Abstract
The articular cartilage does not heal completely after injury, predisposing patients to accelerated progression of degenerative joint disease. While surgical intervention can address chondral defects and yield positive functional outcomes, substantial research has gone into the use of growth factors to augment cartilage repair and preclude or postpone the need for operative management. This chapter describes the growth factors with the most promising in vitro and in vivo data in cartilage repair, namely, bone morphogenetic protein-7, transforming growth factor-β, fibroblast growth factor-18, connective tissue growth factor, insulin-like growth factor-1, and recent advancements with autologous solutions of growth factors, such as platelet-rich plasma. Each section provides a background on mechanism of action, summarizes pivotal basic science research, and describes the results of clinical application in animal and human models of chondral disease.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Connective Tissue Growth Factor
- Cartilage Repair
- International Knee Documentation Committee
- Osteochondral Defect
- Cartilage Regeneration
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
6.1 Introduction
The optimization of cartilage repair both in the setting of acute, post-traumatic chondral injury and in halting the progression of chronic degenerative disease remains a challenge to clinicians and researchers alike. Cartilage tissue is avascular, aneural, and alymphatic and receives all nutrients from the synovium via diffusion. The articular cartilage therefore does not heal completely after traumatic injury. Instead, spontaneous repair of cartilage produces tissue that fails to integrate with surrounding native cartilage and is inferior in both structure and function. The result is a nonuniform articular joint lining with greater susceptibility to inflammation, further injury, and progression of osteoarthritis (OA). Epidemiological consequences are profound, as knee OA affects between 19–28% of Americans over age 45 (Felson et al. 1987; Jordan et al. 2007). Of this number, 13–18% of patients had an identifiable acute trauma to the joint (Kern 1988), and data shows that post-traumatic arthritis can develop within 10 years of the initial insult (Roos et al. 1995). While arthroplasty addresses end-stage disease, there remains a window for intervention with biologic agents to prevent progression of OA after initial chondral injury. Ideally, such intervention must recreate a cartilage that is thin and compression resistant, has a low coefficient of friction, distributes load, and lasts for many years. To date, the use of recombinant growth factors has yielded appreciable cartilage repair both in vitro and in vivo and holds significant clinical promise for patients suffering from chondral injury.
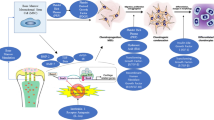
Growth factors are circulating, biologically active polypeptides that stimulate and promote chondrocyte growth and differentiation (Goldring et al. 2006). They drive chondrocyte synthesis of extracellular matrix components, such as proteoglycans, aggrecan, and type II collagen. Aside from anabolic effects, many growth factors also have anti-catabolic effects on cartilage tissue by decreasing expression of local cytokines such as interleukin-1 (IL-1) and matrix metalloproteinases (MMPs) (Fortier et al. 2011; Pascual-Garrido and Chubinskaya 2015; Chubinskaya et al. 2011). Growth factors do not function in a vacuum, but rather have complex signaling interactions with other mediators. As described by Giannoudis, an accurate model for cartilage repair also takes into account chondrocyte interactions with the synovial environment and host bone, all of which are affected by mechanical forces and circulating cytokines (Fig. 6.1) (Giannoudis et al. 2008). For example, the presence of inflammatory cytokines (IL-1) and oxidative stress has recently been shown to decrease chondrocyte responsiveness to growth factor augmentation (Elshaier et al. 2009; Loeser et al. 2014). Thus, maximizing the chondroprotective capacity of growth factors necessitates understanding not only the expression of growth factors in healthy and in diseased cartilage but also their complex interactions with the surrounding synovial environment. The goal of this chapter is to familiarize the reader with growth factors showing the greatest clinical promise in cartilage repair and to provide an update on evidence supporting their use in chondral disease.
6.2 Bone Morphogenetic Proteins
Of all growth factors, bone morphogenetic proteins (BMPs) have the most robust evidence from both in vitro and in vivo studies supporting their use for cartilage regeneration and repair. BMPs are members of the transforming growth factor-β (TGF-β) superfamily that have wide-ranging biological activities, including the regulation of cellular proliferation, apoptosis, differentiation and migration, embryonic development, and the maintenance of tissue homeostasis during adult life (Goumans and Mummery 2000; Massague and Chen 2000; Itoh et al. 2000). In chondrocytes, BMPs stimulate cartilage synthesis and decrease activity of catabolic cytokines, such as IL-1, IL-6, IL-8, MMP-1, and MMP-13 (Badlani et al. 2009; Elshaier et al. 2009; Chubinskaya et al. 2007b, 2008; Im et al. 2003). The ability of BMPs to induce an anabolic response in cartilage in vitro has been documented using different BMPs in multiple species, including human, bovine, rat, rabbit, and mouse, and a variety of culture conditions.
Though the BMP family has several growth factors, most data to date explores the use of BMP-7, also known as osteogenic protein 1 (OP-1). In vitro augmentation of both human and animal chondrocytes with BMP-7 has yielded increased production of cartilage-specific extracellular proteins, such as collagens type II and VI, aggrecan, decorin, fibronectin, and hyaluronan [HA] via upregulation of enzymes such as hyaluronan synthase (Nishida et al. 2000b; Chubinskaya et al. 2007a). When applied to other cell types in the knee, BMP-7 has been shown to increase ECM synthesis in synovial and bone marrow-derived MSCs, both alone and in combination with TGF-β (Miyamoto et al. 2007; Shen et al. 2010). This profound anabolic response stems from BMP-7 regulatory properties as a modulator of other growth factors, such as insulin-like growth factor-1 and fibroblast growth factor, as well as their receptors, kinases involved in signaling, inhibitory binding proteins, and downstream transcription factors (Chubinskaya et al. 2011). Furthermore, BMP has been shown to restore tissue responsiveness to IGF-1 (Chubinskaya et al. 2007a). BMP-7 also downregulates catabolic mediators (IL-1, IL-6, IL-8, IL-11, and tumor necrosis factor [TNF]-α) and inhibits both baseline and cytokine-induced expression of MMP-1 and MMP-13 (Im et al. 2003). Lastly, it modulates expression of receptors for certain matrix components, such as CD44 (Nishida et al. 2000b), and the synthesis of chondrocyte cytoskeleton proteins, such as talin, paxillin, and focal adhesion kinase (Vinall et al. 2002), bolstering the cartilage scaffold and strengthening newly formed tissue. While several growth factors have shown decreased efficacy with aged or diseased chondrocytes, BMP-7 induces an anabolic response across a variety of age groups and different stages of OA (Chubinskaya et al. 2007b). Despite its anabolic capacity, BMP-7 has not been shown to induce chondrocyte hypertrophy or other changes in chondrocytic phenotype, nor have BMP-7-treated animal knees displayed any histological evidence of uncontrolled fibroblast proliferation or radiographically detectable osteophyte formation (Fortier et al. 2011).
