Abstract
While there are numerous options for treatment of small (<4 cm) renal masses, irreversible electroporation (IRE) is a unique method of ablation which is reliable, safe and effective. Unlike other ablation methods, there is no need to cool the ureter or separate the colon from the kidney by injecting fluid into the perinephric space. Even when lesions extend into the central portion of the kidney, IRE is not associated with significant bleeding or haematuria. IRE is especially effective in the elderly or those with impaired renal function.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Surgical excision is still considered the optimal treatment for malignant renal tumours [1, 2]. However, before the advent of computed tomography, renal mass lesions smaller than 4 cm were difficult to detect and TNM staging used 4 cm as the maximum size of T1a lesions. As a result of increased detection of small, often asymptomatic, mass lesions, routine nephrectomy has become less desirable. Nephron-sparing nephrectomy with or without laparoscopy has become a routine procedure for T1a and some T1b lesions. More recently thermal ablative techniques have been employed for T1a lesions particularly when the lesion is exophytic. One of the major limitations of these ablative techniques is thermal damage to adjacent organs and the collecting system and heat sink effects which limit the efficacy of the techniques adjacent to blood vessels. As a result of these limitations, the most suitable lesion for thermal ablation is <4 cm in diameter, polar, cortical and distant from the renal hilum and collecting system. These limitations also apply to a great extent to nephron-sparing surgery.
Percutaneous irreversible electroporation (IRE) is ideally suited to treatment of small renal mass lesions because it is a nonthermal method of soft tissue ablation which is unaffected by local blood flow and it does not disturb the collagenous structure of the target tissue. The kidney is easily accessible by percutaneous image-guided techniques to facilitate the use of IRE, and non-polar, hilar lesions adjacent to the ureter and calyces are not a contraindication to the use of IRE. Provided the lesion is carefully targeted by IRE, lesions larger than 3 cm can be treated effectively and safely.
2 Physiological and Anatomical Considerations
The kidneys are paired retroperitoneal structures surrounded by Gerota’s fascia that is normally located between the transverse processes of T12–L3 vertebrae, with the left kidney typically somewhat more superior in position than the right. The upper poles are normally oriented more medially and posteriorly than the lower poles. The adrenal glands are situated at the upper pole of each kidney and the lower portion of each kidney is closely related to the colon. There are one or more renal arteries to each kidney arising directly from the abdominal aorta and venous drainage is to the inferior vena cava. The left renal vein passes anterior to the aorta about the level of the second part of the duodenum. Urine is secreted into the calyces of the collecting system and transported via the ureter to the urinary bladder by ureteric peristalsis.
The kidneys serve important functions, including filtration and excretion of metabolic waste products, regulation of electrolytes and fluid, acid-base balance and stimulation of red blood cell production. They regulate blood pressure via the renin-angiotensin-aldosterone system, controlling reabsorption of water and maintaining intravascular volume. The kidneys also reabsorb glucose and amino acids and have endocrine functions via erythropoietin, calcitriol and vitamin D activation.
3 Diseases
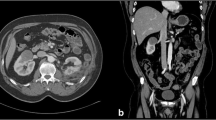
The differential diagnosis of a small (<4 cm) renal mass lesion includes benign cyst, angiomyolipoma, lymphoma, metastasis, benign mesenchymal tumour, renal sarcoma, renal cell carcinoma, adenoma, oncocytoma, abscess and haematoma. The differentiation of these and exclusion of volume averaging artefacts in the renal hilum require high-quality triple phase contrast-enhanced computed tomography (CT) (Fig. 13.1), supplemented by ultrasound, magnetic resonance imaging (MRI)and sometimes biopsy [3,3,7]. Since most lesions are slow growing, short interval repeat imaging may not be helpful. However, in an elderly unfit person with a very small (<2 cm) exophytic renal mass, a repeat CT scan after 12 months is a reasonable approach. In most cases a definitive diagnosis can be made based on the imaging findings (Fig. 13.2), but lesions <1.5 cm are unlikely to cause significant morbidity at least in the short term.
Renal tumour. A solid mass with abnormal vascularity in arterial phase is typical of a renal cell carcinoma. Sometimes, however, the vascularity may be difficult to detect. See Fig. 13.2
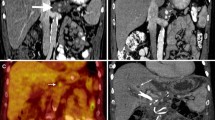
Small indeterminate exophytic lesion. (a) A small poorly enhancing exophytic lesion (arrowhead) in a patient status post liver resection for colon cancer metastasis (2007). (b) By 2014, there was evidence of some growth in size. On biopsy this proved to be a clear cell renal carcinoma. The prior adjacent surgery, diaphragmatic sulcus and proximity of the colon were indications for IRE of this lesion (arrowhead)
Biopsy is recommended before any indeterminate mass lesion is treated by an ablative technique. There is a very small (0.01%) risk of seeding of the biopsy track with malignant tumours and this may limit the enthusiasm for biopsy. It is worth noting that tumour seeding may also occur after laparoscopic nephron-sparing surgery. The electric field immediately adjacent to the active IRE electrode is lethal to cells and this may inhibit seeding.
