Abstract
Glial tumors represent the most common primary central nervous system tumors. They are classified as astrocytomas, oligodendrogliomas, and oligoastrocytomas. They may occur as benign tumors (WHO grade II) or anaplastic tumors (WHO grade III). The most malignant astrocytoma is called glioblastoma and represents WHO grade IV.
With the recent publication of the revised fourth edition of the WHO classification of CNS tumors, the molecular characterization of the tumors becomes mandatory. Elementary investigations include the determination of the mutation status of IDH1/2 and co-deletion of 1p/19q. Additional parameters include mutations in the ATRX gene and the TERT promoter. A variety of genetic alterations have been described. Specific focus was laid on epigenetic changes, i.e., altered methylation patterns. Studies related to gene or microRNA (miR) expression in brain tumors are still scarce. Brain tumors pose a challenging task for the clinician and require further broad-minded molecular investigations at various levels.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
7.1 Introduction
Glial tumors are the most frequently encountered brain tumors. They are tumors of neuroepithelial tissue. Based on the cell of origin, they are classified as astroglial, oligodendroglial, and oligo-astroglial tumors. Other neuroepithelial tumors include ependymal and choroid plexus tumors. Neuronal and mixed neuronal-glial tumors, tumors of the pineal region, and embryonal tumors also belong to the group of tumors of neuroepithelial tissue.
For each tumor entity, predicting the biological behavior by means of histological grading was introduced by the WHO. Thus, (a) grade I tumors have low proliferative potential and the possibility of cure following surgery; (b) grade II tumors are infiltrative in nature and, despite low level of proliferation, often recur. They tend to progress to higher grades; (c) grade III tumors are lesions with histological evidence of malignancy, including nuclear atypia and brisk mitotic activity; and (d) grade IV tumors are cytologically malignant, mitotically active, necrosis-prone neoplasms associated with rapid pre- and postoperative disease evolution and fatal outcome.
According to the Central Brain Tumor Registry of the United States (CBTRUS), the distribution (in %) of primary brain and CNS tumors by histology and by histology subtypes is shown in Table 7.1. The overall distribution is based on an analysis of 356,858 tumors, and the distribution of the histological tumor subtypes is based on an analysis of 97,910 tumors [1,2,3].
7.2 Morphology
7.2.1 Glioblastoma (GBM) (WHO Grade IV)
Glioblastoma or glioblastoma multiforme (GBM) is a highly malignant neuroectodermal tumor composed of densely packed, anaplastic, and highly dedifferentiated tumor cells making the histogenetic typing difficult.
Macroscopically, GBM are tumors of large size which can involve several lobes. GBMs can spread to the contralateral hemisphere through the corpus callosum displaying a symmetrical tumor growth into both hemispheres, i.e., butterfly glioma. The tumor is usually not sharply demarcated, presenting with a broad and diffuse zone of infiltration. The cut surface characteristically shows a varicolored appearance ranging from gray, brown, white, and yellow to dark red. GBMs have a firm consistency. Necroses are present.
At the microscopic level, a high diversity of cell forms is encountered in glioblastoma which encompass anaplastic cells displaying astrocytic features; neoplastic oligodendroglia; high density of small, poorly differentiated cells; marked polymorphism of tumor cells including multinucleated giant cells; areas showing cells with astrocytic, oligodendroglial, and rarely ependymal differentiation; atypical mitoses; vascular endothelial cell proliferation; typical tumor necroses, i.e., palisading with cells arranged side by side in rows and their processes directed toward a central area of necrosis; and large areas of necroses. Based on the predominant features, the following types can be recognized: small cell GBM, classical type, GBM with oligodendroglioma component, and GBM with PNET-like islands.
7.2.2 Astrocytoma (WHO Grade II)
Astrocytoma is a diffusely infiltrating tumor that typically affects young adults and is characterized by a high degree of cellular differentiation and slow growth; the tumor occurs throughout the CNS but is preferentially located supratentorially and has an intrinsic tendency for malignant progression to anaplastic astrocytoma and, ultimately, glioblastoma.
Macroscopically, the borders of the gray or yellow-whitish tumor are blurred. Areas of the tumor tissue might be firm or softened or granular or cystic.
At the microscopic level, astrocytoma is characterized by well-differentiated neoplastic astrocytes in a loosely structured tumor matrix. The neoplastic astrocytes have round to oval nuclei with intermediate-sized masses of chromatin, a distinct nucleolus, and no stainable cytoplasm. There is an absence of mitotic activity, necroses, and microvascular proliferation.
7.2.3 Anaplastic Astrocytoma (AA) (WHO Grade III)
Anaplastic astrocytoma is a diffusely infiltrating, malignant astrocytoma that primarily affects adults, preferentially located in the cerebral hemispheres, which is histologically characterized by nuclear atypia, increased cellularity, and significant proliferative activity. The tumor may arise from diffuse astrocytoma WHO grade II or de novo, i.e., without evidence of a less malignant precursor lesion, and has an inherent tendency to undergo progression.
Macroscopically, AA infiltrates the surrounding brain, accompanied by tissue destruction. It has areas of granularity, opacity, and soft consistency.
At the microscopic level, anaplastic astrocytoma is characterized by an increased cellularity as compared to astrocytoma WHO grade II and distinct nuclear atypia characterized by increased nuclear size, shape, chromatin coarsening, dispersion, and nucleolar prominence. Mitoses might be present as well as multinucleated tumor cells and abnormal mitoses. Microvascular proliferation and necroses are absent.