Animal studies have provided substantial evidence for the use of BMP-7 in vivo. A study of New Zealand white rabbits with femoral condyle defects showed extensive regeneration of both subchondral bone and a hyaline-like cartilage layer when treated with BMP-7 compared to fibrocartilage tissue fill-in defects left empty or treated with collagen only (Grgic et al. 1997). In a sheep study, continuous presence of BMP-7 led to markedly improved gross and microscopic cartilage healing of focal condylar and trochlear defects, suggesting the growth factor attracted mesenchymal-like cells originating from the synovium into the defect area (Jelic et al. 2001). In addition to applications of recombinant BMPs, rabbit studies evaluating the local application of a BMP gene to periosteal-derived allogenic mesenchymal stem cells via a retroviral vector showed complete or near-complete bone and cartilage regeneration of osteochondral defects at 8 and 12 weeks (Mason et al. 2000). In a rabbit model of ACL tears, BMP-7 injections promoted significantly improved tissue healing and prevented progression of OA compared to placebo injection (Hayashi et al. 2008). BMP-7 has also been tested in conjunction with existing cartilage restoration surgery, improving gross and histological outcomes with mosaicplasty (osteochondral autograft) (Shimmin et al. 2003) and with microfracture (Kuo et al. 2006). Though BMP-7 appears effective in several different treatment models, the timing of BMP-7 application after joint injury affects its efficacy. In a model of post-traumatic osteochondral defects, Hurtig and colleagues showed increased cartilage healing and chondroprotective effects of BMP-7 augmentation both immediately and at 3 weeks after injury, but this effect was diminished at 12 weeks after injury (Fig. 6.2) (Hurtig et al. 2009). This finding suggests that a window of opportunity exists shortly after cartilage injury where growth factor intervention used as a disease-modifying agent may prevent progression of post-traumatic OA. At later time points post-injury, the natural history of disease may become more challenging to overcome with growth factor therapy alone.
Timing of the effect of BMP-7 after impact injury in sheep. Growth factor applicationeither immediately (a) or within 1 month (b) of traumatic chondral injury yields excellent cartilage repair on safranin O histology. Delayed growth factor application after 4 months (c) yields suboptimal cartilage repair, suggesting that there is a window for growth factor augmentation of cartilage repair shortly after chondral injury
Human trials of intra-articular BMP-7 injection have been underway over the past half-decade. In 2010, Hunter and colleagues published results of a phase I randomized controlled trial of various dosing regiments of BMP-7 injections in patients over 40 years old with symptomatic knee OA (Hunter et al. 2010). Despite higher rates of injection at the site of pain, there were no differences in toxicity or adverse events between BMP-7 and placebo. Furthermore, patients receiving BMP-7 injections at midrange dose reported a symptomatic improvement and anti-pain effects, though this was not the primary objective of the study. Unfortunately, a phase II clinical OA study did not appear to be successful; however, the reasons for such outcome have yet to be determined.
6.3 Transforming Growth Factor-β
TGF-β is a cytokine secreted by many cell types; it plays crucial roles in cell proliferation, differentiation, development, apoptosis, tissue homeostasis, and the immune system. Signaling occurs primarily through the SMAD pathway, which involves a heterotetrameric complex that acts as a transcription factor via phosphorylation cascades. Among its three common isoforms, TGF-β1 has been mostly studied in chondrogenesis. TGF-β1 has been shown to stimulate chondrocyte synthetic activity and to decrease the catabolic activity of interleukin (IL)-1 and tumor necrosis factor (TNF)-α (Lotz et al. 1995; Lires-Dean et al. 2008). TGF-β1 helps to maintain chondrocyte characteristics during in vitro culture by promoting cell proliferation and extracellular matrix (ECM) protein synthesis and through inhibition of MMPs to protect normal morphology (Blaney Davidson et al. 2007). Furthermore, TGF-β1 stimulates chondrogenesis of synovial lining cells and of bone marrow-derived mesenchymal stem cells (Fan et al. 2010; Kurth et al. 2007). Studies have demonstrated significant enhancement of cartilage repair with TGF-β1 application in scaffolds applied to defects (Abe et al. 2003; Diao et al. 2009) and in human mesenchymal stem cells transfected with TGF-β1 genes via an adenovirus (Qi et al. 2013; Lee et al. 2001).
As with BMP-7, cartilage regeneration efforts utilizing TGF-β have moved forward into human trials over the past few years. Springing from research by Noh and colleagues on the ability of genetically engineered chondrocytes virally transduced with TGF-β1 (GEC-TGF-β1) to repair articular cartilage defects in animals, TissueGene-C has emerged as a leading growth factor in human trials for knee OA (Noh et al. 2010). The safety and biologic activity of injectable GEC-TGF-β1 were evaluated in a 2012 phase I trial by Ha and colleagues in patients with advanced knee OA (Ha et al. 2012). There were no severe adverse effects related to the GEC-TGF-β1 treatment reported, and the most common adverse effect was effusion. Ten of twelve patients showed improvements in clinical scores at 6 months and improvements in range of motion and pain up to 1 year. Since then, two phase II trials have investigated the efficacy and outcomes of intra-articular injectable GEC-TGF-β1 for knee OA. Cherian and colleagues recently conducted a prospective, multicenter, double-blinded, placebo-controlled, randomized study of GEC-TGF-β1 in the knees of patients with grade 3 OA and showed improved responses on the International Knee Documentation Committee (IKDC) score and Visual Analogue Scale (VAS) at 1-year follow-up and decreased need for analgesics (Cherian et al. 2015). Similarly, Lee and colleagues recently published results of another phase II trial of GEC-TGF-β1 injections into the knees of patients with OA yielding improved IKDC and VAS pain scores at 6 months follow-up (Lee et al. 2015). Given such positive results this year, GEC-TGF-β1 appears to hold significant promise as an injection therapy for moderate knee OA. Further, longer follow-up and phase III trials are needed to better define appropriate indications and dosing regimens for patients with chondral disease.
