Abstract
A core feature of ischemic heart disease is injury to cardiomyocytes (CMC). Ischemic CMC manifest the molecular mechanisms to undergo the major forms of cell injury and death, namely, oncotic necrosis, necroptosis, apoptosis and unregulated autophagy. Important modulators of ischemic injury are reperfusion and conditioning. Mitochondria have a major role in mediating the injury to CMC through membrane protein complexes referred to as death channels. Apoptosis is mediated by activation of a channel regulated by the Bcl-2 protein family leading to mitochondrial outer membrane permeabilization (MOMP). Oncotic type injury is mediated by opening of the mitochondrial permeability transition pore (mPTP). Mitochondria also have a reperfusion salvage kinase pathway (RISK). With cyclosporine A serving as a prototype, ongoing research is aimed at developing pharmacological approaches to condition and preserve mitochondrial integrity in order to promote CMC survival during episodes of myocardial ischemia.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiomyocytes
- Myocardial ischemia
- Mitochondria
- Mitochondrial outer membrane permeabilization (MOMP)
- Mitochondrial permeability transition pore (mPTP)
- Reperfusion
- Conditioning
Ischemic heart disease (IHD) is a leading cause of morbidity and mortality worldwide. IHD generally results from pathological interaction between diseased coronary arteries and the myocardium that requires abundant blood flow. Imbalance in coronary blood flow and myocardial demand leads clinically to the development of an acute coronary syndrome (ACS) [1]. At a fundamental level, IHD involves altered biology of the cardiomyocyte (CMC). Increasing evidence has focused on the mitochondrion as a key organelle in the response of the CMC to ischemic injury [2]. This evidence is reviewed in this chapter in the context of the overall pathobiology of IHD.
Basic Concepts of Ischemic Heart Disease
Thrombotic occlusion of an atherosclerotic coronary artery leads to ischemia to the subtended segment of myocardium due to marked reduction in oxygen and metabolic substrate delivery [1]. The ischemic myocardium is subject to complex metabolic and functional changes that lead to progressive injury to CMC and the microvasculature. Contractile function is lost within seconds. Ischemic CMC can become electrically unstable and become the source of an ectopic focus which can lead to ventricular fibrillation and sudden death. With profound ischemia, CMC become progressively impaired and transition from reversible to irreversible injury by 15–20 min. Irreversible injury then progresses in a wavefront pattern extending out from the ischemic subendocardium to the subepicardium leading to a complete myocardial infarct after about 3 h [3,4,5].
Some processes can profoundly alter the response of the myocardium during evolving myocardial infarction [6,7,8,9,10,11]. If instituted in a timely manner, reperfusion significantly limits the magnitude of irreversible myocardial damage. However, there may be some reperfusion injury in the form of impaired contractile function of spared myocardium (stunning) and lethal reperfusion injury to a population of severely impaired CMC at the time of reperfusion. Another process with a major impact on myocardial ischemia is myocardial conditioning. Myocardial preconditioning refers to the retardation of the development of irreversible myocardial injury when brief periods of coronary occlusion and reperfusion precede a prolonged coronary occlusion. After a latent period, the phenomenon returns 24 h later as a second window of protection (SWOP). Post-conditioning also achieves some degree of protection when gradual or intermittent reperfusion rather than immediate full reperfusion is provided after a bout of coronary occlusion. Preconditioning and postconditioning at a distance refer to the salutary effects on the evolution of myocardial ischemic injury produced by episodes of transient ischemia in skeletal muscle.
Myocardium and Its Cardiomyocytes
The myocardium is organized around CMCs and supporting structures [9]. CMCs are large cells (average diameter of 14 μm and length three to four times diameter) which constitute 80% of the volume of the myocardium but 20% of the total number of cells, most of which are non-CMC [9]. The myocardium contains a microvasculature including arterioles, capillaries and venules, all lined by endothelium. The capillaries are tightly aligned with the CMC in a one-to-one ratio. The CMC have one or two (25%) central nuclei containing chromosomes with deoxyribonucleic acid (DNA), abundant myofibrils arranged in sarcomeric contractile units and abundant mitochondria for energy production (Fig. 7.1). The plasma membrane (sarcolemma) has invaginations, the T tubules, at the level of each sarcomere, the T tubules with adjacent elements of smooth endoplasmic reticulum arranged in diads and triads to facilitate calcium flux and excitation-contraction coupling. Adjacent CMC are connected end-to-end by specialized plasma membrane, the intercalated discs, and side-to-side by desmosomes.