4 Current and Future Indications
IRE is indicated for soft tissue ablation and is suited to focal renal masses of malignant potential. IRE does have advantages when compared to partial nephrectomy as the IRE is not dependent on the anatomical relationships of the tumour such as blood supply or venous drainage. However, this is an area with a large number of therapeutic options, and more long-term data is required to establish the final place of IRE in the kidney.
5 Contraindications
Provided the patient is able to tolerate a general anaesthetic with muscle paralysis, there are almost no contraindications to IRE once a tumour of malignant potential has been identified in the kidney. Treatment of nonfocal malignancy such as transitional cell carcinoma is not recommended even though it is probably possible.
Patients with pacemakers and pacemaker-defibrillators should be reviewed by a cardiologist prior to an IRE procedure. We prefer to use cardiac-gated delivery of IRE in all procedures. In some cases the defibrillator may need to be deactivated during the procedure, but a standard demand pacemaker has, in our experience, not provided any problem in terms of aberrant rhythm or generation of abnormal currents through the pacemaker leads.
6 Patient Workup and Treatment Planning (Multidisc Meetings, Histology, Imaging, Lab, Antibiotics, Pre-procedural Safety Procedures, Modelling)
In our institution all renal tract malignancies are reviewed by a multidisciplinary committee comprising urologists, radiologists, radiation oncologists and medical oncologists. Laboratory results, including renal function, cardiorespiratory status, past and recent imaging (CT, ultrasound or magnetic resonance) and histology, are presented and a decision is made on the most appropriate treatment for each particular patient. Lesions which are technically difficult for partial nephrectomy are usually quite accessible for a focal ablative treatment such as IRE.
Once a decision for IRE has been made, the lesion is studied on the available cross-sectional imaging or if necessary further cross-sectional imaging is obtained. The kidney is relatively accessible from a prone or oblique approach for percutaneous procedures like IRE. Because the kidney will move significantly from a supine position to a prone or oblique position, often the final targeting planning will not occur until the patient is positioned and anaesthetised for the IRE procedure. It is, however, still useful to plan the procedure in general terms so that the tumour and a margin of normal tissue can be effectively targeted (Fig. 13.3). Decisions such as the number of electrodes, length of active electrode exposure and distance between electrodes can be made prior to the procedure to ensure that a complete ablation can be achieved. The electrode placement should be designed so that if possible, the distance between electrodes is between 1.0 and 2.5 cm. The effective electroporation field extends about 5 mm from the outer margin of the electrode so that provided adequate current is achieved, a 2.5 cm electrode will cover a 3.0 cm diameter tumour. Ideally the electrodes should sit at the margin of the tumour so that a margin can be achieved with confidence. If desired this planning can be transferred to the AngioDynamics generator to view the electrode siting. This is however only a single-plane diagram. Planning can also be performed using the Visifield planning software which provides a more robust estimate of the electric filed that will be generated [Visifield.com].
Planning the electrode siting. Using axial images select the top (a) and bottom (b) of the tumour, and with a coronal image (c), select the largest region of the tumour. Note the dimensions and calculate what electrode spacing and how many electrodes will be required. Even though the tumour position will change as the patient is turned from supine to prone, the tumour size will be the same
7 Approach and Image Guidance
The major decision to be made at the time of the procedure is whether to place the arms alongside the abdomen or to raise the arms above the imaging field. There is an increased risk of brachial plexus injury if the arms are extended forcibly for long periods even when the patient is in a prone oblique position. In most cases the CT artefact produced by the arms is below the imaging field of the kidneys (Fig. 13.4). We have used the Perfint positioning robot (Fig. 13.5) to aid positioning of IRE electrodes and to assist planning of the electrode array when an oblique electrode path is required, but normally for a renal tumour, the electrode path is in the axial plane and less difficult than, for instance, a placement through the intercostal spaces for a liver tumour.
