthesis in Rhizobium leguminosarum bv. trifolii and its role in desiccation tolerance. Appl Environ Microbiol 73:3984–3992. doi:10.1128/AEM.00412-07
Mergaert P, Uchiumi T, Alunni B, Evanno G, Cheron A, Catrice O, Mausset AE, Barloy-Hubler F, Galibert F, Kondorosi A, Kondorosi E (2006) Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc Natl Acad Sci U S A 103:5230–5235. doi:10.1073/pnas.0600912103
Messina MJ (1999) Legumes and soybeans: overview of their nutritional profiles and health effects. Am J Clin Nutr 70:439–450
Mhamdi R, Nouairi I, ben Hammouda T, Mhamdi R, Mhadhbi H (2015) Growth capacity and biochemical mechanisms involved in rhizobia tolerance to salinity and water deficit. J Basic Microbiol 55:451–461. doi:10.1002/jobm.201400451
Miguel MA, Widrig A, Vieira RF, Brown KM, Lynch JP (2013) Basal root whorl number: a modulator of phosphorus acquisition in common bean (Phaseolus vulgaris). Ann Bot 112:973–982. doi:10.1093/aob/mct164
Minami T, Anda M, Mitsui H, Sugawara M, Kaneko T, Sato S, Ikeda S, Okubo T, Tsurumaro H, Minamisawa K (2016) Metagenomic analysis revealed methylamine and ureide utilization of soybean-associated Methylbacterium. Microbes Environ. doi:10.1264/jsme2.ME16035
Minchin FR, Sheehy JE, Witty JF (1986) Further errors in the acetylene reduction assay: effect of plant disturbance. J Exp Bot 37:1581–1591
Mochida K, Shinozaki K (2011) Advances in omics and bioinformatics tools for systems analyses of plant functions. Plant Cell Physiol 52:2017–2038. doi:10.1093/pcp/pcr153
Molero G, Aranjuelo I, Teixidor P, Araus JL, Nogue S (2011) Measurement of 13C and 15N isotope labeling by gas chromatography/combustion/isotope ratio mass spectrometry to study amino acid fluxes in a plant–microbe symbiotic association. Rapid Commun Mass Spectrom 25:599–607. doi:10.1002/rcm.4895
Moloney AHM, Layzell DB (1993) A model of the regulation of nitrogenase electron allocation in legume nodules (I. The diffusion barrier and H2 inhibition of N2 fixation). Plant Physiol 103:421–428
Moloney AHM, Guy RD, Layzell DB (1994) A model of the regulation of nitrogenase electron allocation in legume nodules (II. Comparison of empirical and theoretical studies in soybean). Plant Physiol 104:541–550
Mori A, Fukuda T, Vejchasarn P, Nestler J, Pariasca-Tanaka J, Wissuwa M (2016) The role of root size versus root efficiency in phosphorus acquisition in rice. J Exp Bot 67:1179–1189. doi:10.1093/jxb/erv557
Mortier V, Holsters M, Goormachtig S (2012) Never too many? How legumes control nodule numbers. Plant Cell Environ 35:245–258. doi:10.1111/j.1365-3040.2011.02406.x
Mus F, Crook MB, Garcia K, Costas AG, Geddes BA, Kouri ED, Paramasivan P, Ryu M-H, Oldroyd GED, Poole PS, Udvardi MK, Voigt CA, Ané J-M, Peters JW (2016) Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl Environ Microbiol 82:3698–3710. doi:10.1128/AEM.01055-16
Nasr Esfahani MN, Kusano M, Nguyen KH, Watanabe Y, Van Ha C, Saito K, Sulieman S, Herrera-Estrella L, Tran L-SP (2016) Adaption of the symbiotic Mesorhizobium –chickpea relationship to phosphate deficiency relies on reprogramming of whole-plant metabolism. Proc Natl Acad Sci 113:4610–4619. doi:10.1073/pnas.1609440113
Negi M, Sanagala R, Rai V, Jain A (2016) Deciphering phosphate deficiency-mediated temporal effects on different root traits in rice grown in a modified hydroponic system. Front Plant Sci 7:550. doi:10.3389/fpls.2016.00550