Ultrastructure of the normal mammalian myocardium. The typical cardiomyocyte has a central nucleus (N), compact cytoplasm, a sarcolemma (plasma membrane) with invaginations called transverse tubules (TT), myofibrils arranged into sarcomeres, a few lipid droplets (LD), glycogen granules and numerous mitochondria (M) containing numerous densely packed invaginations of the inner membranes, the cristae. In close proximity to the cardiomyocytes are capillaries (C). Electron micrograph, ×11,000 (This figure is reproduced from reference [2] with permission)
Because the CMC are specialized for continuous contraction and relaxation, the CMC have a very high energy requirement. This energy requirement is met by the large number of mitochondria which are organelles specialized to conduct oxidative phosphorylation. The mitochondria of CMC have large numbers of tightly paced cristae formed from invaginations of the inner mitochondrial membrane reflecting their high energy output. Maintenance of the electrical potential difference (∆Ψm) across the inner mitochondrial membrane is essential for oxidative phosphorylation and ATP generation [9].
Modes of CMC Injury and Death
In the last several years, research has shown that CMC have the molecular mechanisms to undergo all of the major modes of cell injury and death. These include oncosis, apoptosis and autophagy [9, 10, 12, 13]. The two of major importance in myocardial ischemic injury and infarction are oncosis and apoptosis [9, 10, 12,13,14,15]. Oncosis is manifest by cellular and organellar swelling due to progressive membrane dysfunction and damage due to ischemic or toxic injury coupled with rapid energy depletion reflected by rapid loss of adenosine triphosphate (ATP). Apoptosis is triggered by various physiologic and pathologic stimuli and is characterized by cellular and organellar shrinkage with subsequent fragmentation. Apoptosis is mediated by activation of a cascade of caspase enzymes (cysteine-aspartate proteases) via extrinsic or intrinsic pathways; at least partial preservation of ATP is required. Cell death due to apoptosis is characterized morphologically by cell shrinkage with intact plasma membrane and biochemically by caspase activation and discrete double-stranded DNA breaks. Although oncosis and apoptosis have been termed accidental and programmed cell death, respectively, oncosis can be a relatively uncontrolled process (oncotic necrosis) but often involves signaling pathways that mediate a more regulated process of necroptosis. Many forms of oncosis as well as apoptosis follow programmed patterns mediated by the activation of cell surface receptors, and both involve activation of distinctive gene profiles [14, 15].
Autophagy involves segregation of cellular components, including proteins and mitochondria, in autophagic vacuoles, merger of the autophagic vacuoles with lysosomes to form autophagolysosomes, and subsequent degradation of the constituents in the vacuoles [9]. Depending on whether the autophagic process is regulated or not, autophagy can serve as a mechanism of cell survival following stress or another distinctive form of programmed cell death [9].
Basic Pathobiology of Myocardial Ischemic Injury
Basic information about modes of cell injury and death have been integrated with our knowledge of CMC injury occurring in myocardial infarction. Ischemic cardiomyocyte injury is characterized by progressive membrane damage with a component of cell swelling [3,4,5]. Ischemic membrane damage has been shown to progress from discrete alterations in specific membrane pumps and ion channels to an intermediate stage of less selective and increasing membrane permeability with more severe ionic disturbances including increased influx of calcium ions to a final stage of membrane rupture [12]. The membrane damage is mediated by activation of phospholipases and proteases and accumulation of toxic metabolites. Mitochondrial swelling and calcium accumulation are prominent components of evolving CMC injury (Figs. 7.2 and 7.3). This pattern of cell injury is typical of the oncotic pattern of cell injury and death [12,13,14,15]. With the recognition of apoptosis as another important mode of cell injury and death, the role of apoptosis in ischemic CMC injury also must be considered [12,13,14,15].
