Abstract
Physical activity and especially physical exercise are considered as cornerstones of musculoskeletal health. Indeed, dedicated exercise protocols can affect all fracture parameters, i.e. fall risk, fall impact, and bone strength, and should thus be considered as optimum candidates for non-pharmacological fracture prevention. Some evidence for the general anti-fracture efficacy of exercise was provided by dedicated exercise trials and a corresponding meta-analysis, but the optimum strategy (if there is any) on how to prevent fractures in elderly subjects is still under discussion. Although some researchers postulate to focus more on falls than on osteoporosis to prevent fractures, the most promising and feasible exercise strategy is to select types of exercise that address both factors, falls, and osteoporosis. This approach, however, ought to consider the requirements and determining factors of each individual. That is, the need for fall prevention is higher for elderly subjects with several fall risk factors, while for early postmenopausal women with distinct bone loss, this topic is of lesser relevance. But even with careful adaptation of the exercise program to subjects’ changing bone, health, and fitness status, effectivity may still decrease over the time. This could specifically be the case where the limitations of higher age collide with the specification of the exercise program. In the Erlangen Fitness and Osteoporosis Prevention Study (EFOPS), the overall aim was to evaluate the effect of a multipurpose exercise program on clinical low-trauma fractures in postmenopausal women starting to exercise in their early postmenopausal years. In detail, we intended to answer the following research questions:
-
1.
Can exercise reduce the risk of osteoporotic fractures in postmenopausal women?
-
2.
Is there an optimal exercise program to increase or maintain bone mineral density?
-
3.
Are there temporary limitations on the effectivity of exercise on bone?
-
4.
Can exercise program that focuses on fracture reduction relevantly affect other risk factors with advancing age?
-
5.
Are high-intensity anti-fracture exercise programs attractive and feasible?
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Physical activity and especially physical exercise are considered as cornerstones of musculoskeletal health [1, 2]. Indeed, dedicated exercise protocols can affect all fracture parameters, i.e. fall risk [3], fall impact [4, 5], and bone strength [6, 7], and should thus be considered as optimum candidates for non-pharmacological fracture prevention. Some evidence for the general anti-fracture efficacy of exercise was provided by dedicated exercise trials [8, 9] and a corresponding meta-analysis [10], but the optimum strategy (if there is any) on how to prevent fractures in elderly subjects is still under discussion. Although some researchers postulate to focus more on falls than on osteoporosis to prevent fractures [11], the most promising and feasible exercise strategy is to select types of exercise that address both factors, falls, and osteoporosis. This approach, however, ought to consider the requirements and determining factors of each individual. That is, the need for fall prevention is higher for elderly subjects with several fall risk factors, while for early postmenopausal women with distinct bone loss, this topic is of lesser relevance [12]. But even with careful adaptation of the exercise program to subjects’ changing bone, health, and fitness status, effectivity may still decrease over the time. This could specifically be the case where the limitations of higher age collide with the specification of the exercise program. In the Erlangen Fitness and Osteoporosis Prevention Study (EFOPS), the overall aim was to evaluate the effect of a multipurpose exercise program on clinical low-trauma fractures in postmenopausal women starting to exercise in their early postmenopausal years. In detail, we intended to answer the following research questions:
-
1.
Can exercise reduce the risk of osteoporotic fractures in postmenopausal women?
-
2.
Is there an optimal exercise program to increase or maintain bone mineral density?
-
3.
Are there temporary limitations on the effectivity of exercise on bone?
-
4.
Can exercise program that focuses on fracture reduction relevantly affect other risk factors with advancing age?
-
5.
Are high-intensity anti-fracture exercise programs attractive and feasible?
Methods
The EFOPS is a nonrandomized semi-blinded controlled exercise over 16 years so far. The study complied with the Helsinki Declaration of “Ethical Principles for Medical Research Involving Human Subjects” and was approved by the ethics committee of the University of Erlangen (Ethikantrag 905, 4209, 4914 B) and the Federal Bureau of Radiation Protection (S9108–202/97/1). After detailed information all study participants gave written informed consent. EFOPS was registered under www.clinicaltrials.gov (NCT01177761). In this publication we will present the results and experiences after 16 years of exercise meanwhile organized in the setting of a noncommercial health club (“Sportverein”). Special emphasis is placed on the design of the exercise program and its adaptation to the increasing age and correspondingly changing requirements and determining factors of our cohorts.
Participants
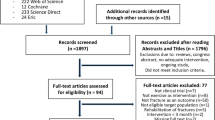
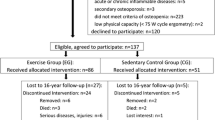
Figure 6.1 shows the participant flow of the study. We queried population registers to contact all women from Erlangen and surroundings in the age between 48 and 60 in the form of individual letters describing the study objectives.
Inclusion criteria were a time window of 1–8-year postmenopause and osteopenia at the total hip or lumbar spine as measured by dual X-ray absorptiometry (DXA) using the WHO T-score definition (−2.5 SD < T-Score ≤ −1.0 SD). Exclusion criteria were diseases and use of medication affecting bone metabolism, known osteoporotic fractures, current or recent athletic activity (defined as participation in sport competitions within two decades before study start), inflammatory diseases, history of cardiovascular disease, and very low physical capacity as defined by ergometry (<75 W).
