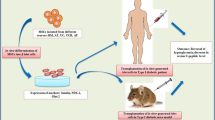
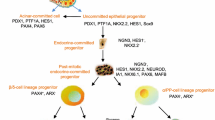
Abstract
Intense research aimed to find solutions for the cure of diabetes mellitus, based on stem cell therapy to replace hypoglycemic drug or insulin administration, has been conducted over the past decades. A variety of stem cells, including induced pluripotent stem cells, embryonic stem cells, or adult stem cells such as bone marrow-, adipose tissue-, and Wharton jelly-derived stem cells, have demonstrated their ability to differentiate into insulin-producing cells and secret insulin in response to high-glucose stimulation. However, achievement of the final goal for routinely clinical application of stem cell therapy is still out of reach. In this chapter, new advances in stem cell therapy for diabetes mellitus were reviewed and discussed in attempt to clarify where we are and how we may go to reach the final goal of the cure of diabetes mellitus.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Diabetes mellitus (DM) is one of the most pandemic chronic diseases that prevail worldwide, and the prevalence has continued to be growing in recent decades. The patients with DM manifest a hyperglycemic state induced by impairments in insulin secretion (type 1 and at the late phase of type 2), insulin sensitivity (type 2), or both. Type 1 diabetes mellitus (T1DM) , which accounts for less than 10% of patients with DM, occurs through mechanisms of an immune-mediated damage and destruction of pancreatic beta cells in the pancreatic islets of Langerhans, leading to absolute insulin deficiency (American Diabetes A 2011). Type 2 diabetes mellitus (T2DM) , which accounts for more than 90% of patients with DM, is characterized by insulin resistance in peripheral tissues and relative insulin deficiency (Groop and Eriksson 1992; D’Souza et al. 2013). Initially, patients with T2DM do not require insulin treatment; however as population and function of pancreatic beta cells declines over time, eventually exogenous insulin supplementation will be required at a late phase. DM is often complicated with major organ damage such as retinopathy, nephropathy, and neuropathy, as well as macrovascular diseases including coronary, cerebral, or peripheral vascular atherosclerosis (Gispen and Biessels 2000).
The cure of DM relies on regenerating functional beta cells, restoring insulin secretion, and relieving abnormal autoimmunity (Wu and Mahato 2014). Allologous whole pancreas or islet transplant has been used to treat DM. The data from the International Pancreatic Transplant Registry showed promising results that clinical hyperglycemia was rapidly controlled in the recipients after transplantation, with consequentially discontinued supplementation of exogenous insulin (Cefalu 2012). However, its clinical application is significantly limited by the lack of organ donors, high risk of major surgery compilations, and the need for lifelong immunosuppressive therapy to prevent graft rejection. In terms of these limitations, currently the allologous whole pancreas or islet transplant is only recommended to insulin-dependent diabetic patients with end-stage renal disease who require kidney co-transplant (Cefalu 2012). Achievement of the cure of DM demands more efficacious and feasible methods.
Stem cells, characterized by the potential of multi-lineage differentiation and self-renewal, have demonstrated their unique roles in functional insulin-producing cell (IPC) regeneration , immune modulation, and other fields of regenerative studies. Compared to organ or tissue transplant, stem cell therapy has the following advantages in the treatment of DM (Lo and Parham 2009; Calafiore and Basta 2015; Matveyenko and Vella 2015). Autologous stem cells can accommodate a long-term stable source and are not limited by the source of donors. Stem cells are also able to secret numerous cytokines, modulate the local inflammation of pancreatic islets, and further improve the microenvironment and autoimmunity. Moreover, stem cell-derived IPCs can avoid graft rejection and eliminate the necessity of immunosuppressive therapy. Prospectively, novel attempts to replenish pancreatic beta cells in DM, with special regard to IPCs for transplant purposes, could be substituted beta cells by allo- or autografted stem cells. Hereby, we reviewed and discussed the recent advances in stem cell therapy for DM in attempt to clarify where we are and how we may go to reach the final goal of the cure of DM.
2 Stem Cell Therapy for Diabetes Mellitus
2.1 Sources of Stem Cells
A variety of stem cells have been isolated from different tissues. Based on sources of origin, biological features, and biochemical markers, they can be grossly classified into embryonic stem cells (ESCs), adult stem cells (ASCs), and induced pluripotent stem cells (iPSCs).
ESCs and ASCs each have advantages and disadvantages in terms of potential use for stem cell therapy (Lo and Parham 2009; Calafiore and Basta 2015; Matveyenko and Vella 2015). One major difference is cell types that they can differentiate into. Theoretically ESCs can differentiate into any cell type since they are pluripotent, whereas ASCs are limited to becoming into cell types of their tissue of origin. Besides, ESCs can be isolated easily and expanded quickly in culture, whereas ASCs are rare in adult tissues, hence isolating them and expanding their numbers are very challenging. Moreover, the likelihood of being rejected after transplantation is different in tissues derived from ESCs and ASCs. Tissues derived from ASCs are less likely to be rejected after transplantation (Lo and Parham 2009; Calafiore and Basta 2015; Matveyenko and Vella 2015), since these cells are isolated from a patient’s own tissue, expanded in culture into presuming a specific cell type, and reintroduced into the same patient.
By being induced to express genes crucial for keeping the properties of ESCs, iPSCs are genetically reprogrammed adult cells maintained in an ESC-like state. Although these cells meet the defining criteria for pluripotent stem cells, it is still unknown if iPSCs and ESCs differ significantly in clinical application. The breakthrough discovery of iPSCs has created a new powerful tool to “dedifferentiate” adult cells and to harvest a large number of target stem cells. Moreover, tissues derived from iPSCs are almost identically matched to the cell donor, thus reducing the likelihood of rejection after transplantation (Lo and Parham 2009; Calafiore and Basta 2015; Matveyenko and Vella 2015). However, the vectors currently being used to introduce the reprogramming factors into adult cells are viruses; therefore, this process must be carefully controlled and evaluated before this technique can be applied on humans.