7.2.4 Pilocytic Astrocytoma (PA) (WHO Grade I)
Pilocytic astrocytoma is a relatively circumscribed, slowly growing, often cystic astrocytoma occurring in children and young adults, histologically characterized by a biphasic pattern with varying proportions of compacted bipolar cells associated with Rosenthal fibers and loose-texture multipolar cells associated with microcysts and eosinophilic granular bodies/hyaline droplets.
Macroscopically, pilocytic astrocytoma is a soft, gray discrete mass with intratumoral and paratumoral cyst formation. Calcifications and hemosiderin deposits might be encountered.
At the microscopic level, PA is characterized by low to moderate cellularity. Heterogeneity of histologic features consists of a biphasic growth pattern of tumor cells with loosely textured multipolar cells (protoplasmic astrocytes), microcysts, and granular bodies/hyaline droplets.
Rosenthal fibers are intracytoplasmic corkscrew-shaped, eosinophilic, and hyaline glial fibrillary acidic protein (GFAP)-positive masses (fibers) which are present in tumor cells. Eosinophilic granular bodies (EGB) are eosinophilic, PAS-positive globular aggregates within astrocytic processes. PAs are highly vascularized tumors. Regressive changes include hyalinized, ectatic vessels (DD cavernous angioma), previous hemorrhage (hemosiderin), calcification, and lymphocytic infiltrates.
7.2.5 Oligodendroglioma (WHO Grade II)
Oligodendroglioma is a diffusely infiltrating, well-differentiated glioma of adults, typically located in the cerebral hemispheres, composed of neoplastic cells morphologically resembling oligodendroglia and often harboring deletions of chromosomal arms 1p and 19q.
Macroscopically, it is a well-defined mass of soft to gelatinous consistency (mucoid degeneration) and grayish-pink color. Calcifications are frequently encountered.
At the microscopic level, oligodendroglioma consists of monomorphic tumor cells with round nuclei, perinuclear halo only seen on paraffin sections (honeycomb appearance), and moderate cellularity.
7.2.6 Anaplastic Oligodendroglioma (WHO Grade III)
Anaplastic oligodendroglioma is an oligodendroglioma with focal or diffuse histological features of malignancy and a less favorable prognosis.
Macroscopically, anaplastic oligodendroglioma presents as a well-defined mass of soft to gelatinous consistency (mucoid degeneration) and grayish-pink color. Calcifications are frequent.
At the microscopic level, anaplastic oligodendroglioma is characterized by diffusely infiltrating tumor cells reminiscent of oligodendrocytes with round hyperchromatic nuclei, perinuclear halo, and scant cellular processes. Tumor cells display marked cellular and nuclear pleomorphism. Multinucleated giant cells might be present. The dense network of branching capillaries (chicken-wire pattern) is still preserved. Necroses, when present, are not indicative of shorter survival.
7.2.7 Oligoastrocytoma (WHO Grade II)
Oligoastrocytoma is a diffusely infiltrating glioma composed of a conspicuous mixture of two distinct neoplastic cell types morphologically resembling the tumor cells in oligodendrogliomas and diffuse astrocytoma of WHO grade II.
Macroscopically, the tumors have blurred borders, enlarge and distort the invaded structures, and are of gray or yellow-whitish color. Areas of the tumor tissue might be firm or softened or granular or cystic.
At the microscopic level, oligoastrocytoma is a tumor of moderate cellularity characterized by the presence of neoplastic glial cells with astrocytic or oligodendroglial phenotypes. Microcalcifications and microcysts might be present. The tumor lacks necroses and microvascular proliferation.
7.2.8 Anaplastic Oligoastrocytoma (WHO Grade III)
Anaplastic oligoastrocytoma is an oligoastrocytoma with histological features of malignancy, such as increased cellularity, nuclear atypia, pleomorphism, and increased mitotic activity.
Macroscopically, the tumor infiltrates the surrounding brain with tissue destruction. The tumor shows areas of granularity, opacity, and soft consistency.
At the microscopic level, anaplastic oligoastrocytoma is characterized by increased cellularity as compared to oligoastrocytoma WHO grade II; distinct nuclear atypia with increased variations of nuclear size, shape, chromatin coarsening, and dispersion; nucleolar prominence; cellular pleomorphism; and high mitotic activity. Tumors with necroses should be classified as “glioblastoma with oligodendroglial component.”
In the revised version of the fourth WHO classification of CNS tumors [4], the above-described tumor entities are labeled as follows:
-
Diffuse astrocytoma, IDH mutant
-
Diffuse astrocytoma, IDH wild type
-
Diffuse astrocytoma, NOS (not otherwise specified)
-
Anaplastic astrocytoma, IDH mutant
-
Anaplastic astrocytoma, IDH wild type
-
Anaplastic astrocytoma, NOS
-
Glioblastoma, IDH wild type
-
Oligodendroglioma, IDH mutant and 1p/19q-co-deleted
-
Oligodendroglioma, NOS
-
Anaplastic oligodendroglioma, IDH-mutant and 1p/19q-co-deleted
-
Anaplastic oligodendroglioma, NOS
-
Oligoastrocytoma, NOS
-
Anaplastic oligoastrocytoma, NOS
The NOS (not otherwise specified) category includes both tumors that have not been tested for the genetic parameter(s) and tumors that have been tested but did not show the diagnostic genetic alterations.
