Abstract
Diabetes mellitus (DM) is reported as the most common metabolic disorder and considered major causes of morbidity and mortality. Type 1 DM (T1DM) is considered an autoimmune disease characterized by deficiency of the insulin secretion due to destruction of specific insulin-producing β-cells. Type 2 DM (T2DM) is defined as an endocrine disease associated with predominantly insulin resistance/metabolically active obese, adipocytokines abnormalities, and secondary development of β-cell dysfunction. With growing understanding of the pathogenesis of DM, alternative approaches aiming at repair and restoration of endogenous insulin production, regulation of metabolic processes with stem cell transplantation are increasingly considered as complements to current diabetes therapy strategies. However, the data on regenerative care in DM are not uniform. There are discrepancies between results received from the animal studies and data obtained by clinical investigations in this field. On the one hand, such inconsistencies have accompanied the fact that several types of stem cells were tested as perspective for regenerative strategy and nor all of stem cells were available in routine clinical practice. On the other hand, patients with different types of diabetes at several stages of evolution of the disease are failed to uniform considered candidates for stem cells transplantation and they probably are required a controversial approaches. Given the conflicting evidence concerning stem cell replacement in DM, the aim of the chapter is to explore, analyze, and summarize the data to clarify current knowledge and identify the future perspectives for regenerative care among DM patients. The present chapter accumulated contemporary knowledge regarding a paradigm of the regenerative therapy in DM. It is discussed the role of use of reprogramming stem cells, bone marrow-derived mononuclear cells, and lineage-specified progenitor cells in modern approaches of DM care.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Diabetes mellitus (DM) is recognized as the most common metabolic disease , which affects more than 347 million people worldwide and is reported as major cause of morbidity and mortality in general population (Scully 2012). Currently, type 1 DM (T1DM) is reported as an autoimmune disease characterized by insulin secretion deficiency due to destruction of insulin-producing β-cells (Ashcroft and Rorsman 2012). It is well established that the type 2 DM (T2DM) associates predominantly with metabolically active obese, insulin resistance, and adipocytokine production imbalance leading secondary to dysfunction/apoptosis of pancreatic β-cell (Paneni et al. 2013). Despite contemporary treatment strategy, T1DM and T2DM are frequently coexisting with major microvascular and macrovascular complications leading to target organ damages, i.e., ischemic tissue injury, retinopathy, nephropathy, and diabetes-related cardiomyopathy (Ali and Dayan 2009). The ability of endogenous repair system to improve ischemic damage of the tissues and to restore of innate mechanisms of cell-to-cell cooperation to attenuate tissue perfusion and endothelial function is sufficiently worse (Cade 2008). As a consequence, intensified tissue remodeling and the extended ischemic injury lead to increased cardiovascular (CV) morbidity and mortality (Berezin 2016a). Finally, DM-related mortality may approximately twofold increase when compared with death rate in DM-free patient population (Nwaneri et al. 2013). Nevertheless, not all complications of DM are considered consequence of ischemic tissue injury, and they may closely relate to metabolic memory phenomenon (Berezin 2016b). Various factors contributed to increased CV risk in DM are determined, i.e., hyperglycemia, lipotoxicity, age-related and diabetes-related comorbidity, and known CV diseases . In fact, they all may lead to malignant evolution of the diabetes associated with poor outcomes and may reflect a shortcoming of current therapies. Currently established therapies molecular targets in diabetic patients affect not only insulin secretion, glucose regulator peptides, hormones, enzymes, and transporters. However, they should also mediate improving hypoglycemia, suppression of oxidative stress and endoplasmic reticulum stress, prevention of atherosclerosis, attenuation of dyslipidemia and endothelial function, modification of coexisting CV risk factors, and adequate control in comorbidities (Howangyin and Silvestre 2014).
Although there is an understanding of the several mechanisms and different phases in pathogenesis of DM, it is unclear whether alternative translational approaches regarding tissue reparation and restoration of failing β-cell function based on attenuation of metabolic processes via stem cell transplantation are effective (Holditch et al. 2014). Indeed, clinical use of pluripotent stem cells (PSCs) , as well as embryonic stem cells (ESCs) and induced PCSs (iPCSs), appears to be promising and safe in a long-term prospective (Liew 2010; Abdelalim et al. 2014). As it is expecting, various types of ESCs and iPSCs lines having a great differential potential may translate into all cell types, and they have a high potency to differentiate into insulin-secreting β-like cells without or very low risk of immune rejection (Abdelalim et al. 2014). However, the results of the recently performed studies regarding regenerative care in DM are controversial and require to be explained in detail (Chidgey et al. 2008). In this context, there are discrepancies between results received in the animal studies and data that have been obtained in the clinical investigations. The first controversy relates to some inconsistencies, which might accompany the fact that several types of stem cells were tested as prospective for regenerative strategy, and not all of stem cells were available in routine clinical practice. The second controversy attached to the patients with different types of DM at several stages of evolution of the disease failed to uniform considered candidates for stem cells transplantation, and they would probably require further investigations (Aguayo-Mazzucato and Bonner-Weir 2010; Anastasia et al. 2010). Given the conflicting evidence concerning stem cell replacement in T2DM and T1DM, the aim of the chapter was to seek, analyzes, and summarize the data to clarify actual knowledge and identify the future perspectives for regenerative therapy in diabetics.
2 The Regenerative Care Paradigm
The paradigm of regenerative therapy bases on novel knowledge of pathogenesis and mechanisms of endogenous reparation mechanisms (Terzic and Behfar 2014). It has been postulated that regenerative therapy based on cell care might attenuate or reverse disease progression. In this context, stem cell therapies are applied essentially as adjuvants to standard of care with the goal of furthering an otherwise limited self-renewal capacity of the disease (Qi et al. 2012).