Patient positioning. In the prone position, the kidney moves forward. As the patient is usually on a support to protect the venous return, the arms may be positioned alongside the patient below the level of interest. In this example the right arm is only just visible and image degradation is acceptable
Perfint robot. (a) CT data is transmitted to the Perfint robot and the electrode placement is planned on the Perfint workstation. Once planning is complete, the robot arm indicates the position at which the electrode should be introduced. The operator inserts the electrode through the guide. (b) The Perfint robot is in position and the last electrode is about to be inserted. CT fluoroscopy is used after placement to confirm accurate positioning
In our experience it is easier to confirm placement of IRE electrodes in the renal target area with CT rather than ultrasound. If ultrasound is used, because the position of the patient is likely to be different to that of the diagnostic CT examinations, CT/ultrasound fusion may not be possible with older fusion technology.
8 Technique (General Technique, Tips and Tricks, Procedural Safety Procedures)
Normally the patient is anaesthetised in the supine position, and ECG and blood pressure monitoring is established, often with a radial arterial pressure line. ECG dots are required for two sets of monitors if cardiac-gated delivery of the electroporation is to be used. A Foley catheter is inserted into the urinary bladder, and the patient is then positioned prone or prone oblique according to the prior plan. All imaging should be performed in expiration as this is the most consistent phase for an anaesthetised and paralysed patient. A contrast-enhanced CT in arterial and excretion phase is obtained with a skin-marking sheet over the area of interest. Once images are obtained, the table position of the upper and lower border of the target zone is recorded and a mark made on the patient’s skin. The medial and lateral borders of the target zone are also marked after calculations from the skin-marking sheet. During placement and adjustment of electrodes, respiration is off so that a consistent respiratory position is achieved.
Electrodes are exposed by withdrawing the insulation sleeve and inserted at sites according to the prior plan to ensure that the target zone is completely covered. As each electrode is placed, the position should be confirmed using a few CT slices or CT fluoroscopy. Each electrode cable should be labelled using the numbered labels supplied with the blue activator electrode.
Once electrode placement is completed, a non-contrast CT through the target kidney is performed and displayed on the workstation. The actual distance between each electrode is determined by a stack of images perpendicular to the long axis of the electrodes (Fig. 13.6). These measurements are then transferred to the AngioDynamics generator. Use of a Faraday probe allows a direct display of the current with each pulse and a more aggressive application of the electroporation energy without causing the generator to shut down because of excessive current (Fig. 13.7). Alternatively a set of ten pulses at a low energy such as 1,200 V/cm can be delivered through each electrode pair and the results viewed on the AngioDynamics generator monitor after the delivery is complete (Fig. 13.8). Low currents require either increase in volts/cm generally or selectively for each electrode pair. High currents or pulses showing spikes require reduction in the energy to be delivered. A further group of about 70–90 pulses are then delivered at the new settings and the results displayed for evaluation. Further adjustments are made as necessary and a final set of 70–90 pulses delivered. The total number of pulses delivered should be about 100 with currents at or above about 35 A. If a single pair of electrodes proves difficult to energise with the rest of the array, they can be deleted and activated separately.
Electrode measurements. (a) Pre-electrode placement. CT sagittal reconstruction showing tumours at the upper pole and the lower pole in a patient with Von Hippel Lindau disease. (b) CT reconstruction in short axis of the electrode to allow accurate measurements of electrode separation. (c) CT reconstruction in long axis of the electrodes to confirm electrodes are truly parallel to one another
Monitoring the IRE pulse. (a) Use of a Faraday current probe. A general view showing multiple electrodes in position. The Faraday probe is switched from one electrode pair to another as the sets of electric pulses are delivered. It is attached to electrode 5 in this image. (b) This oscilloscope display shows a series of pulses around 20 amperes. Each successive pulse in the set of 10 is slightly lower in current as the voltage falls slightly with each discharge from the capacitor in the Nanoknife generator. The shape of this current is ideal
Electric pulse delivery. (a) Abnormal “spikey” current display. Although the current and voltage are well under cut-off levels, the shape of the pulses indicates that the electric field is not uniform. This may be due to very inhomogeneous tumour or due to excessive electrode separation. Repositioning of one or more electrodes may be required. (b) Completion display of IRE electric pulses at the limits of tolerance. Real-time monitoring allows more aggressive current delivery with less chance of overcurrent shutdown as the delivery can be aborted just before the generator shuts down
For each set of activations, the electrode number, volts/cm, and current achieved are recorded on a worksheet (Fig. 13.9). Careful recording is critical when there are a large number of electrodes, and care should be taken to ensure every possible combination of electrodes is activated. These recordings are also important if there is a shutdown because of overvoltage. To force the generator to change voltage, the distance specified on the generator may be increased or decreased without actually changing the relative electrode positions in the patient. The data recorded by the AngioDynamics generator should be saved to a USB only at the end of the procedure.