The participants were free to join the exercise or the control group. On hundred thirty-seven early postmenopausal (1–8 years) women were finally included in the study and 86 women joined the exercise and 51 the control group. Participants of the control group were requested to continue their habitual lifestyle, while participants of the exercise group underwent the training regime described below.
Intervention and Changes of the Exercise Program During the Study Course
Certified trainers monthly or (after year 4) bimonthly briefed by the principal investigator supervised all the joint exercise sessions over the 16-year period. Each subject of the EG kept an individual training log that was checked every 12 weeks in order to determine participants’ attendance and compliance with the exercise protocol. Apart from study years 4 and 5 (see below), the EFOPS protocol scheduled two joint group classes of 60–65 min on nonconsecutive days and two home training sessions of 20–25 min consistently for 49–50 weeks per year. Exercise intensity was regularly adapted (see below) to subjects’ physical performance.
Group Classes
The joint exercise session started with a 20–25-min warm-up/endurance sequence. This sequence focused on running/gaming variations, dancing, and low- and (after 4–5 months of conditioning) high-impact aerobic dance exercises with short intervals (2–3 min) at heart rates (HR) of 80–85% HRmax intermitted by 1–2 min of moderate heart rates (70–80% HRmax). During the high-impact aerobic dance, peak ground reaction forces (peak GRF) of ≈2–3 × body weight were realized. The number of these moderate- to high-impact loads during this session was progressively increased during the first 3–4 study years (up to 150) and decreased (90–110) during the last 3 study years.
While general coordination exercises were consistently applied by traditional dances and aerobic dance during the initial session, the focus slightly shifted from bone to fall-relevant exercises including exercises for dynamic balance. This approach was supported by a dedicated short (3–5 min) sequence that specifically focused on static and dynamic balance exercises introduced after 10 years of exercising.
In order to specifically address bone by high ground reaction forces, a short high-impact sequence (3–5 min) was introduced after a conditioning phase of 6 months. After a 3-month lasting adaption phase of rope skipping, 4 different sets of 15 simple multidirectional jumps/session (e.g., closed leg jumps, lateral one leg jump, jumping jacks) were carried out. Subjects were encouraged to focus on intensive takeoff and soft landing with flexed ankles and knees without heel strikes. Complexity and impact of the prescribed jumping exercises progressively increased up to a peak GRF of ≈4–4.5 × body weight during the first 4 years, while less challenging jumping exercises (peak GRF ≈ 3–3.5 × body weight) with higher demands on balance and coordination (e.g., lateral jumps with predefined rhythm) were introduced during the last 3 study years.
The main part of the exercise program, however, was the resistance sequence that covered 35–40 min of the group session. This training consisted of two different types of resistance training: in one of the two group sessions, exercises were carried out on machines (Techno Gym, Gambettola, Italy), and in the other one isometric exercises, elastic bands, and free weights were used. The following dynamic exercises were performed in the session using resistance machines: horizontal leg press, leg curls, bench press, rowing, leg adduction and abduction, abdominal flexion, back extension, lat pulley, hyperextension, leg extension, shoulder raises, and hip flexion. During the second resistance training session, isometric (12–15 exercises, 2–4 sets, 6–10 s) and elastic band exercises (3 exercises with 2–4 sets and 15–20 reps.) were carried out. In addition, three resistance exercises using free weights (squat/deadlift, one hand dumbbell rowing, and dumbbell chest press) were performed according to the periodized protocol described below.
After 9 months of conditioning, we consistently applied a structured exercise schedule with 12 weeks of linearly or nonlinearly periodized high-intensity resistance training [13] on machines (9–10 exercises, 1–4 sets, 4–12 repetitions with 70–90% one-repetition maximum (1-RM)) and 4–6 weeks of lower intensity (50–55% 1-RM) but higher volume (13 exercises, 2–3 sets, 20–25 repetitions) or correspondingly with free weights. After a dedicated study section during years 4 and 5 [14, 15] that focused on movement velocity (i.e., strain rate [16]) during resistance exercise, applying three-group and one-home training session, movement velocity was also consistently manipulated. Periods of fast (explosive movement during the concentric phase) and slow (up to 4 s during concentric and eccentric phase) velocity were applied, while higher loads (≥1 RM) were always with movement velocities of 2–4 s per movement phase. Exercise intensity prescribed in the participants’ individual training logs was either based on regular 1-RM tests (first 5 years; [13]) or the repetition number combined with the rate of perceived exertion (Borg CR-10 scale, [17]) [13]. Of high importance, although the applied exercise program would be denominated as an “HIT” resistance exercise program nowadays, apart from one 12-week period [13], we did not intend subject’s complete exhaustion by the maximum number of repetitions.