2.2 Embryonic Stem Cells (ESCs)
Mouse ESCs were first differentiated into IPCs, and upon transplantation in streptozotocin (STZ) -induced diabetic mice, those differentiated IPCs led to reversal of hyperglycemia (Soria et al. 2000). Although ESCs have the highest potential of differentiation into IPCs, only a small amount of pancreatic beta-like cells (1–3%) can be identified in vitro (Mfopou et al. 2010a). Subsequently, the findings of D’Amour et al. are a milestone for the differentiation protocol of ESC-derived IPCs. In the study, the differentiation process of ESCs to IPCs was first described in five stages referred to the developmental biology of the pancreas: definitive endoderm, foregut, hindgut, pancreatic endoderm, and then endocrine cells (D’Amour et al. 2006). Since then, strategies of improving the efficiency of differentiation have been developed. Chen et al. found that the differentiation of human ESCs into PDX1-positive cells can be promoted by a small molecule, indolactam V, both in vitro and in vivo, and a large number of ESC-derived cells can be obtained via this method (Chen et al. 2009). Similarly, a new protocol via a nestin expression step has been developed to obtain IPCs from mouse ESCs (Lumelsky et al. 2001).
The ESC-derived IPCs were able to synthesize insulin and expressed voltage- activated calcium channels; however, without the presence of insulin-containing secretary granules, they did not show the exclusive response of insulin secretion to high-glucose stimulation (Sipione et al. 2004). By using stepwise differentiation protocols, several other studies also successfully generated IPCs from human ESCs in vitro, though these generated pancreatic beta-like cells have very low function in terms of glucose responsiveness (Zhang et al. 2009; Nostro et al. 2011; Teo et al. 2013; Kroon et al. 2008; Shim et al. 2007; Mfopou et al. 2010b; Kelly et al. 2011; Xu et al. 2011). The in vivo microenvironment has been proposed as a key element for maturation of pancreatic beta cells intended for transplant. Hence the essential function of beta cells , i.e., the ability to secrete insulin in response to high-glucose stimulation, remains an issue of ESC-derived IPCs (Rolletschek et al. 2006).
Further, the potential tumorigenesis of ESCs would be another hurdle in clinical application. ESCs have the characteristics such as rapid proliferation, self-renewal, lack of contact inhibition, and telomerase activity, resembling cancer cells (Kooreman and Wu 2010). Actually, ESCs were associated with occurrence of teratomas and teratocarcinomas in humans (Blum and Benvenisty 2009). The accumulation of potentially oncogenic chromosomal abnormalities may result from the multiple rounds of cell replication before transplantation (Knoepfler 2009). Increase differentiation status and commitment to the cell type of interest before transplant into patients might reduce the risk of tumor development in future.
2.3 Adult Stem Cells (ASCs)
2.3.1 Bone Marrow: Derived Mesenchymal Stem Cells (BM-MSCs)
The bone marrow contains two different types of stem cells, namely, mesenchymal stem cells (MSCs) and hematopoietic stem cells (HSCs) . Because the bone marrow can be easily obtained by simple procedures, bone marrow stem cells have become one of the focuses of stem cell therapy research for DM (Pileggi 2012). Bone marrow-derived mesenchymal stem cells (BM-MSCs) are multipotent progenitor cells, capable of self-renewal and differentiation into adipogenic, chondrogenic, and osteogenic cell lineages (Oswald et al. 2004). The cells can be isolated from the bone marrow in a low-density cell culture method by removal of nonadherent cells (Pittenger and Martin 2004). BM-MSCs express a typical set of surface markers including CD29, CD44, CD49e, CD51, CD54, CD59, CD71, CD73, CD90, CD105, CD166, and CD200 (Hung et al. 2002; Delorme et al. 2008). Unlike HSCs, BM-MSCs do not express CD14, CD31, CD34, CD45, CD79, CD86, CD117, and glycophorin A (Reger et al. 2008; Turnovcova et al. 2009). In addition, BM-MSCs express markers of class I major histocompatibility complex (MHC) but not class II, which may be very advantageous graft-wise (Weiss et al. 2006).
It has been demonstrated that BM-MSCs are able to differentiate into IPCs in vivo and in vitro, and normalize high blood glucose in diabetic mice (Kim et al. 2012; Ho et al. 2012). Experiments aimed at inducing BM-MSCs to differentiate into IPCs were attempted to properly reprogram these cells by activating “ad hoc” differentiation pathways. Oh et al. suggested that rat BM-MSCs could transdifferentiate into IPCs when cultured in a high-glucose medium. These cells grew into a mixed population of islet-like cells, possessed granules with relatively low insulin content, and expressed typical pancreatic endocrine genes such as insulin, glucagon, and somatostatin. Transplanted these cells into diabetic mice normalized blood glucose levels for over 3 months (Oh et al. 2004). Xie et al. demonstrated a three-step differentiation protocol from human BM-MSCs to IPCs, which activin A as a key differentiating agent was added at the final step. The achievement of IPCs was confirmed by morphological analyses and the expression of typical pancreatic beta cell genes such as Beta2/NeuroD, Glut2, Isl-1, nestin, ngn3, Nkx6.1, Pax6, Pdx-1, insulin, glucagon, and C-peptide. Notably, these IPCs secreted insulin in a glucose-dependent manner and could control hyperglycemia in STZ-induced diabetic rats for more than 1 month (Xie et al. 2009). Further, their ability to escape immune recognition and immunomodulatory potentials is discussed in a different section.
However, because only a small portion of the generated cells was originated from differentiated bone marrow cells, the differentiating ability of BM-MSCs into IPCs is questioned (Hess et al. 2003). Besides, after transplantation, increased levels of insulin and decreased levels of plasma glucose had been observed before the presence of MSC-derived IPCs. Therefore, the success of BM-MSCs treatment is less likely to be obtained by direct differentiation into IPCs but probably by paracrine or endocrine mechanisms (Hess et al. 2003). Finally, BM-MSCs have limitations regarding procured cell mass, requiring in vitro expansion which may increase the risk of microbial contamination, losing stemness properties, and inducing artifactual chromosomal changes (Baksh et al. 2007).