Ultrastructure of ischemic cardiomyocyte with features of contraction band formation and calcium overload as seen in the peripheral zone of an evolving canine myocardial infarct. Note the foci of myofibrils condensed into bands (CB), the lipid droplets (LD), and granular mitochondrial calcium phosphate deposits (MCaD). Electron micrograph, ×6,500 (This figure is reproduced from reference [8] Dialogues in Cardiovascular Medicine with the permission of the publisher Les Laboratories Servier, Suresnes, France)
Ultrastructural detail of ischemic cardiomyocyte with lipid deposits (LD) and swollen mitochondria containing very electron-dense, annular-granular calcium phosphate deposits (MCaD) Electron micrograph, ×26,000. As ATP is reduced, mitochondrial oxidative capacity is decreased, leading to an accumulation of reesterified fatty acids as lipid droplets. As the sarcolemmal function becomes impaired, an increase in calcium influx occurs. With reperfusion, further calcium influx is coupled with an oxidative burst generating toxic oxygen-based radicals. The excess calcium triggers hypercontraction of the myofibrils manifest as contraction bands. The overload of calcium ions and toxic oxygen-based radicals leads to opening of the mitochondrial permeability transition pore (mPTP) , loss of membrane potential, swelling and collapse of ATP generation (This figure is reproduced from reference [8] Dialogues in Cardiovascular Medicine with the permission of the publisher Les Laboratories Servier, Suresnes, France)
CMC have been demonstrated to contain the molecular mechanisms to activate apoptotic pathways as well as pathways leading to progressive membrane damage even though CMC undergoing irreversible ischemic injury do not show the classic morphological features of apoptosis [14,15,16,17,18,19,20,21]. This has led to the conclusion that cardiomyocyte ischemic injury and death is a hybrid form of cell injury in which the terminal events are dominated by oncotic ultrastructure [10, 14, 15]. Perturbation of apoptosis with caspase inhibitors and genetic manipulation produces partial but not complete reduction in infarct size [16]. Thus in evolving myocardial infarction, multiple modes of cell death participate including oncotic necrosis, necroptosis, and apoptosis.
Autophagy has been shown to be capable of modulating cardiomyocyte cell injury and acute myocardial ischemic injury. Autophagy has also been found to have a role in cardiomyocyte survival in the setting of hibernating myocardium, a state of chronic ischemic myocardium associated with decreased myocardial function [22,23,24]. In heart failure, increased loss of CMC occurs by multiple modes of injury, namely, oncotic necrosis, necroptosis, apoptosis and autophagy, and this CMC loss contributes importantly to the progression of heart failure [25,26,27,28]. Both in ischemic injury and heart failure, the rate and magnitude of ATP depletion is a key determinant of the pathway of CMC injury and death [14, 15, 28].
Key Role of Mitochondria in CMC Injury
A number of distinct and overlapping subcellular pathways involving cell membrane death receptors, endoplasmic reticulum and mitochondria can be involved in the development of oncotic and apoptotic cell death [14,15,16,17,18,19,20,21]. Nevertheless, recent studies have implicated the mitochondria have a critical role in the pathogenesis of cell injury (Figs. 7.4 and 7.5) [21,22,23,24,25,26,27,28]. The biochemical and ultrastructural changes occurring in cardiac mitochondria during the evolution of myocardial ischemic injury are well documented [3,4,5]. Mitochondria are dynamic organelles that constantly undergo regulated processes of fusion, fission and substrate trafficking [29, 30]. In stressed cells, mitochondria can be subject to deleterious and beneficial effects due to activation of death channels and salvage pathways, respectively [2, 16,17,18,19,20,21, 28, 31,32,33]. The mitochondrial death channels include the mitochondrial permeability transition pore (mPTP) and a putative mitochondrial apoptosis channel (mAC). The mPTP is a voltage-dependent channel that is regulated by calcium and oxidative stress. The function of the mPTP is influenced by several proteins, especially the voltage-dependent anion channel (VDAC), the adenine nucleotide translocator (ANT) and cyclophilin D (CypD). The VDAC is located in the outer membrane, the ANT in the inner membrane, and CypD on the matrix side of the inner membrane. These proteins span the inner and outer mitochondrial membranes and provide a path for transport between the mitochondrial matrix to the cytoplasm. The opening of the mPTP results in the immediate loss of the electrical potential difference across the inner membrane with resultant cessation of ATP synthesis, influx of solute, and mitochondrial swelling [2, 28, 31,32,33]. Recent studies have determined a key role for another molecule, the F0F1ATPase, in interacting with the other component molecules and leading to the mPTP formation [28]. Modulation of mitochondrial proteins, including a modest increase in expression of uncoupling protein 2 (UCP-2), can cause a rapid decline in mitochondrial membrane potential and ATP level resulting in oncosis [34].