Home Sessions
Except for years 4 and 5 (one-home training session/week only), the 20–25-min home training was consistently prescribed twice per week. The session was structured in a short warm-up sequence including rope skipping (3–5 min) and an isometric and dynamic exercise element. During the latter sequence, isometric exercises primarily focused on trunk stability (e.g., crunches, forearm planks), while dynamic exercises using elastic bands or gravity focused on upper back and upper and lower limbs. Stretching (eight muscle groups, 20 s continuous stretching) was conducted at the end of the resistance exercises. All exercises were carefully practiced in the group sessions beforehand. Home training protocols were changed every 3 (up to year 4) to 6 months.
Calcium and Vitamin D Supplementation
Based on dietary protocols (see below), all study participants received calcium (Ca) and vitamin D (Vit-D) in order to ensure an intake of at least 1000 mg/day Ca (first 10 years, 1500 mg/day) and 500 IU/d Vit-D. Due to funding limitations, after 5 years of free supplementation, participants were directed to resources for low-priced Ca and Vit-D supplements.
Measurements
Except for the assessment of clinical overall fractures, the measurements detailed below were performed at baseline and, during the course of the intervention, repeated after years 1, 2, 3, 4, 5, 8, 12, and 16. All assessments were determined in a blinded fashion, i.e., researchers and research assistants were unaware of the status (EG or CG) of the participant.
Anthropometry
Height, weight, and waist circumference was measured using calibrated devices. Body composition was determined by multi-frequent bio-impedance technique (Tanita BF 305, Tokyo, Japan).
Bone Mineral Density
BMD at the lumbar spine (LS) and the femoral neck (FN) was measured by dual energy X-ray absorptiometry (DXA) using standard protocols of the manufacturer (QDR 4500a; Hologic, Bedford, USA). LS scans (L2–L4) and FN scans were independently analyzed by two experienced researchers. Long-term (16-year) coefficient of variation for BMD at the LS was 0.5% as determined by weekly “spine phantom” measurements.
CHD Risk
The 10-year risk index of myocardial infarction or coronary death was calculated using the algorithm suggested by the NCEP ATP III Panel [18] that includes categories of age, total cholesterol, HDL cholesterol, systolic blood pressure, treatment for hypertension, and smoking status.
Questionnaires
Baseline questionnaires determined demographic parameters, pre-study physical activity and exercise levels, and health risk factors with special regard to bone and quality of life parameters. Follow-up questionnaires conducted after 1, 2, 3, 4, 5, 8, 12, and 16 years were specifically designed to detect changes in confounding parameters that may affect the study endpoints (e.g., medication, diseases, lifestyle, physical activity, exercise, dietary pattern, and Ca−/Vit-D supplementation).
Clinical Overall Fractures
All fractures during the last 16 years were retrospectively determined by questionnaires combined with structured interviews after 4, 8, 12, and 16 years. In order to verify the fracture, subjects were asked to provide a medical report. Low-trauma fracture was defined as a fracture occurring spontaneously without high load or falls from a standing height or lower [19]. Among the low-trauma fractures, we further checked for major osteoporotic fractures (i.e., vertebral, humerus, forearm, proximal femur/hip) according to the WHO Fracture Risk Assessment Tool (FRAX®, [20]). Fractures caused by vehicle/bicycle accidents or bicycle falls, falls from a higher level, or other more serious trauma were excluded from the analysis.
Dietary Intake
The consumed food was weighted precisely and reported by the participants. The analysis of the protocols was performed by research assistants using Prodi-4.5/03 Expert software (Nutri-Science, Hausach, Germany). However, due to participants’ unwillingness to regularly perform this laborious procedure and the minor annual differences for calcium and vitamin D uptake, we decided to stop assessing dietary intake by this method and started to use a standardized calcium and vitamin D questionnaire [21], initially biyearly and later in 4 yearly intervals. A validation of this questionnaire with results of the 5-day dietary assessment resulted in corresponding differences of 10% for calcium and 15% for vitamin D uptake.
Statistical Analysis
Estimated sample size calculation was based on the number of clinical low-trauma fractures. In order to detect a rate ratio of 0.5 for overall fracture rate ratio [22, 23], about 50 patients/group/12 years were required (5% error probability, 80% statistical power). Fisher’s exact test was used to determine differences between EG and CG for the number of subjects with fractures (risk ratio). The total number of fractures (rate ratio) was compared using negative binominal regression. A completer analysis including all subjects with 16-year follow-up data was calculated. However, for research question (3), only subjects with complete BMD values for baseline, years 4, 8, 12, and 16, were considered. According to their distribution, intragroup BMD changes were analyzed by paired t-tests or Wilcoxon rank tests. Differences between the groups were consistently determined using Welch t-tests. Effect sizes (ES) were calculated using Cohen’s d [24]. All tests were two sided with a p-value of less than 0.05 considered as statistically significant.
Results
Can Exercise Reduce the Risk of Osteoporotic Fractures in Postmenopausal Women?