Nova-Franco B, Íñiguez LP, Valdés-López O, ALvarado-Affantranger X, Leija A, Fuentes SI, Ramírez M, Paul S, Reyes JL, Girard L, Hernández G (2015) The micro-RNA172c-APETALA2-1 node as a key regulator of the common bean- Rhizobium etli nitrogen fixation symbiosis. Plant Physiol 168:273–291. doi:10.1104/pp.114.255547
Ohkama-Ohtsu N, Ichida S, Yamaya H, Ohwada T, Itakura M, Hara Y, Mitsui H, Kaneko T, Tabata S, Tejima K, Saeki K, Omori H, Hayashi M, Maekawa T, Murooka Y, Tajima S, Simomura K, Nomura M, Uchiumi T, Suzuki A, Shimoda Y, Abe M, Minamisawa K, Arima Y, Yokoyama T (2015) Peribacteroid solution of soybean root nodules partly induces genomic loci for differentiation into bacteroids of free-living Bradyrhizobium diazoefficiens cells. Soil Sci Plant Nutr 61:461–470. doi:10.1080/00380768.2014.994470
Oldroyd GE, Murray JD, Poole PS, Downie JA (2011) The rules of engagement in the legume-rhizobial symbiosis. Annu Rev Genet 45:119–144. doi:10.1146/annurev-genet-110410-132549
Olivera M, Tejera N, Iribarne C, Ocaña A, Lluch C (2004) Growth, nitrogen fixation and ammonium assimilation in common bean (Phaseolus vulgaris): effect of phosphorus. Physiol Plant 121:498–505. doi:10.1111/j.0031-9317.2004.00355.x
Peláez P, Trejo MS, Iñiguez LP, Estrada-Navarrete G, Covarrubias AA, Reyes JL, Sanchez F (2012) Identification and characterization of microRNAs in Phaseolus vulgaris by high-throughput sequencing. BMC Genomics 13:83. doi:10.1186/1471-2164-13-83
Peleg-Grossman S, Volpin H, Levine A (2007) Root hair curling and Rhizobium infection in Medicago truncatula are mediated by phosphatidylinositide regulated endocytosis and reactive oxygen species. J Exp Bot 58:1637–1649. doi:10.1093/jxb/erm013
Plaxton WC, Tran HT (2011) Metabolic adaptations of phosphate-starved plants. Plant Physiol 156:1006–1015. doi:10.1104/pp.111.175281
Priefer UB, Aurag J, Boesten B, Bouhmouch I, Defez R, Filali-Maltouf A, Miklis M, Moawad H, Mouhsine B, Prell J, Schluter A, Senatore B (2001) Characterisation of Phaseolus symbionts isolated from Mediterranean soils and analysis of genetic factors related to pH tolerance. J Biotechnol 91:223–236. doi:10.1016/S0168-1656(01)00329-7
Qin L, Zhao J, Tian J, Chen L, Sun Z, Guo Y, Lu X, Gu M, Xu G, Liao H (2012) The high-affinity phosphate transporter GmPT5 regulates phosphate transport tonodules and nodulation in soybean. Plant Physiol 159:1634–1643. doi:10.1104/pp.112.199786
Qin S, Tang Y, Chen Y, Wu P, Li M, Wu G, Jiang H (2016) Overexpression of the starch phosphatase-like gene (PHO3) in Lotus japonicus has a profound effect on the growth of plants and reduction of transitory starch accumulation. Front Plant Sci 7:1315. doi:10.3389/fpls.2016.01315
Ramírez M, Flores-Pacheco G, Reyes JL, Luzlvarez A, Drevon JJ, Girard L, Hernández G (2013) Two common bean genotypes with contrasting response to phosphorus deficiency show variations in the microRNA 399-mediated PvPHO2 regulation within the PvPHR1 signaling pathway. Int J Mol Sci 14:8328–8344. doi:10.3390/ijms14048328
Ramos MLG, Gordon AJ, Minchin FR, Sprent JI, Parsons R (1999) Effect of water stress on nodule physiology and biochemistry of a drought tolerant cultivar of common bean (Phaseolus vulgaris L.) Ann Bot 83:57–63
