Abstract
Osteoarthritis and rheumatoid arthritis are the two most common diseases of joints causing cartilage and bone destruction leading to the loss of joint function. Causal therapies are still challenging, and to date various cell biological methods applying chondrocytes or mesenchymal stem cells only generate fibrocartilaginous repair tissue with less good mechano-biological properties.
In osteoarthritis and rheumatoid arthritis, a specific cell type with stem cell characteristics including migratory activity, clonogenicity, and multipotency could be characterized, which we named “chondrogenic progenitor cells.” These cells, involved in regeneration processes, are largely unsuccessful mainly because they produce collagen type I. Manipulation of these progenitor cells, which are already present in diseased cartilage tissue, could be a promising approach for cartilage repair. Several chondrogenic pathways and interacting partners have been already identified. The transcription factors Runx2 and Sox9 play an important role by influencing the collagen II production. Interleukins and TGF-β might also play an important role in the regulation of Runx2. Furthermore, it could be shown that various other factors like mechanical stimulation or components of the pericellular matrix prompt chondrogenic progenitor cells to trigger chondrogenesis. The differentiation potential of chondrogenic progenitor cells seems to be affected by calcium homeostasis, including calcium regulatory mechanisms. However, several challenges remain regarding the elucidation of the regulatory pathways that determine chondrogenic progenitor cells to become more chondrogenic.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
3.1 Osteoarthritis and Rheumatoid Arthritis
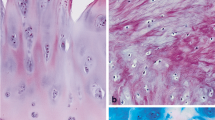
Osteoarthritis (Fig. 3.1) is a whole-organ disease, also affecting the synovium, subchondral bone, and meniscus (Goldring and Otero 2011; Englund et al. 2012; Goldring and Goldring 2010). As a degenerative joint disease, it shows a progressive loss of the articular cartilage (Loeser et al. 2012). The synovium is responsible for producing inflammation mediators, which can also be found in osteoarthritis (Bougault et al. 2012). Modified cell-matrix interactions result in destroyed tissue integrity and primarily affect the hyaline cartilage. However, this also applies to other tissues, especially the subchondral bone. An eburnation of the subchondral bone can arise (Goldring and Otero 2011; Goldring and Goldring 2010). An abnormal remodeling of the subchondral bone often leads to a thicker, but mechanically less stable, tissue (Lories and Luyten 2011). Osteoarthritis often originates from meniscal lesions (Englund et al. 2012). Even when meniscal substitutes, such as allografts or bioengineered substitutes, are used, there is no protective effect against the development of osteoarthritis (Hommen et al. 2007). No markers have been discovered to diagnose early stages of osteoarthritis. At present, no therapy to treat the causes of osteoarthritis is available that could promise a complete cure (Lohmander and Roos 2007; Lohmander et al. 2014; Musumeci et al. 2014). In the late stages of refractory osteoarthritis, knee replacement is the gold standard of treatment (Johnson and Hunter 2014).
(a) Immunohistochemistry for biglycan in healthy human articular cartilage of the knee joint. (b) Histology of late-stage OA, please note the numerous tidemark duplications and the deep surface fissures. (c) Fibrocartilaginous repair tissue, where the chondrogenic progenitor cells are found. (d) Breaks in the tidemark with mesenchymal tissue entering the cartilage tissue
Various cellular mechanisms cause rheumatoid arthritis and result in cartilage and bone destruction. Inflammation plays an important role in the disease process. The inflamed synovium produces a tumor-like pannus tissue, which destroys the cartilage tissue. Proinflammatory cytokines, such as IL-1β, IL-6, TNF-α, and matrix metalloproteinases, are produced by fibroblast-like synoviocytes and synovial macrophages (Karouzakis et al. 2006). The RANKL-dependent induction of osteoclasts is primarily responsible for bone destruction (Kim et al. 2014). In the synovium in rheumatoid arthritis, CD4+ T cells accumulate (Franz et al. 1998; Blaschke et al. 2003; Toh and Miossec 2007).
3.2 Regeneration Attempts in Cartilage Tissue
Chondrocytes are the only cell source found in healthy articular cartilage (Muir 1995; Kock et al. 2012). These produce collagen type II, which together with aggrecan is mainly responsible for the high mechanical resilience of articular cartilage tissue. Cartilage has a very low potential for intrinsic self-repair and regeneration in its mature tissue, because chondrocytes are believed to have no capacity for migration, proliferation, and repair (Tew et al. 2001; Johnstone et al. 2013; Redman et al. 2005).