Only a handful of exercise studies determined fracture risk or rate as a primary or secondary study endpoint (review in [10]). Two of these trials [8, 9] reported significant positive findings. Sinaki et al. [8] detected a significant positive effect for vertebral compression fractures after 2 years of supervised back-strengthening exercises followed by a non-monitored period of 8 years of self-selected physical activity in women aged 58–75 years. Addressing both fall risk and bone strength, Korpelainen et al. [9] reported significant differences between EG and CG concerning “overall fractures” (EG, 6, vs. CG, 16; rate ratio, 0.34; p = 0.019) after 30 months of exercise with 160 women, 70–73 years old. Finally, after 12 months of home exercise with subjects 75 years and older, Robertson et al. [25] observed significant effects for “serious injuries resulting from a fall” in favor of the EG (EG, 2, vs. CG, 9 fractures; rate ratio, 0.25; p = 0.03). However, the same exercise protocol did not result in significant between-group differences (p = 0.26) in somewhat older subjects [26]. However, the limitation of the latter two studies was that their statistical power to address clinical fractures was insufficient; so there is some likelihood that their positive results were promoted by random.
In the present study, however, the high amount of “participant years” (1650) allows us to address clinical fractures, clinical low-trauma fractures, and major osteoporotic fractures according to FRAX® [20]. In summary, risk and rate ratio for all the fracture parameters given above were significantly positive in favor of the exercise group. Most impressive, the number of clinical major osteoporotic fractures decreased by 63% in the EG (rate ratio, 0.37; 95% CI, 0.14–0.88; p = 0.027). Comparable data was observed for total clinical low-trauma fractures; 24 fractures occurred in the CG vs. 13 fractures in the EG (rate ratio, 0.42; 95% CI, 0.20–0.86; p = 0.018).
We are aware that a comparison with pharmacological studies as a benchmark is not fully feasible given the latter’s featured high(er) evidence levels and more dedicated inclusion criteria, but it provides an insight into the dimensions of exercise-induced fracture reduction achieved. Zoledronate, probably the most potent bisphosphonate actually [27], decreases the total clinical fracture rate by 33% [28] which is comparable with the anti-fracture efficacy reported for denosumab (32%) [29] and teriparatide (35%) [30]. However, it would be completely inappropriate to conclude exercise that may be a true alternative to pharmaceutical therapy, since the large proportion of frail elderly, as the classical addressees, are unable or simply unwilling to start and maintain lifelong, frequent, and intense exercise programs [31, 32] comparable to the EFOPS protocol.
Is There an Optimal Exercise Program to Increase or Maintain Bone Mineral Density
In the EFOPS we aimed to transfer approved exercise strategies generated by animal studies [12] and athletic exercise performance to our exercise program. Our exercise strategy was rather pragmatic: since most people are unwilling to spend a lot of time for prevention activities [33], the available time should be used most effectively. In order to optimize training effects under the constraints of a limited exercising volume, we applied modern training strategies [34] developed for athletic performance [35, 36]. One central feature of our exercise protocol was a regular change and adaptation of the training regimen, which required a periodization to structure the macro- as well as the mesocycles [34]. Although this strategy was specifically applied during the resistance sequence, we also periodized the other training sequences by varying the length of the high-impact sequences or the number of jumps per session. However, it is to be emphasized, that despite high exercise intensity, subjects did not exercise until complete exhaustion. Also, during the resistance sequence individual training plans did not call for the maximum possible repetitions for a given workload. We attribute the low injury rate of our study to this “non-exhaustive” strategy as well as to the conditioning period at the start of the study and to the intermediate regeneration phases.
While the effect of exercise types and most strain parameters have been evaluated in the meantime, the optimum design and composition of an exercise program to increase bone strength is still under debate [37, 38]. In this context, one basic question simply is how frequent the exercise sessions should be applied (per week). This decision has a twofold impact on the results of exercise programs: primarily because of its direct impact on the given endpoint of an exercise program and secondarily by affecting feasibility of the program and thus the participant’s compliance [39]. Based on the 12-year results of EFOPS, we structured retrospectively two exercise groups according to the overall exercise frequency. Changes of a BMD at lumbar spine and femoral neck (DXA) were compared between the low-frequency exercise group (LFG, 1.5 to <2 sessions/week) and the high-frequency exercise group (HFG, ≥2 to 3.5 sessions/week). The results showed changes of BMD at lumbar spine (HFG, 1.1 ± 4.7%, vs. LFG, −4.1 ± 3.0%; p = 0.001) and femoral neck (−4.4 ± 3.9% vs. −6.7 ± 3.5%, p = 0.045). Of importance, BMD changes of the LFG did not differ from the data of the non-training control group (LS, −4.4 ± 5.2%; FN, −6.9 ± 5.0%). Although this result might not be generalizable across all exercise types and cohorts, it indicates that an overall exercise frequency of at least two sessions per week may be crucial, even if exercise is applied with high intensity/impact [32].
Another research question that refers to the strain parameter “strain rate” was evaluated during the 4 and 5 study years [14, 15]. In this randomized controlled trial, we evaluated the effect of the movement velocity during the resistance sequence (strength (ST), 4 s (concentric)/4 s (eccentric), vs. power (PT), explosive/4 s). After 2 years of exercise, significant between-group differences were determined for LS-BMD (PT, −0.3%, vs. ST, −2.4%; p = 0.01). Also the incidence of pain indicators at the lumbar spine was more favorable in the PT group.