Reed SC, Cleveland CC, Townsend AR (2011) Functional ecology of free-living nitrogen fixation: a contemporary perspective. Annu Rev Ecol Evol Syst 42:489–512. doi:10.1146/annurev-ecolsys-102710-145034
Reid DE, Heckmann AB, Novák O, Kelly S, Stougaard J (2016) CYTOKININ OXIDASE/DEHYDROGENASE3 maintains cytokinin homeostasis during root and nodule development in Lotus japonicus. Plant Physiol 170:1060–1074. doi:10.1104/pp.15.00650
Rejili M, Mahdhi M, Fterich A, Dhaoui S, Guefrachi I, Andeddayem R, Mars M (2012) Symbiotic nitrogen fixation of wild legumes in Tunisia: soil fertility dynamics, field nodulation and nodules effectiveness. Agric Ecosyst Environ 157:60–69. doi:10.1016/j.agee.2012.01.015
Ribaudo M, Delgado J, Hansen L, Livingston M, Mosheim R, Williamson J (2011) Nitrogen in agricultural systems: implications for conservation policy, USDA Economic Research report, vol 127. BiblioGov, Charleston. ERR-127. U.S. Dept. of Agriculture, Econ. Res. Serv. September 2011
Richardson AE, Simpson RJ (2011) Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol 56:989–996. doi:10.1104/pp.111.175448
Richardson AE, Lynch JP, Ryan PR, Delhaize E, Smith FA, Smith SE, Harvey PR, Ryan MH, Veneklaas EJ, Lambers H, Oberson A, Culvenor RA, Simpson RJ (2011) Plant and microbial strategies to improve the phosphorus efficiency of agriculture. Plant Soil 349:121–156. doi:10.1007/s11104-011-0950-4
Rivas R, Willems A, Subba-Rao NS, Mateos PF, Dazzo FB, Kroppenstedt RM, Martínez-Molina E, Gillis M, Velázquez E (2003) Description of Devosia neptuniae sp. nov. that nodulates and fixes nitrogen in symbiosis with Neptunia natans, an aquatic legume from India. Syst Appl Microbiol 26:47–53. doi:10.1078/072320203322337308
Rodrı́guez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339. doi:10.1016/S0734-9750(99)00014-2
Rozrokh M, Sabaghpour SH, Armin M, Asgharipour M (2012) The effects of drought stress on some biochemical traits in twenty genotypes of chickpea. Eur J Exp Biol 2:1980–1987
Ruiz-Lozano JM (2003) Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 13:309–317. doi:10.1007/s00572-003-0237-6
Rychter AM, Randall DD (1994) The effect of phosphate deficiency on carbohydrate metabolism in bean roots. Physiol Plant 91:383–388. doi:10.1111/j.1399-3054.1994.tb02964.x
Sa T-M, Israel DW (1991) Energy status and functioning of phosphorus-deficient soybean nodules. Plant Physiol 97:928–935
Sahoo RK, Ansari MW, Dangar TK, Mohanty S, Tuteja N (2014) Phenotypic and molecular characterisation of efficient nitrogen-fixing Azotobacter strains from rice fields for crop improvement. Protoplasma 251:511–523. doi:10.1007/s00709-013-0547-2
Saia S, Amato G, Frenda AS, Giambalvo D, Ruisi P (2014) Influence of arbuscular mycorrhizae on biomass production and nitrogen fixation of berseem clover plants subjected to water stress. PLoS One 9:e90738. doi:10.1371/journal.pone.0090738
Salazar-Henao JE, Vélez-Bermúdez IC, Schmidt W (2016) The regulation and plasticity of root hair patterning and morphogenesis. Development 143:1848–1858. doi:10.1242/dev.132845
Sarker BC, Karmoker JL (2011) Effects of phosphorus deficiency on accumulation of biochemical compounds in lentil (Lens culinaris Medik.) Bangladesh J Bot 40:23–27. doi:10.3329/bjb.v40i1.7992