Cell death pathways. (a) Apoptosis. In the extrinsic death receptor pathway, binding of an extracellular ligand such as TNF-α to its death receptor TNFR recruits adaptor molecules to form the death-inducing signaling complex (DISC). Caspase-8 cleaves and activates effector caspase-3 and -7. In the intrinsic mitochondrial pathway, intracellular stress signals such as DNA damage, hypoxia, or glucose deprivation activate the pro-apoptotic Bax and Bak. During mitochondrial outer membrane permeabilization, Bax and Bak form pores that allow the release of apoptogens such as cytochrome c into the cytosol. Cytochrome c and APAF1 form the apoptosome which recruits and activates caspase-9 and leads to caspase-3 and -7 activation. Death-receptor mediated caspase-8 activation can also cleave Bid to form truncated Bid (tBid) and activate the intrinsic pathway. (b) Necrosis/Necroptosis. Excessive influx of Ca2+ into the mitochondria leads to opening of the mitochondrial permeability transition pore (mPTP) and disruption of the proton gradient and ATP synthesis. The influx of water and solutes into mitochondria leads to swelling and membrane rupture. Regulated necrosis, or necroptosis, occurs in the absence of caspase-3 where the RIP1-RIP3 complex facilitates necroptosis (This figure is reproduced from reference [21] with permission)
The mitochondrial death channels. The mPTP involved in oncotic necrosis and necroptosis has several component or associated proteins including the voltage-dependent anion channel (VDAC), adenine nucleotide translocase (ANT), cyclophilin-D (CypD), the benzodiazepine receptor (BDR) and hexokinase. The specific composition of a mitochondrial apoptosis channel (mAC) has not been determined but it is clear that the interactions of the Bcl-2 family of proteins, including Bax, Bak, and tBid with the outer mitochondrial membrane control the mitochondrial outer membrane permeabilization (MOMP). The mPTP and mAC (or Bax channel) are both regulated by Bcl-2 proteins and are opened by calcium ionophores and oxidative stress. When the mAC is opened and cytochrome c is released, this triggers apoptosis. When ischemia-reperfusion leads to an increase in matrix calcium levels above a certain threshold, the mPTP opening occurs. CypD and reactive oxygen species (ROS) can lower this threshold. Once the mPTP is opened, the mitochondrial membrane potential is rapidly lost (∆Ψm) (This figure is reproduced from reference [31] with permission)
Apoptosis can be initiated by an extrinsic pathway involving activation of certain membrane receptors with death domains and intrinsic pathways involving the endoplasmic reticulum and the mitochondria. There are levels of interaction between these pathways. A key event in many forms of apoptosis is mitochondrial outer membrane permeabilization (MOMP) produced by activation of the mAC [2, 16,17,18,19,20,21, 28, 31,32,33]. Outer mitochondrial membrane integrity, including the putative mAC, is regulated by multiple interactions between proteins of the Bcl-2 faimly, including Bax, Bid, Bcl-2 and Bcl-XL. Opening of the MOMP leads to release of cytochrome c and other molecules which join with cytoplasmic components including procaspase 9, apoptotic protease activating factor-1 (Apaf-1) and dATP to form the apoptosome. The apoptosome then triggers the activation of caspase 3 and other effector caspases in the cytoplasm leading to downstream effects on the nucleus [2, 21, 28].
The mitochondrial pathway rather than the extrinsic pathway appears to be dominant in the apoptosis component of myocardial ischemic injury. Confirmatory evidence has been provided to show that the extent of myocardial ischemia and reperfusion damage was reduced in transgenic mice overexpressing the anti-apoptotic Bcl-2 but not in transgenenic mice overexpressing a truncated form of the surface death receptor [35]. The interrelationships between apoptosis and oncosis has been confirmed by models involving initial activation of apoptosis followed by oncosis and, conversely, oncosis followed by apoptosis [16,17,18,19]. At the level of the mitochondria, the oncotic trigger of loss of the mPTP can occur in close temporal relationship to cytochrome c release via the mAC.
