Abstract
Diabetes mellitus type 1 is a form of diabetes mellitus that results from the autoimmune destruction of insulin-producing beta cells in the pancreas. The current gold standard therapy for pancreas transplantation has limitations because of the long list of waiting patients and the limited supply of donor pancreas. Mesenchymal stem cells (MSCs), a relatively new potential therapy in various fields, have already made their mark in the young field of regenerative medicine. Recent studies have shown that the implantation of MSCs decreases glucose levels through paracrine influences rather than through direct transdifferentiation into insulin-producing cells. Therefore, these cells may use pro-angiogenic and immunomodulatory effects to control diabetes following the cotransplantation with pancreatic islets. In this review, we present and discuss new approaches of using MSCs in the treatment of diabetes mellitus type 1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus type 1 (also known as diabetes type 1 or T1DM) is a form of diabetes mellitus that results from the autoimmune destruction of the insulin-producing beta cells in the pancreas. This is a typical chronic condition that demands permanent daily insulin administration to control blood glucose levels in the range of acceptable limits. T1DM affects 5–10 % of the total diabetic population. Insulin injection, the predominant treatment for T1DM, is effective to ameliorate hyperglycemia, but not sufficient to relieve the autoimmunity associated with T1DM or to facilitate the regeneration of lost islets. The resulting lack of insulin leads to hyperglycemia and its devastating complications, including cardiovascular disease, blindness, kidney failure [1], and amputations [2]. One possible treatment option is pancreatic transplantation. Indeed, whole organ pancreas transplantation is very effective in restoring normoglycemia and maintaining long-term physiological glycemic control. Simultaneous pancreas and kidney transplantation is presently considered the standard therapy for patients with T1DM with end-stage renal failure. However, there is a long waitlist of individuals awaiting pancreas transplant, and the procedure itself requires the administration of immunosuppressive therapy, which is associated with several major side effects. Islet transplantation is a less invasive procedure compared to the transplantation of the whole pancreas and is associated with a shorter hospital stay and lower morbidity [3]. Islet cell transplantation is based on the isolation of islets from their surrounding tissue and subsequent implantation in the recipient liver [4, 5]. The method entails the enzymatic and mechanical separation of islets from the rest of the organ [6]. Because of the different densities of islet cells in comparison with acinar tissue, one can use the centrifugation procedure to enrich the layers of high purity that are infused intraportally into the liver of the patients. Here, they lodge and can revascularize in a few weeks [4, 5, 7].
Clinical islet cell transplantation may result in a reduced requirement for insulin injections and may decrease hypoglycemic episodes without the morbidities associated with surgery. Unfortunately, islet cell transplantation was not able to achieve results comparable to those of solid organ transplantation until the Edmonton protocol (steroid-free immunosuppression) was devised; this protocol permitted islet cell transplantation to be an appropriate alternative to pancreatic transplantation. Significant advances in islet purification techniques and novel immunomodulatory agents have since renewed the interest in islet cell transplantation. On the other hand, islet cell transplantation is still limited by problems with the supply of islet cells, inadequate engraftment, and the deleterious effects of chronic immunosuppression [8].
As another alternative, the implantation of stem cells may be a potential treatment of T1DM [9, 10]. Stem cells can be prepared from embryonic or adult stem cells. However, the use of embryonic stem cells is controversial, since the collection of such cells is achieved by killing embryos. For this reason, the acquisition of embryonic stem cells is banned in many states or excluded from public funding. Interestingly, Klimanskaya and co-workers have developed a single-cell biopsy method that will not cause embryonic death [11]. This may provide an alternative method to avoid such regulatory issues. Moreover, while embryonic stem cells have great potential to differentiate into insulin-producing cells, they also have tumorigenic potential when transplanted [12]. Mesenchymal stem cells (MSCs), derived from the bone marrow or other sources, are being extensively investigated in the clinical setting for their immunomodulatory and tissue regenerative properties [13, 14]. MSCs have already been tested in some feasibility studies in the context of islet transplantation [15] and can be used to improve engraftment of pancreatic islets by suppressing inflammatory damage and immune-mediated rejection. In addition to their immunomodulatory effects, MSCs are known to provide a supportive microenvironmental niche by secreting paracrine factors and depositing extracellular matrix [16].
Mesenchymal Stem Cells
MSCs are multipotent, self-renewing cells that not only are primarily localized in the bone marrow but can also be isolated from other tissues, including adipose tissue [17], skeletal muscle [18], amniotic fluid [19], and umbilical cord blood [20]. Moreover, MSCs can be expanded for several passages without losing their self-renewing capacity [21]. The International Society for Cellular Therapy has provided criteria for defining MSCs. MSC populations are able to adhere to plastic in culture and exhibit expression of cell surface markers, such as CD105, CD73, and CD90. MSCs also lack the expression of CD45, CD34, CD14 or CD11b, CD79a, or CD19 and HLA-DR surface molecules [22, 23].
