Abstract
Articular cartilage defects cause pain and progression to osteoarthritis, and there remains a critical need for safe and cost-effective interventions. Historically available treatments for articular cartilage defects are limited in success, in part due to the native biology of the cartilage, which has limited healing potential, as well as due to the inability to create a favorable signaling and cell environment. The evolution of cartilage treatment has been rapid, with emerging options for treatment and repair. Recent advances in platelet-rich plasma (PRP) have demonstrated efficacy in growth factor delivery as well as symptom modulation. Furthermore, cell-based approaches such as autologous chondrocyte implantation (ACI) and stem/stromal cell therapies have ushered in a new era of treatments which can be responsive to and interact with the local intra-articular environment. Finally, the technovolution of this field has led to the development of single-stage combination autologous/allogeneic treatments which have the potential to overcome logistical and recovery limitations of two-stage treatments while providing trophic factors, signaling orchestration, and autologous cells for defect repair.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cartilage
- Repair
- Platelet-rich plasma
- Stem/stromal cell
- Autologous chondrocyte implantation
- Single-stage cartilage repair
24.1 Introduction
Articular cartilage defects cause pain and progression to osteoarthritis (OA), and there exists a critical need for safe and cost-effective interventions. These defects have limited healing potential secondary to the poor regenerative capacity and the avascular nature of cartilage. As a result, chondral lesions can be a source of pain and mechanical symptoms as well as a risk factor for post-traumatic osteoarthritis. Focal cartilage defects impair quality of life in a similar fashion to severe osteoarthritis, causing long-term dysfunction and deterioration of the entire joint [1].
Historical treatment strategies for articular cartilage defects have been limited in success. Whereas palliative treatment options offer limited and short-term symptom relief, articular cartilage restoration has demonstrated effectiveness in reducing pain and functional disability. A variety of surgical options are available to treat cartilage lesions, and these include microfracture, osteochondral allograft transplantation (OCA), and autologous chondrocyte implantation (ACI). Microfracture is the most commonly performed method of cartilage restoration by marrow stimulation for small defects. This technique relies on the influx of marrow products (stem cells, growth factors, and platelets) to form a fibrin clot, which is slowly remodeled into a fibrocartilage scar. This technique has limited outcomes and usefulness due to poor performance in large defects and inferior long-term outcomes compared to more advanced treatment methods. Osteochondral allograft transplantation demonstrates clinically favorable long-term outcomes, with published revision-free survival of 66–69% at 20 years of follow-up [2]. However, the use of OCA remains limited by the fact that these grafts are obtained from young deceased donors, leading to logistical scheduling challenges and lack of scalability of this efficacious resource. While efforts are underway to optimize OCA and expand graft quality availability, autologous approaches remain attractive, given the potential to restore joint biology and repair native tissues [3, 4].
Successful cartilage repair requires an abundance of cells, growth factors, and intricate modulation of the cellular regenerative process. PRP has demonstrated trophic and anti-inflammatory properties utilizing in vitro models [5]. Additionally, PRP has shown favorable outcomes in the treatment of knee osteoarthritis, with the potential to be used synergistically with other surgical products to enhance the healing environment.
Cell-based strategies such as autologous chondrocyte implantation (ACI) have demonstrated better durability over microfracture, due to formation of hyaline-like cartilage over fibrocartilage [6]. However, there are disadvantages of ACI, including costly and logistically challenging need for two-stage surgery with ex vivo expansion of the chondrocytes.
Human mesenchymal stem/stromal cells (MSCs) can also be used to improve cartilage regeneration models. The use of MSCs in cartilage repair is promising, with both small and large animal models as well as pilot studies in man demonstrating safety and efficacy in cartilage regeneration [7, 8].
Finally, the combination of chondrocytes with other cell types has gained recent attention given that cells respond to their environment and can be positively influenced by the presence of other cell types [9, 10]. The combination of cells opens the door to single-stage cartilage repair as both orchestrating [stromal] cells and chondrocyte building blocks can be provided simultaneously, without the need for ex vivo expansion.