Most surgical therapies aim to stimulate cells from the bone marrow by microfracture, abrasion arthroplasty, and Pridie drilling (Buckwalter and Mankin 1998; Hochberg et al. 2012; Lohmander and Roos 2007; Minas 1999; Steadman et al. 2002; Steinwachs et al. 2008; Muller and Kohn 1999). However, these treatments attempt to support fibrocartilaginous repair tissue and cannot induce hyaline cartilage (Ronn et al. 2011; Becher et al. 2010). Other treatment options such as osteochondral autologous transplantation or autologous chondrocyte implantation also try to recover the articular surfaces (Muller et al. 2010; Vasiliadis et al. 2010).
3.2.1 Mesenchymal Stem Cells and Osteoarthritis
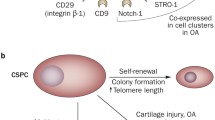
Tissue regeneration should focus on generating a repair tissue, which exerts the same mechano-biological properties and assimilates with the native tissue (Redman et al. 2005). Currently, one focus uses stem cell-based therapies to induce regeneration. The use of mesenchymal stem cells targets restores hyaline articular cartilage. Mesenchymal stem cells can be found in differentiated tissues and fulfil various tasks in the adult human body. They are characterized by their multi-lineage potential, which was demonstrated first by Pittenger et al. (1999), who isolated mesenchymal stem cells from bone marrow. Currently, mesenchymal stem cells can be obtained from diverse adult tissues (Kuhn and Tuan 2010). They have the capability to differentiate into various mesenchymal phenotypes including muscle, ligament, tendon, adipose, stroma, bone, and cartilage (Cai et al. 2004; Caplan 2007). Furthermore, they possess immunomodulating properties, anti-inflammation effects, and self-renewal capacities (Bonfield et al. 2010; Chamberlain et al. 2007; Chen and Tuan 2008). Stem cells are located in a yet-to-be-defined niche and remain quiescent except for rare cell divisions (Fuchs et al. 2004).
One of the first attempts to achieve cartilage repair in osteoarthritis was performed by Wakitani et al. in 1994. Large, full-thickness defects of the articular cartilage in the knees of rabbits were repaired by osteochondral progenitor cells (Wakitani et al. 1994). Later, a clinical trial was designed to investigate the influence of mesenchymal stem cells on damaged human cartilage. Human autologous culture expanded bone marrow mesenchymal cells were transplanted into the osteoarthritic knee joints of patients. A cartilage-like tissue formed after 42 weeks. However, symptoms were not significantly improved (Wakitani et al. 2002).
Further essential findings concerning stem cell therapy of osteoarthritis were obtained by Murphy et al. (2003). They explored the role of implanted adult mesenchymal stem cells in tissue repair and regeneration in an injured joint in a goat model. Regeneration of the meniscal tissue was stimulated, and its progressive destruction was prevented. However, none of the stem cells were located in the diseased cartilage tissue.
Another study investigated the benefit of mesenchymal stem cell treatment in patients suffering from moderate to late-stage osteoarthritis. No complete regeneration of cartilage and no efficient long-term success were obtained (Davatchi et al. 2011; Cucchiarini et al. 2014).
3.2.2 Mesenchymal Stem Cells and Rheumatoid Arthritis
Rheumatoid arthritis involves chronic inflammation of the synovium, which induces cartilage and bone erosion (De Bari 2015). Mesenchymal stem cells can also be found in the synovium (De Bari et al. 2001). In rheumatoid arthritis, the immune modulatory properties of mesenchymal stem cells seem to be very important (El-Jawhari et al. 2014). The effects of mesenchymal stem cells seem to be repressed by the inflammatory milieu. Rheumatoid arthritis patients show a lower prevalence of mesenchymal stem cells than is seen in osteoarthritis patients (Jones et al. 2004). Furthermore, there is a negative correlation between the chondrogenic and clonogenic capacities of synovial mesenchymal stem cells and the magnitude of synovitis in rheumatoid arthritis (Jones et al. 2010). In addition, there is an interdependency between infiltrating inflammatory/immune cells and resident fibroblast-like synoviocytes. A proliferation of fibroblast-like synoviocytes, the major pathogenic component in rheumatoid arthritis, is observed in rheumatoid arthritis (Li and Makarov 2006). These cells support the development of harmful pannus, which results in damaged articular cartilage and bone (Naylor et al. 2013). A recent study demonstrated that interactions between fibroblast-like synoviocytes and mesenchymal stem cells are possible. The placental growth factor, which occurs at higher levels in joints suffering from rheumatoid arthritis, could attract bone marrow mesenchymal stem cells to the synovium, where interactions with the resident fibroblast-like synoviocytes may lead to angiogenesis and chronic synovitis by further enhancing the secretion of placental growth factor (Park et al. 2014). However, the relationship between fibroblast-like synoviocytes and mesenchymal stem cells remains unclear. It might be possible that they represent different functional stages of the same lineage or that they represent the same cell type with functional specialization and diversification according to their positional information and environmental cues (De Bari et al. 2001).