3 Expected Effects of Cell Therapy
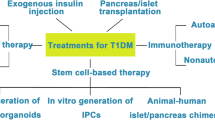
The possible effects of cell therapy have many phases, and they affect several sides of pathophysiological mechanisms of DM evolution (Berezin 2014). The possible therapeutic approaches of cell care in diabetics are presented in Fig. 3.1.
The possible approaches are:
-
Restoring and renewing of pool of the functional β-cells from human stem cells
-
Stimulation of the endogenous reparation processes
-
A main result of stem cell transplantation is regulation of metabolic processes through production of both cytokines and growth factors
3.1 Regeneration of Functional β-Cell Mass
Development of DM is characterized by the loss of functional pool of the pancreatic β-cells due to necrosis/apoptosis and negative autocrine/paracrine regulation that lead to progressive insufficiency in the insulin secretion (Holditch et al. 2014). The attempt to generate surrogate β-cells is widely used technology aim of which was to compensate the short supply of islets for transplantation to diabetics requiring daily short-acting insulin (Bhonde et al. 2014). Unfortunately, the poor availability of donor islets and high risk of rejection even within chronic immune suppression has severely restricted the broad routine clinical use of pancreas islet cell transplantation (Calafiore et al. 2014). Consequently, there is increased interest in islet cell that might obtain through neogenesis from stem cells’ lines originated from embryonic or mesenchymal cells and then used as a translator for favorable responses in diabetics (Burt et al. 2002; Chen et al. 2014; Kojima 2014). The progress in our knowledge ragarding surrogate β-cells and β-cell like cells produced insulin has been mediating a use of ESCs, iPSCs, human perinatal tissues including peripheral blood mononuclears, cord blood, amnion, placenta, umbilical cord, bone marrow, pancreas, and postnatal tissues involving adipose tissue (Bhonde et al. 2014; Rabelink and Little 2013; Bhansali et al. 2009; Abraham et al. 2008; Burt et al. 2002; Lampeter et al. 1998). Although these progresses in derivation of β-cell-like cells from ESCs using reprogramming technology have taken a greater leap, the clinical implementation is limited due to controversies affecting human ESC rejection (Bhonde et al. 2014; Voltarelli et al. 2011).
3.2 Allogenic Pancreatic Islet Transplantation
Currently allogenic pancreatic islet transplantation is considered the most efficient method of regenerative care in DM (Nostro and Keller 2012). Transcriptional signaling mechanisms that are in vivo involved in pancreas reparation and islet pancreatic development are well-structured and closely controlled processes. In this context, up-regulated pancreatic islet transcripts in differentiating β-cell populations are required the formation of β-like cells producing insulin . Several clinical approaches toward reprogramming and differentiation of islet precursor cells from ESC base on regulation of the relevant transcription factor expression (Pdx1, Ngn3, Isl-1, etc.), some extracellular factors (Voltarelli et al. 2011), or lentiviral vectors (Jimenez-Moreno et al. 2015). These approaches might initiate creation of bioartificial pancreas, although a significant translation of similar idea into clinical application for T1DM and T2DM is not evident (Ludwig and Ludwig 2015; Khosravi-Maharlooei et al. 2015). However, the advantages in islet transplantation have exhibited a dramatic improvement in the 5-year insulin independence rates for diabetics (Khosravi-Maharlooei et al. 2015).
It was noted that there is another source for transplantation of exogenous human and nonhuman pancreas/islets or even artificial islets. However, any translational strategies regarding enhancing proliferation and maturation of endogenous β-cells, loss prevention of β-cell and β-like cells, or any methods regarding renewal of β-like-cell populations from ESCs and non-β-cells appear to be promised (Weir et al. 2011). Although currently used methods of regeneration enhancement of functional pool of β-cells from human ESCs appear as the most promising clinical approach for T1DM regenerative therapy (Lindahl et al. 2014), the cell replacement care regarding generation of unlimited sources of β-cells have been met with some sufficient limitations (Calafiore et al. 2014). As one, the purification procedure of desire cell population is a critical step to obtain enough portions of islet precursors needed to further cell-lineage selection (Soria 2001). The prevention of islets’ loss in long-term prospective might need an immunosuppressant use (Jafarian et al. 2014).
3.3 Human Pluripotent Stem Cells
There are various ESC sources, which are considered as an appropriate resource of creating functional β-like cell generations in a safe and efficient manner (Fu 2014; Weir et al. 2011; Pandian et al. 2014; Soria 2001):
-
1.
ESCs derived from surplus blastocysts reprogrammed for fertilization procedures in vitro
-
2.
iPSCs created resulting in the reprogramming method of various somatic cells
The practical value of ESCs has been widely investigated during the last decade. At least two large clinical trials have recently completed, but clinical value of obtained results has become controversial (Philonenko et al. 2011). Although ECSs or adult stem cells, which were derived from various cell lines, may differentiate into β-like cells and restore the insulin production, they have represented the immune effects on the β-cells leading to their direct autoimmune destruction and destroying. (Calafiore 2014).