2.3.2 Adipose Tissue-Derived Mesenchymal Stem Cells (AT-MSCs)
MSCs are multipotent cells existing in several tissues including adipose tissue (Kern et al. 2006). As AT-MSCs can be easily isolated from a patient’s own tissue ex vivo, expanded, differentiated into IPCs, and transplanted back to the same patient, adipose tissue has been gaining increased attention for cell therapy as a primary source of MSCs (Zuk et al. 2002; Schaffler and Buchler 2007). Adipose tissue produces a number of bioactive molecules named adipokines, modulating fat mass, nutrient homeostasis, lipid and glucose metabolism, and blood pressure. Adiponectin, leptin, and visfatin are well-recognized adipokines and play crucial roles in insulin sensitivity and glucose regulation (Kojima et al. 2004). It has also been reported that adipocytes from the carp secreted insulin and the proliferative population of AT-MSCs expressed the transcription factor ISL-1 and PAX-6, which are involve in pancreatic endocrine development (Timper et al. 2006; Chandra et al. 2009). Moreover, several studies have revealed that AT-MSCs have even greater potencies in proliferation, differentiation, and immunomodulatory compared to BM-MSCs (Kern et al. 2006; Kim et al. 2007; Pendleton et al. 2013; Melief et al. 2013; Lee et al. 2004). All these features render AT-MSCs a prominent candidate in stem cell therapies for DM.
Chandra et al. showed that AT-MSCs from murine epididymis could differentiate into IPCs under a 10-day inductive protocol. Differentiated cells expressed Glut2, NeuroD, Ngn3, Pax4, PDX1 and secreted insulin and C-peptide in response to glucose levels. Secretory granules in the cell cytoplasm were confirmed by electron microscopy. Normoglycemic state was restored 2 weeks after intraperitoneal transplant into diabetic mice (Chandra et al. 2009). A recent study demonstrated that AT-MSCs differentiate into IPCs after 38-day co-culture with islet cells. Insulin and C-peptide production were confirmed by ELISA and immunoassaying. After co-transplant of IPCs and islet cells under kidney capsule, hyperglycemic state was recovered in diabetic rats (Karaoz et al. 2013). Moreover, it has been shown that combination of differentiated AT-MSCs and islets resulted in better recovery from diabetes compared to islet transplant alone or co-transplant of islets and differentiated BM-MSCs (Karaoz et al. 2013).
2.3.3 Wharton Jelly-Derived Mesenchymal Stem Cells (WJ-MSCs)
Recent studies suggest that the postpartum umbilical cord-extracted Wharton jelly (WJ) contains adult MSCs that can be successfully expanded ex vivo, cryopreserved, and differentiated into ectodermal, mesodermal, and endodermal cellular lineages (Romanov et al. 2003). The gene expression profile of WJ-derived MSCs (WJ-MSCs) is similar to that of BM-MSCs, although it also expresses additional markers (e.g., CD117) (La Rocca et al. 2009; Montanucci et al. 2011). The immune features of WJ-MSCs resemble those of BM-MSCs, since both do not express type II MHC. Moreover, both also express key molecules associated with immunomodulatory properties (Weiss et al. 2008; Deuse et al. 2011). These characteristics clearly indicated the potential of WJ-MSCs that they can transdifferentiate into IPCs or ancillary cells that may assist IPCs, and they can be transplanted and stay functionally active in a diabetic recipient (Ricordi et al. 2012). As an important requisite for allogeneic graft is low immunogenicity, WJ-MSCs is very competent in allologous cell transplantation. Recently, a study demonstrated long-term effects of WJ-MSC therapy in newly onset T1DM (Hu et al. 2013). All of these evidences supported that WJ-MSCs may be an efficient allologous cell candidate for the cure of DM .
Chao et al. successfully differentiated WJ-MSCs into IPCs through a four-step protocol. They transplanted the IPCs into the liver of diabetic mice (Chao et al. 2008). The results showed insulin and C-peptide secretion in response to plasma physiological glucose levels and pancreas-specific gene expression such as Glut-2, HLXB-9, Nkx2.2, and PDX1 in the transplanted IPCs (Chao et al. 2008; Palmer 2009). In a study by He et al. after infected with PDX1 gene-carrying recombinant adenovirus and then treated with inductive factors, WJ-MSCs differentiated into IPCs in vitro. It showed that the differentiated IPCs expressed beta cell-related genes like PDX1, Ngn3, Glut2, and Nkx6.1 and were able to respond to high-glucose stimulation (He et al. 2011). The beta cell-related genes were expressed in both differentiated cells and beta like-cells transplanted into the liver of STZ-induced diabetic rats through the portal vein. As a result, blood glucose levels were significantly reduced 4 weeks after transplantation (Tsai et al. 2012). Wang et al. also differentiated WJ-MSCs into IPCs with an inductive medium. They confirmed that differentiated IPCs responded to the glucose challenge test in vitro. After retro-orbital injection of IPCs into nonobese diabetic (NOD) mice, they found that IPCs containing human nuclei and human C-peptide were located in the liver. They concluded that differentiated IPCs from human WJ-MSCs can alleviate hyperglycemia in NOD mice (Wang et al. 2011). These promising data indicated that WJ-MSCs possess the ability to differentiate into IPCs, both in vivo and in vitro. With respect to the outstanding differentiation and immunomodulatory capacities, WJ-MSCs should be considered as a potential cell therapy option.
2.3.4 Pancreatic Stem Cells
It has been shown that pancreatic tissues of adult rat can regenerate efficiently even after resection of 90% of the pancreas (Bonner-Weir et al. 1993). Physiological changes such as pregnancy and obesity also promote pancreatic cell proliferation in great numbers. These suggested that pancreatic cells are capable of self-renewal. However, it remains uncertain whether new regenerated cells are derived from the differentiation of pancreatic stem cells or the proliferation of existing mature cells. By cell sorting and lineage tracing, Simon et al. isolated PDX1+/insulin+/GLUT2 cells from rodent pancreatic islets and pancreatic duct (Smukler et al. 2011). In vitro these cells could expand clonally and differentiate into different types of pancreatic cells. Transplant of these cells reduced high blood glucose levels in STZ-induced diabetic mice. Therefore, this group of cells was considered as pancreatic stem cells (Smukler et al. 2011). However, several experiments were performed to stimulate pancreatic regeneration in vivo, and no pancreatic stem cells could be traced (Furuyama et al. 2011). Bonner et al. concluded that the process of cell growth and regeneration is likely from slow replication of mature cells rather than pancreatic stem cells (Bonner-Weir et al. 2010; Brennand et al. 2007).