7.3 Genetics
In addition to “classical” histopathology, molecular genetic markers have found their way into diagnostic schemes designed for glioma classification and prognosis. Of particular interest are marke rs which allow an unambiguous distinction of tumor subtypes. Ideally, the requirements for such markers are their significant prevalence in some tumor subtypes and their low frequency, or even virtual absence, in others. In routine diagnostics, comprehensive genetic testing includes the synopsis of all analyzed markers to obtain a reliable classification of the investigated tumor(s).
Some molecular markers that have emerged as powerful tools in recent years are IDH1/IDH2 mutations, ATRX mutations, TERT promoter mutations, and a chromosomal aberration typically found in oligodendrogliomas, the 1p/19q co-deletion [reviewed in [5]].
7.3.1 IDH Mutations
Important genetic traits in glial tumors are recurrent point mutations in the IDH1 and IDH2 genes. IDH1 is located on chromosome 2q33.3 and encodes the NADP+-dependent cytosolic enzyme isocitrate dehydrogenase 1 (IDH1) [6]. The IDH2 gene at chromosome locus 15q26.1 encodes the NADP+-dependent mitochondrial enzyme isocitrate dehydrogenase 2 (IDH2).
A frequently observed aberration is a missense IDH1 point mutation, resulting in the transition of arginine to histidine at amino acid position 132 (R132H) which is located in the enzyme’s substrate binding site. Wild-type IDH1 is involved in cytosolic NADPH production, but to date, the role of the mutated gene in gliomagenesis is not yet fully understood. It is important to note that the IDH1 mutation is found at a high frequency in secondary GBM (~80%) but only rarely in primary GBM. Moreover, the IDH1-R132H mutation was also observed in up to 80% of grade II and grade III astrocytomas. The detection of mutated IDH1 (R132H) is therefore routinely used as a specific diagnostic marker in these tumors, and it supports discrimination between primary and secondary GBM.
At a much lower frequency, functional mutations are also reported in the IDH2 gene; here, the major target is codon 172 which corresponds to an arginine residue in the wild-type enzyme. R172 represents the site analogous to R132 in IDH1. Noteworthy, IDH1 and IDH2 mutations appear to occur mutually exclusive in glioma patients.
7.3.2 ATRX Mutations
The ATRX gene (α-thalassemia/mental retardation syndrome X-linked) is located at Xq21.1 on the long arm of the X chromosome. ATRX is expressed exclusively in the nucleus where it plays an important role in telomere stabilization and chromatin remodeling. Inactivating mutations in the ATRX gene result in loss of functional protein which in turn triggers a mechanism known as the ALT (alternative lengthening of telomeres) phenotype. ATRX mutations are frequently found in astrocytomas WHO grades II and III as well as in oligoastrocytomas, and with a lower incidence in secondary glioblastomas and oligodendrogliomas [7].
7.3.3 TERT Promoter Mutations
The TERT gene (telomerase reverse transcriptase) encodes a subunit of the telomerase complex which is crucial for maintaining telomere length and stability. In dividing cells, telomeres become shorter with each division cycle which eventually leads to cellular senescence. In proliferating cells, e.g., during developmental or regenerative processes, telomerase activity counteracts telomere shortening, thus maintaining the cells’ replicative potential, a feature that is also characteristic for tumor tissue. Mutations in the TERT promoter resulting in increased expression of the gene have been described in a variety of cancer cells. High mutation frequencies were observed in adult primary glioblastomas (83%) and oligodendrogliomas (78%); lower frequencies were seen in oligoastrocytomas (25%) and astrocytomas WHO grades II and III (~10%) [8].
7.3.4 1p/19q Co-deletion
The most commonly detected genomic aberration in oligodendrogliomas is a heterozygous loss (LOH) of the short arm of chromosome 1 associated with LOH of the long arm of chromosome 19 (1p/19q co-deletion). This genetic anomaly is observed in the vast majority of oligodendrogliomas, with incidences of up to 90% reported for grade II and somewhat lower for grade III tumors (50–70%). Moreover, the 1p/19q co-deletion apparently occurs mutually exclusive of the TP53 mutations and chromosome 17p losses which are more common in astrocytic tumors.
Table 7.2 illustrates the mutual relationship of the presence of IDH mutations, ATRX mutations, and TERT promoter mutations in astrocytic tumors; “+” indicates high prevalence of the mutations; “−” refers to very low frequencies/absence of the mutations.
7.3.5 Glioblastoma (WHO Grade IV)
Genome-wide analyses revealed that basically three major signaling cascades are affected by genetic aberrations in GBM: (a) the TP53 (tumor protein 53) pathway; (b) the RTK (receptor tyrosine kinase)/RAS/PI3K (phosphoinositide 3-kinase) pathway, both involved in the regulation of cellular growth, apoptosis, and proliferation; and (c) the RB1 (retinoblastoma) pathway, controlling the G1 to S phase transition in the cell cycle.
Genes which are mutated in GBM are involved in the regulation of cell signaling, cell proliferation and survival, cell cycle, apoptosis, and NADPH production.
7.3.5.1 Pathway-Related Genes
The compilation outlined below (Table 7.3) contains a selection of pathway-related genes which are commonly altered in GBM, with respect to their properties, chromosomal location, and nature of pathogenic changes.