In ischemic CMC, when the electron transport system in mitochondria stops functioning due to lack of oxygen, this state leaves various components of the electron transport chain primed to generate oxygen free radicals when oxygen returns. Indeed with reperfusion a massive diversion of electrons from the electron transport system leads to the generation of oxygen radicals, the oxidative burst. Simultaneously, a large influx of calcium occurs. When the mitochondria are exposed to excess oxygen radicals and calcium, the result is opening of the mPTP and collapse of mitochondrial function [11]. This is a key event in ischemic reperfusion injury. A dual role for autophagy also has been identified. Activation of autophagy during the early phase of ischemia is cardioprotective, whereas delated or late activation of autophagy during reperfusion is detrimental and promotes cell death [28].
Conditioning is a complex phenomenon initiated by activation of various G-protein coupled receptors by various autocoids, including adenosine, bradykinin, and opioids, that are released during the brief periods of ischemia and reperfusion [36,37,38]. Activation of these receptors is followed by activation of a complex signaling cascade, including multiple kinases, that leads to opening of potassium channels in the mitochondrial membrane and maintenance of the mPTP and the electrical potential of the inner mitochondrial membrane. Preservation of mitochondrial function and ATP production is an absolute requirement for the protective effect of conditioning.
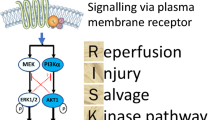
A component of the protective effect of conditioning involves the activation of a reperfusion injury salvage kinase (RISK) pathway in the mitochondria [10, 11, 39, 40]. One component of the pathway involves phosphatidylinositol-3 kinase (PI-3 K) acting on Akt (protein kinase B) and the mammalian target of rapamycin (mTOR). The other component involves mitogen-associated protein kinase (MAPK) and p42/p44 extracellular signal-related kinase (ERK). The two arms of the pathway converge on p70s6 kinase to activate glycogen synthase kinase β which acts to prevent opening of the mPTP. An isoform of the ATP-sensitive potassium channel (K+ ATP) also regulates mPTP opening. A component of autophagy, mitophagy, contributes to the protective effect of ischemic preconditioning [28,29,30].
Therapeutic Considerations
Because of the clinical importance of preserving myocardium, extensive effort has been aimed at developing pharmacological approaches to limiting myocardial ischemic damage. This area of research has been frustrated by initially promising experimental work being followed by negative or equivocal clinical trials in patients. Although myocardial ischemia – reperfusion injury is clearly mediated by calcium overload, toxic oxygen-based molecules, inflammatory mediators and neutrophils, clinical trials of agents aimed against these components of ischemic injury have not been found to be convincingly effective [10, 11]. Deficiencies in the design of the experimental work and complexities of confirming efficacy in clinical trials are important factors for these results [10, 11, 41]. The major advances in salvage of ischemic myocardium have come from the introduction of thrombolytic therapy and percutaneous coronary interventions (PCI) rather than from pharmacological treatments. Nevertheless, increased understanding of the pathobiology of myocardial ischemic injury and the key role of the mitochondria has led to renewed interest and promise in novel therapeutic approaches, used alone or in combination with PCI, for the treatment of patients with acute ischemic heart disease [9,10,11, 28, 31,32,33, 42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58].
The prototype for a pharmacological approach targeting the mitochondria is cyclosporine A (CsA) (Table 7.1) [28]. CsA is known to inhibit the formation and opening of the mPTP by binding to CypD. Positive results were obtained in a proof-of-concept small clinical trial of patients with evolving myocardial infarction [48, 49]. Other pharmacological agents are being designed and tested for their ability to activate the RISK pathway in the mitochondria and to enhance mitochondrial energy metabolism [11, 40, 54, 55]. Pharmacological agents under recent or active investigation include adenosine, atrial naturetic peptide, beta adrenergic blocking drugs, nitrates, phosphodiesterase-5 inhibitors, and supersaturated oxygen therapy, and antioxidant peptides targeted at mitochondria [54,55,56,57,58].
In experimental models, caspase inhibitors and other supressors of apoptosis have been shown to reduce myocardial infarct size [50]. However, approaches to retard apoptosis during evolving myocardial ischemic injury in patients have not yet been demonstrated. Other agents, designated as necrostatins, are being developed for activity against receptors involved in the hybrid necroptosis form of injury [50, 51]. Other approaches directed to the non-myocytic components of the myocardium, including endothelial cells, fibroblasts, and extracellular matrix are being explored [52].