6.4 Fibroblast Growth Factors
The fibroblast growth factor (FGF) family plays important roles in human embryonic development, cell growth, morphogenesis, tissue repair, tumor growth, and invasion. FGFs are heparin-binding proteins and interact with heparan sulfate proteoglycans on the cell surface for signal transduction (Friedl et al. 1997). In total, 22 members of the FGF family have been identified in humans. The FGF receptor family has four members: FGFR1, FGFR2, FGFR3, and FGFR4. Human articular chondrocytes express all four receptors but contain significantly higher concentrations of FGFR1 and FGFR3 compared with FGFR2 and FGFR4 (Yan et al. 2011). FGFs are important regulators of cartilage development and homeostasis (Ellman et al. 2008). Three members of FGF family, FGF-2, FGF-8, and FGF-18, have been investigated for their role in cartilage homeostasis.
The role of FGF-2 in the production of the ECM in cartilage is controversial. FGF-2 was initially suggested to stimulate a robust cartilage repair response (Henson et al. 2005). However, strong mitogenic effects of FGF-2 lead to clustering of chondrocytes and uneven production of ECM due to low levels of collagen type II (Ellman et al. 2008). Furthermore, FGF-2 has been shown to suppress aggrecan and type II collagen and to promote the expression of aggrecanase and TNF-α receptors (Ellman et al. 2008; Im et al. 2008). FGF-2 also inhibits the stimulatory effect of bone morphogenetic protein-7 (BMP-7) and insulin-like growth factor-1 (IGF-1) on chondrocyte proteoglycan synthesis (Loeser et al. 2003, 2005). Ultimately, these findings have precluded FGF-2 from further consideration as a biological treatment in OA (Ellman et al. 2013).
While FGF-2 is mostly known for its pro-catabolic responses in cartilage, FGF-18 has repeatedly been shown to exert strong anabolic effects in chondrocytes and chondroprogenitor cells, leading to enhanced chondrogenic cell differentiation and type II collagen production (Ellsworth et al. 2002; Moore et al. 2005; Bradley et al. 2015). FGF-18 signaling through FGFR3 promotes chondrocyte proliferation at early embryonic stages. When development is complete, signaling through the same receptor works to suppress chondrocyte proliferation and prevent hypertrophic differentiation (Liu et al. 2002, 2007). The ability of FGF-18 to promote chondrocyte proliferation and the production of type II collagen and proteoglycan has been shown in vitro using adenovirus-mediated transfer of FGF-18 into the pinnae of nude mice (Ellsworth et al. 2002). Similarly, Barr et al. demonstrated an anabolic in vitro effect of recombinant human FGF-18 (rhFGF-18) on damaged cartilage, as rhFGF-18 increased aggrecan synthesis and reduced collagen breakdown in response to damage (Barr et al. 2014). In vivo studies began in rats, with Moore and colleagues investigating the utility of FGF-18 injections in a rat meniscal tear model of OA (Moore et al. 2005). Intra-articular injection of FGF-18 provided substantial cartilage production and reduced cartilage degeneration in OA. In an ovine model of chondral defects, augmentation of microfracture surgery with intra-articular injection of rhFGF-18 improved quality and quantity of repair tissue in chondral defects (Power et al. 2014).
Over the last decade, FGF-18 injection trials have commenced in humans (Fig. 6.3). Two phase II trials investigating the use of FGF-18 in patients with focal chondral defects were closed due to low enrollment. However, a randomized, double-blind, placebo-controlled, proof-of-concept trial recently investigated the efficacy and safety of recombinant human FGF-18 (sprifermin) in the treatment of symptomatic knee OA (Lohmander et al. 2014). There were no reports of local or systemic safety problems associated with any dose of sprifermin, and systemic levels of sprifermin were below detectable levels. Sprifermin was associated with statistically significant, dose-dependent reductions in loss of both total and lateral femorotibial cartilage thickness and volume on quantitative MRI, as well as reductions in radiographic joint space narrowing in the lateral femorotibial compartment. There was no significant relationship between treatment group and reduction in central medial femorotibial compartment cartilage thickness as measured by quantitative MRI. However, while all groups had improved WOMAC pain scores, patients receiving the 100 μg dose of sprifermin had significantly less improvement at 12 months compared with those receiving placebo. Despite this, positive imaging results and lack of toxicity in this phase I study suggest that FGF-18 may be a promising therapy for OA.
6.5 Connective Tissue Growth Factor
Connective tissue growth factor (CTGF, also known as CCN2) is an extracellular protein of the CCN family. Together, this group comprises extracellular matrix-associated heparin-binding proteins that play an important role in the regulation of cellular proliferation, migration, adhesion, survival, differentiation, and synthesis of extracellular matrix proteins. Among these members, CTGF has been studied widely in chondrogenesis. CTGF plays a crucial role in the development of skeletal tissues, namely, by driving condensation of mesenchymal cells and regulating chondrocyte proliferation and differentiation (Nishida et al. 2000a; Song et al. 2007). CTGF promotes proliferation and cartilage matrix formation in the growth plate of the cartilage (Nakanishi et al. 2000) but has not been shown to cause hypertrophy or calcification in articular cartilage chondrocytes (Nishida et al. 2002).
In vivo studies exploring the use of CTGF in animal models of OA have emerged over the past decade. Such studies have further illustrated its role in promoting chondrocyte proliferation and differentiation during chondrogenesis, as CTGF knockout mice exhibited an expanded growth plate hypertrophic zone and impaired endochondral ossification (Ivkovic et al. 2003; Kawaki et al. 2008). Conversely, Tomita and colleagues demonstrated that overexpression of CTGF accelerated the process of endochondral ossification by promoting the proliferation and differentiation of growth plate chondrocytes (Tomita et al. 2013). Furthermore, cartilage-specific overexpression of CTGF in transgenic mouse studies slowed progression of age-related osteoarthritic changes in articular cartilage (Itoh et al. 2013). When applied to a full-thickness cartilage defect model, Nishida and colleagues showed that local administration of recombinant CTGF with gelatin hydrogel stimulated cartilage repair in a rat model (Nishida et al. 2004). Similarly, bone marrow mesenchymal stem cells transfected with CTGF recently provided hyaline-like cartilage regeneration similar to normal cartilage in a rabbit model of focal articular cartilage defects (Zhu et al. 2014). Such studies suggest a critical role for CTGF in both the protection and regeneration of articular cartilage. Though further testing is needed to clarify the safety and efficacy of this growth factor in cartilage disease, CTGF remains a promising therapeutic target for cartilage regeneration.