7.3.5.2 IDH1 Mutations
In addition to the pathway-related gene alterations, point mutations in the IDH1 gene are a prominent feature in GBM. As described above, the IDH1-R132H mutation is detected predominantly in secondary GBM (~80%) but only rarely in primary GBM. Analysis of the IDH1 mutation is therefore routinely used as a supporting parameter to differentiate between GBM subtypes (Table 7.2).
7.3.5.3 TERT Promoter Mutations
Unlike the IDH1 mutation, TERT promoter mutations are found in the vast majority of adult primary glioblastomas (~80%) (Table 7.2). On the contrary, very low mutation frequencies were observed in secondary GBM [9].
7.3.5.4 ATRX Mutations
ATRX mutations were frequently detected in secondary glioblastomas (57%) and rarely in primary glioblastomas (4%) [10], an observation which provides an additional tool for the characterization of GBM subtypes (Table 7.2).
7.3.5.5 Mutations Affecting Oncogenes/Tumor Suppressor Genes
Somatic mutations frequently found to cause activation of oncogenes and/or inactivation of tumor suppressor genes are listed in Table 7.4 (mutation frequencies compiled from [11, 12]).
7.3.5.6 Chromosomal Aberrations
Apart from somatic mutations, genomic instability resulting in somatic copy number alterations (SCNAs) is a major determining factor in GBM. The most important chromosomal abnormalities are depicted in Table 7.5 (LOH = loss of heterozygosity).
7.3.5.7 Summary of Genetic Characteristics of Primary and Secondary Glioblastomas
The differentiation between primary and secondary GBM is reflected by distinct pathogenetic patterns in the two tumor subtypes. Major differences that are consistently observed mainly involve key regulatory genes such as EGFR, PTEN, TP53, and, as described earlier, IDH1, ATRX, and TERT promoter mutations. In the following, the most significant differences are summarized.
Primary GBM is characterized by:
-
(a)
Amplification of the EGFR oncogene accompanied by LOH on chromosome 10q where the tumor suppressor gene PTEN is located.
-
(b)
Complete loss of chromosome 10.
-
(c)
Mutated PTEN in about one third of primary GBMs (not seen in secondary glioblastoma).
-
(d)
TP53 mutations found at a significantly lower rate in primary than in secondary GBM.
-
(e)
A preferred correlation pattern of EGFR amplification and mutations in PTEN and TP53, i.e., EGFR amplification and PTEN mutations are associated with low TP53 mutation frequencies.
-
(f)
Absence of IDH1 mutations.
-
(g)
A high frequency of TERT promoter mutations (~80%).
Secondary GBM is characterized by:
-
(a)
A high frequency of TP53 mutations, often occurring together with LOH in chromosome 17p.
-
(b)
The occurrence of high TP53 mutation rates which is largely complemented by a lack of EGFR amplification, probably indicating a mutually exclusive relationship.
-
(c)
A chromosomal aberration associated predominantly with secondary GBM is LOH in chromosome 19q (54%), in contrast to primary GBM (6%).
-
(d)
A high frequency of IDH1 mutations (~80%).
-
(e)
ATRX mutations (57%).
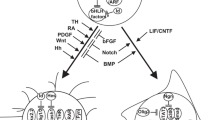
A graphical representation of molecular alterations occurring in primary GBM as compared to secondary GBM is illustrated as follows:

7.3.5.8 Novel Molecular Classification of GBM into Four Distinct Subtypes
Based entirely on genomic and gene expression profiling, a new classification model was recently proposed for glioblastoma. This scheme suggests to replace the currently accepted primary and secondary GBM subtypes with four redefined subgroups: proneural, neural, classical, and mesenchymal [13]. The essential intention of this approach was to relate the specific molecular signature of each tumor subgroup to the progenitor cell type from which it may have developed, thus providing a basis for better and more specific therapeutic strategies.
Each of the four subtypes was shown to display characteristic features:
-
(a)
Proneural subtype: amplification of platelet-derived growth factor receptor, alpha polypeptide (PDGFRA) and high levels of PDGFRA expression, frequent IDH1 and TP53 mutations, and TP53 LOH, all features which are reminiscent of secondary GBM.
-
(b)
Neural subtype: an expression pattern of neuronal markers very similar to that in normal tissue was observed, indicating that this subtype is not defined by a specific pathogenetic signature.
-
(c)
Classical subtype: chromosome 7 amplification (EGFR) with corresponding enhanced EGFR expression, chromosome 10 loss (PTEN), and loss of chromosome 9p regions (CDKN2A); no abnormalities were detected in PDGFRA, IDH1, TP53, and NF1.
-
(d)
Mesenchymal subtype: loss of the NF1 locus on chromosome 17q11.2, correlating with low NF1 expression levels.
7.3.6 Anaplastic Astrocytoma (WHO Grade III)
Anaplastic astrocytomas frequently evolve from diffuse astrocytomas (WHO grade II) and eventually progress further to secondary glioblastomas (WHO grade IV). Accordingly, the genetic background of anaplastic astrocytomas includes features that are also found in astrocytic tumors of both WHO grades II and IV.