The resurgence of interest in reducing ischemic and reperfusion injury has led to the formation of an NIH-sponsored consortium for preclinical assessment of cardioprotective therapies (CAESAR) [59,60,61]. Recently, however, several larger multicenter trials have reported lack of protective effect with CsA and remote ischemic pre-conditioning [62,63,64,65,66]. It has been pointed out that the effect of CsA is indirect and mediated by binding of CsA to the matrix protein CyP-D with the net effect being desensitization rather than true block of the pore. This has given rise to strong interest in developing potentially more effective inhibitors of the mPTP (Table 7.1) [28].
Conclusions
The cumulative effects of episodes of ACS and subsequent extent of myocardial infarction have major significance for the prognosis of patients with ischemic heart disease. Acutely, extensive myocardial infarction is associated with acute heart failure, cardiogenic shock and malignant arrhythmias [1]. Chronically, the cumulative effect of ischemic damage leads to chronic heart failure [9]. Chronic heart failure is mediated not only by the loss of myocardium but by progressive pathological remodeling in the viable myocardium [6,7,8,9,10]. Coronary artery stenting and coronary artery bypass grafting, unless introduced early, may not be effective in stabilizing or reversing the process which leads to fixed structural dilatation of the heart and is manifest clinically as ischemic cardiomyopathy [9]. Contradictory or negative results of interventional studies highlight the difficulties of translating basic knowledge of pathobiology into effective clinical therapy and the need for continuing effort in that regard [67, 68]. Nevertheless, continued effort at developing pharmacological and pathophysiological approaches to reducing ischemic damage is worthy of pursuit. Further development of more effective approaches to preserving mitochondrial integrity should continue to be pursued.
References
Buja LM, Vander Heide RS. Pathobiology of ischemic heart disease: past, present and future. Cardiovasc Pathol. 2016;25:214–20.
Buja LM. The pathobiology of acute coronary syndromes. Clinical implications and central role of the mitochondria. Tex Heart Inst J. 2013;40:221–8.
Reimer KA, Ideker RE. Myocardial ischemia and infarction: anatomic and biochemical substrates for ischemic cell death and ventricular arrhythmias. Hum Pathol. 1987;18:462–75.
Reimer KA, Jennings RB. Myocardial ischemia, hypoxia, and infarction. In: Fozzard HA, Haber E, Jennings RB, Katz AM, Morgan HE, editors. The heart and cardiovascular system: scientific foundations, vol. 2. 2nd ed. New York: Raven Press; 1992. p. 1875–973.
Jennings RB, Reimer KA. The cell biology of acute myocardial ischemia. Annu Rev Med. 1991;42:225–46.
Buja LM. Modulation of the myocardial response to ischemia. Lab Investig. 1998;78:1345–73.
Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005;14:170–5.
Willerson JT, Buja LM. Myocardial reperfusion: biology, benefits and consequences. Dialogues Cardiovasc Med. 2006;11:267–78.
Buja LM, Vela D. Cardiomyocyte death and renewal in the normal and diseased heart. Cardiovasc Pathol. 2008;17:349–74.
Buja LM, Weerasinghe P. Unresolved issues in myocardial reperfusion injury. Cardiovasc Pathol. 2010;19:29–35.
Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–35.
Buja LM, Eigenbrodt ML, Eigenbrodt EH. Apoptosis and necrosis. Basic types and mechanisms of cell death. Arch Pathol Lab Med. 1993;117:1208–14.
Majno G, Joris I. Apoptosis, oncosis and necrosis. An overview of cell death. Am J Pathol. 1995;146:3–15.
Weerasinghe P, Hallock S, Brown R, Loose DS, Buja LM. A model for cardiomyocyte cell death: insights into mechanisms of oncosis. Exp Mol Pathol. 2013;94:289–300.
Weerasinghe P, Buja LM. Oncosis: an important non-apoptotic mode of cell death. Exp Mol Pathol. 2012;93:302–8.
Foo RS, Mani K, Kitsis RN. Death begets failure in the heart. J Clin Invest. 2005;115:565–71.