Schaller GE, Bishopp A, Kieber JJ (2015) The yin-yang of hormones: cytokinin and auxin interactions in plant development. Plant Cell 27:44–63. doi:10.1105/tpc.114.133595
Schulze J (2004) How are nitrogen fixation rates regulated in legumes? J Plant Nutr Soil Sci 167:125–137. doi:10.1002/jpln.200320358
Schulze J, Drevon J-J (2005) P-deficiency increases the O2 uptake per N2 reduced in alfalfa. J Exp Bot 56:1779–1784. doi:10.1093/jxb/eri166
Schulze J, Temple G, Temple SJ, Beschow H, Vance CP (2006) Nitrogen fixation by white lupin under phosphorus deficiency. Ann Bot 98:731–740. doi:10.1093/aob/mcl154
Seefeldt LC, Hoffman BH, Dean DR (2009) Mechanism of Mo-dependent nitrogenase. Annu Rev Biochem 78:701–722. doi:10.1146/annurev.biochem.78.070907.103812
Serraj R, Vadez V, Sinclair TR (2001) Feedback regulation of symbiotic N2 fixation under drought stress. Agronomie 21:621–626. doi:10.1051/agro:2001153
Sha A, Li M, Yang P (2016) Identification of phosphorus deficiency responsive proteins in a high phosphorus acquisition soybean (Glycine max) cultivar through proteomic analysis. Biochim Biophys Acta 1864:427–434. doi:10.1016/j.bbapap.2016.02.001
Shane MW, Lambers H (2005) Cluster roots: a curiosity in context. Plant Soil 274:101–125. doi:10.1007/s11104-004-2725-7
Shearer G, Kohl DH (1986) N2 fixation in field settings: estimations based on natural 15N abundance. Aust J Plant Physiol 13:699–756. doi:10.1071/PP9860699
Singh DK, Sale PWG, McKenzie BM (1997) Water relations of white clover (Trifolium repens L.) in a drying soil, as a function of phosphorus supply and defoliation frequency. Aust J Agric Res 48:675–681. doi:10.1071/A96156
Singh JS, Kumar A, Rai AN, Singh DP (2016) Cyanobacteria: a precious bio-resource in agriculture, ecosystem, and environmental sustainability. Front Microbiol 7:529. doi:10.3389/fmicb.2016.00529
Smith FW, Jackson WA (1987) Nitrogen enhancement of phosphate transport in roots of Zea mays L. (I. Effects of ammonium and nitrate pretreatment). Plant Physiol 84:1314–1318
Smith FA, Jakobsen I, Smith SE (2000) Spatial differences in acquisition of soil phosphate between two arbuscular mycorrhizal fungi in symbiosis with Medicago truncatula. New Phytol 147:357–366. doi:10.1046/j.1469-8137.2000.00695.x
Sprent JI, James EK (2007) Legume evolution: where do nodules and mycorrhizas fit in? Plant Physiol 144:575–581. doi:10.1104/pp.107.096156
Staudinger C, Mehmeti-Tershani V, Gil-Quintana E, Gonzalez EM, Hofhansl F, Bachmann G, Wienkoop S (2016) Evidence for a rhizobia-induced drought stress response strategy in Medicago truncatula. J Proteome 136:202–213. doi:10.1016/j.jprot.2016.01.006
Streeter JG (2003) Effect of trehalose on survival of Bradyrhizobium japonicum during desiccation. J Appl Microbiol 95:484–491. doi:10.1046/j.1365-2672.2003.02017.x
Sulieman S, Tran L-SP (2015) Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci 239:36–43. doi:10.1016/j.plantsci.2015.06.018
Sulieman S, Schulze J, Tran LS (2013a) Comparative analysis of the symbiotic efficiency of Medicago truncatula and Medicago sativa under phosphorus deficiency. Int J Mol Sci 14:5198–5213. doi:10.3390/ijms14035198
Sulieman S, Van Ha C, Schulze J, Tran LSP (2013b) Growth and nodulation of symbiotic Medicago truncatula at different levels of phosphorus availability. J Exp Bot 64:2701–2712. doi:10.1093/jxb/ert122