MSCs have been well characterized for their ability to differentiate into several cell types of mesenchymal origin, such as osteoblasts, adipocytes, and chondrocytes [22]. However, MSCs have also been shown to be able to differentiate into cell types of endodermal and ectodermal lineages [23], including renal tubular cells [24], lung epithelial cells [25], skin [26], neural cells [27], hepatocytes [28], and insulin-producing cells [29]. Despite this multipotency, the contribution of MSCs to the formation of functional tissue through transdifferentiation processes is still controversial [30].
Recently published studies have shown promising results with intraportal transplantation of bone marrow MSCs. Indeed, MSCs have been shown to stimulate liver regeneration after extensive liver resection, thereby ameliorating liver fibrosis and acute liver failure [31–33]. Because the harvesting of bone marrow MSCs is relatively simple, these MSCs have tremendous therapeutic potential in tissue repair and organ regeneration [34, 35]. Therefore, MSCs can also exert important reparative effects. These effects are often achieved through migration to the site of injury [36] and the release of paracrine factors that affect the migration, proliferation, and survival of the surrounding cells [37].
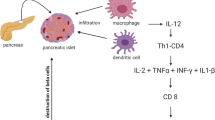
In addition, MSCs have been shown to contribute to repair processes through the secretion of pro-angiogenic molecules, thus promoting the formation of new blood vessels in vivo [38]. MSCs have emerged as a useful cell population for immunomodulation therapy due to their ability to secrete a large amount of bioactive molecules that affect immune and inflammatory responses [23, 39, 40]. The combination of immunomodulatory or immunosuppressive activity and tissue regenerative potential remains a significant scientific and clinical interest, and β-cell replacement therapy via stem cell transplantation may be a promising treatment for T1DM (Fig. 1).
Mesenchymal Stem Cell Sources and Safety
In order to utilize MSCs in a translational fashion, it is important to expand these cells ex vivo in order to obtain sufficient quantities for therapeutic use. Compared to other stem cell sources, such as hematopoietic stem cells (HSCs) or stem cell populations from the liver, spleen, and pancreas, MSCs seem to be a promising source for overcoming autoimmunity in T1DM because of their ability to transdifferentiate into various different lineages, including endodermal lines, and their particular immunosuppressive capacities [41]. MSCs from human bone marrow and adipose tissue (AT) represent very similar cell populations with comparable phenotypes [42]. The discovery of multipotent MSCs within AT has established a second major source of MSCs. Thus, MSCs with the potential to adopt a pancreatic endocrine phenotype could also exist in human AT. Besides having a comparable degree of mesodermal differentiation potential, AT-derived MSCs also appear to have higher frequencies and a high potential for angiogenesis or vasculogenesis compared to that of bone-marrow-derived MSCs [43]. Although these were only preliminary results, Timper et al. reported that insulin-producing cells could be generated from adipose-derived MSCs [44]. Finally, the human umbilical cord blood is another source of stem cells with the potential to develop into insulin-producing cells, even in vivo [20].
Human MSC transplantation could be practiced with minimal risks compared with HSC or bone marrow transplantation due to the hypo-immunogenicity of MSCs. This supports the hypothesis that MSCs are immune-privileged, which may be explained by their low expression of MHC proteins and T cell costimulatory molecules [45]. Allogeneic MSCs also have some advantages, such as immediate availability, no limitations in numbers, the possibility of donor selection based on different parameters, rapid performance of quality control tests, and product stability. Ongoing clinical trials are evaluating the effects of bone-marrow-derived MSCs for engraftment and survival of transplanted islets as well as the possibly of preventing complications from type 1 and type 2 diabetes [46]. In addition to their immunomodulatory effects, MSCs are able to participate in islet regeneration on the basis of their capacity to generate insulin-producing cells [47, 48]. Thus, the regenerative and immunomodulatory properties of allogeneic MSCs make them natural candidates for the treatment of diabetes [40].
Stem cells possess some features of cancer cells, including long lifespan, apoptosis resistance, and the ability to replicate for extended periods of time with the same control mechanisms. Therefore, stem cells may undergo malignant transformation, which is often a key obstacle to the safe use of stem-cell-based medicinal products. MSC-based therapy has also already been used in human disease settings, such as graft-versus-host and cardiac disease, with initial reports indicating a good safety profile. No tumors have been found in human recipients of MSCs thus far, and remarkably, even aneuploid MSCs have not been shown to give rise to tumors [49]. These findings indicate the lack of solid evidence for malignant transformation in vivo following the implantation of MSCs for clinical use. Further studies will be required to determine whether MSCs can prevent tumor formation and related mechanisms. MSCs with chromosomal alterations did not show any sign of malignant transformation either in vitro or in vivo [49]. However, the effects of MSCs on the regulation of tumor growth in animal and in vitro models are not as clear [50, 51]. Thus, whether MSCs stimulate growth of other tumors is still controversial, and no clinical evidence has validated this hypothesis [52].