Worksheet for renal tumour. It is important to record the voltage, separation, number of pulses delivered and the current achieved for each pair of electrodes each time any parameters are changed. Ideally an increase in current of 15–20 amperes is desirable for IRE. Note that in this example, the highlighted distance between electrodes 2 and 3 in sequence #3 has been increased artificially to force a higher voltage. The electrode was not physically moved
Once the ablation is considered complete, a final CT scan with electrodes in position is performed. For best evaluation, contrast is required but this depends on the renal function. The ablation zone is of lower attenuation than normal kidney and contains a few bubbles of gas. There will be normally minor soft tissue change in the perinephric fat as a result of electrode placement. If there is any doubt the ablation is incomplete, the electrodes should be repositioned or some of the electroporation repeated.
After the patient is conscious, a trial of void is performed and the indwelling urinary catheter removed. If there is a history of prostatism, the catheter is left in position until the following day. Even in cases of electroporation reaching the hilum of the kidney, significant haematuria is unusual.
9 Complications (Related to Needles, Related to Energy Delivery or Related to the General Procedure)
Complications after renal electroporation are very infrequent in our experience. The most surprising outcome is that haematuria is most uncommon and has been very limited even with deep insertion of the IRE electrodes. The incidence of bleeding in our experience, even though it is limited, is equal to or less than the risk after renal biopsy.
Even with cardiac gating, long sequences of electroporation pulses may result in an increase in blood pressure during the procedure. This is possibly related to diaphragmatic contractions and increased venous return to the heart but in truth we have no exact cause. The blood pressure is easily controlled by the anaesthetist during the procedure and we have not seen persistent hypertension after the procedure.
In one case the left adrenal gland was unintentionally included in the electroporation field, and this resulted in severe hypertension easily controlled with alpha and beta blockade without late sequelae.
Direct puncture damage caused by an electrode is possible with any IRE procedure, but we do not need to introduce fluids to separate adjacent organs and we have not employed urinary cooling to protect the collecting system and ureter.
The major complication is an incomplete ablation, but this is part of a learning curve in planning deployment of electrodes and ensuring the ablative electric field encompasses the entire tumour and a margin.
10 (Neo)adjuvant Treatment and Supportive Procedures (Renal Failure Support, Urine Cooling, etc.)
Although there is theoretically some advantage in adjuvant chemotherapy with IRE in other regions, principally skin tumours, this is not routine practice due to the lack of an effective therapeutic agent. Adequate hydration and minimization of contrast media is employed as with any CT contrast procedure as the aim of the IRE procedure is to preserve renal function. The lack of thermal effects makes urine cooling and tissue separation unnecessary.
11 Follow-Up and Response Evaluation (Imaging, Lab)
Standard follow-up protocols as for any other treatment method for renal tumour are used. This may be CT or ultrasound as desired. We performed a contrast CT or an ultrasound at 1–2 months post IRE in all our patients with further follow-up at 6- and then 12-month intervals. Where renal function was reduced, contrast-enhanced MR imaging is also suitable (Fig. 13.10).
Follow-up. (a) Prone CT image at completion of IRE and removal of electrodes. Gas in the line of the electrodes is related to hydrolysis. (b) MR with contrast (axial 3 phase LAVA sequence) in the same patient at 6 months post IRE. The tumour mass has decreased in size and is non-enhancing. Note the IRE ablation extended to the central portion of the left kidney
12 Disease Recurrence (Residual Disease, Local Site Recurrence or Distant Disease Recurrence)
Residual disease indicates a failure of the original index IRE procedure and is more frequent in the early experience of the operator. Following successful (complete) ablation, we have not seen evidence of local or distal site recurrence. One of our patients has Von Hippel Lindau disease and develops tumours in other renal sites. We have performed three procedures and she has had two partial nephrectomies for separate tumours.
Our results were presented at SIR 2016 in Vancouver, BC. Nineteen patients of ages ranging from 43 to 85 years (mean 70 years) with a total of 27 tumours were treated between 2008 and 2015. Six of the 19 patients were undertaken on a salvage compassionate basis under an ethics-approved trial to evaluate the safety of the IRE technique. Of the 13 other patients, there has been no recurrence of ablated tumours where a complete ablation was achieved. Overall, including the salvage patients, this experience resulted in an ablation rate of 88% for tumours <3 cm and 63% for tumours >3 cm. However seven of the tumours without recurrence were >3 cm in diameter. There was no difference in results by site (central or peripheral).