3.4 Induced Pluripotent Stem Cells
In this context, human iPSCs are discussed the most promising source for transplantation, because contemporary cellular reprogramming technology did not associate with immune modulatory ability of iPSCs did not represented immune modulatory ability (Bar-Nur et al. 2011). First iPSCs have been successfully derived from human somatic cells, i.e., dermal fibroblasts and keratinocytes (Soejitno and Prayudi 2011). It is known that redifferentiation of iPSCs into functional maturated pancreatic islets may modify disease progression through an increase of failing islet survival (Kudva et al. 2012). Importantly, the contemporary technology of iPSC derivation from adult somatic cells completely excludes the exposure of embryonic cells. Currently, iPSCs have been derived from diabetics using integrating retroviral vectors that incorporate directly in the host genome (Reiland et al. 2011; Ma et al. 2013). In fact, various reprogramming systems are different in their ability to biosafety, and integration-free reprogramming systems are more modern and hazardless (Kudva et al. 2012; Sommer et al. 2012).
The discovery of novel technologies suggests seeking of possible approaches regarding generation of patient-specific iPSCs. Various reprogramming factors have been now identified as functionally acting molecules – the main role of which was to significantly improve the results of iPSC reprogramming procedure. However, prior to a clinical implementation of the iPSCs, a strong concerns about their specificity, kinetics, and safety is required. (Bai et al. 2013).
3.5 Trans-differentiation Procedure
Yet one of the attractive strategies for regenerative care is the trans-differentiation procedure based on the direct conversion of the single somatic cell to another type of cell (Ma et al. 2013). Recent studies have shown a new paradigm of trans-differentiation, i.e., using specific transcriptional factors to induce novel PSC generation through trans-differentiation or induce PSC trans-differentiation through transcription factors. The trans-differentiation procedure allows generating plastic intermediates synthesis, which may attenuate iPSC reprogramming and a wide range of tissue-specific precursor cells (Ma et al. 2013; Bai et al. 2013). Clinically based evidences are required to be disseminating knowledge about novel methods of iPSC trans-differentiation on routine clinical practice.
The results of the investigations regarding an ability of surrogate cells derived from ESCs to produce insulin in vivo have appeared to be controversial. It particularly relates to absence of the commonly used pretty accurate standard protocol. The currently implemented protocol represents the requirement regarding methods of derivation of the pancreatic progenitors from PSCs (Bar-Nur et al. 2011). Moreover, there are no commonly used essential criteria in helping determine number of in vitro generated β-like cells enough to further transplantation (Naujok et al. 2011; Naujok and Lenzen 2012). Therefore, human-derived bone marrow-originated mesenchymal stem cells (hBM-MSCs) may be considered as a source for reprogramming procedure of insulin-producing β-like cells. A specific protocol regarding the generation of insulin-producing islet-like clusters derived from hBM-MSCs has now been produced. (Jafarian et al. 2014). On this way, the platelet-rich plasma might attenuate the environment for further BMSC development and differentiation (Lian et al. 2014). It has been postulated that hBM-MSCs may be considered an optimal source for an appropriate transplantation procedure compared with iPSC, while large clinical investigations are required to obtain strong evidence regarding this item.
3.6 The Endogenous Reparation Process Stimulation
Recent studies have shown that the development of DM-related complications, i.e., critical limb ischemia, vasculopathy, peripheral neuropathy, accelerating atherosclerosis, and neuropathic diabetic foot, closely associated with dramatic decline of number of PSCs, circulating precursor cells (CPCs), and endothelial progenitor cells (PCs) (Russ et al. 2015; Berezin 2016c). Stem cells (SCs) play a central role in the precise regulation and an appropriate provision of the organism development at the embryonic stage. Therefore, SCs represent their direct potency in tissue regeneration and an ability to regulate innate mechanisms of endogenous reparation in adult life period (Sener and Albeniz 2015). Indeed, SCs have exhibited a high potency to differentiate into a wide spectrum of the cells and also to provide several soluble circulating transcriptional factors, which are required for tissue regeneration and maintenance of repair systems (Sener and Albeniz 2015). In fact, there are evidence that the SCs exposure might be effective through modulating inflammatory changes, tissue remodeling, extracellular matrix renewal, attenuation of cell migration, and maintenance of angiogenesis/neogenesis. It is reported that EPCs with immune phenotypes CD34 + KDR+(VEGFR1) and CD31 + 133+ might have high therapeutic value in the healing process of neuropathic and ischemic lesions in DM (Sambataro et al. 2014; Shi and VandeBerg 2015). As it is expected, direct derivation of embryonic SCs to CD34+ cells might give a source for regenerative care in the future (Shi and VandeBerg 2015). The favorable effects of EPCs on target cells may associate with stimulation of the endogenous repair systems especially affecting the endothelium . The restoring of the endothelium structure and function may be deemed as a basis to improve natural evolution of DM and clinical outcomes in DM-related diseases, such as peripheral neuropathy, atherosclerosis, and critical limb ischemia (Berezin 2016d). In this context, the modulation of EPC-related signaling pathways may be useful for supporting trans-differentiation of the endogenous human SCs into functional β-like cells and mature endothelial cells (Mayhew and Wells 2010).
3.7 Regulation of Metabolic Processes via SCs
Replacing β-cells is frequently considered a simple way to enhance the population of pre-existing β-cells, stimulate angiogenesis, and reprogram pancreatic exocrine cells to β-like cells with ability to produce insulin (Berezin 2014). Cellular approaches based on SCs transplantation may also be useful for restoring the immune system response in T1DM or to attenuate insulin resistance and adipocytokines’ abnormalities in T2DM. It has been predisposed that identification of novel transcription factors and development of strategies for their modulation could lead to effective regeneration of functioning pool of pancreatic β-cells (Soria 2001; Pandian et al. 2014; Weir et al. 2011). Other promising soluble circulating factors, which could translate the effects on SCs, are cytokines and growth factors, but their clinical significance in diabetics is not yet clear and requires more investigations.