2.3.5 Hepatic Stem Cells
It has been assumed that liver cells can be an alternative source of IPCs, because in developmental biology, both the liver and pancreas originate from the endoderm, and they share common progenitor cells (Zaret and Grompe 2008). After exogenous PDX1 and NGN3 were transducted into the mouse liver, pancreatic endocrine and exocrine gene expression was substantially induced in liver cells. These transdifferentiated cells were able to survive in the liver and form new pancreatic tissue clusters around the central veins. Besides, they did not affect the normal liver function but could secret insulin, which had normalized blood glucose levels for 8 months in STZ-induced diabetic mice (Ber et al. 2003; Yechoor et al. 2009). Yang et al. further demonstrated that the differentiation of PDX1-reprogrammed liver tissue into functional IPCs only occurred under a state of high-glucose stimulation in vivo and in vitro (Yang 2006 ). However, so far no experiment could obtain sufficient number of functional IPCs for transplantation via the in vitro proliferation of reprogrammed liver cells.
2.4 Induced Pluripotent Stem Cells (iPSCs)
Induced pluripotent stem cells (iPSCs) are ESC-like cells from reprogrammed adult somatic cells by the introduction of embryonic genes. Thus, a large number of cells specific to the donor can be obtained, thereby reducing the likelihood of rejection when these cells are transplanted back. Human iPSCs were successfully achieved by reprogrammed somatic cells like fibroblasts (Takahashi et al. 2007), hepatocytes and stomach cells (Aoi et al. 2008), blood cells, and keratinocytes (Hanna et al. 2008), with a cocktail of key transcription factors including cMYC, KLF4, LIN28, NANOG, OCT4, and SOX2 (Group CR 2009). IPSCs are able to differentiate into IPCs by stepwise differentiation protocols including SOX17-positive cells, PDX1-positive cells (pancreatic progenitors), and NGN3-positive cells (endocrine progenitors) (D’Amour et al. 2006; Maehr et al. 2009; Hua et al. 2013; Thatava et al. 2013), which are similar to those applied to ESCs (D’Amour et al. 2006; Maehr et al. 2009). However, like human ESCs, it is still under investigation by which methods iPSCs can be committed to proper cells of interest effectively and reproducibly.
The differentiation of human iPSCs into pancreatic beta cells was first reported in 2008 (Tateishi et al. 2008). By using a four-stage differentiation protocols, IPCs with glucose responsiveness were differentiated from skin fibroblast-derived iPSCs (Thatava et al. 2013). Furthermore, human iPSC clones showed the variations in pancreatic differentiation abilities into IPCs, which was more prominent at the final stage of differentiation (Thatava et al. 2011; Liew et al. 2008). However it is controversial in terms of the differentiation of iPSCs into fully functional IPCs with glucose responsiveness (Teo et al. 2013). The iPSC-derived IPCs had the expression of multi-hormones such as insulin, C-peptide, and glucagon, but they did not express the specific markers for mature pancreatic beta cells such as MAFA and NKX6-1 (Tateishi et al. 2008).
Transplantation of pancreatic progenitor cells or immature pancreatic beta cells into experimental animals may lead to the maturation of iPSC-derived IPCs. In both T1DM and T2DM mouse models, the transplanted iPSC-derived beta cells were able to proficiently secrete insulin with glucose responsiveness and improve hyperglycemia (Alipio et al. 2010). Furthermore, transplantation of monkey iPSC-derived beta cells into diabetic mouse models could correct their hyperglycemia (Zhu et al. 2011). IPCs from the differentiation of beta cell- derived iPSCs can release insulin upon glucose stimulation and after transplantation into T2DM mice can normalize high blood glucose and reduce glycated hemoglobin levels (Bar-Nur et al. 2011). The transplantation of iPSC-derived IPCs obtained from pancreatic epithelial cells into a kidney led to a functional response to glucose stimulation in NOD mice (Jeon et al. 2012). These findings indicated that in vivo maturation is the key of the functionality of iPSC-derived IPCs .
Currently, viral transfection of transcription factors is a major step in the reprogramming of somatic cells into iPSCs. The viral backbone and transgenes are permanently integrated into the genome of transfected cells, and this integration may expose the iPSCs to the risk of mutations, tumorigenesis, dysfunction, or reduced differentiation ability (Okita et al. 2007). With the modification of differentiating protocols, the safety of iPSCs has been greatly improved. By using an adenoviral reprogramming protocol to generate iPSCs, exogenous genes (Oct4, Sox2, Klf4, and c-Myc) are highly and transiently expressed within the cells, but adenovirus does not integrate into the genome (Stadtfeld et al. 2008; Okita et al. 2008). Further, some transfected genes used to generate iPSCs are tumorigenic genes like MYC, with which iPSCs were granted pluripotency and tumorigenicity (Yamanaka 2007; Knoepfler 2008). Recently, to exclude the oncogene MYC so as to prevent genetic modifications, new techniques have been developed by the use of microribonucleic acids (Nakagawa et al. 2008; Wernig et al. 2008). By using only three factors (OCT4, SOX2, and KLF4), and excluding the oncogene MYC, iPSCs have also been produced from patients with T1DM (Maehr et al. 2009). Another method to generated iPSCs is the supplementation of appropriate growth factors into the medium of cultured spermatogonial stem cells that can reprogram iPSCs into cells with three germ layers (Golestaneh et al. 2009). As exogenous genes are not used in the reprogramming process, related risks to exogenous gene integration are eliminated. Certainly, to enhance the efficiency of these methods, further studies are warranted.
3 Immune-Modulation of Stem Cell Therapy
MSCs have been shown to have presumptive plasticity potential to differentiate into multiple lineages, and their ability to escape immune recognition and potent immunomodulatory properties have received great interest in regenerative medicine (Anzalone et al. 2011; Abdi et al. 2008; Larijani et al. 2012; Fiorina et al. 2011). Currently, research focusing on the treatment of diabetes with MSCs has led to the following findings. Several experimental studies showed that allogeneic or syngeneic BM-MSCs could prevent or reverse autoimmune diabetes in diabetic animals (Hess et al. 2003; Lee et al. 2006; Ezquer et al. 2008; Boumaza et al. 2009; Zhao et al. 2008; Jurewicz et al. 2010; Fiorina et al. 2009). Urban et al. demonstrated that the immunomodulation of MSCs is one of the mechanisms that support cell regeneration (Urban et al. 2008). In mice with STZ/radiation-induced diabetes, a mixture of HSCs and BM-MSCs were injected into the bone marrow, where these cells inhibited the proliferation of pancreatic cell-specific T cells, reduced the damage caused by T cells, and increased new cell regeneration to a certain extent. The transplantation successfully enabled the blood glucose control in the mice (Urban et al. 2008). Bassi et al. reported that administrated AT-MSCs reversed hyperglycemia in NOD mice. The underlying mechanisms were induction of regulatory T cells and reduction of CD4+ Type 1 T helper cell response as well as decrease in interferon gamma levels (Bassi et al. 2012). Madec et al. revealed that MSCs could induce interleukin 10-producing regulatory T cells and suppress beta cell- specific T cell responses in vitro and in NOD mice (Madec et al. 2009).