Sullivan BW, Smith WK, Townsend AR, Nasto MK, Reed SC, Chazdon RL, Cleveland CC (2014) Spatially robust estimates of biological nitrogen (N) fixation imply substantial human alteration of the tropical N cycle. Proc Natl Acad Sci U S A 111:8101–8106. doi:10.1073/pnas.1320646111
Suter M, Connolly J, Finn JA, Loges R, Kirwan L, Sebastia M-T, Lüscher A (2015) Nitrogen yield advantage from grass-legume mixtures is robust over a wide range of legume proportions and environmental conditions. Glob Chang Biol 21:2424–2438. doi:10.1111/gcb.12880
Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T (2007) Root tip contact with low-phosphate media reprograms plant root architecture. Nat Genet 39:792–796. doi:10.1038/ng2041
Tatsukami Y, Nambu M, Morisaka H, Kuroda K, Ueda M (2013) Disclosure of the differences of Mesorhizobium loti under the free-living and symbiotic conditions by comparative proteome analysis without bacteroid isolation. BMC Microbiol 13:180. doi:10.1186/1471-2180-13-180
Teijma K, Arima Y, Yokoama T, Sekimoto H (2003) Composition of amino acids, organic acids, and sugars in the peribacteroid space of soybean root nodules. Soil Sci Plant Nutr 49:239–247. doi:10.1080/00380768.2003.10410003
Tesfaye M, Liu J, Allan DL, Vance CP (2007) Genomic and genetic control of phosphate stress in legumes. Plant Physiol 144:594–603. doi:10.1104/pp.107.097386
Thuynsma R, Valentine A, Kleinert A (2014) Phosphorus deficiency affects the allocation of below-ground resources to combined cluster roots and nodules in Lupinus albus. J Plant Physiol 171:285–291. doi:10.1016/j.jplph.2013.09.001
Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S (2004) Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. Plant J 37:801–814. doi:10.1111/j.1365-313X.2004.02005.x
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418:671–677. doi:10.1038/nature01014
Toth K, Stacey G (2015) Does plant immunity play a critical role during initiation of the legume-rhizobium symbiosis? Front Plant Sci 6:401. doi:10.3389/fpls.2015.00401
Tredeser KK, Vitousek PM (2001) Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82:946–954. doi:10.1890/0012-9658(2001)082[0946:EOSNAO]2.0.CO;2
Uhde-Stone C, Zinn KE, Ramírez-Yañez M, Li A, Vance CP, Allan DL (2003) Nylon filter arrays reveal differential gene expression in proteoid roots of white lupin in response to phosphorus deficiency. Plant Physiol 131:1064–1079. doi:10.1104/pp.102.016881
Valdés-López O, Hernández G (2008) Transcriptional regulation and signaling in phosphorus starvation: what about legumes? J Integr Plant Biol 5:1213–1222. doi:10.1111/j.1744-7909.2008.00758.x
Valdés-López O, Yang SS, Aparicio-Fabre R, Graham PH, Reyes JL, Vance CP, Hernández G (2010) MicroRNA expression profile in common bean (Phaseolus vulgaris) under nutrient deficiency stresses and manganese toxicity. New Phytol 187:805–818. doi:10.1111/j.1469-8137.2010.03320.x
van Berkum PR, Tully E, Keister DL (1995) Nonpigmented and bacteriochlorophyll-containing bradyrhizobia isolated from Aeschynomene indica. Appl Environ Microbiol 61:623–629
van Noorden EE, Verbeek R, Dinh QD, Jin J, Green A, Ng JLP, Mathesius U (2016) Molecular signals controlling the inhibition of nodulation by nitrate in Medicago truncatula. Int J Mol Sci 17:1060. doi:10.3390/jims17071060