4 Results of Preclinical Studies of Stem Cell-Based Therapy
The first experience in the treatment of DM with using cell technologies was based on employment of the native SCs, as well as unfractionated or enriched in subpopulation PCs, while the next generations of cell delivery, i.e., directly reprogramming SCs, human bone marrow-derived mononuclear cells; lineage-specified PCs, are discussed as a more prospective cell resource, which appears to be much more promised for cell therapy.
4.1 Reprogramming Stem Cells
Therapeutic cloning of cells has now entered a new era in cell recruiting and reprogramming procedures in clinical settings. The novel approach has become affordable and assessable in the treatment of DM (Kang et al. 2010). By now commonly used essential requirements and regulatory approvals regarding clear design of SC use and recruitment of SCs for further reprogramming have been created (Zhou and Ding 2010). Apart from SCs suitable for reprogramming, it discussed ESCs and multipotent adult SCs/PCs derived from various tissues, i.e., pancreas, peripheral blood, intestine, liver, bone marrow, brain, etc. (Nsair and MacLellan 2011).
There is a large body of evidence regarding clinical exposure of some recombinant proteins and/or several pharmacological-active drugs that might initiate and then support the reprogramming process in the target cell population (Burns et al. 2006; Tancos et al. 2012). Various approaches including nonintegrating, nonviral, and nongenetic methods have been developed for generating clinically compatible iPSCs (Tancos et al. 2012). The are some conditions that have now been determined in vitro in which cell pluripotency is maintained, and even an ability to differentiate to specific somatic cells is desired (Lu and Zhao 2013). Hindley and Philpott (2013), summarizing our knowledge of the possible links between the core cell cycle machinery and the maintenance of iPSCs pluripotency, have emphasized that some advantages of therapeutic non-β-cell cloning includes low rate of the autoimmune reaction after transplantation (Teng et al. 2013). Although a strict similarity of iPSCs with ESCs is determined, the efficacy of reprogramming procedure is now low. Recent study performed by Soejitno and Prayudi (2011) has revealed that a typical reprogramming event may count only 0.01–0.1% of the entire cultured cell population. In fact, the establishment and design of SCs bank is discussed widely (Taylor et al. 2011). However, human iPSCs have demonstrated enormous clinical potency, which may affect their unique capability to self-renew and their innate ability to self-differentiate into all cell types when compared with human ESCs (Yeo amd Ng 2011). However, whether or not reprogrammed iPSCs have remained fully pluripotent at long time, it is not yet clear (Kang et al. 2010).
Little is known about the importance of the abovementioned advances of iPSCs for developing a new treatment strategy in DM (Fu and Xu 2012; Jiang et al. 2014). It has been postulated that the nature of the pluripotency is under a tight mutual counter-regulated control of genetic and epigenetic mechanisms. It is unclear whether the pluripotency is essential for an increase in the efficacy of cell transplantation or there is no usefulness in pluripotency in safety at long time (Kao et al. 2008). All these unresolved issues should be addressed to further large investigations.
4.2 Bone Marrow-Derived Mesenchymal Stem Cell Replacement
Since the first derivation of ESCs and iPSCs, the safety of clinical application of the cell therapy has been come under watchful gaze. Recent benefits in reprogramming regarding transgene-free iPSC have taken into consideration the potential of implementation of the PSC differentiated from different populations (Jung et al. 2012). Care with mesenchymal-originated PSCs is an extremely fast-growing method of regenerative medicine that has now proven their high safety and efficacy in the therapy of various states and diseases. Human bone marrow mesenchymal SCs (hBM-MSCs) are a self-renewing pool of the multipotent cells that is able to migrate to the sites of the pathological process and then mediate regenerative effect in situ. Animal model of T2DM showed that a 6-week period after successful hBM-MSC transplantation was associated with a sufficient decrease of the fasting blood glucose and lipid plasma levels. Additionally, the circulating C-peptide level was significantly increased (Pan et al. 2014). El-Tantawy and Haleem (2014) have reported that use of the autologous BM-MSCs has significantly prevented alterations of tissue and markedly attenuated alloxan-induced oxidative stress in albino rats with DM. Authors believed that BM-MSCs may be helpful in the prevention of diabetic complications associated with oxidative stress.
Tang et al. (2014) have studied the effect of autologous BM-MSCs in miniature pigs with established streptozotocin-induced DM. The obtained results have showed that BM-MSCs transplantation may prevent natural evolution of DM in animals that is associated with restoring blood glucose, serum insulin, and C-peptide levels, as well as attenuation of the oral glucose tolerance test and an increase in the islet numbers. These data suggested the implantation of autologous BM-MSCs for T1DM may partially improve a glucose homeostasis through restoration of the pool/function of β-cells and attenuation of the pancreatic microcirculation. Overall, the majority of the investigators believe that the BM-MSC transplantation is a safe and an effective procedure with great long-term prospective regarding clinical evolution of DM (El-Tantawy and Haleem 2014; Tang et al. 2014).