Moreover, BM-MSCs have been shown to inhibit both alloimmunization and autoimmunization (Hashemian et al. 2015). Combining pancreatic islets with MSCs transplantation enables the graft to escape immune surveillance and improves the survival rate of the graft (Hashemian et al. 2015). Ding et al. also exploited this feature of MSCs to transplant pancreatic islets and BM-MSCs, the transplanted islets had prolonged survival time, and blood glucose levels were normalized for a significant period of time (Ding et al. 2009). It was speculated that synthesis and secretion of matrix metalloproteinase 2 and 9 into the extracellular matrix play a role in the immune evasion effects of MSCs (Ding et al. 2009).
However, in different microenvironments, MSCs utilize different mechanisms to exert immunosuppressive function. Thus, it should be taken into consideration that animal models are not exactly equal to their human counterparts, and, in different species, MSCs have different functions (Ren et al. 2009). Once safety issues of iPSC were solved, combined transplantation of recipient-specific iPSC and MSCs could be promising (Calafiore and Basta 2015). Another ultimate frontier to cure T1DM is, by proper stimuli of MSCs, to eliminate the autoimmune destruction of pancreatic beta cells; hence the original beta cell reservoir could be regenerated to reconstitute a sufficient mass of IPCs (Calafiore and Basta 2015).
4 Future Direction
There still have a number of challenges for the real clinical application of stem cell therapy for DM. One of these challenges is the cell source. Each transplantation needs approximately 100,000 pancreatic islets, and a larger number of alternative cells have to be produced in vitro (Liu et al. 2013 ). Although a variety of methods to differentiate various stem cells into IPCs have been developed, in terms of clinical application, these methods have relatively low differentiation efficiency, and their generated cell numbers are far from meeting transplant requirements. Therefore, a more effective method for enhancing the differentiation of stem cells into functional IPCs should be developed.
Second, they have several potential critical risks associated with stem cell therapy. Kroon et al. have shown that after human ESC-derived IPCs were transplanted into mice, a 15% of generated cells had components of teratomas or other tissue (Kroon et al. 2008). In vitro proliferation of MSCs was shown to increase the risk of tumor formation and metastasis (Tang et al. 2012; Vajdic and van Leeuwen 2009). These safety concerns must be cautiously evaluated and prevented before stem cell therapy can be used in humans.
Finally, the immune rejection is an issue associated with stem cell therapy (Halban et al. 2010). After receiving allogeneic cell therapy, patients must receive lifelong immunosuppression to avoid graft rejection. Immunosuppressive agents may inhibit insulin secretion or exacerbate insulin resistance, thus counteract the insulin-producing effects of implanting cells. Meanwhile, immunosuppressive agents may increase the risk of malignancy. Further, for the treatment of T1DM, even the autologous cells can be rapid damaged by autoimmunity. In the scenario of the dysfunction of beta cells that is related to genetic abnormalities or changes, transplantation of these autologous cells may not be able to be functioning as well (Liu et al. 2013).
5 Conclusion
To find a cure of DM, various scientific areas of research have been extensively explored, with stem cell therapy being one of them. In the present chapter, we have reviewed the current basic and clinical research regarding the development of stem cell therapy for DM. An effective cell transplantation and therapeutic immunomodulatory strategy are required in stem cell therapy for DM (Li and Ikehara 2013). Various stem cells are capable to generate functional IPCs and to improve diabetes in animals and humans. MSCs can inhibit the T cell- mediated autoimmune against newly generated IPCs and prevent destruction of beta cells in DM (Li and Ikehara 2013). Therefore, a blended strategy that combines reliable existing therapies such as islet and pancreas transplantation, the latest bioengineering techniques, and novel immunosuppressive and immunomodulatory agents, with an effective and safe stem cell protocol would secure an optimistic approach for successful translation of stem cell therapy into a cure of DM (Chhabra and Brayman 2013). Overall, for clinical application of stem cell therapy in DM, more studies with various sources of stem cells, larger population of patients undergoing transplantation, and longer monitoring duration are needed to testify the efficacy and safety of this auspicious therapeutic approach.
Disclosure Summary
The authors have nothing to disclose.