Genetic aberrations observed in anaplastic astrocytoma WHO grade III include gains of chromosome 7; losses of chromosome 17p; mutations in the tumor suppressor gene TP53 (tumor protein 53); the IDH1 point mutation (IDH1-R132H) in up to 80% of cases; LOH on chromosomes 6, 10q, 11p, 19q, and 22q; deletions of the chromosome 9p21 region carrying the tumor suppressor genes CDKN2A (cyclin-dependent kinase inhibitor 2A) and CDKN2B (cyclin-dependent kinase inhibitor 2B); and mutations in the RB1 gene (retinoblastoma 1) (~25% of cases).
7.3.7 Diffuse Astrocytoma (WHO Grade II)
In line with the pronounced tendency of astrocytoma WHO grade II tumors to progress to higher grade gliomas (anaplastic astrocytoma, grade III, and secondary glioblastoma, grade IV), some of the genetic traits associated with these malignant tumors are already laid out in diffuse astrocytoma. In particular, gains of chromosome 7 and losses of chromosome 17p which occur at high frequencies should be noted.
Genes affected in astrocytomas WHO grade II include:
-
(a)
TP53 (tumor suppressor gene, on chromosome 17p13.1, encoding tumor protein 53):
In more than 60% of diffuse astrocytomas, monoallelic deletion (loss of heterozygosity, LOH) of the chromosome 17p region harboring TP53 has been described; moreover, in the majority of cases, mutations in the remaining TP53 allele ultimately result in a total lack of the functional gene product.
-
(b)
PDGFRA (oncogenic; encoding platelet-derived growth factor receptor, α-peptide):
In WHO grade II astrocytomas, upregulation of PDGFRA expression can be observed, although supporting evidence is based on relatively low sample sizes. In addition, elevated PDGFRA levels are more frequently correlated with higher grade gliomas. Taken together, it therefore remains uncertain to which extent PDGFRA overexpression contributes to tumorigenesis in diffuse astrocytoma.
-
(c)
IDH1/2 (isocitrate dehydrogenase 1, isocitrate dehydrogenase 2):
The IDH1-R132H mutation was found in up to 80% of grade II gliomas and, at a much lower frequency, functional mutations in the IDH2 gene affecting codon 172 (R172).
7.3.8 Pilocytic Astrocytoma (WHO Grade I)
Patients suffering from neurofibromatosis type 1 (NF1), a hereditary tumor syndrome, frequently develop pilocytic astrocytoma. About one third of pilocytic astrocytomas are observed in NF1 patients, whereas the sporadic types of this tumor are NF1 independent.
NF1-associated pilocytic astrocytoma: Neurofibromin, encoded by the NF1 gene on chromosome 17q11.2, functions as a tumor suppressor by inhibiting oncogenic Ras (=Rat sarcoma) signaling. In neurofibromatosis type 1 and in NF1-associated pilocytic astrocytoma, NF1 gene deletions and mutations result in loss of functional neurofibromin.
NF1-independent pilocytic astrocytoma: In sporadic pilocytic astrocytoma, typical genetic aberrations (>60%) are duplications at chromosome region 7q34, affecting the BRAF gene (v-raf murine sarcoma viral oncogene homolog B). These duplications create in-frame fusions of BRAF with the upstream KIAA1549 gene. The resulting aberrant fusion proteins contain the BRAF kinase domain and were shown to exhibit constitutive BRAF kinase activity which in turn activates the oncogenic MAPK (mitogen-activated protein kinase) signaling pathway.
Oncogenic BRAF activation not only occurs via gene duplication but may also be the result of mutations occurring around codon 600: (a) a T > A mutation at nucleotide position 1799, creating the replacement of the wild-type valine 600 by a glutamate residue in the protein (referred to as the BRAFV600E mutation), and (b) two different 3bp insertions, both resulting in an additional threonine residue at amino acid position 599. These activating mutations occur at a much lower frequency than the KIAA1549/BRAF fusions (~9%).
Similar to the BRAF fusions, albeit less common, are fusions on chromosome 3p25 between the SRGAP3 (SLIT-ROBO Rho GTPase-activating protein 3) gene and the RAF1 (v-raf-1 murine leukemia viral oncogene homolog 1) gene. RAF1 is a positive regulator of the oncogenic MAPK signaling pathway; in SRGAP3/RAF1 fusions, the auto-inhibitory region of RAF1 is lost, leading to a constitutive activation of the MAPK pathway.
Somatic mitochondrial mutations, mostly single nucleotide exchanges, were recently reported in pilocytic astrocytoma. Some of the mutations resided in coding regions, causing amino acid alterations. The affected gene products were identified as proteins involved in electron transport/oxidative phosphorylation [14].
7.3.9 Oligodendroglioma (WHO Grade II) and Anaplastic Oligodendroglioma (WHO Grade III)
As described above, about 90% of WHO grade II and 50–70% of WHO grade III oligodendrogliomas exhibit the heterozygous 1p/19q co-deletion. Thus, this chromosomal aberration represents a genetic hallmark in oligodendrogliomas.
Mutations in the IDH1 and IDH2 genes are another characteristic feature in oligodendrogliomas. The IDH1-R132H mutation is most frequently observed (>70%). In IDH2, the homologous site (R172) was found to be mutated, however, in only a small fraction of the tumors [6]. In oligodendrogliomas, IDH1/2 mutations appear to be strongly associated with TERT promoter mutations and the 1p/19q co-deletion.