Mesenchymal Stem Cells in Treatment of Diabetes Mellitus Type 1
One strategy for treating T1DM is to induce the differentiation of stem cells into insulin-producing cells in vitro; such a strategy may include the use of specific growth factors and an analogous microenvironment in vitro [53]. Recent research results on stem cells have shown that stem cell functions are controlled by intrinsic genetic programs and extracellular cues [54, 55]. Because the tissue-specific microenvironment plays a major role in the differentiation of pluripotent cells, MSCs found in the pancreas could represent an ideal source for β-cell generation. During the last few years, many research reports have described the existence of pancreatic tissue-specific MSCs having the general characteristic properties of MSCs, including the ability to differentiate into osteocytes, adipocytes, and neural cells on treatment with appropriate induction media [56]. However, several studies have presented controversial data regarding the origin of pancreatic MSCs and their ability to differentiate into insulin-producing cells (IPCs) [36, 57, 58]. Several problems prevent the translation of MSC-derived IPCs to clinical use, including the low proportion of differentiation to IPCs, restricted proliferation of IPCs, and lack of glucose responsively. While a few groups have claimed that these MSCs originated from the bone marrow, many reports have suggested that these cells originated from the pancreas itself. Moreover, one report demonstrated that mesenchymal proliferating cells are derived from a reversible epithelial-mesenchymal transition of pancreatic cells and can be redirected to IPCs in vitro [59]. However, using cell lineage tracing experiments in mice, several groups have published contradictory data, attributing the origin of these MSCs to preexisting pancreatic connective tissue rather than the epithelial-mesenchymal transition process [60, 61].
Many research groups have been working on the generation of IPCs from the transdifferentiation of MSCs in vitro [62]. Moreover, crosstalk has been observed between MSCs and the pancreas in diabetic animals, although the mechanisms mediating this process are unclear. MSCs express a set of chemokine receptors that may play critical roles in pancreatic homing/regeneration [36]. Systemic administration of MSCs increases β-cell mass and reverts hyperglycemia in streptozotocin-induced diabetic mice [63]. However, it is not clear whether MSCs can directly transform into β-cells. Dor and co-workers suggested that new β-cells can only be generated from preexisting β-cells instead of MSCs and concluded that MSCs only served as a “tropic mediator” to support islet function in an indirect manner, such as promoting angiogenesis [64]. However, some reports did show that IPCs can be regenerated from the transdifferentiation of other cells, pancreatic duct cells, and acinar cells [65–67].
Chen and collaborators [53] first reported the in vitro transdifferentiation of MSCs from rats into the functional insulin-producing islet-like cells that actively control blood glucose level in diabetic rats. Pancreatic and duodenal homeobox 1 (Pdx-1) is one of the most critical factors involved in the transdifferentiation of MSCs to β-cells [68, 69]. Other transcription or soluble factors, such as neurogenin 3 (Ngn3), paired box gene 4 (Pax4), aristaless related homeobox (Arx), glucagon-like peptide-1 (GLP-1), and epidermal growth factor (EGF), have also been shown to play a role in the regeneration of β-cells [56, 70, 71]. Further studies have demonstrated that MSCs also have the potential to transdifferentiate into glucagon- and somatostatin-expressing cells [44]. Several groups have also reported the formation of islet-like clusters from in vitro-cultured MSCs, given proper stimulation [72]. On the other hand, these reports failed to confirm the regeneration of whole functional islets, containing α-cells, β-cells, other cell types, and functional vasculature. Moreover, most of these studies failed to generate a sufficient amount of islets for human transplantation and long-term stability. Recent progress in tissue engineering suggested that a biocompatible scaffold may be necessary for the in vitro generation of artificial islets with functional vasculature from stem cells [73]. In our recent study [74], we confirmed that the administration of MSCs into the pancreatic microenvironment can indeed improve the symptoms of diabetes. We also aimed to determine whether different pancreatic microenvironments influenced the improvement of hyperglycemia and insulin deficiency. At 28 days after MSC transplantation, we observed a statistically significant decrease in blood glucose levels in rats and found that diabetic rats gained more weight than control rats during the course of the study. However, diabetic rats treated with MSCs did not exhibit significantly different blood glucose levels or body weight. Consequently, our results suggested that the transplantation of MSCs can improve diabetes in the pancreatic microenvironment in an animal model with streptozotocin (STZ)-induced diabetes. The different pancreatic areas into which the MSCs were implanted had no significant influence on the improvement of hyperglycemia and insulin deficiency (Fig. 2).