References
Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH (2008) Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 57(7):1759–1767
Alipio Z, Liao W, Roemer EJ, Waner M, Fink LM, Ward DC et al (2010) Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc Natl Acad Sci U S A 107(30):13426–13431
American Diabetes A (2011) Diagnosis and classification of diabetes mellitus. Diabetes Care 34(Suppl 1):S62–S69
Anzalone R, Lo Iacono M, Loria T, Di Stefano A, Giannuzzi P, Farina F et al (2011) Wharton’s jelly mesenchymal stem cells as candidates for beta cells regeneration: extending the differentiative and immunomodulatory benefits of adult mesenchymal stem cells for the treatment of type 1 diabetes. Stem Cell Rev 7(2):342–363
Aoi T, Yae K, Nakagawa M, Ichisaka T, Okita K, Takahashi K et al (2008) Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science 321(5889):699–702
Baksh D, Yao R, Tuan RS (2007) Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 25(6):1384–1392
Bar-Nur O, Russ HA, Efrat S, Benvenisty N (2011) Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 9(1):17–23
Bassi EJ, Moraes-Vieira PM, Moreira-Sa CS, Almeida DC, Vieira LM, Cunha CS et al (2012) Immune regulatory properties of allogeneic adipose-derived mesenchymal stem cells in the treatment of experimental autoimmune diabetes. Diabetes 61(10):2534–2545
Ber I, Shternhall K, Perl S, Ohanuna Z, Goldberg I, Barshack I et al (2003) Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem 278(34):31950–31957
Blum B, Benvenisty N (2009) The tumorigenicity of diploid and aneuploid human pluripotent stem cells. Cell Cycle 8(23):3822–3830
Bonner-Weir S, Baxter LA, Schuppin GT, Smith FE (1993) A second pathway for regeneration of adult exocrine and endocrine pancreas. A possible recapitulation of embryonic development. Diabetes 42(12):1715–1720
Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A (2010) Beta-cell growth and regeneration: replication is only part of the story. Diabetes 59(10):2340–2348
Boumaza I, Srinivasan S, Witt WT, Feghali-Bostwick C, Dai Y, Garcia-Ocana A et al (2009) Autologous bone marrow-derived rat mesenchymal stem cells promote PDX-1 and insulin expression in the islets, alter T cell cytokine pattern and preserve regulatory T cells in the periphery and induce sustained normoglycemia. J Autoimmun 32(1):33–42
Brennand K, Huangfu D, Melton D (2007) All beta cells contribute equally to islet growth and maintenance. PLoS Biol 5(7):e163
Calafiore R, Basta G (2015) Stem cells for the cell and molecular therapy of type 1 diabetes mellitus (T1D): the gap between dream and reality. Am J Stem Cells 4(1):22–31
Cefalu WT (2012) American diabetes association-European association for the study of diabetes position statement: due diligence was conducted. Diabetes Care 35(6):1201–1203
Chandra V, Swetha G, Phadnis S, Nair PD, Bhonde RR (2009) Generation of pancreatic hormone-expressing islet-like cell aggregates from murine adipose tissue-derived stem cells. Stem Cells 27(8):1941–1953
Chao KC, Chao KF, Fu YS, Liu SH (2008) Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One 3(1):e1451
Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L et al (2009) A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol 5(4):258–265
Chhabra P, Brayman KL (2013) Stem cell therapy to cure type 1 diabetes: from hype to hope. Stem Cells Transl Med 2(5):328–336
D’Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG et al (2006) Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 24(11):1392–1401
D’Souza DM, Al-Sajee D, Hawke TJ (2013) Diabetic myopathy: impact of diabetes mellitus on skeletal muscle progenitor cells. Front Physiol 4:379
Delorme B, Ringe J, Gallay N, Le Vern Y, Kerboeuf D, Jorgensen C et al (2008) Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood 111(5):2631–2635
Deuse T, Stubbendorff M, Tang-Quan K, Phillips N, Kay MA, Eiermann T et al (2011) Immunogenicity and immunomodulatory properties of umbilical cord lining mesenchymal stem cells. Cell Transplant 20(5):655–667
Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ (2009) Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes 58(8):1797–1806
Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA (2008) Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant: J Am Soc Blood Marrow Transplant 14(6):631–640
Fiorina P, Jurewicz M, Augello A, Vergani A, Dada S, La Rosa S et al (2009) Immunomodulatory function of bone marrow-derived mesenchymal stem cells in experimental autoimmune type 1 diabetes. J Immunol 183(2):993–1004
Fiorina P, Voltarelli J, Zavazava N (2011) Immunological applications of stem cells in type 1 diabetes. Endocr Rev 32(6):725–754
Furuyama K, Kawaguchi Y, Akiyama H, Horiguchi M, Kodama S, Kuhara T et al (2011) Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet 43(1):34–41
Gispen WH, Biessels GJ (2000) Cognition and synaptic plasticity in diabetes mellitus. Trends Neurosci 23(11):542–549
Golestaneh N, Kokkinaki M, Pant D, Jiang J, DeStefano D, Fernandez-Bueno C et al (2009) Pluripotent stem cells derived from adult human testes. Stem Cells Dev 18(8):1115–1126
Groop LC, Eriksson JG (1992) The etiology and pathogenesis of non-insulin-dependent diabetes. Ann Med 24(6):483–489
Group CR (2009) 2007 update on allogeneic islet transplantation from the Collaborative Islet Transplant Registry (CITR). Cell Transplant 18(7):753–767
Halban PA, German MS, Kahn SE, Weir GC (2010) Current status of islet cell replacement and regeneration therapy. J Clin Endocrinol Metab 95(3):1034–1043
Hanna J, Markoulaki S, Schorderet P, Carey BW, Beard C, Wernig M et al (2008) Direct reprogramming of terminally differentiated mature B lymphocytes to pluripotency. Cell 133(2):250–264
Hashemian SJ, Kouhnavard M, Nasli-Esfahani E (2015) Mesenchymal stem cells: rising concerns over their application in treatment of type one diabetes mellitus. J Diabetes Res 2015:675103
He D, Wang J, Gao Y, Zhang Y (2011) Differentiation of PDX1 gene-modified human umbilical cord mesenchymal stem cells into insulin-producing cells in vitro. Int J Mol Med 28(6):1019–1024
Hess D, Li L, Martin M, Sakano S, Hill D, Strutt B et al (2003) Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol 21(7):763–770
Ho JH, Tseng TC, Ma WH, Ong WK, Chen YF, Chen MH et al (2012) Multiple intravenous transplantations of mesenchymal stem cells effectively restore long-term blood glucose homeostasis by hepatic engraftment and beta-cell differentiation in streptozotocin-induced diabetic mice. Cell Transplant 21(5):997–1009
Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H et al (2013) Long term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J 60(3):347–357