24.2 Key Concepts
-
Evolving use of Platelet-Rich Plasma for Cartilage Treatment
-
Emergence and growth of cell-based therapies:
-
Autologous Chondrocyte Implantation
-
Stem/Stromal Cell-Based Therapeutics
-
Single-Stage Auto/Allo Cartilage Repair
-
24.2.1 Platelet-Rich Plasma for Cartilage Treatment
Platelet-rich plasma (PRP) has received significant attention in recent years as a potential treatment for knee osteochondral defects and osteoarthritis. PRP contains growth factors, modulating local inflammatory responses as well as cellular proliferation and differentiation involved in healing processes [5].
The literature has demonstrated that PRP studies are quite varied with regard to their processing, composition, timing, and indications. A recent systematic review showed that only 10%, or 11/105 studies, provided a comprehensive report with clear description of preparation in composition of the PRP investigated [11]. Furthermore, PRP varies significantly with regard to its platelet, growth factor, and leukocyte composition from patient to patient [11]. Recent efforts have shown that leukocyte composition may be a key factor in the treatment efficacy of knee osteoarthritis [12]. There does appear to be a delicate balance with leukocytes and platelets, as each has important catabolic properties, but, in excess have the ability to upregulate certain proteins such as matrix metalloproteinases, leading to detrimental changes to the surrounding tissues.
Favorable outcomes of intra-articular injections of PRP when compared to saline [13], corticosteroids [14], and hyaluronic acid (HA) [15] have been reported in several blinded, randomized controlled trials (RCT). Other RCTs have demonstrated significant improvement but similar results between PRP and HA [16, 17]. In vitro studies and early clinical observations have also shown a potential synergistic interaction between PRP and HA [18, 19].
The utilization of PRP as an augmentation in the treatment of chondral defects is currently evolving. PRP as an augmentation to microfracture in the treatment of small chondral lesions may provide additional benefit at short-term follow-up, even up to 12 months [20], but did not reach the minimally clinically important difference (MCID) in a recent meta-analysis [21]. While the use of PRP for the treatment of knee cartilage defects and osteoarthritis is becoming increasing popular, its implementation and outcomes remain under scientific debate, in particular due to its heterogeneous nature and preparation. Furthermore, PRP provides a one-time dose of factors which does not have the capacity to for long-term modulation and feedback regulation-inhibition. Given this, emerging treatment options for cartilage repair increasingly involve cell-based therapies that open the door for sustained, modulated healing and regeneration.
24.2.2 Autologous Chondrocyte Implantation
Cell-based strategies have demonstrated better durability over microfracture, due to the formation of hyaline-like cartilage over fibrocartilage [6]. Indeed, a growing body of evidence suggests that microfracture does no better than debridement alone [22, 23]. Given this, we increasingly recommend consideration of debridement for small chondral defects, to better preserve the subchondral plate, should future ACI or other biologic therapy be warranted. Furthermore, this approach has been postulated to limit the occurrence of intralesional osteophyte formation or subchondral plate fracture as well as allow for cell-based biologic intervention without having to treat the full-depth osteochondral unit, such as with OCA.
In several RCTs, we have demonstrated cell-based ACI has superior clinical outcomes and better structure repair compared to scar formation after microfracture [24,25,26,27]. Technically, ACI requires precise debridement to stable defect edges as well as close matching of defect geography to the implanted membrane. For this, we prefer to use a cookie cutter technique in order to provide efficient operative workflow, precisely cut defect edges, and a form-fitting ACI membrane [28].
It is important to note that there are several disadvantages of ACI, including the need for two-stage surgery with ex vivo expansion of the chondrocytes. This delays the final rehabilitation of the patients, and in some cases makes quadriceps atrophy and deconditioning of the affected extremity worse over time. In addition, this procedure is very costly, and is continuously challenged by payers.