Gao and collaborators described the paracrine mechanism of MSCs [75] by testing the cytoprotective effects of conditioned medium from cultured MSCs on isolated islets exposed to STZ in vitro and in mice islets after the experimental induction of diabetes in vivo. The authors found that the transplantation of MSCs can ameliorate hyperglycemia in diabetic mice by promoting the regeneration of β-cells. Both β-cell replication and islet progenitor differentiation contribute to β-cell regeneration. MSC transplantation results in an increased phosphorylation of Akt and Erk by islets in vivo, and treatment with MSC-conditioned media promotes islet cell proliferation, concurrent with the increased phosphorylation of Akt and Erk in vitro. The induction of β-cell proliferation by MSC-conditioned media is completely blocked by treatment with the PI3K/Akt inhibitor LY294002 but not by treatment with the MEK/Erk inhibitor PD98059. Together, these data suggest that the PI3K/Akt signal pathway plays a critical role in β-cell proliferation after MSC transplantation [75].
Potential Role of Mesenchymal Stem Cells in Pancreatic Islet Transplantation
In a recent study [23], Figliuzii and collaborators analyzed an approach via which MSC may help the success of pancreatic islet transplantation and addressed some of the current problems associated with islet transplantation. MSCs may exert pro-angiogenic and immunomodulatory effects if they are transplanted together with pancreatic islets. The observed pro-angiogenic effects result from the release of angiogenic factors from MSCs that have been shown to improve islet vascularization and graft function in islet transplantation. The immunomodulatory possibilities of MSCs may help to reduce inflammatory damage to the islets. MSCs may also reduce autoimmunity through their capacity to inhibit T cell proliferation and suppress the differentiation and maturation of dendritic cells [23]. Several other approaches have been attempting to identify immune privileged sites, in order to minimize the role of autoimmunity [62, 76].
As reported by Sakata and collaborators, bone marrow can promote angiogenesis following the cotransplantation with islets. The authors found that blood glucose was lower and serum insulin was higher in mice administered both islet cells and bone marrow MSCs. Moreover, significantly more new peri-islet vessels were detected in these mice as compared with mice implanted with islet cells alone. Vascular endothelial growth factor (VEFG) was expressed at higher levels in mice implanted with bone marrow MSCs than in mice implanted with islets. PDX-1-positive areas were identified in bone marrow cells with increased staining over time. However, there were no normoglycemic mice and no insulin-positive cells in mice implanted with bone marrow alone. These results suggest that the cotransplantation of bone marrow cells with islets is associated with enhanced islet graft vascularization and function [15].
Based on our current understanding, bone marrow stem cells are perhaps the most enticing candidates of non-pancreatic origin for various reasons; in particular, clinical protocols for their collection and use have already been established, they have been shown to differentiate into pancreatic β-cells, and they have been proven to be effective at stimulating β-cell regeneration in damaged pancreatic tissue. In vivo murine studies have consistently shown that, even without differentiating into β-cells, bone marrow stem cell transplantation causes a reduction in plasma glucose levels and an increase in systemic insulin through a variety of mechanisms. Because a crucial concern for any cell-based T1DM therapy is preventing the autoimmune destruction of β-cells that create the original pathology and because bone-marrow-derived MSCs have been shown to inhibit T cell-mediated immune responses against newly formed β-cells, bone marrow stem cell therapy may provide the best treatment [9].
Human islet transplantation following the Edmonton protocol may be a permanent treatment for T1DM if the transplanted islets were not subjected to blood-mediated inflammatory reactions [77], acute rejection [78], islet toxicity by immunosuppressive agents [79], and ischemia caused by poor vascularity at transplantation [15]. As MSCs are well documented to possess better immunosuppressive and angiogenic properties, many studies have examined the effects of cotransplanting islets with MSCs and have found improved islet function and recovery [80–82].
Clinical Applications of Mesenchymal Stem Cells in Diabetes Mellitus Type 1
The first clinical trial using MSCs was carried out in 1995 [83]. In this study, 15 hemato-oncology patients became the recipients of autologous bone-marrow-derived MSCs. Since then, the use of MSCs has been further explored. By October 2014, the clinical trial database (http://www.clinicaltrials.gov) showed 352 clinical trials using MSCs for a variety of clinical disorders, including acute myocardial infarction, stroke, liver cirrhosis, amyotrophic lateral sclerosis, graft-versus-host disease (GVHD), solid organ transplant rejection, and autoimmune disorders. MSCs were used for the treatment of diabetes in 13 clinical trials, including eight trials investigating T1DM. In general, MSCs appear to be well tolerated, with most trials reporting lack of adverse effects in the medium term, although a few showed mild and transient peri-injected effects [84].