Hua H, Shang L, Martinez H, Freeby M, Gallagher MP, Ludwig T et al (2013) iPSC-derived beta cells model diabetes due to glucokinase deficiency. J Clin Invest 123(7):3146–3153
Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH (2002) Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells 20(3):249–258
Jeon K, Lim H, Kim JH, Thuan NV, Park SH, Lim YM et al (2012) Differentiation and transplantation of functional pancreatic beta cells generated from induced pluripotent stem cells derived from a type 1 diabetes mouse model. Stem Cells Dev 21(14):2642–2655
Jurewicz M, Yang S, Augello A, Godwin JG, Moore RF, Azzi J et al (2010) Congenic mesenchymal stem cell therapy reverses hyperglycemia in experimental type 1 diabetes. Diabetes 59(12):3139–3147
Karaoz E, Okcu A, Unal ZS, Subasi C, Saglam O, Duruksu G (2013) Adipose tissue-derived mesenchymal stromal cells efficiently differentiate into insulin-producing cells in pancreatic islet microenvironment both in vitro and in vivo. Cytotherapy 15(5):557–570
Kelly OG, Chan MY, Martinson LA, Kadoya K, Ostertag TM, Ross KG et al (2011) Cell-surface markers for the isolation of pancreatic cell types derived from human embryonic stem cells. Nat Biotechnol 29(8):750–756
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K (2006) Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24(5):1294–1301
Kim Y, Kim H, Cho H, Bae Y, Suh K, Jung J (2007) Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem: Int J Exp Cell Physiol Biochem Pharmacol 20(6):867–876
Kim SJ, Choi YS, Ko ES, Lim SM, Lee CW, Kim DI (2012) Glucose-stimulated insulin secretion of various mesenchymal stem cells after insulin-producing cell differentiation. J Biosci Bioeng 113(6):771–777
Knoepfler PS (2008) Why myc? An unexpected ingredient in the stem cell cocktail. Cell Stem Cell 2(1):18–21
Knoepfler PS (2009) Deconstructing stem cell tumorigenicity: a roadmap to safe regenerative medicine. Stem Cells 27(5):1050–1056
Kojima H, Fujimiya M, Matsumura K, Nakahara T, Hara M, Chan L (2004) Extrapancreatic insulin-producing cells in multiple organs in diabetes. Proc Natl Acad Sci U S A 101(8):2458–2463
Kooreman NG, Wu JC (2010) Tumorigenicity of pluripotent stem cells: biological insights from molecular imaging. J R Soc Interface/R Soc 7(Suppl 6):S753–S763
Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S et al (2008) Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 26(4):443–452
La Rocca G, Anzalone R, Corrao S, Magno F, Loria T, Lo Iacono M et al (2009) Isolation and characterization of Oct-4+/HLA-G+ mesenchymal stem cells from human umbilical cord matrix: differentiation potential and detection of new markers. Histochem Cell Biol 131(2):267–282
Larijani B, Esfahani EN, Amini P, Nikbin B, Alimoghaddam K, Amiri S et al (2012) Stem cell therapy in treatment of different diseases. Acta Med Iran 50(2):79–96
Lee RH, Kim B, Choi I, Kim H, Choi HS, Suh K et al (2004) Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem: Int J Exp Cell Physiol Biochem Pharmacol 14(4–6):311–324
Lee RH, Seo MJ, Reger RL, Spees JL, Pulin AA, Olson SD et al (2006) Multipotent stromal cells from human marrow home to and promote repair of pancreatic islets and renal glomeruli in diabetic NOD/scid mice. Proc Natl Acad Sci U S A 103(46):17438–17443
Li M, Ikehara S (2013) Bone marrow stem cell as a potential treatment for diabetes. J Diabetes Res 2013:329596
Liew CG, Shah NN, Briston SJ, Shepherd RM, Khoo CP, Dunne MJ et al (2008) PAX4 enhances beta-cell differentiation of human embryonic stem cells. PLoS One 3(3):e1783
Liu X, Wang Y, Li Y, Pei X (2013) Research status and prospect of stem cells in the treatment of diabetes mellitus. Sci China Life Sci 56(4):306–312
Lo B, Parham L (2009) Ethical issues in stem cell research. Endocr Rev 30(3):204–213
Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R (2001) Differentiation of embryonic stem cells to insulin-secreting structures similar to pancreatic islets. Science 292(5520):1389–1394
Madec AM, Mallone R, Afonso G, Abou Mrad E, Mesnier A, Eljaafari A et al (2009) Mesenchymal stem cells protect NOD mice from diabetes by inducing regulatory T cells. Diabetologia 52(7):1391–1399
Maehr R, Chen S, Snitow M, Ludwig T, Yagasaki L, Goland R et al (2009) Generation of pluripotent stem cells from patients with type 1 diabetes. Proc Natl Acad Sci U S A 106(37):15768–15773
Matveyenko A, Vella A (2015) Regenerative medicine in diabetes. Mayo Clin Proc 90(4):546–554
Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H (2013) Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med 2(6):455–463
Mfopou JK, Chen B, Sui L, Sermon K, Bouwens L (2010a) Recent advances and prospects in the differentiation of pancreatic cells from human embryonic stem cells. Diabetes 59(9):2094–2101
Mfopou JK, Chen B, Mateizel I, Sermon K, Bouwens L (2010b) Noggin, retinoids, and fibroblast growth factor regulate hepatic or pancreatic fate of human embryonic stem cells. Gastroenterology 138(7):2233–2245. 45 e1–14
Montanucci P, Basta G, Pescara T, Pennoni I, Di Giovanni F, Calafiore R (2011) New simple and rapid method for purification of mesenchymal stem cells from the human umbilical cord Wharton jelly. Tissue Eng Part A 17(21–22):2651–2661
Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T et al (2008) Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol 26(1):101–106
Nostro MC, Sarangi F, Ogawa S, Holtzinger A, Corneo B, Li X et al (2011) Stage-specific signaling through TGFbeta family members and WNT regulates patterning and pancreatic specification of human pluripotent stem cells. Development 138(5):861–871
Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE (2004) Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest; J Tech Methods Pathol 84(5):607–617
Okita K, Ichisaka T, Yamanaka S (2007) Generation of germline-competent induced pluripotent stem cells. Nature 448(7151):313–317
Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S (2008) Generation of mouse induced pluripotent stem cells without viral vectors. Science 322(5903):949–953
Oswald J, Boxberger S, Jorgensen B, Feldmann S, Ehninger G, Bornhauser M et al (2004) Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells 22(3):377–384
Palmer JP (2009) C-peptide in the natural history of type 1 diabetes. Diabetes Metab Res Rev 25(4):325–328
Pendleton C, Li Q, Chesler DA, Yuan K, Guerrero-Cazares H, Quinones-Hinojosa A (2013) Mesenchymal stem cells derived from adipose tissue vs bone marrow: in vitro comparison of their tropism towards gliomas. PLoS One 8(3):e58198