Recent clinical studies have explored the benefit of mesenchymal stem cell treatment in rheumatoid arthritis patients. The intravenous injection of umbilical cord mesenchymal stem cells in addition to disease-modifying antirheumatic drugs induced a significant clinical improvement in patients suffering from active rheumatoid arthritis and in whom conventional treatment was ineffective (Wang et al. 2013). However, larger multicenter clinical studies are needed to provide safe treatment recommendations.
3.3 Chondrogenic Progenitor Cells
Restoring fully functional hyaline cartilage has not been achieved to date via chondrocytes or mesenchymal stem cells. Various experiments and methods have only generated fibrocartilaginous repair tissue instead of stable hyaline cartilage (Cucchiarini et al. 2014). Fibrocartilaginous repair tissue shows morphologically distinct cell types (Kouri et al. 1996). In the late stages of osteoarthritis, single chondrocytes and cells, which are organized in aggregates, are found. Furthermore, comprised chondrocytes undergoing a degenerative process exist in all zones of the cartilage. The most common cells are elongated secretory type 2 cells, which display a secretory phenotype (Kouri et al. 1996). These cells have been named fibroblast-like chondrocytes (Tesche and Miosge 2005). Koelling et al. (2009) were able to demonstrate that these cells show typical stem cell characteristics, including clonogenicity, multipotency, and migratory activity, and named them chondrogenic progenitor cells. They are also referred to as osteochondroprogenitor cells (Khan et al. 2009). In osteoarthritis and rheumatoid arthritis, these chondrogenic progenitor cells (Table 3.1) are involved in regeneration efforts that are largely unsuccessful in diseased cartilage tissue (Schminke and Miosge 2014).
Recently the presence of migratory progenitor cells in diseased tissues has been explored because they may play an important role in tissue regeneration and could be a promising target for cell-based therapy. Migratory progenitor cells show stem cell characteristics and possess great chondrogenic potential. The migratory potential is an important feature. The mechanism of cell migration can be found in numerous biological processes (Theveneau and Mayor 2013). Migration is a relevant characteristic of epithelial cells, i.e., during wound healing of the skin (Blanpain and Fuchs 2014). Migration is also indispensable to mesenchymal stem cells. Diverse repair processes in one’s lifetime are dependent on the migration of these cells (Sohni and Verfaillie 2013). Hematopoiesis and bone regeneration require cell migration (Sahin and Buitenhuis 2012; Pignolo and Kassem 2011). Additionally, progenitor cells, which are involved in basic biological processes of the stem cell niche, migrate (Augello et al. 2010). Progenitor cell populations that were generated from patient tissue from late stages of osteoarthritis possessed great migratory potential, at least in vitro and ex vivo (Schminke and Miosge 2014; Muhammad et al. 2013).
Chondrogenic progenitor cells show stem cell marker positivity for Stro-1 and CD29 and also for CD13, CD44, CD73, and CD90. They are negative for CD31, CD34, CD117, and CD271. Cells isolated from the superficial zone of healthy cartilage tissue in vivo also show stem cell marker positivity and could be related to chondrogenic progenitor cells. So far, this observation has not been confirmed (Dowthwaite et al. 2004). Chondrogenic progenitor cells in the late stages of osteoarthritis exhibit multi-differentiation potential to become adipocytes, cells of the osteoblastic lineage and chondrocytes. They can be cloned and expanded for up to 60 population doublings. Chondrogenic progenitor cells can exist as cells of the chondrogenic lineage if they are simply placed in 3D alginate culture (Koelling and Miosge 2009).
3.3.1 Chondrogenic Progenitor Cells from Osteoarthritic Patients (CPCs)
Chondrogenic progenitor cells are a subpopulation of cells that are localized in the repair tissue of advanced stages of osteoarthritis (Koelling et al. 2009). The compositions of collagens change in the late stages of osteoarthritis. Collagen types I and III are found in the fibrocartilaginous cartilage (Sandell and Aigner 2001; Poole 1999). Furthermore, a reduction of collagen type II is observed by quantitative immunohistochemistry (Miosge et al. 1998). In contrast to the microarray experiments, mRNAs of cartilage-specific collagens are upregulated, and increased anabolism is observed (Aigner et al. 2006). The change of the matrix composition may have a distinct influence on the deficient functioning of the repair tissue. Further investigations showed an increased expression level of proteoglycans such as biglycan, decorin, and perlecan (Tesche and Miosge 2005; Bock et al. 2001). This might reflect compensation for the loss of matrix molecules and the stabilization of the extracellular matrix (Tesche and Miosge 2005).
The transcription factors runt-related transcription factor 2 (Runx2) and sex-determining region Y-box 9 (Sox9) play an important role in the regulation mechanisms of chondrogenic progenitor cells (Koelling et al. 2009; Koelling and Miosge 2010). Sox9 is involved in the development of chondrocytes, operates the synthesis of cartilage-specific matrix components, and inhibits the beginning of chondral ossification (de Crombrugghe et al. 2001). Runx9 coordinates the development of osteoblasts and is essential for bone formation (Stein et al. 2004).