24.2.3 Stem/Stromal Cell-Based Therapeutics
Stem/stromal cells represent a population of cells that demonstrate the ability for self-renewal, long-term viability, and multilinear culture [29]. Embryonically, mesenchymal stem/stromal cells (MSCs) are derived from the mesoderm and are distinguished by their capacity to divide into connective tissues including ligament, bone, and cartilage leading to evolving interest in their therapeutic use for orthopedic care [29, 30].
Stem/stromal cell preparations exist in varying formulations spanning from point-of-care aspirates to culture-expanded and characterized cell populations. Classic stem/stromal investigations in musculoskeletal repair were centered initially about bone marrow mesenchymal stem/stromal cells (BMSCs) [31]. While bone marrow is relatively enriched in MSCs as compared to other adult tissues, we caution efforts to employ bone marrow aspirate as a robust source of stem/stromal cells given that MSCs comprise only 0.01–0.001% of the harvested cell population [30, 32]. In contrast, the stromal vascular fraction (SVT) of adipose tissue contains approximately 500-fold the stem/stromal cell concentration of bone marrow [33, 34].
Adipose-derived mesenchymal stem/stromal cells (AMSCs) have also demonstrated growing interest and promise in regenerative therapeutics including cartilage repair. AMSCs differ from BMSCs in the relative ease of adipose isolation, both in clinic and in the OR, as well as the quantity of tissue that can be readily harvested in most patients depending on habitus. AMSCs have been demonstrated to differentiate into fibrocytes and tenocytes in addition to adipogenic, myogenic, and chondrogenic tissues and are therefore a natural target for tendon repair/regeneration studies [35,36,37]. In a recent RNA sequencing analysis of AMSCs and BMSCs obtained from the same human donors, Zhou et al. found that AMSCs demonstrated lower expression of Human Leukocyte Antigen I (HLA I) as well as higher immunosuppression capacity when compared with the BMSC population [38]. This is desirable given that limitations in HLA effect can enable allogeneic stem cell application, easing logical preparations, especially as they relate to culture-expanded formulations [39, 40]. Furthermore, the immunomodulatory effect of stem cells may also play a key role in ligament healing given that multiple groups have proposed and reported on the positive histologic effects and recreation of native-like tendon-bone interfaces with immunosuppression and macrophage inhibition [41,42,43].
24.2.4 Single-Stage Auto/Allo Cartilage Repair
Finally, the combination of chondrocytes with other cell types has also gained attention as others showed that cells respond to their environment and can be positively influenced by the presence of other cell types [9, 10]. Indeed, direct contact between MSCs and dedifferentiated articular chondrocytes recently showed improvement of the cartilage phenotype of dedifferentiated articular chondrocytes [44, 45]. Therefore, combining articular chondrocytes with other cell types can help us overcome barriers and improve the traditional ACI-approach.
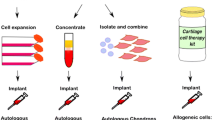
In their first-in-man trial, de Windt et al. demonstrated that one-stage application of allogeneic BMSCs mixed with 10–20% defect-derived autologous chondrons resulted in significant improvements in Knee Injury and Osteoarthritis Outcome Score (KOOS) as well as visual analog scale (VAS) which was durable at 18 months of follow-up [39, 46]. Furthermore, MRI demonstrated complete defect filling as well as integration with host tissue, while 32 s-look arthroscopies with tissue biopsy demonstrated that the regenerate contained only autologous DNA, supporting that MSCs provide a transient orchestrating effect whereas autologous cells are needed for defect healing.
Recently, our team has initiated an analogous trial under US Clinical trial NCT03672825. Preliminary results using allogeneic AMSCs mixed with defect-derived autologous chondrocytes demonstrate no significant adverse events and satisfactory outcomes at 3–18 months of follow-up. Formal results of this 25 patient Phase I Clinical Trial are forthcoming.
24.3 Conclusions
Cartilage defects substantially affect patient quality of life, and there remains a critical need for safe and cost-effective interventions. The recent technovolution of cartilage treatment has been rapid, with newly emerging options for repair. Methods of PRP preparation are increasingly nuanced and demonstrate promise in growth factor delivery and use as an adjuvant to advanced biologic therapies. Cell-based approaches represent the latest in emerging cartilage repair options. The latest in the evolutionary line of cell-based therapies is represented by single-stage combination autologous/allogeneic treatments which increasingly address and overcome the logistical challenges of two-stage treatments while providing the signal orchestration and autologous cells needed for defect repair.