6.6 Insulin-Like Growth Factor-1
Insulin-like growth factor-1 (IGF-1) is a growth factor that is essential in embryonic tissue development and for growth and maintenance of mass throughout all stages of human life. Similar to BMP-7, IGF-1 has been shown to have robust anabolic and anti-catabolic effects. IGF-1 is required to maintain integrity of healthy articular cartilage, as rats with IGF-1 deficiency develop greater articular osteoarthritic cartilage degeneration in the knee than controls (Ekenstedt et al. 2006). The role of IGF-1 in healing of cartilage defects has best been demonstrated in a horse model, both by supplementing chondrocytes with IGF-1 (Fortier et al. 2002) and by genetically modifying chondrocytes with an IGF-1-encoded adenovirus (Goodrich et al. 2007). Unlike BMP-7, however, human chondrocytes have demonstrated a decreased response to IGF-1 with increasing age (Loeser et al. 2000, 2002) and with advanced OA (Schalkwijk et al. 1989). IGF-1 also appears to be more affective as a driver of synthetic function and less effective at preventing catabolism in advanced disease (Morales 2008). As such, there have not been any trials to date in humans with IGF-1 for the treatment of OA or focal chondral defects.
6.7 Platelet-Rich Plasma
While most studies to date have focused on individual growth factors, the use of combined autologous solutions of multiple growth factors, namely, in the form of platelet-rich plasma (PRP), has gained enormous popularity in the past decade. PRP is defined as plasma with a minimum twofold increase in platelet concentration above baseline levels or greater than 1.1 × 106 platelets/μL (Miller et al. 2007). Its use stems from the well-defined role of platelets in wound healing, initially through clot formation with fibrin to close wounds and ending with the release of factors involved in angiogenesis, inflammation, and the immune response (Nurden et al. 2008). Densely packed α-granules in platelets release a multitude of growth factors, including FGF, TGF-β, platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF), that modulate inflammation, attract local stem cells and fibroblasts, and trigger a regulated proliferative healing response (Smyth et al. 2009). In vitro studies of PRP on cartilage have yielded extremely positive results, as stem cells buffered in PRP had increased proliferation and chondrogenic differentiation (Mishra et al. 2009). Akeda and colleagues showed that porcine chondrocytes cultured in PRP displayed increased DNA content and proteoglycan and collagen type II synthesis (Akeda et al. 2006). Importantly, PRP did not affect the types of proteoglycans or collagen produced, suggesting that chondrocytes remain phenotypically stable when enriched with PRP. When compared to hyaluronic acid in co-culture with osteoarthritic chondrocytes, PRP-enriched samples resulted in a significant reduction in MMP expression, increased hyaluronan (HA) synthase expression by synoviocytes, and increased aggrecan production (Sundman et al. 2014). Aside from aiding in tissue healing, this data suggests that PRP may play a chondroprotective role as well (for details see Chap. 7).
With such overwhelmingly positive in vitro results, animal and human studies utilizing PRP have progressed rapidly. In rabbits, PRP-treated osteochondral defects showed greater cartilage regeneration and production of ECM than placebo (Sun et al. 2010). Early human knee injection trials with PRP showed improved pain and patient-reported outcome scores when compared with HA (Kon et al. 2010). In 2012, Sanchez and colleagues conducted a randomized, controlled trial comparing PRP to HA and achieved the same results at 6 months post-injection (Sanchez et al. 2012). In longer follow-up studies (12 months), symptomatic improvements in patients with knee OA have been also documented (Gobbi et al. 2012). Multiple randomized controlled trials have since shown benefits of PRP compared to other available therapies (Cerza et al. 2012). Furthermore, PRP has not been limited to the knee, as injections were shown to be effective in osteochondral lesions of the talus compared to HA (Mei-Dan et al. 2012). Unfortunately, latest studies have yielded mixed results, as a randomized controlled trial by Filardo and colleagues found no difference in outcomes between HA and PRP (Filardo et al. 2015). This finding is in agreement with an earlier study by the same group showing no superiority of PRP over HA (Filardo et al. 2012). However, a recent systematic review of PRP injections in the knee found that intra-articular injections are overall a viable therapy for patients with mild OA (Campbell et al. 2015). This group found that PRP injections carry a slightly higher risk of local adverse reactions after multiple injections and work best in patients with early degenerative disease. Ultimately, more trials are needed to determine the proper patient cohort, dosing strategy, and injection frequency of PRP.
Conclusion
In summary, tremendous progress has been made in harnessing the potential of growth factors for cartilage repair, both in regard to halting deterioration following post-traumatic focal defects and in preserving cartilage from long-term degenerative changes. Of the individual growth factors, BMP-7, TGF-β, and FGF-18 appear to have the most short-term promise for future clinical studies. However, it has become increasingly clear that complete cartilage repair will not stem from the addition of a single growth factor, but rather a combination of growth factors (Fortier et al. 2011). This has been shown by the fact that BMP-7 produces better cartilage repair when applied in combination with TGF-β or IGF-1 than it does on its own (Loeser et al. 2003; Chubinskaya et al. 2007a) and by in vitro evidence that IGF-1, TGF-β, and FGF-2 regulate each other’s gene expression and subsequent protein production (Shi 2009). Thus, it comes as no surprise that combinations of growth factors, such as those in platelet-rich plasma, may yield better and more sustainable clinical outcomes in patients with focal cartilage defects or OA, especially at early stages of disease.
Several unanswered questions remain in regard to the use of growth factors for cartilage repair. Aside from which growth factors produce optimal regeneration, it is unclear whether clinicians and researchers should strive to boost endogenous growth factor production or augment with recombinant, exogenous growth factors. Future short-term goals include obtaining a better understanding of the pathophysiology of cartilage degeneration so that growth factor therapy can be tailored to various stages of the healing process. Optimal doses and formulations must be determined in order to maximize clinical response and minimize side effects. Growth factors must be studied further in hostile, inflammatory environments to better understand their efficacy in disease states. This will likely underscore a difference in potential therapy for post-traumatic chondral defects versus therapy for chronic degenerative joint disease. Relationships between industry and academia must be fostered transparently, as previous clinical trials have come to a halt after suboptimal results. Ultimately, the solution will likely involve partnerships with regulatory agencies to move new technologies forward efficiently. Future clinical trials must be conducted with carefully selected patient cohorts. Lastly, costs of therapies will have to be reduced so that these may become financially feasible options for patients.