Although final evidence had been generated by another study of our group (TRACE study, [40]), we also addressed the relevance of block periodization in the EFOPS. Shortly, block periodization bases on two main determinants. From a scientific point of view, there is some experimental evidence that regular “unloading periods” (4 weeks within a 12-week exercise program) may be even more effective to increase bone strength than continuously applied loading [41] because of bone desensitization to frequent and high mechanical stimuli. From a pragmatic point of view, these “bone unloading periods” can be used to address other relevant training aims/risk factors of the elderly (i.e., falls, cardiometabolic risk factors) more specifically. Even though we do not directly compare the effects of a block periodized vs. a non-block periodized study group (but block periodized (EG) vs. sedentary control (CG)), the study results for LS-BMD as determined by QCT (total BMD: EG, −0.3 ± 2.1%, vs. CG, −2.1 ± 2.2%; p = 0.015; trabecular BMD: −0.7 ± 3.4% vs. −4.7 ± 4.9%; p = 0.001) and DXA (−0.1 ± 2.2% vs. −2.0 ± 2.0%, p = 0.002) were promising.
In summary, our appraisal on how to increase or maintain bone mineral density most favorably included the following items:
-
1.
General application of multipurpose exercise program with special regard to bone.
-
2.
Application of exercise type with relevant joint and (if applicable) high ground reaction forces.
-
3.
High-intensity (HIT) strategy, however, without work to failure.
-
4.
Implementation of 6 months of conditioning before HIT.
-
5.
Progression of the exercise program with special regard to intensity.
-
6.
Consequent and consistent variation/manipulation of the exercise program with respect to exercise type and parameters (e.g., intensity, movement velocity).
-
7.
Regular rest periods and (if applicable) the implementation of block periodization.
-
8.
Regular tests to monitor changes of short- and long-term aims of the exercise program, use of corresponding feedback to define next trainings, aims, and steps
Are There Temporary Limitations of Effectivity of Exercise on Bone?
Another aspect of our research was whether exercise consistently prevents loss of bone mineral density or whether there are periods of reduced effectiveness. To answer this question, we structured EFOPS in four periods of 4 years. As given, we only included participants with complete BMD data for baseline 4-, 8-, 12-, and 16-year follow-up. Figure 6.2 gives the result of the corresponding comparison for LS and FN.
In summary, after a slight increase of BMD at LS and FN in the EG during the first 2 years [42], we observed a largely linear decrease of BMD during the study course. Finally, we observed an overall decrease of −2.2 ± 3.1% (p = 0.02) after 16 years of continuous, supervised exercise with an average training frequency of 2.2 ± 0.4 sessions/week. However, BMD changes were quite homogeneous in the EG with reductions ranging between 0.49 and 0.61%. BMD reductions in the CG (6.6 ± 3.1, p < 0.001) were most pronounced during the first period with a gradual leveling-off afterward. About half of the total LS-BMD reduction took place during the first 4 years (i.e., during early menopause).
During the first 4 years, reductions of FN-BMD (EG, 0.862 ± 0.077 to 0.810 ± 0.074, vs. CG, 0.853 ± 0.088 to 0.775 ± 0.090 g/cm2; p < 0.001) were less distinct than at the LS, but increased significantly (p ≤ 0.022) during phase 2 (5–8 years). While the CG demonstrated a constant BMD decrease between 2.4 and 3.1% during periods 2–4, a reduction of BMD loss (p = 0.051) during the last 4 years (i.e., phase 4 compared with phase 3) was observed for the EG.
In summary, the BMD gap between EG and CG increased progressively throughout the study course, although we failed to determine significant differences for all isolated 4-year periods (LS-BMD, first and final period only; FN-BMD, second and final period). We are unable to refer these periods of (slightly) reduced effectivity to changes of our exercise program. Even the most pronounced reduction of bone-specific contents realized after year 12 was not related to a decreased effectivity during the final period. In conclusion, our sophisticated exercise program adapted to subjects’ priorities demonstrated a highly significant and clinically relevant long-term effect on BMD at lumbar spine and femoral neck. However, compared with the comfortable option of pharmaceutical intervention, more time and effort have to be invested in order to favorably affect BMD through exercise. With respect to the generalizability of our results, this suggests that “exercise” will be still reserved for motivated postmenopausal females.
Which Impact on Cardiometabolic Risk Factors Features an Exercise Program That Focuses on Fracture Reduction in Postmenopausal Women?
Multi-morbidity of the elderly is an increasing problem in the Western world [43]. Besides musculoskeletal problems, metabolic and cardiac diseases largely contribute to the high morbidity of our elderly population [44, 45]. Uniquely “exercise” represents a complex agent that in general affects most, if not all, of the relevant risk factors and diseases of the elderly [1, 46, 47]. However, it is not trivial to design a multipurpose exercise training that favorably affects the most relevant early menopausal risk factors (i.e., bone loss and cardiometabolic diseases) that may fundamentally differ with respect to their sport-scientific addressing. Examining our exercise protocol, the endurance/jumping sequence can be considered as a high-intensity interval training (HIIT) [48], a method with high relevance for cardiometabolic prevention and rehabilitation [49,50,51]. Further, the high relevance of resistance training for cardiometabolic is also accepted [52]. Both components were regularly and frequently applied in the EFOPS trial; thus a positive effect on relevant cardiometabolic markers should be achieved.