References
Heir S, Nerhus TK, Rotterud JH, Loken S, Ekeland A, Engebretsen L, et al. Focal cartilage defects in the knee impair quality of life as much as severe osteoarthritis: a comparison of knee injury and osteoarthritis outcome score in 4 patient categories scheduled for knee surgery. Am J Sports Med. 2010;38(2):231–7.
Familiari F, Cinque ME, Chahla J, Godin JA, Olesen ML, Moatshe G, et al. Clinical outcomes and failure rates of osteochondral allograft transplantation in the knee: a systematic review. Am J Sports Med. 2017; https://doi.org/10.1177/0363546517732531.
Denbeigh JM, Hevesi M, Paggi CA, Resch ZT, Bagheri L, Mara K, et al. Modernizing storage conditions for fresh osteochondral allografts by optimizing viability at physiologic temperatures and conditions. Cartilage. 2019; https://doi.org/10.1177/1947603519888798.
Hevesi M, Denbeigh JM, Paggi CA, Galeano-Garces C, Bagheri L, Larson AN, et al. Fresh osteochondral allograft transplantation in the knee: a viability and histologic analysis for optimizing graft viability and expanding existing standard processed graft resources using a living donor cartilage program. Cartilage. 2019; https://doi.org/10.1177/1947603519880330.
Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M. Platelet-rich plasma: why intra-articular? A systematic review of preclinical studies and clinical evidence on PRP for joint degeneration. Knee Surg Sports Traumatol Arthrosc. 2015;23(9):2459–74.
Pareek A, Carey JL, Reardon PJ, Peterson L, Stuart MJ, Krych AJ. Long-term outcomes after autologous chondrocyte implantation: a systematic review at mean follow-up of 11.4 years. Cartilage. 2016;7(4):298–308.
Mokbel A, El-Tookhy O, Shamaa AA, Sabry D, Rashed L, Mostafa A. Homing and efficacy of intra-articular injection of autologous mesenchymal stem cells in experimental chondral defects in dogs. Clin Exp Rheumatol. 2011;29(2):275–84.
Marquass B, Schulz R, Hepp P, Zscharnack M, Aigner T, Schmidt S, et al. Matrix-associated implantation of predifferentiated mesenchymal stem cells versus articular chondrocytes: in vivo results of cartilage repair after 1 year. Am J Sports Med. 2011;39(7):1401–12.
Fischer J, Dickhut A, Rickert M, Richter W. Human articular chondrocytes secrete parathyroid hormone-related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010;62(9):2696–706.
Hwang NS, Varghese S, Puleo C, Zhang Z, Elisseeff J. Morphogenetic signals from chondrocytes promote chondrogenic and osteogenic differentiation of mesenchymal stem cells. J Cell Physiol. 2007;212(2):281–4.
Chahla J, Cinque ME, Piuzzi NS, Mannava S, Geeslin AG, Murray IR, et al. A call for standardization in platelet-rich plasma preparation protocols and composition reporting: a systematic review of the clinical orthopaedic literature. J Bone Joint Surg Am. 2017;99(20):1769–79.
Riboh JC, Saltzman BM, Yanke AB, Fortier L, Cole BJ. Effect of leukocyte concentration on the efficacy of platelet-rich plasma in the treatment of knee osteoarthritis. Am J Sports Med. 2016;44(3):792–800.
Smith PA. Intra-articular autologous conditioned plasma injections provide safe and efficacious treatment for knee osteoarthritis: an FDA-sanctioned, randomized, double-blind, placebo-controlled clinical trial. Am J Sports Med. 2016;44(4):884–91.