References
Abe T, Yamada H, Nakajima H, Kikuchi T, Takaishi H, Tadakuma T, Fujikawa K, Toyama Y (2003) Repair of full-thickness cartilage defects using liposomal transforming growth factor-beta1. J Orthop Sci 8(1):92–101. doi:10.1007/s007760300016
Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJ, Lenz ME, Sah RL, Masuda K (2006) Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthritis Cartilage 14(12):1272–1280. doi:10.1016/j.joca.2006.05.008
Badlani N, Oshima Y, Healey R, Coutts R, Amiel D (2009) Use of bone morphogenetic protein-7 as a treatment for osteoarthritis. Clin Orthop Relat Res 467(12):3221–3229. doi:10.1007/s11999-008-0569-9
Barr L, Getgood A, Guehring H, Rushton N, Henson FM (2014) The effect of recombinant human fibroblast growth factor-18 on articular cartilage following single impact load. J Orthop Res 32(7):923–927. doi:10.1002/jor.22622
Blaney Davidson EN, van der Kraan PM, van den Berg WB (2007) TGF-beta and osteoarthritis. Osteoarthritis Cartilage 15(6):597–604. doi:10.1016/j.joca.2007.02.005
Bradley EW, Carpio LR, Newton AC, Westendorf JJ (2015) Deletion of the PH-domain and Leucine-rich repeat protein phosphatase 1 (Phlpp1) increases fibroblast growth factor (Fgf) 18 expression and promotes chondrocyte proliferation. J Biol Chem 290(26):16272–16280. doi:10.1074/jbc.M114.612937
Campbell KA, Saltzman BM, Mascarenhas R, Khair MM, Verma NN, Bach BR Jr, Cole BJ (2015) Does intra-articular platelet-rich plasma injection provide clinically superior outcomes compared with other therapies in the treatment of knee Osteoarthritis? A systematic review of overlapping meta-analyses. Arthroscopy. doi:10.1016/j.arthro.2015.03.041
Cerza F, Carni S, Carcangiu A, Di Vavo I, Schiavilla V, Pecora A, De Biasi G, Ciuffreda M (2012) Comparison between hyaluronic acid and platelet-rich plasma, intra-articular infiltration in the treatment of gonarthrosis. Am J Sports Med 40(12):2822–2827. doi:10.1177/0363546512461902
Cherian JJ, Parvizi J, Bramlet D, Lee KH, Romness DW, Mont MA (2015) Preliminary results of a phase II randomized study to determine the efficacy and safety of genetically engineered allogeneic human chondrocytes expressing TGF-beta1 in patients with grade 3 chronic degenerative joint disease of the knee. Osteoarthritis Cartilage 23(12):2109–2118. doi:10.1016/j.joca.2015.06.019
Chubinskaya S, Hakimiyan A, Pacione C, Yanke A, Rappoport L, Aigner T, Rueger DC, Loeser RF (2007a) Synergistic effect of IGF-1 and OP-1 on matrix formation by normal and OA chondrocytes cultured in alginate beads. Osteoarthritis Cartilage 15(4):421–430. doi:10.1016/j.joca.2006.10.004
Chubinskaya S, Hurtig M, Rueger DC (2007b) OP-1/BMP-7 in cartilage repair. Int Orthop 31(6):773–781. doi:10.1007/s00264-007-0423-9
Chubinskaya S, Segalite D, Pikovsky D, Hakimiyan AA, Rueger DC (2008) Effects induced by BMPS in cultures of human articular chondrocytes: comparative studies. Growth Factors 26(5):275–283. doi:10.1080/08977190802291733
Chubinskaya S, Otten L, Soeder S, Borgia JA, Aigner T, Rueger DC, Loeser RF (2011) Regulation of chondrocyte gene expression by osteogenic protein-1. Arthritis Res Ther 13(2):R55. doi:10.1186/ar3300
Diao H, Wang J, Shen C, Xia S, Guo T, Dong L, Zhang C, Chen J, Zhao J, Zhang J (2009) Improved cartilage regeneration utilizing mesenchymal stem cells in TGF-beta1 gene-activated scaffolds. Tissue Eng Part A 15(9):2687–2698. doi:10.1089/ten.TEA.2008.0621
Ekenstedt KJ, Sonntag WE, Loeser RF, Lindgren BR, Carlson CS (2006) Effects of chronic growth hormone and insulin-like growth factor 1 deficiency on osteoarthritis severity in rat knee joints. Arthritis Rheum 54(12):3850–3858. doi:10.1002/art.22254
Ellman MB, An HS, Muddasani P, Im HJ (2008) Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene 420(1):82–89. doi:10.1016/j.gene.2008.04.019
Ellman MB, Yan D, Ahmadinia K, Chen D, An HS, Im HJ (2013) Fibroblast growth factor control of cartilage homeostasis. J Cell Biochem 114(4):735–742. doi:10.1002/jcb.24418
Ellsworth JL, Berry J, Bukowski T, Claus J, Feldhaus A, Holderman S, Holdren MS, Lum KD, Moore EE, Raymond F, Ren H, Shea P, Sprecher C, Storey H, Thompson DL, Waggie K, Yao L, Fernandes RJ, Eyre DR, Hughes SD (2002) Fibroblast growth factor-18 is a trophic factor for mature chondrocytes and their progenitors. Osteoarthritis Cartilage 10(4):308–320. doi:10.1053/joca.2002.0514
Elshaier AM, Hakimiyan AA, Rappoport L, Rueger DC, Chubinskaya S (2009) Effect of interleukin-1beta on osteogenic protein 1-induced signaling in adult human articular chondrocytes. Arthritis Rheum 60(1):143–154. doi:10.1002/art.24151
Fan J, Gong Y, Ren L, Varshney RR, Cai D, Wang DA (2010) In vitro engineered cartilage using synovium-derived mesenchymal stem cells with injectable gellan hydrogels. Acta Biomater 6(3):1178–1185. doi:10.1016/j.actbio.2009.08.042
Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF (1987) The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum 30(8):914–918
Filardo G, Kon E, Di Martino A, Di Matteo B, Merli ML, Cenacchi A, Fornasari PM, Marcacci M (2012) Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet Disord 13:229. doi:10.1186/1471-2474-13-229
Filardo G, Di Matteo B, Di Martino A, Merli ML, Cenacchi A, Fornasari P, Marcacci M, Kon E (2015) Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med 43(7):1575–1582. doi:10.1177/0363546515582027