Whelan RS, Kaplinskiy V, Kitsis RN. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol. 2010;72:19–44.
Kung G, Konstantinidis K, Kitsis RN. Programmed necrosis, not apoptosis, in the heart. Circ Res. 2011;108:1017–36.
Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012;32:1552–65.
Gottlieb RA. Cell death pathways in acute I/R injury. J Cardiovasc Pharmacol Ther. 2011;16:233–8.
Orogo AM, Gustafsson AB. Cell death in the myocardium: my heart won’t go on. IUBMB Life. 2013;65:651–6.
Gottlieb RA, Mentzer Jr RM. Autophagy: an affair of the heart. Heart Fail Rev. 2013;18:575–84.
Hickson-Bick DLM, Jones C, Buja LM. Stimulation of mitochondrial biogenesis and autophagy by lipopolysaccharide in the neonatal rat cardiomyocyte protects against programmed cell death. J Mol Cell Cardiol. 2008;44:411–8.
Yan L, Sadoshima J, Vatner DE, Vatner SV. Autophagy in ischemic preconditioning and hibernating myocardium. Autophagy. 2009;5:709–12.
Marín-García J. Cell death in the pathogenesis and progression of heart failure. Heart Fail Rev. 2016;21:117–21.
Moe GW, Marín-García J. Role of cell death in the progression of heart failure. Heart Fail Rev. 2016;21:157–67.
Adameova A, Goncalvesova E, Szobi A, Dhalla NS. Necroptotic cell death in heart failure: relevance and proposed mechanisms. Heart Fail Rev. 2016;21:213–21.
Goldenthal MJ. Mitochondrial involvement in myocyte death and heart failure. Heart Fail Rev. 2016;21:137–55.
Marín-García J, Akhmedov AT, Moe GW. Mitochondria in heart failure: the emerging role of mitochondrial dynamics. Heart Fail Rev. 2013;18:439–56.
Mariín-García J, Akhmedov AT. Mitochondrial dynamics and cell death in heart failure. Heart Fail Rev. 2016;21:123–36.
Webster KA. Programmed death as a therapeutic target to reduce myocardial infarction. Trends Pharmacol Sci. 2007;9:492–9.
Webster KA. Mitochondrial death channels. Am Sci. 2009;97:384–91.
Webster KA. Mitochondrial membrane permeabilization and cell death during myocardial infarction: roles of calcium and reactive oxygen species. Futur Cardiol. 2012;8:863–84.
Mills EM, Xu D, Fergusson MM, Combs CA, Xu Y, Finkel T. Regulation of cellular oncosis by uncoupling protein 2. J Biochem. 2002;277:27385–92.
Kristen AV, Ackermann K, Buss S, Lehmann L, Schnabel PA, Katus HA, Hardt S. Inhibition of apoptosis by the intrinsic but not the extrinsic apoptotic pathway in myocardial ischemia-reperfusion. Cardiovasc Pathol. 2013;22:280–6.
Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–51.
Downey JM, Davis AM, Cohen MV. Signaling pathways in ischemic preconditioning. Heart Fail Rev. 2007;12:181–8.
Yang X, Cohen MV, Downey JM. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc Drugs Ther. 2010;24:225–34.
Skyschally A, Gent S, Amanakis G, Schulte C, Kleinbongard P, Heusch G. Across-species transfer of protection by remote ischemic preconditioning with species-specific myocardial signal transduction by reperfusion injury salvage kinase and survival activating factor enhancement pathways. Circ Res. 2015;117:279–88.
Smith CCT, Yellon DM. Adipocytokines, cardiovascular pathophysiology and myocardial protection. Pharmacol Therap. 2011;129:206–19.
Miura T, Miki T. Limitation of myocardial infarct size in the clinical setting: current status and challenges in translating animal experiments into clinical therapy. Basic Res Cardiol. 2008;103:501–13.
Vander Heide R. Clinically useful cardioprotection: ischemic preconditioning then and now. J Cardiovasc Pharmacol Ther. 2011;16:251–4.
Vander Heide RS, Steenbergen C. Cardioprotection and myocardial reperfusion: pitfalls to clinical application. Circ Res. 2013;113:464–77.