13 Results from Literature
Pech et al. first reported human experience of IRE for renal tumour in an “ablate and resect” research project, but the interval between ablation and nephrectomy was too short to establish any IRE effect [8, 10]. A new Dutch study protocol has been proposed by Wagstaff et al. where the interval between IRE and radical nephrectomy will be 4 weeks which will provide data on the histologic changes after IRE [9].
Trimmer et al. reported a small cohort of renal tumours treated by IRE, but their selection was for tumours of a smaller size (average 2.9 cm) and a peripheral location distant from critical structures [11]. Their study reported no complications and ablation rates similar to those achieved with thermal ablation.
Wehle et al. considered that “watchful waiting” and serial CT scan management of small renal masses (average 1.83 cm) in patients unsuitable for surgery or thermal ablation are appropriate [12]. This is a widely held view among urologists, but “watchful waiting” as a management strategy has not been thoroughly evaluated.
Health technology assessment (www.nice.org.uk) of rena l IRE is that current evidence on the safety and efficacy of IRE for treating renal cancer is inadequate in quality and quantity and that this procedure should only be used in the context of research. NICE also stated that studies should report the effect of the procedure on local tumour control and patient survival.
Our opinion is that IRE, in experienced hands, is as effective as other ablative methods but has far wider application within the renal parenchyma with less restrictions on its use.
References
American Cancer Society. Cancer facts and figures 2000. Atlanta: American Cancer Society; 2000. p. 4.
Russo P. Renal cell carcinoma: presentation, staging, and surgical treatment. Semin Oncol. 2000;27:160–76. Medline.
Cohan RH, Sherman LS, Korobkin M, Bass JC, Francis IR. Renal masses: assessment of corticomedullary-phase and nephrographic-phase CT scans. Radiology. 1995;196:445–51. Link.
Kopka L, Fischer U, Zoeller G, Schmidt C, Ringert RH, Grabbe E. Dual-phase helical CT of the kidney: value of the corticomedullary and nephrographic phase for evaluation of renal lesions and preoperative staging of renal cell carcinoma. AJR Am J Roentgenol. 1997;169:1573–8. CrossRef, Medline.
Szolar DH, Kammerhuber F, Altziebler S, et al. Multiphasic helical CT of the kidney: increased conspicuity for detection and characterization of small (<3-cm) renal masses. Radiology. 1997;202:211–7. Link.
Yuh BI, Cohan RH. Different phases of renal enhancement: role in detecting and characterizing renal masses during helical CT. AJR Am J Roentgenol. 1999;173:747–55. CrossRef, Medline.
Silverman SG, Lee BY, Seltzer SE, Bloom DA, Corless CL, Adams DF. Small (<3-cm) renal masses: correlation of spiral CT features and pathologic findings. AJR Am J Roentgenol. 1994;163:597–605. Cros allsRef, Medline.
Pech M1, Janitzky A, Wendler JJ, Strang C, Blaschke S, Dudeck O, Ricke J, Liehr UB. Irreversible electroporation of renal cell carcinoma: a first-in-man phase I clinical study. Cardiovasc Intervent Radiol. 2011;34:132–8.
Wagstaff P, de Bruin DM, Zondervan PJ, Heijink CD, Engelbrecht MRW, van Delden OM, van Leeuwen TG, Wijkstra H, de la Rosette JJ, Pes MP. The efficacy and safety of irreversible electroporation for the ablation of renal masses: a prospective, human, in-vivo study protocol. BMC Cancer. 2015;15:165.
Deodhar A, Monette S, Single GW Jr, Hamilton WC Jr, Thornton R, Maybody M, Coleman JA, Solomon SB. Renal tissue ablation with irreversible electroporation. Preliminary results in a porcine model. Urology. 2011;77:754–60.
Trimmer CK, Khosla A, Morgan M, Stephenson SL, Ozayar A, Cadeddu JA. Minimally invasive percutaneous treatment of small renal tumours with irreversible electroporation: a single centre experience. JVIR. 2015;26:1465–71.
Wehle MJ, Thiel DD, Petrou SP, Young PR, Frank I, Karsteadt N. Conservative management of incidental contrast-enhancing renal masses as safe alternative to invasive therapy. Urology. 2004;64:49–52.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG
About this chapter
Cite this chapter
Koukounaras, J., Kavnoudias, H., Thomson, K.R. (2018). Irreversible Electroporation of Kidney Tumours. In: Meijerink, M., Scheffer, H., Narayanan, G. (eds) Irreversible Electroporation in Clinical Practice. Springer, Cham. https://doi.org/10.1007/978-3-319-55113-5_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-55113-5_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-55112-8
Online ISBN: 978-3-319-55113-5
eBook Packages: MedicineMedicine (R0)