Novel genetic anomalies have recently been described in oligodendrogliomas and encompass point mutations in the CIC (capicua transcriptional repressor) gene, located at chromosome 19q13.2; its gene product acts as a transcriptional repressor downstream of the receptor tyrosine kinase (RTK) pathway. Interestingly, CIC mutations occur in the majority (~70%) of oligodendrogliomas exhibiting the 1p/19q co-deletion plus IDH mutations and point mutations in the FUBP1 (far upstream element binding protein 1) gene on chromosome 1p31.1; FUBP1 is a transcriptional regulator of the c-Myc oncogene. Most of the FUBP1 mutations (>70%) are found in oligodendrogliomas that also carry CIC mutations.
7.3.10 Oligoastrocytoma (WHO Grade II)
Similar to oligodendrogliomas, albeit at a lower frequency, heterozygous chromosome 1p/19q co-deletions have been described in oligoastrocytomas (30–50% of the cases). Notably, 19q deletion without 1p loss is often observed in these tumors.
Several genetic aberrations which are reminiscent of astrocytic tumors can be detected in about 30% of cases, e.g., (a) mutations in the tumor suppressor gene TP53, (b) LOH of chromosome 17p, (c) anomalies of chromosome 10, and (d) amplification of the EGFR (epidermal growth factor receptor) gene.
7.3.11 Genetic Markers
Taken together, the determination of the following genetic markers is requested/useful:
-
Astrocytomas and/or glioblastomas:
Mutation status of IDH1 and IDH2 (mutated versus wild type), allelic loss of chromosomes 1p and 19q (co-deletion), loss-of-function mutations in the TP53 and ATRX genes. Additional genes include EGFR, PTEN, PDGFRA, MET, PI3K, chromatin-related genes (H3F3A, HIST1H3B/C), and TERT promoter mutations.
-
Oligodendrogliomas and/or oligoastrocytomas:
Mutation status of IDH1 and IDH2 (mutated versus wild type), allelic loss of chromosomes 1p and 19q (co-deletion), and mutations in the CIC and FUBP1 genes as well as in the TERT promoter region.
7.4 Epigenetics
7.4.1 DNA Methylation
7.4.1.1 Glioblastoma (WHO Grade IV)
DNA hypermethylation of promoter regions is a frequently observed mechanism in glioblastomas by which tumor suppressor genes (such as TP53, PTEN, or CDKN2A) are silenced.
In recent years, the MGMT gene, encoding the DNA repair enzyme O6-methylguanine-DNA methyltransferase, has received particular attention. Guanine alkylated at its O6 position represents a mutagenic DNA lesion that is normally repaired by the MGMT enzymatic activity. It transfers the methyl group from the nucleobase to an active cysteinyl residue in its own sequence. Thus, by removing methyl groups from mutagenic O6-methylguanine residues, the enzyme contributes to genome integrity.
In glioblastoma chemotherapy, alkylating drugs like the widely used temozolomide (TMZ) are employed to introduce DNA damage in tumor cells with the intention to trigger apoptosis and cell death. Active MGMT counteracts this mechanism, thus conferring resistance to the treatment. However, hypermethylation of the MGMT promoter, abolishing the transcription of the gene, was demonstrated in a high percentage of GBMs, i.e., in up to 75% of secondary and approximately 35% of primary glioblastomas. It is obvious that patients with glioblastoma lacking MGMT expression show better responsiveness to TMZ chemotherapy which also has been implicated with improved prognosis.
7.4.1.2 Diffuse Astrocytoma (WHO Grade II)
So far, the best studied epigenetic feature associated with grade II astrocytomas is promoter hypermethylation of two tumor suppressor genes, ARF and MGMT:
-
The ARF gene codes for the tumor suppressor protein p14ARF which acts as a supporting factor in the TP53 pathway.
-
The MGMT gene encodes the DNA repair enzyme O6-methylguanine-DNA methyltransferase.
-
Promoter hypermethylation results in reduced expression of the tumor suppressor genes and, as a result, in diminished protein function.
7.4.1.3 Oligodendroglioma (WHO Grade II)
Several genes were shown to be affected by promoter hypermethylation, resulting in reduced expression levels: (a) the MGMT gene, encoding the DNA repair enzyme O6-methylguanine-DNA methyltransferase, (b) CDKN2A (cyclin-dependent kinase inhibitor 2A) and CDKN2B (cyclin-dependent kinase inhibitor 2B), (c) RB1 (retinoblastoma 1), (d) DAPK1 (death-associated protein kinase 1), and ESR1 (estrogen receptor 1).
7.4.2 MicroRNAs
7.4.2.1 Glioblastoma (WHO Grade IV)
In GBM, most of the microRNAs (miRNAs) surveyed were shown to be overexpressed, and some have been functionally studied, e.g., miR-10b, miR-17, miR-21, miR-93, miR-221, and miR-222. The smaller cluster of downregulated miRNAs includes, among others, miR-7, miR-34a, miR-128, and miR-137. For a detailed update on miRNAs and their specific targets in glioblastoma, the reader is referred to a systematic review [15].
7.4.2.2 Pilocytic Astrocytoma (WHO Grade I)
To date, epigenomic investigations in pilocytic astrocytoma are still sparse. One study on microRNA (miR) expression [16] revealed overexpression of miR-29a, miR-34a, miR-138, miR-299–5p, and miR-432 and underexpression of miR-93, miR-106b, miR-129, miR-135a, and miR-135b.