Fortier LA, Mohammed HO, Lust G, Nixon AJ (2002) Insulin-like growth factor-I enhances cell-based repair of articular cartilage. J Bone Joint Surg Br 84(2):276–288
Fortier LA, Barker JU, Strauss EJ, McCarrel TM, Cole BJ (2011) The role of growth factors in cartilage repair. Clin Orthop Relat Res 469(10):2706–2715. doi:10.1007/s11999-011-1857-3
Friedl A, Chang Z, Tierney A, Rapraeger AC (1997) Differential binding of fibroblast growth factor-2 and -7 to basement membrane heparan sulfate: comparison of normal and abnormal human tissues. Am J Pathol 150(4):1443–1455
Giannoudis PV, Einhorn TA, Schmidmaier G, Marsh D (2008) The diamond concept--open questions. Injury 39(Suppl 2):S5–S8. doi:10.1016/s0020-1383(08)70010-x
Gobbi A, Karnatzikos G, Mahajan V, Malchira S (2012) Platelet-rich plasma treatment in symptomatic patients with knee osteoarthritis: preliminary results in a group of active patients. Sports Health 4(2):162–172. doi:10.1177/1941738111431801
Goldring MB, Tsuchimochi K, Ijiri K (2006) The control of chondrogenesis. J Cell Biochem 97(1):33–44. doi:10.1002/jcb.20652
Goodrich LR, Hidaka C, Robbins PD, Evans CH, Nixon AJ (2007) Genetic modification of chondrocytes with insulin-like growth factor-1 enhances cartilage healing in an equine model. J Bone Joint Surg Br 89(5):672–685. doi:10.1302/0301-620x.89b5.18343
Goumans MJ, Mummery C (2000) Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol 44(3):253–265
Grgic M, Jelic M, Basic V, Basic N, Pecina M, Vukicevic S (1997) Regeneration of articular cartilage defects in rabbits by osteogenic protein-1 (bone morphogenetic protein-7). Acta Med Croatica 51(1):23–27
Ha CW, Noh MJ, Choi KB, Lee KH (2012) Initial phase I safety of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 in degenerative arthritis patients. Cytotherapy 14(2):247–256. doi:10.3109/14653249.2011.629645
Hayashi M, Muneta T, Ju YJ, Mochizuki T, Sekiya I (2008) Weekly intra-articular injections of bone morphogenetic protein-7 inhibits osteoarthritis progression. Arthritis Res Ther 10(5):R118. doi:10.1186/ar2521
Henson FM, Bowe EA, Davies ME (2005) Promotion of the intrinsic damage-repair response in articular cartilage by fibroblastic growth factor-2. Osteoarthritis Cartilage 13(6):537–544. doi:10.1016/j.joca.2005.02.007
Hunter DJ, Pike MC, Jonas BL, Kissin E, Krop J, McAlindon T (2010) Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet Disord 11:232. doi:10.1186/1471-2474-11-232
Hurtig M, Chubinskaya S, Dickey J, Rueger D (2009) BMP-7 protects against progression of cartilage degeneration after impact injury. J Orthop Res 27(5):602–611. doi:10.1002/jor.20787
Im HJ, Pacione C, Chubinskaya S, Van Wijnen AJ, Sun Y, Loeser RF (2003) Inhibitory effects of insulin-like growth factor-1 and osteogenic protein-1 on fibronectin fragment- and interleukin-1beta-stimulated matrix metalloproteinase-13 expression in human chondrocytes. J Biol Chem 278(28):25386–25394. doi:10.1074/jbc.M302048200
Im HJ, Li X, Muddasani P, Kim GH, Davis F, Rangan J, Forsyth CB, Ellman M, Thonar EJ (2008) Basic fibroblast growth factor accelerates matrix degradation via a neuro-endocrine pathway in human adult articular chondrocytes. J Cell Physiol 215(2):452–463. doi:10.1002/jcp.21317
Itoh S, Itoh F, Goumans MJ, Ten Dijke P (2000) Signaling of transforming growth factor-beta family members through Smad proteins. Eur J Biochem 267(24):6954–6967
Itoh S, Hattori T, Tomita N, Aoyama E, Yutani Y, Yamashiro T, Takigawa M (2013) CCN family member 2/connective tissue growth factor (CCN2/CTGF) has anti-aging effects that protect articular cartilage from age-related degenerative changes. PLoS One 8(8):e71156. doi:10.1371/journal.pone.0071156
Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM (2003) Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development 130(12):2779–2791
Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Maticic D, McCartney J, Yin S, Rueger D, Vukicevic S (2001) Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors 19(2):101–113
Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, Fang F, Schwartz TA, Abbate LM, Callahan LF, Kalsbeek WD, Hochberg MC (2007) Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol 34(1):172–180
Kawaki H, Kubota S, Suzuki A, Lazar N, Yamada T, Matsumura T, Ohgawara T, Maeda T, Perbal B, Lyons KM, Takigawa M (2008) Cooperative regulation of chondrocyte differentiation by CCN2 and CCN3 shown by a comprehensive analysis of the CCN family proteins in cartilage. J Bone Miner Res 23(11):1751–1764. doi:10.1359/jbmr.080615
Kon E, Buda R, Filardo G, Di Martino A, Timoncini A, Cenacchi A, Fornasari PM, Giannini S, Marcacci M (2010) Platelet-rich plasma: intra-articular knee injections produced favorable results on degenerative cartilage lesions. Knee Surg Sports Traumatol Arthrosc 18(4):472–479. doi:10.1007/s00167-009-0940-8
Kern D, Zlatkin MB, Dalinka MK (1988) Occupational and post-traumatic arthritis. Radiol Clin North Am 26(6):1349–1358
Kuo AC, Rodrigo JJ, Reddi AH, Curtiss S, Grotkopp E, Chiu M (2006) Microfracture and bone morphogenetic protein 7 (BMP-7) synergistically stimulate articular cartilage repair. Osteoarthritis Cartilage 14(11):1126–1135. doi:10.1016/j.joca.2006.04.004
Kurth T, Hedbom E, Shintani N, Sugimoto M, Chen FH, Haspl M, Martinovic S, Hunziker EB (2007) Chondrogenic potential of human synovial mesenchymal stem cells in alginate. Osteoarthritis Cartilage 15(10):1178–1189. doi:10.1016/j.joca.2007.03.015