5 Clinical Efficacy of Stem Cell-Based Regenerative Therapy
There are controversial results regarding clinical efficacy of SC therapy in T1DM and T2DM patients (Matveyenko and Vella 2015; Moore et al. 2015). In prospective, open-labeled, two-armed study in T1DM (n = 20), Thakkar et al. (2015) have infused allogenic and autologous adipose-derived insulin-secreting mesenchymal stromal cells (IS-AD-MSCs) in combination with bone marrow-derived hematopoietic stem cells (BM-HSCs). Authors have concluded that co-infusion with the use of autologous IS-AD-MSCs and BM-HSCs have appeared to be a better method for long-term control of hyperglycemia when compared with isolated allogenic SC therapy. Surprisingly, allogenic SCs infusion has exhibited very varied effects on fasting glucose level, while safety of the treatment was good. In similar small studies, it was shown that the implantation of mesenchymal stem cells may lower glucose levels via paracrine-mediated influences rather than through direct trans-differentiation of transplanted precursors into β-like insulin-producing cells (Katuchova et al. 2015; Dave 2014; Ezquer et al. 2008). As it is expected, BM-HSCs may represent pro-angiogenic and immunomodulatory effects that might be useful to improve metabolic control in DM, especially when there is coadministrated transplantation BM-HSCs with pancreatic islets (Trivedi et al. 2008). Probably similar approaches might have more efficacy and safety.
6 Limitation of the Regenerative Therapy
Because T1DM is considered a chronic metabolic disorder characterized by targeted autoimmune-mediated β-cell destruction, there are some limitations regarding successful transplantation of both pancreatic islets and SCs (Xiao et al. 2014). By now, the islet transplantation in T1DM has been determined as the curative therapy only. Yet, there are several technical limitations regarding donor shortages and cellular damage that may appear within the isolation process and critically limit the further exposure of cultured cells (Jun et al. 2014). However, there is a method created for successful islet transplantation and based on coculturing single primary islet cells with adipose-derived stem cells (ADSCs) in concave micro wells. It has suggested that ADSCs may protect islet cells from damage and increase their survival in the culture prior to transplantation. In animal model xenotransplantation of microfiber-encapsulated spheroids has shown that coculture-transplanted mice maintained their blood glucose levels better than monoculture-transplanted mice. Moreover, it needed sufficiently less islet mass to reverse DM. Jun et al. (2014) have reported that the method for culturing islet spheroids might became a novel step in creating bioartificial pancreas. However, exaggerated immunogenic capacity and potent tumor-induced capability of transplanted cells have remained serious reasons for clinical implementation, while iPSCs, ADSCs, and BM-MSCs might be considered as a future of regenerative care (Schuetz and Markmann 2015).
7 Expectancies of Cell Replacement Care in Diabetics: From Bench to Bedside
It is well known that all forms of DM associate with the loss of pool of insulin-produced cells. In this context, the replacement of β-like cells, pancreatic islet, and iPSCs might be argued as a way to attenuate the natural evolution of the DM through improvement of metabolic control (Schroeder 2012). On the other hand, the metabolic abnormalities in DM do not limit a loss of insulin production. The results of the preclinical studies have supported an idea that the cell replacement might improve the entire regenerative potency including islet restoration and prevention of the metabolic memory phenomenon in target cells. Interestingly, the regenerative paradigm has been involved in various clinical settings appearing to be related to cultured SC platforms (Kojima 2014), while contemporary techniques for human ESC generation are known as genetically diverse, patient-specific, or disease-related SCs (Deng 2010). However, the efficacy of the several methods regarding standardization of cell isolation, the conversional nuclear transfer and delivery protocols have now assayed as very low, and the safety of the procedure has remained under discussion and evoked a serious concern even for iPSCs (Hao et al. 2009; Naujok and Lenzen 2012). Moreover, Soejitno and Prayudi (2011), thinking about SC application in several clinical setting, have determined limiting factors, which explain an increased risk of complications after cell therapy. Consequently, the use of retroviral or lentiviral vectors , coadministration of cMyc oncogene, may associate with low efficiency of reprogramming, allogeneic immune rejection, other autoimmune response, and tumor formation resulting in pre-existing epigenetic signature of the target cells (Fu 2014). The resolve of these issues might be mitigated by a breakthrough in the contemporary technology in iPSCs (Fu 2014; Li et al. 2010).
8 Future Perspectives of Regenerative Therapy
The shortcoming benefits of the cell therapy in DM relate to implement in routine clinical practice the own patient cells, which are directly differentiated into β-like cells under influence of the specifically created reprogramming technique (Chen et al. 2014). On this way, the correct choosing of the cell source (i.e., adult cells of intestine, pancreas, liver, bone morrow) is discussed as a limiting factor which contributed in successful transplantation of functional insulin-producing cells because no transplantable pancreatic islets were now found (Kojima 2014). The next expectation is creating appropriate techniques that could help to seek the personally patient-specific transcriptional factors required for islet regeneration and trans-differentiation of the target cells into β-like cells suitable for adequate insulin production (Jang et al. 2012; Boland et al. 2012). Currently synthetic DNA-based small molecule, which do not directly affect genome manipulation and allow to regulate and trigger epigenetic mechanisms, i.e., epigenetic enzymes or signaling pathways, of trans-differentiation of own cells into β-like cells with desired phenotype, have been found and broadly investigated (Sohn et al. 2012; Zou et al. 2012; Dadheech et al. 2015). Finally, protection of transplanted cells or renewal β-cells/pancreatic islets from destruction by immune reaction via discover of potent pharmacological agents appears to be promised (Lysy et al. 2012; Kim 2014).
9 Conclusion
Cell replacement therapy is considered a promising approach in the combined therapy of DM at the different stages of its evolution. The implementation of the novel technologies regarding isolation, sorting, culture, reprogramming, and trans-differentiation of SCs open serious prospective for achieving adequate control under metabolic abnormalities in all types of the DM, while several coexisting ethical and practical concerns require resolving in large clinical trials in the future.