An ex vivo experiment using siRNA in three-dimensional culture downregulation of the osteogenic transcription factor Runx2 resulted in a simultaneous upregulation of the chondrogenic transcription factor Sox9. Hence, COL2A1 mRNA was detected (Koelling and Miosge 2010; Koelling et al. 2009; Muhammad et al. 2014). A Sox9 knockdown results in reduced aggrecan and Runx2 expression (unpublished observation). Mass spectrometry analysis was used to identify proteins, which are involved in the signal transduction and transcription of Sox9 and Runx2. Overexpression of DDX5, HSPA8, RAB5C, and YWHAE resulted in enhanced gene expression of Sox9. HSPA8 also enhanced the gene expression of Runx2, which was downregulated by YWHAE, and the chondrogenic potential of the chondrogenic progenitor cells was increased. A knockdown of LEMD2 and TMPO leads to an upregulation of Sox9. Further indicators of increased chondrogenic potential were the enhanced expression of the extracellular component ACAN and the decreased expression of COL1A1.
The pericellular matrix with laminins and nidogen-2 may also play an important role in the regulation mechanisms of chondrogenic progenitor cells. It has been shown that chondrogenic progenitor cells produce high levels of laminin-α1, laminin-α5, and nidogen-2 in their pericellular matrix. Laminin-α1 regulates collagen expression by enhancing collagen type II and decreasing collagen type I expression. Nidogen-2 upregulates Sox9 expression. A knockdown of nidogen-2 results in reduced Sox9 expression and enhanced Runx2 expression. Laminins and nidogen-2 guide chondrogenic progenitor cells toward chondrogenesis (Schminke et al. 2016a). This fact highlights the importance of the extracellular matrix components on chondrogenic progenitor cells and in stem cell biology (Fuchs et al. 2004; Fuchs 2008). The population of chondrogenic progenitor cells is not present in healthy cartilage.
Mechanical stimulation has an influence on chondrocytes. The primary cilium, a mechanosensor, also seems to be involved in mechano-transduction in chondrocytes. The mechanical load enhances ciliogenesis in the growth plate. The expression and localization of key members of the Ihh-PTHrP loop is altered, resulting in decreased proliferation and a switch from proliferation to differentiation. Abnormal chondrocyte morphology and organization is also observed (Rais et al. 2015; Muhammad et al. 2012).
Furthermore, calcium signaling is important for chondrogenesis. A recent study suggested that calcium homeostasis, including calcium regulatory mechanisms, has an influence on the differentiation potential of chondrogenic progenitor cells. An autocrine/paracrine purinergic mechanism plays an important role in driving calcium oscillations in these cells (Matta et al. 2015). Furthermore, the external influence of the sympathetic nervous system on chondrogenic progenitor cell-dependent chondrogenesis is important. A norepinephrine-dependent inhibition of chondrogenesis and acceleration of hypertrophic differentiation was recently discovered (Jenei-Lanzl et al. 2014).
3.3.2 Chondrogenic Progenitor Cells from Rheumatoid Arthritis Patients
Chondrogenic progenitor cells can also be isolated from diseased cartilage tissue of patients who suffer from rheumatoid arthritis. Interleukins, which are commonly found in the inflamed rheumatoid tissue of affected joints, exert a negative influence on these cells. This results in a less chondrogenic phenotype. High levels of matrix metalloproteinases and proinflammatory cytokines, such as tumor necrosis factor α (TNF-α), which are influenced by IL-17, are produced by these chondrogenic progenitor cells. IL-17 A/F leads to the upregulation of Runx2 protein and enhanced IL-6 protein and MMP3 mRNA levels. Blocking antibodies against IL-17 improved the repair potential of the progenitor cells. When chondrogenic progenitor cells are treated with the antihuman IL-17 antibody secukinumab or the anti-TNF-α-antibody adalimumab, a reduction of the proinflammatory IL-6 protein levels and a positive influence on the secretion of anti-inflammatory IL-10 protein are observed. Runx2 protein is also reduced by the same antibodies, which promote chondrogenesis. The chondrogenic capacity of the chondrogenic progenitor cells can be improved again by anti-inflammatory agents. Again, progenitor cells are distinguished by their high migration potential. They are able to repopulate diseased cartilage tissue ex vivo. Inflammatory mediators have a remarkable influence on these progenitor cells and their ability to migrate (Schminke et al. 2016b).