Lee KH, Song SU, Hwang TS, Yi Y, Oh IS, Lee JY, Choi KB, Choi MS, Kim SJ (2001) Regeneration of hyaline cartilage by cell-mediated gene therapy using transforming growth factor beta 1-producing fibroblasts. Hum Gene Ther 12(14):1805–1813. doi:10.1089/104303401750476294
Lee MC, Ha CW, Elmallah RK, Cherian JJ, Cho JJ, Kim TW, Bin SI, Mont MA (2015) A placebo-controlled randomised trial to assess the effect of TGF-ss1-expressing chondrocytes in patients with arthritis of the knee. Bone Joint J 97-B(7):924–932. doi:10.1302/0301-620x.97b7.35852
Lires-Dean M, Carames B, Cillero-Pastor B, Galdo F, Lopez-Armada MJ, Blanco FJ (2008) Anti-apoptotic effect of transforming growth factor-beta1 on human articular chondrocytes: role of protein phosphatase 2A. Osteoarthritis Cartilage 16(11):1370–1378. doi:10.1016/j.joca.2008.04.001
Liu Z, Xu J, Colvin JS, Ornitz DM (2002) Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev 16(7):859–869. doi:10.1101/gad.965602
Liu Z, Lavine KJ, Hung IH, Ornitz DM (2007) FGF18 is required for early chondrocyte proliferation, hypertrophy and vascular invasion of the growth plate. Dev Biol 302(1):80–91. doi:10.1016/j.ydbio.2006.08.071
Loeser RF, Shanker G, Carlson CS, Gardin JF, Shelton BJ, Sonntag WE (2000) Reduction in the chondrocyte response to insulin-like growth factor 1 in aging and osteoarthritis: studies in a non-human primate model of naturally occurring disease. Arthritis Rheum 43(9):2110–2120. doi:10.1002/1529-0131(200009)43:9<2110::aid-anr23>3.0.co;2-u
Loeser RF, Carlson CS, Del Carlo M, Cole A (2002) Detection of nitrotyrosine in aging and osteoarthritic cartilage: correlation of oxidative damage with the presence of interleukin-1beta and with chondrocyte resistance to insulin-like growth factor 1. Arthritis Rheum 46(9):2349–2357. doi:10.1002/art.10496
Loeser RF, Pacione CA, Chubinskaya S (2003) The combination of insulin-like growth factor 1 and osteogenic protein 1 promotes increased survival of and matrix synthesis by normal and osteoarthritic human articular chondrocytes. Arthritis Rheum 48(8):2188–2196. doi:10.1002/art.11209
Loeser RF, Chubinskaya S, Pacione C, Im HJ (2005) Basic fibroblast growth factor inhibits the anabolic activity of insulin-like growth factor 1 and osteogenic protein 1 in adult human articular chondrocytes. Arthritis Rheum 52(12):3910–3917. doi:10.1002/art.21472
Loeser RF, Gandhi U, Long DL, Yin W, Chubinskaya S (2014) Aging and oxidative stress reduce the response of human articular chondrocytes to insulin-like growth factor 1 and osteogenic protein 1. Arthritis Rheumatol 66(8):2201–2209. doi:10.1002/art.38641
Lohmander LS, Hellot S, Dreher D, Krantz EF, Kruger DS, Guermazi A, Eckstein F (2014) Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 66(7):1820–1831. doi:10.1002/art.38614
Lotz M, Rosen F, McCabe G, Quach J, Blanco F, Dudler J, Solan J, Goding J, Seegmiller JE, Terkeltaub R (1995) Interleukin 1 beta suppresses transforming growth factor-induced inorganic pyrophosphate (PPi) production and expression of the PPi-generating enzyme PC-1 in human chondrocytes. Proc Natl Acad Sci U S A 92(22):10364–10368
Mason JM, Breitbart AS, Barcia M, Porti D, Pergolizzi RG, Grande DA (2000) Cartilage and bone regeneration using gene-enhanced tissue engineering. Clin Orthop Relat Res (379 Suppl):S171–S178
Massague J, Chen YG (2000) Controlling TGF-beta signaling. Genes Dev 14(6):627–644
Mei-Dan O, Carmont MR, Laver L, Mann G, Maffulli N, Nyska M (2012) Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med 40(3):534–541. doi:10.1177/0363546511431238
Miller Y, Bachowski G, Benjamin R, Eklund D, Hibbard A, Lightfoot T (2007) Practice guidelines for blood transfusion: a compilation from recent peer-reviewed literature, vol 56, 2nd edn. American Red Cross, Washington
Mishra A, Tummala P, King A, Lee B, Kraus M, Tse V, Jacobs CR (2009) Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C Methods 15(3):431–435. doi:10.1089/ten.tec.2008.0534
Miyamoto C, Matsumoto T, Sakimura K, Shindo H (2007) Osteogenic protein-1 with transforming growth factor-beta1: potent inducer of chondrogenesis of synovial mesenchymal stem cells in vitro. J Orthop Sci 12(6):555–561. doi:10.1007/s00776-007-1176-4
Moore EE, Bendele AM, Thompson DL, Littau A, Waggie KS, Reardon B, Ellsworth JL (2005) Fibroblast growth factor-18 stimulates chondrogenesis and cartilage repair in a rat model of injury-induced osteoarthritis. Osteoarthritis Cartilage 13(7):623–631. doi:10.1016/j.joca.2005.03.003
Morales TI (2008) The quantitative and functional relation between insulin-like growth factor-I (IGF) and IGF-binding proteins during human osteoarthritis. J Orthop Res 26(4):465–474. doi:10.1002/jor.20549
Nakanishi T, Nishida T, Shimo T, Kobayashi K, Kubo T, Tamatani T, Tezuka K, Takigawa M (2000) Effects of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture. Endocrinology 141(1):264–273. doi:10.1210/endo.141.1.7267
Nishida T, Nakanishi T, Asano M, Shimo T, Takigawa M (2000a) Effects of CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, on the proliferation and differentiation of osteoblastic cells in vitro. J Cell Physiol 184(2):197–206. doi:10.1002/1097-4652(200008)184:2<197::aid-jcp7>3.0.co;2-r