During the first study years, we focused on isolated cardiometabolic risks (e.g., blood lipids, blood pressure, waist circumference) which were consistently positively affected [42, 53]. However, in parallel to the osteoporotic fracture issue, the large number of participant years enabled us to select more meaningful cardiometabolic endpoints. Thus, finally (years 12 and 16, respectively), we addressed the metabolic syndrome according to the International Diabetes Federation (IDF) [54] and the “hard coronary heart disease” risk (i.e., risk of myocardial infarction and coronary death during the next 10 years [18]). The latter parameter significantly deteriorated in both study arms (p < 0.001); however, the changes were significantly less unfavorable in the EG, compared with the CG (5.00 ± 2.94% vs. 6.90 ± 3.98%; p = 0.017). Ignoring the subjects’ increasing age, which is however considered as a core risk factor by the hard CHD risk score, changes were no more significantly negative in the EG, contrarily to the CG. In parallel, metabolic syndrome Z-score [55] that did not include the variables sex and age and may be thus more sensible for “true” changes of cardiometabolic risk did not relevantly change in the EG but significantly deteriorate in the CG (EG, −0.42 ± 1.03%, p = 0.003, vs. CG, 1.61 ± 1.88%, p = 0.001).
In summary, the EFOPS strategy of a consistently applied high-intensity training program complies with our philosophy of multipurpose exercise programs, able to favorably address the most important risk factors of the menopause and of increasing age.
Are High-Intensity Exercise Programs for Osteoporosis Safe, Attractive, and Feasible?
Finally, we aimed to clarify an important issue with respect to high-intensity exercise training programs. Reviewing the literature, there is some evidence that high-impact [56]/high-intensity training [57] may lead to joint and/or low back pain.
In summary, we cannot verify this estimation; in fact, we determined positive effects on pain frequency and intensity for the lumbar spine and main joints that, however, reached significance only for the LS region [58]. This result is not trivial since LS and joint pain incidence in (early) postmenopausal females is very high. In a study by Raspe et al. [59], 35–40% of German women between the age of 50 and 59 years reported back pain and 45–50% reported joint pain. In our cohort pain incidence was even higher, 60% suffer from back pain (cervical, thoracic, and lumbar spine) and 68% from joint pain (knee, hip, and shoulder). Thus, exercise programs that generate pain reduction are of high relevance for this cohort. Our result that high-intensity exercise training, even with intermitted “power” training (i.e., high movement velocity) phases, did not lead to complication but even improve dedicated pain parameters has been recently confirmed by an exercise trial with subjects with hip arthritis [60]. Thus, our results do not support the statement “what is good for the bones is bad for the joints” [56], at least if certain rules are adhered to. These include: (1) careful incrementing of exercise intensity and impact, (2) avoiding complete exhaustion from maximizing the number of repetitions under a given load, (3) including intermittent “recreational exercise periods,” (4) proper variation of intensity and volume within the heavy-load periods, and (5) replacing exercises with very high ground reaction forces by less challenging high-impact movements in the course of advancing age. However, the latter was not applied for intense joint reaction forces reported to be less critical for elderly subjects [61, 62].
The attractiveness of the exercise program is easy to determine since subjects “vote with their feet”; thus, high attendance and low dropout rates indicate a high attractiveness of the program. The overall dropout rate of the EFOPS exercise group (Fig. 6.1) was 28%; however, taken into account that only nine subjects quit the study due to study-related reasons (loss of interest!), compared with other much shorter exercise trials [6, 63], the commitment was very impressive. The attendance rate per se averaged only 57%, but, taking into account that four sessions per week were prescribed by the EFOPS protocol, the average number of sessions effectively conducted per week was 2.15 ± 0.40. In total about two third of the EG participants exercised more than two sessions/week/year. Of importance, the number of group session attended (≈1.6) per week did not change during the 16-year period.
Besides effectiveness and attractiveness, feasibility is a further determinant of successful exercise programs. In general, we consider that the application of high-intensity training is feasible at least if educated trainers lead the sessions. However, even if this might be a specific problem of the complex German rehabilitation exercise practice, the requirement of resistance machines is problematic. Of lesser relevance, the use of resistance machines may increase the organizational and financial expenditure for the exercise groups. More important, the application of resistance machines within the framework of institutional rehabilitation by exercise according to SGB IX (Social Security Code) § 44 is not allowed. Since the vast majority of corresponding groups were largely co-financed by public health funds, the corresponding use of resistance machine is rare. This determinant, however, prevents the broad application of the EFOPS protocol that based on dedicated resistance exercise training on machines, at least in osteoporosis rehabilitation groups in Germany.
References
Börjesson M, Hellenius ML, Jansson E, et al. Physical activity in the prevention and treatment of disease. Stockholm: Swedish Institute of Health; 2010.