Vance CP, Uhde-Stone C, Allan D (2003) Phosphorus acquisition and use: critical adaptation by plants for securing non-renewable resources. New Phytol 157:423–447. doi:10.1046/j.1469-8137.2003.00695.x
Vanderlinde EM, Harrison JJ, Muszyński A, Carlson RW, Turner RJ, Yost CK (2010) Identification of a novel ABC transporter required for desiccation tolerance, and biofilm formation in Rhizobium leguminosarum bv. viciae 3841. FEMS Microbiol Ecol 71:327–340. doi:10.1111/j.1574-6941.2009.00824.x
Vessey JK (1994) Measurement of nitrogenase activity in legume root nodules: in defense of the acetylene reduction assay. Plant Soil 158:151–162. doi:10.1007/BF00009490
Vitousek PM, Menge DNL, Reed SC, Cleveland CC (2013) Biological nitrogen fixation: rates, patterns and ecological controls in terrestrial ecosystems. Philos Trans R Soc Lond B Biol Sci 368:20130119. doi:10.1098/rstb.2013.0119
Volpe V, Giovannetti M, Sun X-G, Fiorilli V, Bonfante P (2016) The phosphate transporters LjPT4 and MtPT4 mediate early root responses to phosphate status in non mycorrhizal roots. Plant Cell Environ 39:660–671. doi:10.1111/pce.12659
Vriezen JA, de Bruijn FJ, Nüsslein K (2007) Responses of rhizobia to desiccation in relation to osmotic stress, oxygen, and temperature. Appl Environ Microbiol 73:3451–3459. doi:10.1128/AEM.02991-06
Wang X, Pan QA, Chen FX, Yan XL, Liao H (2011) Effects of co-inoculation with arbuscolar mychorrizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza 21:173–181. doi:10.1007/s00572-010-0319-1
Wang X, Veneklaas EJ, Pearse SJ, Lambers H (2015) Interactions among cluster-root investment, leaf phosphorus concentration, and relative growth rate in two Lupinus species. Am J Bot 102:1529–1537. doi:10.3732/ajb.1500268
Watanabe T, Osaki M, Tadano T (1998) Effects of nitrogen source and aluminum on growth of tropical tree seedlings adapted to low pH soils. Soil Sci Plant Nutr 44:655–656. doi:10.1080/00380768.1998.10414489
Weaver RW, Danso SKA (1994) Dinitrogen fixation. In: Weaver RW, Angle S, Bottomley P, Bezdicek D, Tabatabai A, Wollum A (eds) Methods of soil analysis. Part 2, Microbiological and biochemical properties. Soil Sci Soc Am, Madison, pp 1019–1045. doi:10.2136/sssabookser5.2
Weese DJ, Heath KD, Dentinger BT, Lau JA (2015) Long-term nitrogen addition causes the evolution of less-cooperative mutualists. Evolution 69:631–642. doi:10.1111/evo.12594
Wei H, Atkins CA, Layzell DB (2004) Adenylate gradients and Ar:O2 effects on legume nodules (II. Changes in the subcellular adenylate pools). Plant Physiol 134:1775–1783. doi:10.1104/pp.103.038547
Xu J, Xiao-Lin L, Luo L (2012) Effects of engineered Sinorhizobium meliloti on cytokinin synthesis and tolerance of alfalfa to extreme drought stress. Appl Environ Microbiol 78:8056–8061. doi:10.1128/AEM.01276-12
Xue Y, Xia H, Christie P, Zhang Z, Li L, Tang C (2016) Crop acquisition of phosphorus, iron and zinc from soil in cereal/legume intercropping systems: a critical review. Ann Bot 117:363–377. doi:10.1093/aob/mcv182
Yanni YG, Rizk RY, Corich V, Squartini A, Ninke K, Philip-Hollingsworth S, Orgambide G, de Bruijn F, Stoltzfus J, Buckley D, Schmidt TM, Mateos PF, Ladha JK, Dazzo FB (1997) Natural endophytic association between Rhizobium leguminosarum bv. trifolii and rice roots and assessment of its potential to promote rice growth. Plant Soil 194:99–114. doi:10.1023/A:1004269902246