Nishida Y, Knudson CB, Eger W, Kuettner KE, Knudson W (2000b) Osteogenic protein 1 stimulates cells-associated matrix assembly by normal human articular chondrocytes: up-regulation of hyaluronan synthase, CD44, and aggrecan. Arthritis Rheum 43(1):206–214. doi:10.1002/1529-0131(200001)43:1<206::aid-anr25>3.0.co;2-1
Nishida T, Kubota S, Nakanishi T, Kuboki T, Yosimichi G, Kondo S, Takigawa M (2002) CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, stimulates proliferation and differentiation, but not hypertrophy of cultured articular chondrocytes. J Cell Physiol 192(1):55–63. doi:10.1002/jcp.10113
Nishida T, Kubota S, Kojima S, Kuboki T, Nakao K, Kushibiki T, Tabata Y, Takigawa M (2004) Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor). J Bone Miner Res 19(8):1308–1319. doi:10.1359/jbmr.040322
Noh MJ, Copeland RO, Yi Y, Choi KB, Meschter C, Hwang S, Lim CL, Yip V, Hyun JP, Lee HY, Lee KH (2010) Pre-clinical studies of retrovirally transduced human chondrocytes expressing transforming growth factor-beta-1 (TG-C). Cytotherapy 12(3):384–393. doi:10.3109/14653240903470639
Nurden AT, Nurden P, Sanchez M, Andia I, Anitua E (2008) Platelets and wound healing. Front Biosci 13:3532–3548
Pascual-Garrido C, Chubinskaya S (2015) Potential targets for pharmacologic therapies. In: Olson S, Guilak F (eds) Post-traumatic arthritis: pathogenesis, diagnosis, and management. Springer, pp 331–342
Power J, Hernandez P, Guehring H, Getgood A, Henson F (2014) Intra-articular injection of rhFGF-18 improves the healing in microfracture treated chondral defects in an ovine model. J Orthop Res 32(5):669–676. doi:10.1002/jor.22580
Qi BW, Yu AX, Zhu SB, Zhou M, Wu G (2013) Chitosan/poly(vinyl alcohol) hydrogel combined with Ad-hTGF-beta1 transfected mesenchymal stem cells to repair rabbit articular cartilage defects. Exp Biol Med (Maywood) 238(1):23–30. doi:10.1258/ebm.2012.012223
Roos H, Adalberth T, Dahlberg L, Lohmander LS (1995) Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage 3(4):261–267
Sanchez M, Fiz N, Azofra J, Usabiaga J, Aduriz Recalde E, Garcia Gutierrez A, Albillos J, Garate R, Aguirre JJ, Padilla S, Orive G, Anitua E (2012) A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthroscopy 28(8):1070–1078. doi:10.1016/j.arthro.2012.05.011
Schalkwijk J, Joosten LA, van den Berg WB, van de Putte LB (1989) Chondrocyte nonresponsiveness to insulin-like growth factor 1 in experimental arthritis. Arthritis Rheum 32(7):894–900
Shen B, Wei A, Whittaker S, Williams LA, Tao H, Ma DD, Diwan AD (2010) The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J Cell Biochem 109(2):406–416. doi:10.1002/jcb.22412
Shi S, Mercer S, Eckert GJ, Trippel SB. Growth factor regulation of growth factors in articular chondrocytes. J Biol Chem 2009;284:6697–6704
Shimmin A, Young D, O’Leary S, Shih MS, Rueger DC, Walsh WR (2003) Growth factor augmentation of an ovine mosaicplasty model. Trans ICRS 4th Symposium, no. 16, Toronto
Smyth SS, McEver RP, Weyrich AS, Morrell CN, Hoffman MR, Arepally GM, French PA, Dauerman HL, Becker RC (2009) Platelet functions beyond hemostasis. J Thromb Haemost 7(11):1759–1766. doi:10.1111/j.1538-7836.2009.03586.x
Song JJ, Aswad R, Kanaan RA, Rico MC, Owen TA, Barbe MF, Safadi FF, Popoff SN (2007) Connective tissue growth factor (CTGF) acts as a downstream mediator of TGF-beta1 to induce mesenchymal cell condensation. J Cell Physiol 210(2):398–410. doi:10.1002/jcp.20850
Sun Y, Feng Y, Zhang CQ, Chen SB, Cheng XG (2010) The regenerative effect of platelet-rich plasma on healing in large osteochondral defects. Int Orthop 34(4):589–597. doi:10.1007/s00264-009-0793-2
Sundman EA, Cole BJ, Karas V, Della Valle C, Tetreault MW, Mohammed HO, Fortier LA (2014) The anti-inflammatory and matrix restorative mechanisms of platelet-rich plasma in osteoarthritis. Am J Sports Med 42(1):35–41. doi:10.1177/0363546513507766
Tomita N, Hattori T, Itoh S, Aoyama E, Yao M, Yamashiro T, Takigawa M (2013) Cartilage-specific over-expression of CCN family member 2/connective tissue growth factor (CCN2/CTGF) stimulates insulin-like growth factor expression and bone growth. PLoS One 8(3):e59226. doi:10.1371/journal.pone.0059226
Vinall RL, Lo SH, Reddi AH (2002) Regulation of articular chondrocyte phenotype by bone morphogenetic protein 7, interleukin 1, and cellular context is dependent on the cytoskeleton. Exp Cell Res 272(1):32–44. doi:10.1006/excr.2001.5395
Yan D, Chen D, Cool SM, van Wijnen AJ, Mikecz K, Murphy G, Im HJ (2011) Fibroblast growth factor receptor 1 is principally responsible for fibroblast growth factor 2-induced catabolic activities in human articular chondrocytes. Arthritis Res Ther 13(4):R130. doi:10.1186/ar3441
Zhu S, Zhang B, Man C, Ma Y, Liu X, Hu J (2014) Combined effects of connective tissue growth factor-modified bone marrow-derived mesenchymal stem cells and NaOH-treated PLGA scaffolds on the repair of articular cartilage defect in rabbits. Cell Transplant 23(6):715–727. doi:10.3727/096368913x669770
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Meyer, M.A., Urita, A., Cole, B.J., Chubinskaya, S. (2017). Growth Factors in Cartilage Repair. In: Grässel, S., Aszódi, A. (eds) Cartilage. Springer, Cham. https://doi.org/10.1007/978-3-319-53316-2_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-53316-2_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53314-8
Online ISBN: 978-3-319-53316-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)