7.5 Gene Expression
7.5.1 Glioblastoma (WHO Grade IV)
Microarray-based analyses of differentially expressed genes have lately been used to characterize GBM subtypes correlating with patients’ response to treatment and prognosis of survival. Recently, an intensified search for a “consensus” expression profile from several independent GBM data sets produced a limited number of statistically robust marker panels [17,18,19]. Each proposed panel contains only a handful of marker genes which were validated for their potential to support classification of GBM subtypes for predicting clinical outcome.
In summary, the marker genes included in the panels described above are POLD2, CYCS, MYC, AKR1C3, YME1L1, ANXA7, and PDCD4 [17]; EDNRB, CHAF1B, PDLIM4, and HJURP [19]; and AQP1, CHI3L1, EMP3, GPNMB, IGFBP2, LGALS3, OLIG2, PDPN, and RTN1 [18]. Genes associated with a better prognosis of survival were EDNRB, OLIG2, and RTN1. Genes associated with worse prognosis include CHAF1B, PDLIM4, HJURP, AQP1, CHI3L1, EMP3, GPNMB, IGFBP2, LGALS3, and PDPN.
7.5.2 Diffuse Astrocytoma (WHO Grade II)
Several genes differentially expressed in astrocytomas grade II, as compared to controls, were identified in recent years. Upregulated expression was described for CD9; CSPG2, also known as VCAN (versican); NTF3 (neurotrophin 3); EGFR (epidermal growth factor receptor); PDGFRA (platelet-derived growth factor receptor, alpha polypeptide); and TIMP3 (TIMP metallopeptidase inhibitor 3). Downregulated expression was noted for TYRO3 (TYRO3 protein tyrosine kinase).
7.5.3 Oligodendroglioma (WHO Grade II)
Commonly observed in oligodendrogliomas are elevated expression levels of PDGFA (platelet-derived growth factor, alpha polypeptide), PDGFB (platelet-derived growth factor, beta polypeptide), PDGFRA (platelet-derived growth factor receptor, alpha polypeptide), and PDGFRB (platelet-derived growth factor receptor, beta polypeptide).
7.6 Pathogenesis
The pathogenesis of glial tumors is not well understood. Most of the tumors might arise de novo, i.e., astrocytomas (WHO grade II), anaplastic astrocytoma (WHO grade III), glioblastoma (WHO grade IV), pilocytic astrocytoma (WHO grade I), oligodendroglioma (WHO grade II), anaplastic oligodendroglioma (WHO grade III), oligoastrocytoma (WHO grade II), and anaplastic oligoastrocytoma (WHO grade III).
Malignant progression from WHO grade II to WHO grade III occurs in anaplastic astrocytoma, oligodendroglioma, and oligoastrocytoma.
Pilocytic astrocytoma (WHO grade I) derived from piloid cells, i.e., cells similar to those found around chronic lesions of the hypothalamus, cerebellum, spinal cord, or glial stromal cells of the pineal gland. Oligodendroglioma (WHO grade II) arises from oligodendrocytes or other glial precursor cells.
Glioblastoma (WHO grade IV) arises via de novo genesis of highly anaplastic tumor cells (primary glioblastoma) or from anaplastic areas which develop rapidly within astrocytomas, oligodendrogliomas, or ependymomas and overgrow the primary less anaplastic areas (secondary glioblastoma). This pathogenic mechanism becomes obvious when the clinical history is of long duration with several surgical interventions. With progression of the disease, the grade of anaplasia of the tumors removed is also progressing.
7.7 Further Reading
For more detailed information on the different tumor entities, the following articles are suggested: astrocytoma [20, 21], anaplastic astrocytoma [22, 23], glioblastoma [11,12,13, 24,25,26,27,28], pilocytic astrocytoma [29, 30], oligodendroglioma [20, 31,32,33], anaplastic oligodendroglioma WHO grade III [20, 31,32,33], and oligoastrocytoma [20, 32].
References
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, Stroup NE, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15(Suppl 2):ii1–56. doi:10.1093/neuonc/not1151.
Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. doi:10.1093/neuonc/nov1189.
Ostrom QT, Gittleman H, Liao P, Rouse C, Chen Y, Dowling J, Wolinsky Y, Kruchko C, Barnholtz-Sloan J. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol. 2014;16(Suppl 4):iv1–63. doi:10.1093/neuonc/nou1223.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger D, Perry A, Reifenberger G, Von Deimling A. WHO classification of tumours of the central nervous system. Revised 4th ed. Lyon: WHO/IARC; 2016.
Rodriguez FJ, Vizcaino MA, Lin MT. Recent advances on the molecular pathology of glial neoplasms in children and adults. J Mol Diagn. 2016;18(5):620–34. doi:10.1016/j.jmoldx.2016.1005.1005.
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–73. doi:10.1056/NEJMoa0808710.
Watson LA, Goldberg H, Berube NG. Emerging roles of ATRX in cancer. Epigenomics. 2015;7(8):1365–78. doi:10.2217/epi.1315.1382.
Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz Jr LA, Friedman AH, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci U S A. 2013;110(15):6021–6. doi:10.1073/pnas.1303607110.
Koelsche C, Sahm F, Capper D, Reuss D, Sturm D, Jones DT, Kool M, et al. Distribution of TERT promoter mutations in pediatric and adult tumors of the nervous system. Acta Neuropathol. 2013;126(6):907–15. doi:10.1007/s00401-00013-01195-00405.