In an ongoing phase I–II clinical trial, patients with T1DM received umbilical MSC (UC-MSC) infusions through the pancreatic artery. The investigators hypothesized that the infusion of UC-MSCs may provide multiple signals for β-cell regeneration and even redifferentiate into local tissues in patients with diabetes mellitus. The use of autologous umbilical cord stem cells in children with T1DM resulted in insignificant differences in daily insulin doses and caused a decline in C-peptide levels after 1 year of follow-up [85]. In another study, Hu et al. assessed the long-term effects of the implantation of Wharton’s jelly-derived MSCs (WJ-MSCs) from the umbilical cords of 29 patients with newly onset T1DM. The authors demonstrated that this therapy can restore the function of islet β-cells over a longer time, although the precise mechanisms are still unknown. Thus, these data suggested that the implantation of WJ-MSCs may be an effective strategy for the treatment of T1DM [86]. Another form of cell therapy was performed by Mesples and co-workers using stem cells from the patient’s own bone marrow, including a conglomerate of MSCs and HSCs [87]. Bone marrow mononuclear cells were injecting by arterial catheterization directly into the patient’s pancreas. This therapy was performed in 20 T1DM patients, and the follow-up results showed a significant increase in pancreatic secretion of C-peptide and a decrease in the daily dose of exogenous insulin. This effect partially disappeared by the 3-year follow-up visit without increases in the levels of ICA and GAD antibodies [87].
There are several challenges in the application of MSCs in patients with newly diagnosed T1DM, the most important of which is that the length of the clinical response (insulin independence) and the relapse mechanism must still be investigated.
The Limitations of Mesenchymal Stem Cell-Based Therapy in Diabetes Mellitus Type 1
Promising preliminary and preclinical studies have led to phase I and phase II clinical trials with a therapeutic use of MSCs for T1DM treatment. However, some reports suggest that stem cells are unlikely to be used as a stand-alone therapy, because it may not be efficiently reverse autoimmunity of T1DM. They could be applied only for reducing immunosuppressant doses [88, 89]. The timing of cell delivery, number of cells, engraftment, and cell manufacture are very important; however, there is still a lack of information in this regard [90]. There is a need to tightly control the microenvironment and engraftment of the transplanted cell. Detailed investigations of how the microenvironment affects the homing and immunosuppressive effects of MSC are still lacking. An added complication in the scale-up of MSC-based technologies in T1DM is the lack of an effective route of cell administration. It was demonstrated that 70 % of MSCs injected intravenously stopped in the lungs, and only a small amount of cells arrived to the heart, kidneys, and liver [91]. The choice of an autologous or allogeneic approach is also an important consideration, and the option may be limited by disease-induced cell dysfunction and by an immune response to the transplanted cells. Another challenge in cell therapy is the large-scale production of MSCs under GMP conditions to prevent contamination and avoid MSC heterogeneity. Furthermore, the methods for the transportation of MSC-based products without affecting their viability and efficacy are an important subject, along with the issues related to cryopreservation.
Conclusions
The main goal of the present review was to present recent studies on the use of MSCs in the treatment of T1DM. We hypothesized that the paracrine function of transplanted MSCs rather than cell transdifferentiation may play a crucial role in hyperglycemia reversal in diabetic animal models. Through this paracrine action, the transplantation of MSCs would ameliorate T1DM. The best results have been obtained by the cotransplantation of MSCs with islet cells. These new innovative tools have provided tremendous opportunities for moving from the bench to the clinic in achieving successful long-term functional islet graft survival.
There is an increased need to describe the mechanisms of MSC-mediated cell therapy in detail, and challenges are still present in terms of engraftment, persistence, tissue targeting, and cell manufacture. Successful management of these challenges and the outcome of clinical trials using MSCs to treat T1DM or their complications will decide the future of cell-based therapy for this devastating disease.
References
Liao YH, Verchere CB, Warnock GL: Adult stem or progenitor cells in treatment for type 1 diabetes: current progress. Can J Surg 2007;50:137-42.
Pataky Z, Allet L, Golay A: Biofeedback: a new method for the prevention of amputations in patients with diabetes. Rev Med Suisse 2014;10:82-6.
Hatziavramidis DT, Karatzas TM, Chrousos GP: Pancreas islet cell transplantation: An Update. Ann Biomed Eng 2013;41:469-76.
Domíniguez-Bendala J, Riccordi C: Present and future cell therapies for pancreatic beta cell replenishment. World J Gastroenterol 2012;18:6876-84.
Domíniguez-Bendala J, Lanzoni G, Inverardi L, Riccordi C: Concise review: Mesenchymal stem cells for diabetes. Stem Cells Transl Med 2012;1:59-63.
O’Sullivan ES, Vegas A, Anderson DG, Weir GC: Islets transplanted in immunoisolation devices: a review of the progress and the challenges that remain. Endocrine Rev 2011;32:827-44.
Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000;343:230-8.
Agarwal A, Brayman KL: Update on islet cell transplantation for type 1 diabetes. Semin Intervent Radiol 2012;29:90-8.
Godfrey KJ, Mathew B, Bulman JC, Shah O, Clement S, Gallicano GI: Stem cell-based treatments for Type 1 diabetes mellitus: bone marrow, embryonic, hepatic, pancreatic and induced pluripotent stem cells. Diabet Med 2012;29:14-23.