3.3.3 Meniscus Progenitor Cells (MPCs)
In the inner, avascular part of diseased human menisci, meniscus progenitor cells can be found. They are normally distinguished by the production of collagen type I, and they display a fibrocartilaginous nature and high migration potential (Muhammad et al. 2014). During the investigation of diseased human menisci from patients who were suffering from advanced stages of osteoarthritis, strongly affected menisci exhibit a downregulation of TGF-β and Smad2, resulting in the upregulation of Runx2. These facts support the assumption that meniscus progenitor cells also underlie a fine-tuned interaction between Runx2 and Sox9. Chondrogenic differentiation can be initiated by a knockdown of Runx2, which enhances p-Smad2. On the other hand, BMP2 stimulation of meniscus progenitor cells results in lower Smad2 levels and supports a change of the cells toward the osteogenic lineage (Muhammad et al. 2014). Additionally, a study in mice ascertained that TGF-β signaling directs knee morphogenesis and is important for meniscus development (Pazin et al. 2012).
Conclusion
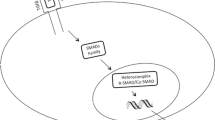
Regenerative therapies aim to substitute diseased tissue with native-like functional tissue. Until now, nearly all attempts have only managed to achieve the generation of a fibrocartilaginous repair tissue instead of fully functional, collagen type II-enriched hyaline cartilage. To date, treatment approaches using mesenchymal stem cells have not obtained satisfying long-term results. A new strategy is not to transplant stem cells into diseased cartilage tissue but to manipulate resident cells with stem cell characteristics, which are already present in situ and are active in their physiological response to the cell biological stimuli of the diseased tissue (Muhammad et al. 2013). Chondrogenic progenitor cells could be a promising target for cartilage repair (Fig. 3.2).
The concept of chondrogenic progenitor cells in situ. Cells derived from the stem cell in its niche are already pre-determined as osteochondroprogenitor cells. In OA cartilage, under the control of runx2, they become collagen type I producing cells. If these cells would be targeted in situ to switch to collagen type II production by enhancing Sox9 expression, they would help to regenerate a more hyaline-like cartilage tissu
Published successes provide insight into the chondrogenic pathways and interacting partners of chondrogenic progenitor cells. Two master regulators, Runx2 and Sox9, have already been identified. A knockdown of Runx2 leads to the upregulation of Sox9, aggrecan, and collagen type II (Koelling et al. 2009). The pericellular matrix with laminins and nidogen-2 is also involved in regulation mechanisms. Laminin-α1 and nidogen-2 guide chondrogenic progenitor cells toward chondrogenesis (Schminke et al. 2016a). Furthermore, mechanical stimulation has an influence on the chondrogenic differentiation potential of chondrogenic progenitor cells. An important role is played here by the primary cilium, which is necessary for mechano-transduction in chondrocytes (Rais et al. 2015; Muhammad et al. 2012). Calcium homeostasis may have autocrine/paracrine purinergic mechanisms that affect the calcium oscillations in these cells (Matta et al. 2015). In addition, the sympathetic nervous system was induced to have a norepinephrine-dependent inhibition of chondrogenesis (Jenei-Lanzl et al. 2014). Interleukins, especially IL-17, influence chondrogenic progenitor cells through the upregulation of Runx2 and drive them toward a less chondrogenic phenotype. Repair potential can be improved again by blocking antibodies against IL-17 in rheumatoid arthritis (Schminke et al. 2016b). The downregulation of TGF-β is also associated with an upregulation of Runx2 in meniscus progenitor cells (Muhammad et al. 2014).
Against the background outlined here, further investigations should focus on manipulating chondrogenic progenitor cells in situ with the help of small modifying molecules to improve their chondrogenic potential. It remains to be demonstrated whether manipulated chondrogenic progenitor cells produce an extracellular matrix, which provides repair tissue with better mechanical stress resistance than fibrocartilaginous tissue. Moreover, there is still a lack of knowledge about the behavior of manipulated chondrogenic progenitor cells in vivo. It has to be shown that they survive and maintain their favorable characteristics in the hostile microenvironment of the diseased organ (Koelling and Miosge 2009). In addition, the influence of age, gender, and body weight should not be disregarded (Gharibi et al. 2014; Murphy et al. 2002). Overall, further knowledge and understanding of the mechanisms that contribute to the regulation of stemness, multipotency, and differentiation have to be accomplished to allow a “restitutio ad integrum” in diseased cartilage tissue.