Abbreviations
- ABMSCs:
-
Autologous bone marrow-derived mesenchymal stem cells
- ADSCs:
-
Adipose-derived stem cells
- CPC:
-
Circulating precursor cells
- DM:
-
Diabetes mellitus
- EPC:
-
Endothelial progenitor cells
- ESCs:
-
Embryonic stem cells
- PSCs:
-
Pluripotent stem cells
- SCs:
-
Stem cells
- T1DM:
-
Type one diabetes mellitus
- T2DM:
-
Type two diabetes mellitus
References
Abdelalim EM, Bonnefond A, Bennaceur-Griscelli A, Froguel P (2014) Pluripotent stem cells as a potential tool for disease modelling and cell therapy in diabetes. Stem Cell Rev 10(3):327–337
Abraham NG, Li M, Vanella L et al (2008) Bone marrow stem cell transplant into intra-bone cavity prevents type 2 diabetes: role of heme oxygenase-adiponectin. J Autoimmun 30:128–135
Aguayo-Mazzucato C, Bonner-Weir S (2010) Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol 6:139–148
Ali MA, Dayan CM (2009) The importance of residual endogenous beta-cell preservation in type 1 diabetes. Br J Diabetes Vasc Dis 9:6
Anastasia L, Pelissero G, Venerando B, Tettamanti G (2010) Cell reprogramming: expectations and challenges for chemistry in stem cell biology and regenerative medicine. Cell Death Differ 17:1230–1237
Ashcroft FM, Rorsman P (2012) Diabetes mellitus and the beta cell: the last ten years. Cell 148:1160–1171
Bai Q, Desprat R, Klein B et al (2013) Embryonic stem cells or induced pluripotent stem cells? A DNA integrity perspective. Curr Gene Ther 13(2):93–98
Bar-Nur O, Russ HA, Efrat S et al (2011) Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell 9(1):17–23
Berezin AE (2014) Diabetes mellitus and cellular replacement therapy: expected clinical potential and perspectives. World J Diabetes 5(6):777–786
Berezin AE (2016a) Endothelial progenitor cells dysfunction and impaired tissue reparation: the missed link in diabetes mellitus development. Diabetes Metab Syndr Clin Res Rev. [ahead of print]. doi:10.1016/j.dsx.2016.08.007
Berezin AE (2016b) Metabolic memory phenomenon in diabetes mellitus: achieving and perspectives. Diabetes Metab Syndr Clin Res Rev 10(2 Suppl 1):S176–S183. doi:10.1016/j.dsx.2016.03.016
Berezin A (2016c) Biomarkers for cardiovascular risk in diabetic patients. Heart. [epub ahead of print]. doi:10.1136/heartjnl-2016-310197
Berezin (2016d) Endothelial repair in diabetes: the causative role of progenitor cells dysfunction? J Clin Epigenet 2(2):22–24
Bhansali A, Upreti V, Khandelwal N et al (2009) Efficacy of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cells Dev 18:1407–1415
Bhonde RR, Sheshadri P, Sharma S et al (2014) Making surrogate β-cells from mesenchymal stromal cells: perspectives and future endeavors. Int J Biochem Cell Biol 46:90–102
Boland MJ, Hazen JL, Nazor KL et al (2012) Generation of mice derived from induced pluripotent stem cells. J Vis Exp 69:e4003
Burns CJ, Persaud SJ, Jones PM (2006) Diabetes mellitus: a potential target for stem cell therapy. Curr Stem Cell Res Ther 1(2):255–266
Burt RK, Oyama Y, Traynor A et al (2002) Hematopoietic stem cell therapy for type 1 diabetes: induction of tolerance and islet cell neogenesis. Autoimmune Rev 1:133–138
Cade WT (2008) Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther 88(11):1322–1335
Calafiore R, Montanucci P, Basta G (2014) Stem cells for pancreatic β-cell replacement in diabetes mellitus: actual perspectives. Curr Opin Organ Transplant 19(2):162– 168.
Chen YJ, Finkbeiner SR, Weinblatt D et al (2014) De novo formation of insulin-producing “neo-β cell islets” from intestinal crypts. Cell Rep 6(6):1046–1058
Chidgey AP, Layton D, Trounson A, Boyd RL (2008) Tolerance strategies for stem-cell-based therapies. Nature 453:330–337
Dadheech N, Srivastava A, Paranjape N et al (2015) Swertisin an anti-diabetic compound facilitate islet neogenesis from pancreatic stem/progenitor cells via p-38 MAP kinase-SMAD pathway: an in-vitro and in-vivo study. PLoS One 10(6):e0128244
Dave S (2014) Mesenchymal stem cells derived in vitro transdifferentiated insulin-producing cells: a new approach to treat type 1 diabetes. Adv Biomed Res 3:266
Deng W (2010) Exploiting pluripotency for therapeutic gain. Panminerva Med 52(2):167–173
El-Tantawy WH, Haleem EN (2014) Therapeutic effects of stem cell on hyperglycemia, hyperlipidemia, and oxidative stress in alloxan-treated rats. Mol Cell Biochem 391(1–2):193–200
Ezquer FE, Ezquer ME, Parrau DB et al (2008) Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 14:631–640
Fu X (2014) The immunogenicity of cells derived from induced pluripotent stem cells. Cell Mol Immunol 11(1):14–16
Fu X, Xu Y (2012) Challenges to the clinical application of pluripotent stem cells: towards genomic and functional stability. Genome Med 4(6):55
Hao J, Zhu W, Sheng C et al (2009) Human parthenogenetic embryonic stem cells: one potential resource for cell therapy. Sci China C Life Sci 52(7):599–602
Hindley C, Philpott A (2013) The cell cycle and pluripotency. Biochem J 451(2):135–143