Forogh B, Mianehsaz E, Shoaee S, Ahadi T, Raissi GR, Sajadi S. Effect of single injection of platelet-rich plasma in comparison with corticosteroid on knee osteoarthritis: a double-blind randomized clinical trial. J Sports Med Phys Fitness. 2016;56(7–8):901–8.
Dai WL, Zhou AG, Zhang H, Zhang J. Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy. 2017;33(3):659–70, e1.
Filardo G, Di Matteo B, Di Martino A, Merli ML, Cenacchi A, Fornasari P, et al. Platelet-rich plasma intra-articular knee injections show no superiority versus viscosupplementation: a randomized controlled trial. Am J Sports Med. 2015;43(7):1575–82.
Di Martino A, Di Matteo B, Papio T, Tentoni F, Selleri F, Cenacchi A, et al. Platelet-rich plasma versus hyaluronic acid injections for the treatment of knee osteoarthritis: results at 5 years of a double-blind, randomized controlled trial. Am J Sports Med. 2019;47(2):347–54.
Chen WH, Lo WC, Hsu WC, Wei HJ, Liu HY, Lee CH, et al. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials. 2014;35(36):9599–607.
Saturveithan C, Premganesh G, Fakhrizzaki S, Mahathir M, Karuna K, Rauf K, et al. Intra-articular hyaluronic acid (HA) and platelet rich plasma (PRP) injection versus hyaluronic acid (HA) injection alone in patients with grade III and IV knee osteoarthritis (OA): a retrospective study on functional outcome. Malays Orthop J. 2016;10(2):35–40.
Manunta AF, Manconi A. The treatment of chondral lesions of the knee with the microfracture technique and platelet-rich plasma. Joints. 2013;1(4):167–70.
Boffa A, Previtali D, Altamura SA, Zaffagnini S, Candrian C, Filardo G. Platelet-rich plasma augmentation to microfracture provides a limited benefit for the treatment of cartilage lesions: a meta-analysis. Orthop J Sports Med. 2020;8(4):2325967120910504.
Hevesi M, Bernard C, Hartigan DE, Levy BA, Domb BG, Krych AJ. Is microfracture necessary? Acetabular chondrolabral debridement/abrasion demonstrates similar outcomes and survival to microfracture in hip arthroscopy: a multicenter analysis. Am J Sports Med. 2019;47(7):1670–8.
Gudas R, Gudaite A, Mickevicius T, Masiulis N, Simonaityte R, Cekanauskas E, et al. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: a prospective study with a 3-year follow-up. Arthroscopy. 2013;29(1):89–97.
Saris D, Price A, Widuchowski W, Bertrand-Marchand M, Caron J, Drogset JO, et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am J Sports Med. 2014;42(6):1384–94.
Saris DB, Vanlauwe J, Victor J, Almqvist KF, Verdonk R, Bellemans J, et al. Treatment of symptomatic cartilage defects of the knee: characterized chondrocyte implantation results in better clinical outcome at 36 months in a randomized trial compared to microfracture. Am J Sports Med. 2009;37(Suppl 1):10s–9s.
Saris DB, Vanlauwe J, Victor J, Haspl M, Bohnsack M, Fortems Y, et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36(2):235–46.
Brittberg M, Recker D, Ilgenfritz J, Saris DBF. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: five-year follow-up of a prospective randomized trial. Am J Sports Med. 2018;46:1343–51.
Hevesi M, Krych AJ, Saris DBF. Treatment of cartilage defects with the matrix-induced autologous chondrocyte implantation cookie cutter technique. Arthrosc Tech. 2019;8(6):e591–e6.
Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95.
Sullivan MO, Gordon-Evans WJ, Fredericks LP, Kiefer K, Conzemius MG, Griffon DJ. Comparison of mesenchymal stem cell surface markers from bone marrow aspirates and adipose stromal vascular fraction sites. Front Vet Sci. 2016;2:82.
Bianco P. “Mesenchymal” stem cells. Annu Rev Cell Dev Biol. 2014;30:677–704.