Chang C, Niu D, Zhou H, Zhang Y, Li F, Gong F: Mesenchymal stroma cells improve hyperglycemia and insulin deficiency in the diabetic porcine pancreatic microenvironment. Cytotherapy 2008;10:796-805.
Klimanskaya I, Chung Y, Becker S, Lu SJ, Lanza R: Human embryonic stem cell lines derived from single blastomeres. Nature 2006;444:481-5.
Fujikawa T, Oh SH, Pi L, Hatch HM, Shupe T, Petersen BE: Teratoma formation leads to failure of treatment for type 1 diabetes using embryonic stem cell-derived insulin-producing cells. Am J Pathol 2005;166:1781-91.
Kode JA, Mukherjee S, Joglekar MV, Hardikar AA: Mesenchymal stem cells: immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009;11:377-91.
Hanson SE, Gutowski KA, Hematti P: Clinical applications of mesenchymal stem cells in soft tissue augmentation. Aesthet Surg J 2010;30:838-42.
Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E: Bone marrow cell co-transplantation with islets improves their vascularization and function. Transplantation 2010;89:686-693.
Hematti P, Jaehyup K, Stein AP, Kaufman D: Potential role of mesenchymal stromal cells in pancreatic islet transplantation. Transplant Rev 2013;27:21-9.
Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 2001;7(2):211-28.
Williams JT, Southerland SS, Souza J, Calcutt AF, Cartledge RG: Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg 1999;65:22-6.
In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, Noort WA, Claas FH, Willemze R, et al. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 2003;102:1548-9.
Reddi AS, Kuppasani K, Ende N: Human umbilical cord blood as an emerging stem cell therapy for diabetes mellitus. Curr Stem Cell Res Ther 2010;5:356-61.
Polak JM, Bishop AE: Stem cells and tissue engineering: past, present, and future. Ann N Y Acad Sci 2006;1068:352-66.
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8: 315-7.
Figliuzzi M, Bonandrini B, Silvani S, Remuzzi A: Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes. World J Stem Cells 2014;6:163-72.
Morigi M, Imberti B, Zoja C, Corna D, Tomasoni S, Abbate M, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol 2004;15:1794-804.
Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci U S A 2003;100:8407-11.
Nakagawa H, Akita S, Fukui M, Fujii T, Akino K: Human mesenchymal stem cells successfully improve skin-substitute wound healing. Br J Dermatol 2005;153:29-36.
Muñoz-Elias G, Marcus AJ, Coyne TM, Woodbury D, Black IB: Adult bone marrow stromal cells in the embryonic brain: engraftment, migration, differentiation, and long-term survival. J Neurosci 2004;24:4585-95.
Schwartz RE, Reyes M, Koodie L, Jiang Y, Blackstad M, Lund T, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest 2002;109:1291-302.
Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, et al. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes 2004;53:1721-32.
Phinney DG, Prockop DJ: Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells 2007;25:2896-2902.
Zheng JF, Liang LJ: Intra-portal transplantation of bone marrow stromal cells ameliorates liver fibrosis in mice. Hepatobiliary Pancreat Dis Int 2008;7:264-70.
Kaibori M, Adachi Y, Shimo T, Ishizaki M, Matsui K, Tanaka Y, et al. Stimulation of liver regeneration after hepatectomy in mice by injection of bone marrow mesenchymal stem cell via portal vein. Transplant Proc 2012;44:1107-9.
Yu J, Yin S, Zhang W, Gao F, Liu Y, Chen Z, et al. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther 2013;4:83.
Christ B, Stock P: Mesenchymal stem cell-derived hepatocytes for functional liver replacement. Front Immunol 2012;3:168.
Gruttadauria S, Grosso G, Pagano D, Biondi A, Echeverri GJ, Seria E, et al. Marrow-derived mesenchymal stem cells restore biochemical markers of acute liver injury in experimental model. Transplant Proc 2013;45:480-6.
Sordi, V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, et al. Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 2005;106:419-27.
Caplan AI, Dennis JE: Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006;98:1076-84.
Chen L, Tredget EE, Wu PY, Wu Y: Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3:e1886.
Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F: Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation 2007;83:71-6.
Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH: Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes 2008;57:1759-67.
Murphy MB, Moncivais K, Caplan AI: Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 2013;45:e54.
Dicker A, Le Blank K, Aström G, van Harmelen V, Götherström C, Blomqvist L, et al. Functional studies of mesenchymal stem cells derived from adult human adipose tissue. Exp Cell Res 2005;308:283-90.
Lin G, Wang G, Liu G, Yang LJ, Chang LJ, Lue TF, et al. Treatment of type 1 diabetes with adipose tissue-derived stem cells expressing pancreatic duodenal homeobox 1. Stem Cells Dev 2009;18:1399-406.
Timper K, Seboek D, Eberhardt M, Linscheid P, Christ-Crain M, Keller U, et al. Human adipose tissue-derived mesenchymal stem cells differentiate into insulin, somatostatin, and glucagon expressing cells. Biochem Biophys Res Commun 2006;341:1135-40.