Holditch SJ, Terzic A, Ikeda Y (2014) Concise review: pluripotent stem cell-based regenerative applications for failing β-cell function. Stem Cells Transl Med 3(5):653–661
Howangyin KY, Silvestre JS (2014) Diabetes mellitus and ischemic diseases: molecular mechanisms of vascular repair dysfunction. Arterioscler Thromb Vasc Biol 34(6):1126–1135
Jafarian A, Taghikhani M, Abroun S et al (2014) Generation of high-yield insulin producing cells from human bone marrow mesenchymal stem cells. Mol Biol Rep 41(7):4783–4794
Jang J, Yoo JE, Lee JA et al (2012) Disease-specific induced pluripotent stem cells: a platform for human disease modeling and drug discovery. Exp Mol Med 44(3):202–213
Jiang Z, Han Y, Cao X (2014) Induced pluripotent stem cell (iPSCs) and their application in immunotherapy. Cell Mol Immunol 11(1):17–24
Jimenez-Moreno CM, de Gracia Herrera-Gomez I, Lopez-Noriega L et al (2015) A simple high efficiency intra-islet transduction protocol using lentiviral vectors. Curr Gene Ther 15 (4): 436–446
Jun Y, Kang AR, Lee JS et al (2014) Microchip-based engineering of super-pancreatic islets supported by adipose-derived stem cells. Biomaterials 35(17):4815–4826
Jung Y, Bauer G, Nolta JA (2012) Concise review: induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells 30(1):42–47
Kang L, Kou Z, Zhang Y et al (2010) Induced pluripotent stem cells (iPSCs) – a new era of reprogramming. J Genet Genomics 37(7):415–421
Kao CF, Chuang CY, Chen CH et al (2008) Human pluripotent stem cells: current status and future perspectives. Chin J Physiol 51(4):214–225
Katuchova J, Harvanova D, Spakova T et al (2015) Mesenchymal stem cells in the treatment of type 1 diabetes mellitus. Endocr Pathol 26(2):95–103
Khosravi-Maharlooei M, Hajizadeh-Saffar E, Tahamtani Y, Basiri M, Montazeri L, Khalooghi K, et al (2015) Therapy of Endocrine Disease: Islet transplantation for type 1 diabetes: so close and yet so far away. Eur J Endocrinol 173(5):R165– R183
Kim C (2014) Disease modeling and cell based therapy with iPSC: future therapeutic option with fast and safe application. Blood Res 49(1):7–14
Kojima N (2014) In vitro reconstitution of pancreatic islets. Organogenesis 10(2):225–230
Kudva YC, Ohmine S, Greder LV et al (2012) Transgene-free disease-specific induced pluripotent stem cells from patients with type 1 and type 2 diabetes. Stem Cells Transl Med 1(6):451–461
Lampeter EF, McCann SR, Kolb H (1998) Transfer of insulin-dependent diabetes by bone marrow transplantation. Lancet 351:568–569
Li M, Chen M, Han W et al (2010) How far are induced pluripotent stem cells from the clinic? Ageing Res Rev 9(3):257–264
Lian Z, Yin X, Li H et al (2014) Synergistic effect of bone marrow-derived mesenchymal stem cells and platelet-rich plasma in streptozotocin-induced diabetic rats. Ann Dermatol 26(1):1–10
Liew CG (2010) Generation of insulin-producing cells from pluripotent stem cells: from the selection of cell sources to the optimization of protocols. Rev Diabet Stud 7(2):82–92
Lindahl M, Danilova T, Palm et al (2014) MANF is indispensable for the proliferation and survival of pancreatic β cells. Cell Rep 7(2):366–375
Lu X, Zhao T (2013) Clinical therapy using iPSCs: hopes and challenges. Genomics Proteomics Bioinforma 11(5):294–298
Ludwig B, Ludwig S (2015) Transplantable bioartificial pancreas devices: current status and future prospects. Langenbecks Arch Surg 400(5):531–540
Lysy PA, Weir GC, Bonner-Weir S (2012) Concise review: pancreas regeneration: recent advances and perspectives. Stem Cells Transl Med 1(2):150–159
Ma T, Xie M, Laurent T, Ding S (2013) Progress in the reprogramming of somatic cells. Circ Res 112(3):562–574
Matveyenko A, Vella A (2015) Regenerative medicine in diabetes. Mayo Clin Proc 90(4):546–554
Mayhew CN, Wells JM (2010) Converting human pluripotent stem cells into beta-cells: recent advances and future challenges. Curr Opin Organ Transplant 15(1):54–60
Moore SJ, Gala-Lopez BL, Pepper AR et al (2015) Bioengineered stem cells as an alternative for islet cell transplantation. World J Transplant 5(1):1–10. doi:10.5500/wjt.v5.i1.1
Naujok O, Lenzen S (2012) Pluripotent stem cells for cell replacement therapy of diabetes. Dtsch Med Wochenschr 137(20):1062–1066
Naujok O, Burns C, Jones PM et al (2011) Insulin-producing surrogate β-cells from embryonic stem cells: are we there yet? Mol Ther 19(10):1759–1768
Nostro MC, Keller G (2012) Generation of beta cells from human pluripotent stem cells: potential for regenerative medicine. Semin Cell Dev Biol 23(6):701–710
Nsair A, MacLellan WR (2011) Induced pluripotent stem cells for regenerative cardiovascular therapies and biomedical discovery. Adv Drug Deliv Rev 63(4–5):324–330
Nwaneri C, Cooper H, Bowen-Jones D (2013) Mortality in type 2 diabetes mellitus: magnitude of the evidence from a systematic review and meta-analysis. Brit J Diabetes Vasc Dis 13(4):192–207
Pan XH, Song QQ, Dai JJ et al (2014) Transplantation of bone marrow mesenchymal stem cells for the treatment of type 2 diabetes in a macaque model. Cells Tissues Organs 198(6):414–427