Jiao Y, Killela PJ, Reitman ZJ, Rasheed AB, Heaphy CM, de Wilde RF, Rodriguez FJ, et al. Frequent ATRX, CIC, FUBP1 and IDH1 mutations refine the classification of malignant gliomas. Oncotarget. 2012;3(7):709–22.
Bleeker FE, Molenaar RJ, Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neurooncol. 2012;108(1):11–27. doi:10.1007/s11060-11011-10793-11060.
Dunn GP, Rinne ML, Wykosky J, Genovese G, Quayle SN, Dunn IF, Agarwalla PK, et al. Emerging insights into the molecular and cellular basis of glioblastoma. Genes Dev. 2012;26(8):756–84. doi:10.1101/gad.187922.187112.
Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, et al. An integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR and NF1. Cancer Cell. 2010;17(1):98.
Lueth M, Wronski L, Giese A, Kirschner-Schwabe R, Pietsch T, von Deimling A, Henze G, Kurtz A, Driever PH. Somatic mitochondrial mutations in pilocytic astrocytoma. Cancer Genet Cytogenet. 2009;192(1):30–5. doi:10.1016/j.cancergencyto.2009.1003.1002.
Moller HG, Rasmussen AP, Andersen HH, Johnsen KB, Henriksen M, Duroux M. A systematic review of microRNA in glioblastoma multiforme: micro-modulators in the mesenchymal mode of migration and invasion. Mol Neurobiol. 2013;47(1):131–44. doi:10.1007/s12035-12012-18349-12037.
Birks DK, Barton VN, Donson AM, Handler MH, Vibhakar R, Foreman NK. Survey of MicroRNA expression in pediatric brain tumors. Pediatr Blood Cancer. 2011;56(2):211–6. doi:10.1002/pbc.22723.
Bredel M, Scholtens DM, Harsh GR, Bredel C, Chandler JP, Renfrow JJ, Yadav AK, et al. A network model of a cooperative genetic landscape in brain tumors. JAMA. 2009;302(3):261–75. doi:10.1001/jama.2009.1997.
Colman H, Zhang L, Sulman EP, McDonald JM, Shooshtari NL, Rivera A, Popoff S, et al. A multigene predictor of outcome in glioblastoma. Neuro Oncol. 2010;12(1):49–57. doi:10.1093/neuonc/nop1007.
de Tayrac M, Aubry M, Saikali S, Etcheverry A, Surbled C, Guenot F, Galibert MD, et al. A 4-gene signature associated with clinical outcome in high-grade gliomas. Clin Cancer Res. 2011;17(2):317–27. doi:10.1158/1078-0432.CCR-1110-1126.
Marko NF, Weil RJ. The molecular biology of WHO Grade II gliomas. Neurosurg Focus. 2013;34(2):E1. doi:10.3171/2012.3112.FOCUS12283.
Ohgaki H, Kleihues P. Genetic profile of astrocytic and oligodendroglial gliomas. Brain Tumor Pathol. 2011;28(3):177–83. doi:10.1007/s10014-10011-10029-10011.
Riemenschneider MJ, Reifenberger G. Astrocytic tumors. In: von Deimling A, editor. Gliomas, Recent results cancer res, vol. 171. Berlin: Springer; 2009. p. 3–24.
Sarkar C, Jain A, Suri V. Current concepts in the pathology and genetics of gliomas. Indian J Cancer. 2009;46(2):108–19.
Gupta K, Salunke P. Molecular markers of glioma: an update on recent progress and perspectives. J Cancer Res Clin Oncol. 2012;138(12):1971–81. doi:10.1007/s00432-00012-01323-y.
Martinez R. Beyond genetics in glioma pathways: the ever-increasing crosstalk between epigenomic and genomic events. J Signal Transduct. 2012;2012:519807.
Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007;170(5):1445–53.
Purow BW, Schiff D. Glioblastoma genetics: in rapid flux. Discov Med. 2010;9(45):125–31.
Westermark B. Glioblastoma--a moving target. Ups J Med Sci. 2012;117(2):251–6. doi:10.3109/03009734.03002012.03676574.
Marko NF, Weil RJ. The molecular biology of WHO grade I astrocytomas. Neuro Oncol. 2012;14(12):1424–31. doi:10.1093/neuonc/nos1257.
Pfister S, Witt O. Pediatric gliomas. In: von Deimling A, editor. Gliomas, Recent results cancer res, vol. 171. Berlin: Springer; 2009. p. 67–81.
Alentorn A, Sanson M, Idbaih A. Oligodendrogliomas: new insights from the genetics and perspectives. Curr Opin Oncol. 2012;24(6):687–93. doi:10.1097/CCO.1090b1013e328357f328354ea.
Harris BT, Hattab EM. Molecular pathology of the central nervous system. In: Cheng L, Eble JN, editors. Molecular surgical pathology. New York, NY: Springer Science+Business Media; 2013. p. 357–405.
Hartmann C, von Deimling A. Molecular pathology of oligodendroglial tumors. In: von Deimling A, editor. Gliomas, Recent results cancer res, vol. 171. Berlin: Springer; 2009. p. 25–49.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Strasser, P., Weis, S. (2017). Molecular Carcinogenesis of Glial Brain Tumors. In: Haybaeck, J. (eds) Mechanisms of Molecular Carcinogenesis – Volume 1. Springer, Cham. https://doi.org/10.1007/978-3-319-53659-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-53659-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53657-6
Online ISBN: 978-3-319-53659-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)