Vuori I. Exercise and physical health: musculoskeletal health and functional capacities. Res Q Exerc Sport. 1995;66:276–85.
Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146.
Groen BE, Smulders E, de Kam D, et al. Martial arts fall training to prevent hip fractures in the elderly. Osteoporos Int. 2010;21:215–21.
Weerdesteyn V, Groen BE, van Swigchem R, et al. Martial arts fall techniques reduce hip impact forces in naive subjects after a brief period of training. J Electromyogr Kinesiol. 2008;18:235–42.
Marques EA, Mota J, Carvalho J. Exercise effects on bone mineral density in older adults: a meta-analysis of randomized controlled trials. Age. 2011;34:1493–515.
Nikander R, Sievanen H, Heinonen A, et al. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47.
Sinaki M, Itoi E, Wahner HW, et al. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone. 2002;30:836–41.
Korpelainen R, Keinanen-Kiukaanniemi S, Heikkinen J, et al. Effects of impact exercise on bone mineral density in elderly women with low BMD: a population based randomized controlled 30-month intervention. Osteoporos Int. 2006;17:109–18.
Kemmler W, Haberle L, von Stengel S. Effects of exercise on fracture reduction in older adults: a systematic review and meta-analysis. Osteoporos Int. 2013;24:1937–50.
Jarvinen TL, Sievanen H, Khan KM, et al. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ. 2008;336:124–6.
Kemmler W, von Stengel S. Exercise and osteoporosis-related fractures: perspectives and recommendations of the sports and exercise scientist. Phys Sportsmed. 2011;39:142–57.
Kemmler W, Lauber D, Von Stengel S, et al. Developing maximum strength in older adults—a series of studies. In: Gießing J, Fröhlich M, Preuss P, editors. Current results of strength training research. Göttingen: Cuvillier Verlag; 2005. p. 114–33.
von Stengel S, Kemmler W, Lauber D, et al. Power training is more effective than strength training to maintain bone mineral density in postmenopausal woman. J Appl Physiol. 2005;99:181–8.
von Stengel S, Kemmler W, Kalender WA, et al. Differential effects of strength versus power training on bone mineral density in postmenopausal women: a 2-year longitudinal study. Br J Sports Med. 2007;41:649–55; discussion 55.
Mosley JR, Lanyon LE. Strain rate as a controlling influence on adaptive modeling in response to dynamic loading of the ulna in growing male rats. Bone. 1998;23:313–8.
Borg E, Kaijser L. A comparison between three rating scales for perceived exertion and two different work tests. Scand J Med Sci Sports. 2006;16:57–69.
Expert-Panel. Executive summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97.
DVO. DVO-Leitlinien 2014 zur Prophylaxe, Diagnostik und Therapie der Osteoporose bei Männern ab dem 60. Lebensjahr und postmenopausalen Frauen. Stuttgart: Schattauer; 2014.
Kanis JA, McCloskey EV, Johansson H, et al. Development and use of FRAX in osteoporosis. Osteoporos Int. 2010;21(Suppl 2):S407–13.
Salamone LM, Dallal GE, Zantos D, et al. Contributions of vitamin D intake and seasonal sunlight exposure to plasma 25-hydroxyvitamin D concentration in elderly women. Am J Clin Nutr. 1994;59:80–6.
Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009;(2):CD007146.
Karinkanta S, Piirtola M, Sievanen H, et al. Physical therapy approaches to reduce fall and fracture risk among older adults. Nat Rev Endocrinol. 2010;6(7):396-407 (online first).
Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Earlbaum Associates; 1988.
Robertson MC, Devlin N, Gardner MM, et al. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 1: Randomised controlled trial. BMJ. 2001;322:697–701.
Robertson MC, Gardner MM, Devlin N, et al. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 2: Controlled trial in multiple centres. BMJ. 2001;322:701–4.
Jansen JP, Bergman GJ, Huels J, et al. The efficacy of bisphosphonates in the prevention of vertebral, hip, and nonvertebral-nonhip fractures in osteoporosis: a network meta-analysis. Semin Arthritis Rheum. 2011;40:275–84 e1–2.
Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22.
McCloskey EV, Johansson H, Oden A, et al. Denosumab reduces the risk of osteoporotic fractures in postmenopausal women, particularly in those with moderate to high fracture risk as assessed with FRAX. J Bone Miner Res. 2012;27:1480–6.
Neer RM, Arnaud CD, Zanchetta JR. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41.
Kemmler W, von Stengel S. Exercise frequency, health risk factors, and diseases of the elderly. Arch Phys Med Rehabil. 2013;94:2046–53.
Kemmler W, von Stengel S. Dose-response effect of exercise frequency on bone mineral density in post-menopausal, osteopenic women. Scand J Med Sci Sports. 2014;24:526–34.
Rütten A, Abu-Omar K, Lampert T, et al. Körperliche Aktivität [Physical activity]. Report. Berlin: Statistisches Bundesamt; 2005.
ACSM. Progression models in resistance training for healthy adults. Med Sci Sports. 2002;34:364–80.