References
Aigner T, Fundel K, Saas J, Gebhard PM, Haag J, Weiss T, Zien A, Obermayr F, Zimmer R, Bartnik E (2006) Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum 54(11):3533–3544. doi:10.1002/art.22174
Augello A, Kurth TB, De Bari C (2010) Mesenchymal stem cells: a perspective from in vitro cultures to in vivo migration and niches. Eur Cell Mater 20:121–133
Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H (2010) Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc 18(5):656–663. doi:10.1007/s00167-009-1036-1
Blanpain C, Fuchs E (2014) Stem cell plasticity. Plasticity of epithelial stem cells in tissue regeneration. Science 344(6189):1242281. doi:10.1126/science.1242281
Blaschke S, Middel P, Dorner BG, Blaschke V, Hummel KM, Kroczek RA, Reich K, Benoehr P, Koziolek M, Muller GA (2003) Expression of activation-induced, T cell-derived, and chemokine-related cytokine/lymphotactin and its functional role in rheumatoid arthritis. Arthritis Rheum 48(7):1858–1872. doi:10.1002/art.11171
Bock HC, Michaeli P, Bode C, Schultz W, Kresse H, Herken R, Miosge N (2001) The small proteoglycans decorin and biglycan in human articular cartilage of late-stage osteoarthritis. Osteoarthritis Cartilage 9(7):654–663. doi:10.1053/joca.2001.0420
Bonfield TL, Nolan Koloze MT, Lennon DP, Caplan AI (2010) Defining human mesenchymal stem cell efficacy in vivo. J Inflamm 7:51. doi:10.1186/1476-9255-7-51
Bougault C, Gosset M, Houard X, Salvat C, Godmann L, Pap T, Jacques C, Berenbaum F (2012) Stress-induced cartilage degradation does not depend on the NLRP3 inflammasome in human osteoarthritis and mouse models. Arthritis Rheum 64(12):3972–3981. doi:10.1002/art.34678
Buckwalter JA, Mankin HJ (1998) Articular cartilage repair and transplantation. Arthritis Rheum 41(8):1331–1342. doi:10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J
Cai J, Weiss ML, Rao MS (2004) In search of “stemness”. Exp Hematol 32(7):585–598. doi:10.1016/j.exphem.2004.03.013
Caplan AI (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213(2):341–347. doi:10.1002/jcp.21200
Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 25(11):2739–2749. doi:10.1634/stemcells.2007-0197
Chen FH, Tuan RS (2008) Mesenchymal stem cells in arthritic diseases. Arthritis Res Ther 10(5):223. doi:10.1186/ar2514
de Crombrugghe B, Lefebvre V, Nakashima K (2001) Regulatory mechanisms in the pathways of cartilage and bone formation. Curr Opin Cell Biol 13(6):721–727
Cucchiarini M, Madry H, Guilak F, Saris DB, Stoddart MJ, Koon Wong M, Roughley P (2014) A vision on the future of articular cartilage repair. Eur Cell Mater 27:12–16
Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B (2011) Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis 14(2):211–215. doi:10.1111/j.1756-185X.2011.01599.x
De Bari C (2015) Are mesenchymal stem cells in rheumatoid arthritis the good or bad guys? Arthritis Res Ther 17:113. doi:10.1186/s13075-015-0634-1
De Bari C, Dell’Accio F, Tylzanowski P, Luyten FP (2001) Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44(8):1928–1942. doi:10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P
Dowthwaite GP, Bishop JC, Redman SN, Khan IM, Rooney P, Evans DJ, Haughton L, Bayram Z, Boyer S, Thomson B, Wolfe MS, Archer CW (2004) The surface of articular cartilage contains a progenitor cell population. J Cell Sci 117(Pt 6):889–897. doi:10.1242/jcs.00912
El-Jawhari JJ, El-Sherbiny YM, Jones EA, McGonagle D (2014) Mesenchymal stem cells, autoimmunity and rheumatoid arthritis. QJM 107(7):505–514. doi:10.1093/qjmed/hcu033
Englund M, Roemer FW, Hayashi D, Crema MD, Guermazi A (2012) Meniscus pathology, osteoarthritis and the treatment controversy. Nat Rev Rheumatol 8(7):412–419. doi:10.1038/nrrheum.2012.69
Franz JK, Kolb SA, Hummel KM, Lahrtz F, Neidhart M, Aicher WK, Pap T, Gay RE, Fontana A, Gay S (1998) Interleukin-16, produced by synovial fibroblasts, mediates chemoattraction for CD4+ T lymphocytes in rheumatoid arthritis. Eur J Immunol 28(9):2661–2671. doi:=10.1002/(SICI)1521-4141(199809)28:09<2661::AID-IMMU2661>3.0.CO;2-N
Fuchs E (2008) Skin stem cells: rising to the surface. J Cell Biol 180(2):273–284. doi:10.1083/jcb.200708185
Fuchs E, Tumbar T, Guasch G (2004) Socializing with the neighbors: stem cells and their niche. Cell 116(6):769–778