Yao Z-F, Liang C-Y, Zhang Q, Chen Z-Y, Xiao B-I, Tian J, Liao H (2014) SPX1 is an important component in the phosphorus signalling network of common bean regulating root growth and phosphorus homeostasis. J Exp Bot 12:3299–3310. doi:10.1093/jxb/eru183
Yuan P, Ding G-D, Cai H-M, Jin K-M, Broadley MR, Xu F-S, Shi L (2016) A novel Brassica-rhizotron system to unravel the dynamic changes in root system architecture of oilseed rape under phosphorus deficiency. Ann Bot 118:173–184. doi:10.1093/aob/mcw083
Zehr JP, Ward BB (2002) Nitrogen cycling in the ocean: new perspectives on processes and paradigms. Appl Environ Microbiol 68:1015–1024. doi:10.1128/AEM.68.3.1015-1024.2002
Zeng HQ, Zhu YY, Huang SQ, Yang ZM (2010) Analysis of phosphorus-deficient responsive miRNAs and cis-elements from soybean (Glycine max L.) J Plant Physiol 167:1289–1297. doi:10.1016/j.jplph.2010.04.017
Zeng HQ, Liu G, Kinoshita T, Zhang R, Zhu Y, Shen Q, Xu G (2012) Stimulation of phosphorus uptake by ammonium nutrition involves plasma membrane H+ ATPase in rice roots. Plant Soil 357:205–214. doi:10.1007/s11104-012-1136-4
Zhang J, Yin B, Xie Y, Li J, Yang Z, Zhang G (2015) Legume-cereal intercropping improves forage yield, quality and degradability. PLoS One 10:e0144813. doi:10.1371/journal.pone.0144813
Zhao XQ, Shen RF, Sun QB (2009) Ammonium under solution culture alleviates aluminum toxicity in rice and reduces aluminum accumulation in roots compared with nitrate. Plant Soil 315:107–121. doi:10.1007/s11104-008-9736-8
Zhu CQ, Zhu XF, Hu AY, Wang C, Wang B, Dong XY, Shen RF (2016) Differential effects of nitrogen forms on cell wall phosphorus remobilization in rice (Oryza sativa) are mediated by nitric oxide, pectin content, and phosphate transporter expression. Plant Physiol 171:1407–1417. doi:10.1104/pp.16.00176
Acknowledgments
The financial support from the University of Pavia (Italy) and Fundação para a Ciência e a Tecnologia (Lisbon, Portugal) is acknowledged through the research unit “GREEN-it: Bioresources for Sustainability” (UID/Multi/04551/2013), GREEN-IT fellowship of D.M (036/BTI/2016), PhD grant (PD/BD/128498/2017) of D.M. within the scope of the PhD program Plants for Life (PD/00035/2013) and S.S.A. postdoctoral grant (SFRH/BPD/108032/2015). This work has been partially supported by Reg.CE n. 1698/2005 Programma di Sviluppo rurale per la Puglia 2007/2013 (Misura 214 – Azione 4 Sub azione a) “SaVeGraINPuglia – Progetti integrati per la Biodiversità- Recupero, caratterizzazione, salvaguardia e valorizzazione di leguminose, cereali da granella e foraggio in Puglia.”
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Martins, D., Macovei, A., Leonetti, P., Balestrazzi, A., Araújo, S. (2017). The Influence of Phosphate Deficiency on Legume Symbiotic N2 Fixation. In: Sulieman, S., Tran, LS. (eds) Legume Nitrogen Fixation in Soils with Low Phosphorus Availability. Springer, Cham. https://doi.org/10.1007/978-3-319-55729-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-55729-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55728-1
Online ISBN: 978-3-319-55729-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)