Pandian GN, Taniguchi J, Sugiyama H (2014) Cellular reprogramming for pancreatic β-cell regeneration: clinical potential of small molecule control. Clin Translat Med 3:6
Paneni F, Beckman JA, Creager MA et al (2013) Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J 34(31):2436–2443
Philonenko ES, Shutova MV, Chestkov IV et al (2011) Current progress and potential practical application for human pluripotent stem cells. Int Rev Cell Mol Biol 292:153–196
Qi SD, Smith PD, Choong PF (2012) Nuclear reprogramming and induced pluripotent stem cells: a review for surgeons. ANZ J Surg 84(6):E1–11
Rabelink TJ, Little MH (2013) Stromal cells in tissue homeostasis: balancing regeneration and fibrosis. Nat Rev Nephrol 9(12):747–753
Reiland S, Salekdeh GH, Krijgsveld J (2011) Defining pluripotent stem cells through quantitative proteomic analysis. Expert Rev Proteomics 8(1):29–42
Russ HA, Parent AV, Ringler JJ et al (2015) Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J 34:1759–1772
Sambataro M, Seganfreddo E, Canal F et al (2014) Prognostic significance of circulating and endothelial progenitor cell markers in type 2 diabetic foot. Int J Vasc Med 2014:589412
Schroeder IS (2012) Potential of pluripotent stem cells for diabetes therapy. Curr Diab Rep 12(5):490–498
Schuetz C, Markmann JF (2015) Immunogenicity of β-cells for autologous transplantation in type 1 diabetes. Pharmacol Res 98:60–68
Scully T (2012) Diabetes in numbers. Nature 485:S2–S3
Sener LT, Albeniz I (2015) Challenge of mesenchymal stem cells against diabetic foot ulcer. Curr Stem Cell Res Ther 10:530–534
Shi Q, VandeBerg JL (2015) Experimental approaches to derive CD34+ progenitors from human and nonhuman primate embryonic stem cells. Am J Stem Cells 4(1):32–37
Soejitno A, Prayudi PK (2011) The prospect of induced pluripotent stem cells for diabetes mellitus treatment. Ther Adv Endocrinol Metab 2(5):197–210
Sohn YD, Han JW, Yoon YS (2012) Generation of induced pluripotent stem cells from somatic cells. Prog Mol Biol Transl Sci 111:1–26
Sommer AG, Rozelle SS, Sullivan S et al (2012) Generation of human induced pluripotent stem cells from peripheral blood using the STEMCCA lentiviral vector. J Vis Exp 68:pii: 4327
Soria B (2001) In-vitro differentiation of pancreatic beta-cells. Differentiation 68(4–5):205–219
Tancos Z, Nemes C, Polgar Z et al (2012) Generation of rabbit pluripotent stem cell lines. Theriogenology 78(8):1774–1786
Tang K, Xiao X, Liu D et al (2014) Autografting of bone marrow mesenchymal stem cells alleviates streptozotocin-induced diabetes in miniature pigs: real-time tracing with MRI in vivo. Int J Mol Med 33(6):1469–1476
Taylor CJ, Bolton EM, Bradley JA (2011) Immunological considerations for embryonic and induced pluripotent stem cell banking. Philos Trans R Soc Lond Ser B Biol Sci 366(1575):2312–2322
Teng S, Liu C, Krettek C et al (2013) The application of induced pluripotent stem cells for bone regeneration: current progress and prospects. Tissue Eng Part B Rev 20(4):328–339
Terzic A, Behfar A (2014) Regenerative heart failure therapy headed for optimization. Eur Heart J 35:1231–1234
Thakkar UG, Trivedi HL, Vanikar AV et al (2015) Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy 17(7):940–947
Trivedi HL, Vanikar AV, Thakker U et al (2008) Human adipose tissue-derived mesenchymal stem cells combined with hematopoietic stem cell transplantation synthesize insulin. Transplant Proc 40:1135–1139
Voltarelli JC, Couri CE, Oliveira MC et al (2011) Stem cell therapy for diabetes mellitus. Kidney Int 1(3):94–98
Weir GC, Cavelti-Weder C, Bonner-Weir S (2011) Stem cell approaches for diabetes: towards beta cell replacement. Genome Med 3(9):61
Xiao F, Ma L, Zhao M et al (2014) Ex vivo expanded human regulatory T cells delay islet allograft rejection via inhibiting islet-derived monocyte chemoattractant protein-1 production in CD34+ stem cells-reconstituted NOD-scid IL2rγnull mice. PLoS One 9(3):e90387
Yeo JC, Ng HH (2011) Transcriptomic analysis of pluripotent stem cells: insights into health and disease. Genome Med 3(10):68
Zhou H, Ding S (2010) Evolution of induced pluripotent stem cell technology. Curr Opin Hematol 17(4):276–280
Zou C, Chou BK, Dowey SN et al (2012) Efficient derivation and genetic modifications of human pluripotent stem cells on engineered human feeder cell lines. Stem Cells Dev 21(12):2298–2311
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Berezin, A.E. (2017). New Trends in Stem Cell Transplantation in Diabetes Mellitus Type I and Type II. In: Pham, P. (eds) Pancreas, Kidney and Skin Regeneration. Stem Cells in Clinical Applications. Springer, Cham. https://doi.org/10.1007/978-3-319-55687-1_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-55687-1_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55686-4
Online ISBN: 978-3-319-55687-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)