Oedayrajsingh-Varma MJ, van Ham SM, Knippenberg M, Helder MN, Klein-Nulend J, Schouten TE, et al. Adipose tissue-derived mesenchymal stem cell yield and growth characteristics are affected by the tissue-harvesting procedure. Cytotherapy. 2006;8(2):166–77.
Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150–4.
Tremolada C, Colombo V, Ventura C. Adipose tissue and mesenchymal stem cells: state of the art and Lipogems® technology development. Curr Stem Cell Rep. 2016;2(3):304–12.
Norelli JB, Plaza DP, Stal DN, Varghese AM, Liang H, Grande DA. Tenogenically differentiated adipose-derived stem cells are effective in Achilles tendon repair in vivo. J Tissue Eng. 2018;9:2041731418811183.
Torres-Torrillas M, Rubio M, Damia E, Cuervo B, Del Romero A, Peláez P, et al. Adipose-derived mesenchymal stem cells: a promising tool in the treatment of musculoskeletal diseases. Int J Mol Sci. 2019;20(12):3105.
Hevesi M, LaPrade M, Saris DBF, Krych AJ. Stem cell treatment for ligament repair and reconstruction. Curr Rev Musculoskelet Med. 2019;12(4):446–50.
Zhou W, Lin J, Zhao K, Jin K, He Q, Hu Y, et al. Single-cell profiles and clinically useful properties of human mesenchymal stem cells of adipose and bone marrow origin. Am J Sports Med. 2019;47(7):1722–33.
de Windt TS, Vonk LA, Slaper-Cortenbach IC, van den Broek MP, Nizak R, van Rijen MH, et al. Allogeneic mesenchymal stem cells stimulate cartilage regeneration and are safe for single-stage cartilage repair in humans upon mixture with recycled autologous chondrons. Stem Cells. 2017;35(1):256–64.
Dalle JH, Balduzzi A, Bader P, Lankester A, Yaniv I, Wachowiak J, et al. Allogeneic stem cell transplantation from HLA-mismatched donors for pediatric patients with acute lymphoblastic leukemia treated according to the 2003 BFM and 2007 International BFM studies: impact of disease risk on outcomes. Biol Blood Marrow Transplant. 2018;24(9):1848–55.
Hays PL, Kawamura S, Deng XH, Dagher E, Mithoefer K, Ying L, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90(3):565–79.
Hu J, Yao B, Yang X, Ma F. The immunosuppressive effect of Siglecs on tendon-bone healing after ACL reconstruction. Med Hypotheses. 2015;84(1):38–9.
Kawamura S, Ying L, Kim HJ, Dynybil C, Rodeo SA. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23(6):1425–32.
Chen WH, Lai MT, Wu AT, Wu CC, Gelovani JG, Lin CT, et al. In vitro stage-specific chondrogenesis of mesenchymal stem cells committed to chondrocytes. Arthritis Rheum. 2009;60(2):450–9.
Tsuchiya K, Chen G, Ushida T, Matsuno T, Tateishi T. The effect of coculture of chondrocytes with mesenchymal stem cells on their cartilaginous phenotype in vitro. Mater Sci Eng C. 2004;24(3):391–6.
de Windt TS, Vonk LA, Slaper-Cortenbach ICM, Nizak R, van Rijen MHP, Saris DBF. Allogeneic MSCs and recycled autologous chondrons mixed in a one-stage cartilage cell transplantion: a first-in-man trial in 35 patients. Stem Cells. 2017;35(8):1984–93.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 ISAKOS
About this chapter
Cite this chapter
Hevesi, M., Kruckeberg, B.M., Krych, A.J., Saris, D.B.F. (2021). Emerging Cartilage Repair Options. In: Krych, A.J., Biant, L.C., Gomoll, A.H., Espregueira-Mendes, J., Gobbi, A., Nakamura, N. (eds) Cartilage Injury of the Knee. Springer, Cham. https://doi.org/10.1007/978-3-030-78051-7_24
Download citation
DOI: https://doi.org/10.1007/978-3-030-78051-7_24
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-78050-0
Online ISBN: 978-3-030-78051-7
eBook Packages: MedicineMedicine (R0)