Frohlich GM, Meier P, White SK, Yellon DM, Hausenloy DJ. Myocardial reperfusion injury: looking beyond primary PCI. Eur Heart J. 2013;34:1714–22.
Perricone AJ, Vander Heide RS. Novel therapeutic strategies for ischemic heart disease. Pharmacol Res. 2014;89:36–45.
Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. 2015;116:674–99.
Ibanez B, Heusch G, Ovize M, Van de Werf F. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol. 2015;65:1454–71.
Piot C, Croisille P, Gahide C, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–81.
Morel O, Perret T, Delarche N, Labèque JN, Jouve B, Elbaz M, Piot C, Ovize M. Pharmacologic approaches to reperfusion therapy. Cardiovasc Res. 2012;94:246–52.
Oerlemans MI, Koudstall S, Chamuleau SA, de Kleijn DP, Doevendans PA, Sluijter JP. Targeting cell death in the reperfused heart: pharmacological approaches for cardioprotection. Int J Cardiol. 2013;165:410–22.
Koudstall S, Oerlemans MI, Van der Spoel TI, Janssen AW, Hoefer IE, Doevendans PA, Sluijter JP, Chamuleau SA. Necrostatin-1 alleviates reperfusion injury following acute myocardial infarction in pigs. Eur J Clin Investig. 2015;45:150–9.
Bell RM, Yellon DM. Conditioning the whole heart – not just the cardiomyocyte. J Mol Cell Cardiol. 2012;53:24–32.
Hausenloy DJ, Boston-Griffiths E, Yellon DM. Cardioprotection during cardiac surgery. Cardiovasc Res. 2012;94:253–65.
Sharma V, Bell RM, Yellon DM. Targeting reperfusion injury in acute myocardial infarction: a review of reperfusion injury pharmacotherapy. Expert Opin Pharmacother. 2012;12:1153–75.
Gerczuk PZ, Kloner RA. An update on cardioprotection: a review of the latest adjunctive therapies to limit myocardial infarction size in clinical trials. J Am Coll Cardiol. 2012;59:969–78.
Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci USA. 2015;112:11389–94.
Rocha M, Hernandez-Mijares A, Garcia-Malpartida K, Bañuls C, Bellod L, Victor VM. Mitochondria-targeted antioxidant peptides. Curr Pharm Des. 2010;16:3124–31.
Szeto HH, Schiller PW. Novel therapies targeting inner mitochondrial membrane – from discoveries to clinical development. Pharm Res. 2011;28:2669–79.
Kloner RA, Schwartz Longacre L. State of the science of cardioprotection: challenges and opportunities – proceedings of the 2010 NHLBI workshop on cardioprotection. J Cardiovasc Pharmacol Ther. 2011;16:223–32.
Schwartz Longacre L, Kloner RA, Arai AE, et al. New horizons in cardioprotection: Recommendations from the 2010 national heart, lung, and blood institute workshop. Circulation. 2011;124:1172–9.
Fernández-Jiménez R, Ibanez B. CAESAR: one step beyond in the construction of a translational bridge for cardioprotection. Circ Res. 2015;116:554–6.
Cung TT, Morel O, Cayla G, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–31.
Hausenloy DJ, Kharbanda R, Rahbek Schmidt M, et al. Effect of remote ischaemic conditioning on clinical outcomes in patients presenting with an ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J. 2015;36:1846–8.
Meybohm P, Bein B, Brosteanu O, et al. A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–407.
Hausenloy DJ, Candilio L, Evans R, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–17.
Zaugg M, Lucchinetti E. Remote ischemic preconditioning in cardiac surgery – ineffective and risky? N Engl J Med. 2015;373:1470–2.
Bulluck H, Yellon DM, Hausenloy DJ. Reducing myocardial infarct size: challenges and future opportunities. Heart. 2016;102:341–8.
Hausenloy DJ, Yellon DM. Ischaemic conditioning and reperfusion injury. Nature Rev/Cardiol. 2016;13:193–209.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Maximilian Buja, L. (2017). Mitochondria in Ischemic Heart Disease. In: Santulli, G. (eds) Mitochondrial Dynamics in Cardiovascular Medicine. Advances in Experimental Medicine and Biology, vol 982. Springer, Cham. https://doi.org/10.1007/978-3-319-55330-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-55330-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55329-0
Online ISBN: 978-3-319-55330-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)