Pileggi A (2012) Mesenchymal stem cells for the treatment of diabetes. Diabetes 61(6):1355–1356
Pittenger MF, Martin BJ (2004) Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res 95(1):9–20
Reger RL, Tucker AH, Wolfe MR (2008) Differentiation and characterization of human MSCs. Methods Mol Biol 449:93–107
Ren G, Su J, Zhang L, Zhao X, Ling W, L’Huillie A et al (2009) Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 27(8):1954–1962
Ricordi C, Inverardi L, Dominguez-Bendala J (2012) From cellular therapies to tissue reprogramming and regenerative strategies in the treatment of diabetes. Regen Med 7(6 Suppl):41–48
Rolletschek A, Kania G, Wobus AM (2006) Generation of pancreatic insulin-producing cells from embryonic stem cells – ‘proof of principle’, but questions still unanswered. Diabetologia 49(11):2541–2545
Romanov YA, Svintsitskaya VA, Smirnov VN (2003) Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells 21(1):105–110
Schaffler A, Buchler C (2007) Concise review: adipose tissue-derived stromal cells – basic and clinical implications for novel cell-based therapies. Stem Cells 25(4):818–827
Shim JH, Kim SE, Woo DH, Kim SK, Oh CH, McKay R et al (2007) Directed differentiation of human embryonic stem cells towards a pancreatic cell fate. Diabetologia 50(6):1228–1238
Sipione S, Eshpeter A, Lyon JG, Korbutt GS, Bleackley RC (2004) Insulin expressing cells from differentiated embryonic stem cells are not beta cells. Diabetologia 47(3):499–508
Smukler SR, Arntfield ME, Razavi R, Bikopoulos G, Karpowicz P, Seaberg R et al (2011) The adult mouse and human pancreas contain rare multipotent stem cells that express insulin. Cell Stem Cell 8(3):281–293
Soria B, Roche E, Berna G, Leon-Quinto T, Reig JA, Martin F (2000) Insulin-secreting cells derived from embryonic stem cells normalize glycemia in streptozotocin-induced diabetic mice. Diabetes 49(2):157–162
Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K (2008) Induced pluripotent stem cells generated without viral integration. Science 322(5903):945–949
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Tang DQ, Wang Q, Burkhardt BR, Litherland SA, Atkinson MA, Yang LJ (2012) In vitro generation of functional insulin-producing cells from human bone marrow-derived stem cells, but long-term culture running risk of malignant transformation. Am J Stem Cells 1(2):114–127
Tateishi K, He J, Taranova O, Liang G, D’Alessio AC, Zhang Y (2008) Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem 283(46):31601–31607
Teo AK, Wagers AJ, Kulkarni RN (2013) New opportunities: harnessing induced pluripotency for discovery in diabetes and metabolism. Cell Metab 18(6):775–791
Thatava T, Nelson TJ, Edukulla R, Sakuma T, Ohmine S, Tonne JM et al (2011) Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny. Gene Ther 18(3):283–293
Thatava T, Kudva YC, Edukulla R, Squillace K, De Lamo JG, Khan YK et al (2013) Intrapatient variations in type 1 diabetes-specific iPS cell differentiation into insulin-producing cells. Mol Ther: J Am Soc Gene Ther 21(1):228–239
Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U et al (2006) Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun 341(4):1135–1140
Tsai PJ, Wang HS, Shyr YM, Weng ZC, Tai LC, Shyu JF et al (2012) Transplantation of insulin-producing cells from umbilical cord mesenchymal stem cells for the treatment of streptozotocin-induced diabetic rats. J Biomed Sci 19:47
Turnovcova K, Ruzickova K, Vanecek V, Sykova E, Jendelova P (2009) Properties and growth of human bone marrow mesenchymal stromal cells cultivated in different media. Cytotherapy 11(7):874–885
Urban VS, Kiss J, Kovacs J, Gocza E, Vas V, Monostori E et al (2008) Mesenchymal stem cells cooperate with bone marrow cells in therapy of diabetes. Stem Cells 26(1):244–253
Vajdic CM, van Leeuwen MT (2009) Cancer incidence and risk factors after solid organ transplantation. Int J Cancer J Int Cancer 125(8):1747–1754
Wang HS, Shyu JF, Shen WS, Hsu HC, Chi TC, Chen CP et al (2011) Transplantation of insulin-producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell Transplant 20(3):455–466
Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S et al (2006) Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 24(3):781–792
Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, Vander Werff I et al (2008) Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells 26(11):2865–2874
Wernig M, Meissner A, Cassady JP, Jaenisch R (2008) c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2(1):10–12
Wu H, Mahato RI (2014) Mesenchymal stem cell-based therapy for type 1 diabetes. Discov Med 17(93):139–143
Xie QP, Huang H, Xu B, Dong X, Gao SL, Zhang B et al (2009) Human bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitro. Differ Res Biol Divers 77(5):483–491
Xu X, Browning VL, Odorico JS (2011) Activin, BMP and FGF pathways cooperate to promote endoderm and pancreatic lineage cell differentiation from human embryonic stem cells. Mech Dev 128(7–10):412–427
Yamanaka S (2007) Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell 1(1):39–49
Yang LJ (2006) Liver stem cell-derived beta-cell surrogates for treatment of type 1 diabetes. Autoimmun Rev 5(6):409–413
Yechoor V, Liu V, Espiritu C, Paul A, Oka K, Kojima H et al (2009) Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev Cell 16(3):358–373
Zaret KS, Grompe M (2008) Generation and regeneration of cells of the liver and pancreas. Science 322(5907):1490–1494
Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S et al (2009) Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res 19(4):429–438
Zhao W, Wang Y, Wang D, Sun B, Wang G, Wang J et al (2008) TGF-beta expression by allogeneic bone marrow stromal cells ameliorates diabetes in NOD mice through modulating the distribution of CD4+ T cell subsets. Cell Immunol 253(1–2):23–30
Zhu FF, Zhang PB, Zhang DH, Sui X, Yin M, Xiang TT et al (2011) Generation of pancreatic insulin-producing cells from rhesus monkey induced pluripotent stem cells. Diabetologia 54(9):2325–2336
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H et al (2002) Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 13(12):4279–4295
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Lei, L., Mao, Y. (2017). New Advances in Stem Cell Therapy for Diabetes Mellitus. In: Pham, P. (eds) Pancreas, Kidney and Skin Regeneration. Stem Cells in Clinical Applications. Springer, Cham. https://doi.org/10.1007/978-3-319-55687-1_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-55687-1_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55686-4
Online ISBN: 978-3-319-55687-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)