Gharibi B, Farzadi S, Ghuman M, Hughes FJ (2014) Inhibition of Akt/mTOR attenuates age-related changes in mesenchymal stem cells. Stem Cells 32(8):2256–2266. doi:10.1002/stem.1709
Goldring MB, Goldring SR (2010) Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci 1192:230–237. doi:10.1111/j.1749-6632.2009.05240.x
Goldring MB, Otero M (2011) Inflammation in osteoarthritis. Curr Opin Rheumatol 23(5):471–478. doi:10.1097/BOR.0b013e328349c2b1
Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, Towheed T, Welch V, Wells G, Tugwell P, American College of Rheumatology (2012) American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 64(4):465–474
Hommen JP, Applegate GR, Del Pizzo W (2007) Meniscus allograft transplantation: ten-year results of cryopreserved allografts. Arthroscopy 23(4):388–393. doi:10.1016/j.arthro.2006.11.032
Jenei-Lanzl Z, Grassel S, Pongratz G, Kees F, Miosge N, Angele P, Straub RH (2014) Norepinephrine inhibition of mesenchymal stem cell and chondrogenic progenitor cell chondrogenesis and acceleration of chondrogenic hypertrophy. Arthritis Rheumatol 66(9):2472–2481. doi:10.1002/art.38695
Johnson VL, Hunter DJ (2014) The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol 28(1):5–15. doi:10.1016/j.berh.2014.01.004
Johnstone B, Alini M, Cucchiarini M, Dodge GR, Eglin D, Guilak F, Madry H, Mata A, Mauck RL, Semino CE, Stoddart MJ (2013) Tissue engineering for articular cartilage repair--the state of the art. Eur Cell Mater 25:248–267
Jones EA, English A, Henshaw K, Kinsey SE, Markham AF, Emery P, McGonagle D (2004) Enumeration and phenotypic characterization of synovial fluid multipotential mesenchymal progenitor cells in inflammatory and degenerative arthritis. Arthritis Rheum 50(3):817–827. doi:10.1002/art.20203
Jones E, Churchman SM, English A, Buch MH, Horner EA, Burgoyne CH, Reece R, Kinsey S, Emery P, McGonagle D, Ponchel F (2010) Mesenchymal stem cells in rheumatoid synovium: enumeration and functional assessment in relation to synovial inflammation level. Ann Rheum Dis 69(2):450–457. doi:10.1136/ard.2008.106435
Karouzakis E, Neidhart M, Gay RE, Gay S (2006) Molecular and cellular basis of rheumatoid joint destruction. Immunol Lett 106(1):8–13. doi:10.1016/j.imlet.2006.04.011
Khan IM, Williams R, Archer CW (2009) One flew over the progenitor’s nest: migratory cells find a home in osteoarthritic cartilage. Cell Stem Cell 4(4):282–284. doi:10.1016/j.stem.2009.03.007
Kim HR, Kim KW, Kim BM, Jung HG, Cho ML, Lee SH (2014) Reciprocal activation of CD4+ T cells and synovial fibroblasts by stromal cell-derived factor 1 promotes RANKL expression and osteoclastogenesis in rheumatoid arthritis. Arthritis Rheumatol 66(3):538–548. doi:10.1002/art.38286
Kock L, van Donkelaar CC, Ito K (2012) Tissue engineering of functional articular cartilage: the current status. Cell Tissue Res 347(3):613–627. doi:10.1007/s00441-011-1243-1
Koelling S, Miosge N (2009) Stem cell therapy for cartilage regeneration in osteoarthritis. Expert Opin Biol Ther 9(11):1399–1405. doi:10.1517/14712590903246370
Koelling S, Miosge N (2010) Sex differences of chondrogenic progenitor cells in late stages of osteoarthritis. Arthritis Rheum 62(4):1077–1087. doi:10.1002/art.27311
Koelling S, Kruegel J, Irmer M, Path JR, Sadowski B, Miro X, Miosge N (2009) Migratory chondrogenic progenitor cells from repair tissue during the later stages of human osteoarthritis. Cell Stem Cell 4(4):324–335. doi:10.1016/j.stem.2009.01.015
Kouri JB, Jimenez SA, Quintero M, Chico A (1996) Ultrastructural study of chondrocytes from fibrillated and non-fibrillated human osteoarthritic cartilage. Osteoarthritis Cartilage 4(2):111–125
Kuhn NZ, Tuan RS (2010) Regulation of stemness and stem cell niche of mesenchymal stem cells: implications in tumorigenesis and metastasis. J Cell Physiol 222(2):268–277. doi:10.1002/jcp.21940
Li X, Makarov SS (2006) An essential role of NF-kappaB in the “tumor-like” phenotype of arthritic synoviocytes. Proc Natl Acad Sci U S A 103(46):17432–17437. doi:10.1073/pnas.0607939103
Loeser RF, Goldring SR, Scanzello CR, Goldring MB (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 64(6):1697–1707. doi:10.1002/art.34453
Lohmander LS, Roos EM (2007) Clinical update: treating osteoarthritis. Lancet 370(9605):2082–2084. doi:10.1016/S0140-6736(07)61879-0