Baechle TR. Essentials of strength training and conditioning. Champaign: Human Kinetics; 1994.
Bompa TO. Periodization. Theorie and methodology of training. Champaign: Human Kinetics; 1999.
Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;(7):CD000333.
Sherrington C, Whitney JC, Lord SR, et al. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc. 2008;56:2234–43.
Wagner P. Aussteigen oder Dabeibleiben? [dissertation]. Darmstadt: Technical University Darmstadt; 2000.
Kemmler W, Bebenek M, von Stengel S, et al. Effect of block-periodized exercise training on bone and coronary heart disease risk factors in early post-menopausal women: a randomized controlled study. Scand J Med Sci Sports. 2013;23:121–9.
Saxon LK, Robling AG, Alam IM, et al. Mechanosensitivity of the rat skeleton decreases after a long period of loading, but is improved with time off. Bone. 2005;36:454–64.
Kemmler W, Lauber D, Weineck J, et al. Benefits of 2 years of intense exercise on bone density, physical fitness, and blood lipids in early postmenopausal osteopenic women: results of the Erlangen Fitness Osteoporosis Prevention Study (EFOPS). Arch Intern Med. 2004;164:1084–91.
Tesch-Römer C, Engstler H, Wurm S. Altwerden in Deutschland. Sozialer Wandel und individuelle Entwicklung in der zweiten Lebenshälfte. Wiesbaden: VS-Verlag für Sozialwissenschaften; 2006.
Löwel H. Koronare Herzkrankheit und akuter Myokardinfarkt. Berlin: Robert-Koch-Institut; 2006.
Roger VL, Go AS, Lloyd-Jones DM, et al. Executive summary: heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:188–97.
Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–30.
Pedersen BK, Saltin B. Evidence for prescribing exercise as a therapy in chronic disease. Scand J Med Sci Sports. 2006;16:3–63.
Gibala MJ, Little JP, Macdonald MJ, et al. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J Physiol. 2012;590:1077–84.
Guiraud T, Nigam A, Gremeaux V, et al. High-intensity interval training in cardiac rehabilitation. Sports Med. 2012;42:587–605.
Hansen D, Dendale P, van Loon LJ, et al. The impact of training modalities on the clinical benefits of exercise intervention in patients with cardiovascular disease risk or type 2 diabetes mellitus. Sports Med. 2010;40:921–40.
Haykowsky MJ, Timmons MP, Kruger C, et al. Meta-analysis of aerobic interval training on exercise capacity and systolic function in patients with heart failure and reduced ejection fractions. Am J Cardiol. 2013;111:1466–9.
Strasser B, Siebert U, Schobersberger W. Resistance training in the treatment of the metabolic syndrome: a systematic review and meta-analysis of the effect of resistance training on metabolic clustering in patients with abnormal glucose metabolism. Sports Med. 2010;40:397–415.
Kemmler W, von Stengel S, Weineck J, et al. Exercise effects on menopausal risk factors of early postmenopausal women: 3-yr Erlangen fitness osteoporosis prevention study results. Med Sci Sports Exerc. 2005;37:194–203.
Alberti KG, Zimmet P, Shaw J. Metabolic syndrome—a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80.
Johnson JL, Slentz CA, Houmard JA, et al. Exercise training amount and intensity effects on metabolic syndrome (from studies of a targeted risk reduction intervention through defined exercise). Am J Cardiol. 2007;100:1759–66.
Turner CH. Exercise as a therapy for osteoporosis: the drunk and the street lamp, revisited. Bone. 1998;23:83–5.
Vuori IM. Dose-response of physical activity and low back pain, osteoarthritis, and osteoporosis. Med Sci Sports Exerc. 2001;33:S551–S86.
Kemmler W, Bebenek M, Kohl M, et al. Long-term exercise effects on health parameters in postmenopausal females. Final Results of the Erlangen Fitness and Osteoporosis Prevention Study (EFOPS). Menopause 2017;24:45–51.
Raspe H, Kohlmann T. Rückenschmerzen—eine Epidemie unserer Tage. Dtsch Arztebl. 1993;90:B2165–9.
Fukumoto Y, Tateuchi H, Ikezoe T, et al. Effects of high-velocity resistance training on muscle function, muscle properties, and physical performance in individuals with hip osteoarthritis: a randomized controlled trial. Clin Rehabil. 2014;28:48–58.
Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;(3):CD002759.
Mangione KK, Miller AH, Naughton IV. Cochrane review: improving physical function and performance with progressive resistance strength training in older adults. Phys Ther. 2010;90:1711–5.
Wolff I, van Croonenborg JJ, Kemper HC, et al. The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int. 1999;9:1–12.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Kemmler, W., von Stengel, S. (2017). Exercise for Prevention of Bone Loss: The Role of Sports Medicine. In: Sinaki, M., Pfeifer, M. (eds) Non-Pharmacological Management of Osteoporosis. Springer, Cham. https://doi.org/10.1007/978-3-319-54016-0_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-54016-0_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-54014-6
Online ISBN: 978-3-319-54016-0
eBook Packages: MedicineMedicine (R0)