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O: HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol 2003;31:890-6.
Fotino C, Ricordi C, Lauriola V, Alejandro R, Pileggi A: Bone marrow derived stem cell transplantation for the treatment of insulin-dependent diabetes. Rev Diabet Stud 2010;7:144-157.
Xie QP, Huang H, Xu B, Dong X, Gao SL, Zhang B, et al. Human bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitro. Differentiation 2009;77:483-91.
Sun Y, Chen L, Hou XG, Hou WK, Dong JJ, Sun L, et al. Differentiation of bone marrow-derived mesenchymal stem cells from diabetic patients into insulin-producing cells in vitro. Chin Med J 2007;120:771-6.
Tarte K, Gaillard J, Lataillade JJ, Fouillard L, Becker M, Mossafa H, et al. Clinical-grade production of human mesenchymal stromal cells: occurrence of aneuploidy without transformation. Blood 2010;115:1549-53.
Li X, Ling W, Pennisi A, Wang Y, Khan S, Heidaran M, et al. Human Placenta-Derived Adherent Cells Prevent Bone Loss, Stimulate Bone Formation, and Suppress Growth of Multiple Myeloma in Bone. Stem Cells 2011;29:263-73.
Tian LL, Yue W, Zhu F, Li S, Li W: Human mesenchymal stem cells play a dual role on tumor cell growth in vitro and in vitro. J Cell Physiol 2011;226:1860-7.
Zhu Y, Sun Z, Han Q, Liao L, Wang J, Bian C, et al. Human mesenchymal stem cells inhibit cancer cell proliferation by secreting DKK-1. Leukemia 2009;23:925-933.
Chen LB, Jiang XB, Yang L: Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastoenterol 2004;10:3016-20.
Gregory CA, Ylostalo J, Prockop DJ: Adult bone marrow stem/ progenitor cells(MSCs) are preconditioned by microenvironmental niches in culture: a two-stage hypothesis for regulation of MSC fate. Sci STKE 2005;294:pe37.
Li L, Xie T: Stem cell niche: structure and function. Annu Rev Cell Dev Biol 2005;21:605-31.
Limbert C1, Päth G, Ebert R, Rothhammer V, Kassem M, Jakob F, et al. PDX1- and NGN3-mediated in vitro reprogramming of human bone marrow-derived mesenchymal stromal cells into pancreatic endocrine lineages. Cytotherapy 2011;13:802-13.
Ianus A, Holz GG, Theise ND, Hussain MA: In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest 2003;111:843-50.
Lechner A, Yang YG, Blacken RA, Wang L, Nolan AL, Habener JF. No evidence for significant transdifferentiation of bone marrow into pancreatic ß-cells in vivo. Diabetes 2004;53:616-23.
Gershengorn MC, Geras-Raaka E, Hardikar AA, Raaka BM: Are better islet cell precursors generated by epithelial-to-mesenchymal transition? Cell Cycle 2005;4:380-2.
Chase LG, Ulloa-Montoya F, Kidder BL, Verfaillie CM: Islet-derived fibroblast-like cells are not derived via epithelial-mesenchymal transition from Pdx-1 or insulin-positive cells. Diabetes 2007;56:3-7.
Morton RA, Geras-Raaka E, Wilson LM, Raaka BM, Gershengorn MC: Endocrine precursor cells from mouse islets are not generated by epithelial-to-mesenchymal transition of mature beta cells. Mol Cell Endocrinol 2007;270:87-93.
Wu H, Mahato RI: Mesenchymal stem cell-based therapy for type 1 diabetes. Discov Med 2014;17:139-43.
Ezquer FE, Ezquer ME, Parrau DB, Carpio D, Yanez AJ, Conget PA: Systemic administration of multipotent mesenchymal stromal cells reverts hyperglycemia and prevents nephropathy in type 1 diabetic mice. Biol Blood Marrow Transplant 2008;14:631-40.
Dor Y, Brown J, Martinez OI, Melton DA: Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004;429:41-6.
Furuya F, Shimura H, Asami K, Ichijo S, Takahashi K, Kaneshige M, et al. Ligand-bound thyroid hormone receptor contributes to reprogramming of pancreatic acinar cells into insulin-producing cells. J Biol Chem 2013;288:16155-66.
Kopp JL, Dubois CL, Schaffer AE, Hao E, Shih HP, Seymour PA, et al. Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 2011;138:653-665.
Thorell F, Népote V, Avril I, Kohno K, Desgraz R, Chera S, et al. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010;464:1149-54.
Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S: Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells 2007;25:2837-44.
Li Y, Zhang R, Qiao H, Zhang H, Wang Y, Yuan H, et al. Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells. J Cell Physiol 2007;211:36-44.
Blyszczuk P, Czyz J, Kania G, Wagner M, Roll U, St-Onge L, et al. Expression of Pax4 in embryonic stem cells promotes differentiation of nestin-positive progenitor and insulin-producing cells. Proc Natl Acad Sci U S A 2003;100:998-1003.
Bonner-Weir S, Weir GC: New sources of pancreatic beta-cells. Nat Biotechnol 2005;23:857-861.
Chao KC, Chao KF, Fu YS, Liu SH: Islet-like clusters derived from mesenchymal stem cells in Wharton’s Jelly of the human umbilical cord for transplantation to control type 1 diabetes. PLoS One 2008;3:e1451.
Aloysious N, Nair PD: Enhanced survival and function of islet like clusters differentiated from adipose stem cells on a three dimensional natural polymeric scaffold: An in vitro study. Tissue Eng Part A 2013;20:1508-22.
Katuchova J, Tothova T, FarkasovaIannaccone S, Toporcer T, Harvanova D, Hildebrand T, et al. Impact of different pancreatic microenvironments on improvement in hyperglycemia and insulin deficiency in diabetic rats after transplantation of allogeneic mesenchymal stromal cells. J Surg Res 2012;178:188-95.
Gao X, Song L, Shen K, Wang H, Qian M, Niu W, et al. Bone marrow mesenchymal stem cells promote the repair of islets from diabetic mice through paracrine actions. Mol Cell Endocrinol 2014;388: 41-50.
Fadini GP, Ferraro F, Quaini F, Asahara T, Madeddu P: Concise review: diabetes, the bone marrow niche, and impaired vascular regeneration. Stem Cells Transl Med 2014; 3(8):949-957.
Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B: Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes 2002;51:1779-84.
Berman A, Pawelec K, Fiedor P: Allogeneic transplantation of isolated islet cells in clinical practice. Pol Arch Med Wewn 2009;119:326-32.
Desai NM, Goss JA, Deng S, Wolf BA, Markmann E, Palanjian M, et al. Elevated portal vein drug levels of sirolimus and tacrolimus in islet transplant recipients: local immunosuppression or islet toxicity? Transplantation 2003;76:1623-5.
Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, et al. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation 2010;89:1438-45.
Ohmura Y, Tanemura M, Kawaguchi N, Machida T, Tanida T, Deguchi T, et al. Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice. Transplantation 2012;90:1366-73.
Bhonde RR, Sheshadri P, Sharma S, Kumar A: Making surrogate ß-cells from mesenchymal stromal cells: Perspectives and future endeavors. Int J Biochem Cell Biol 2014;46:90-102.
Lazarus HM, Haynesworth SE, Gerson SL, Rosenthal NS, Caplan AI: Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells: implications for therapeutic use. Bone Marrow Transplant 1995;16:557-64.
Otto WR, Wright NA: Mesenchymal stem cells: from experiment to clinic. Fibrogenesis Tissue Repair 2011;4:20.
Haller MJ, Wasserfall CH, Hulme MA, Cintron M, Brusko TM, McGrail KM, et al. Autologous umbilical cord blood infusion followed by oral docosahexaenoic acid and vitamin D supplementation for C-peptide preservation in children with Type 1 diabetes. Biol Blood Marrow Transplant 2013;19:1126-9.
Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H, et al. Long term effects of the implantation of Wharton's jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J 2013;60:347-57.
Mesples A, Jiang S, Zhang Y, Luo Z, Hu X: C-peptide increase in chronic type 1 diabetic patients treated with autologous bone marrow cell transplantation through pancreatic artery catheterization: Three years follow-up. Stem Cell Discovery 2013;3:56-63.
Xu DM, Yu XF, Zhang D, Zhang MX, Zhou JF, Tan PH, et al. Mesenchymal stem cells differentially mediate regulatory T cells and conventional effector T cells to protect fully allogeneic islet grafts in mice. Diabetologia 2012;55:1091-1102.
Kim YH, Wee YM, Choi MY, Lim DG, Kim SC, Han DJ: Interleukin (IL)-10 induced by CD11b(+) cells and IL-10-activated regulatory T cells play a role in immune modulation of mesenchymal stem cells in rat islet allografts. Mol Med 2011;17:697-708.
Wang S, Qu X, Chunhua R: Clinical applications of mesenchymal stem cells. J Hematol Oncol 2012;5:19.
Herreros J, Chaques J, Trainini J, Ponton A, Sarralde A, Genovese J: Cardiac cell regeneration. Circle Cardiovascular 2011;18:207-215.
Acknowledgments
This work was supported by the Grant of the European Regional Development Fund—Project FNUSA-ICRC (No. CZ.1.05/1.1.00/02.0123) and VEGA grant 1/0592/13 and 1/0754/15 from the Ministry of Education, Science, Research and Sport of the Slovak Republic.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katuchova, J., Harvanova, D., Spakova, T. et al. Mesenchymal Stem Cells in the Treatment of Type 1 Diabetes Mellitus. Endocr Pathol 26, 95–103 (2015). https://doi.org/10.1007/s12022-015-9362-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-015-9362-y