Lohmander LS, Hellot S, Dreher D, Krantz EF, Kruger DS, Guermazi A, Eckstein F (2014) Intraarticular sprifermin (recombinant human fibroblast growth factor 18) in knee osteoarthritis: a randomized, double-blind, placebo-controlled trial. Arthritis Rheumatol 66(7):1820–1831. doi:10.1002/art.38614
Lories RJ, Luyten FP (2011) The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol 7(1):43–49. doi:10.1038/nrrheum.2010.197
Matta C, Fodor J, Miosge N, Takacs R, Juhasz T, Rybaltovszki H, Toth A, Csernoch L, Zakany R (2015) Purinergic signalling is required for calcium oscillations in migratory chondrogenic progenitor cells. Pflugers Arch 467(2):429–442. doi:10.1007/s00424-014-1529-8
Minas T (1999) The role of cartilage repair techniques, including chondrocyte transplantation, in focal chondral knee damage. Instr Course Lect 48:629–643
Miosge N, Waletzko K, Bode C, Quondamatteo F, Schultz W, Herken R (1998) Light and electron microscopic in-situ hybridization of collagen type I and type II mRNA in the fibrocartilaginous tissue of late-stage osteoarthritis. Osteoarthritis Cartilage 6(4):278–285. doi:10.1053/joca.1998.0121
Muhammad H, Rais Y, Miosge N, Ornan EM (2012) The primary cilium as a dual sensor of mechanochemical signals in chondrocytes. Cell Mol Life Sci 69(13):2101–2107. doi:10.1007/s00018-011-0911-3
Muhammad H, Schminke B, Miosge N (2013) Current concepts in stem cell therapy for articular cartilage repair. Expert Opin Biol Ther 13(4):541–548. doi:10.1517/14712598.2013.758707
Muhammad H, Schminke B, Bode C, Roth M, Albert J, von der Heyde S, Rosen V, Miosge N (2014) Human migratory meniscus progenitor cells are controlled via the TGF-beta pathway. Stem Cell Rep 3(5):789–803. doi:10.1016/j.stemcr.2014.08.010
Muir H (1995) The chondrocyte, architect of cartilage. Biomechanics, structure, function and molecular biology of cartilage matrix macromolecules. Bioessays 17(12):1039–1048. doi:10.1002/bies.950171208
Muller B, Kohn D (1999) Indication for and performance of articular cartilage drilling using the Pridie method. Orthopade 28(1):4–10
Muller S, Breederveld RS, Tuinebreijer WE (2010) Results of osteochondral autologous transplantation in the knee. Open Orthop J 4:111–114. doi:10.2174/1874325001004020111
Murphy JM, Dixon K, Beck S, Fabian D, Feldman A, Barry F (2002) Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum 46(3):704–713. doi:10.1002/art.10118
Murphy JM, Fink DJ, Hunziker EB, Barry FP (2003) Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum 48(12):3464–3474. doi:10.1002/art.11365
Musumeci G, Castrogiovanni P, Leonardi R, Trovato FM, Szychlinska MA, Di Giunta A, Loreto C, Castorina S (2014) New perspectives for articular cartilage repair treatment through tissue engineering: a contemporary review. World J Orthod 5(2):80–88. doi:10.5312/wjo.v5.i2.80
Naylor AJ, Filer A, Buckley CD (2013) The role of stromal cells in the persistence of chronic inflammation. Clin Exp Immunol 171(1):30–35. doi:10.1111/j.1365-2249.2012.04634.x
Park SJ, Kim KJ, Kim WU, Cho CS (2014) Interaction of mesenchymal stem cells with fibroblast-like synoviocytes via cadherin-11 promotes angiogenesis by enhanced secretion of placental growth factor. J Immunol 192(7):3003–3010. doi:10.4049/jimmunol.1302177
Pazin DE, Gamer LW, Cox KA, Rosen V (2012) Molecular profiling of synovial joints: use of microarray analysis to identify factors that direct the development of the knee and elbow. Dev Dyn 241(11):1816–1826. doi:10.1002/dvdy.23861
Pignolo RJ, Kassem M (2011) Circulating osteogenic cells: implications for injury, repair, and regeneration. J Bone Miner Res 26(8):1685–1693. doi:10.1002/jbmr.370
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147
Poole AR (1999) An introduction to the pathophysiology of osteoarthritis. Front Biosci 4:D662–D670
Rais Y, Reich A, Simsa-Maziel S, Moshe M, Idelevich A, Kfir T, Miosge N, Monsonego-Ornan E (2015) The growth plate’s response to load is partially mediated by mechano-sensing via the chondrocytic primary cilium. Cell Mol Life Sci 72(3):597–615. doi:10.1007/s00018-014-1690-4
Redman SN, Oldfield SF, Archer CW (2005) Current strategies for articular cartilage repair. Eur Cell Mater 9:23–32. discussion 23-32
Ronn K, Reischl N, Gautier E, Jacobi M (2011) Current surgical treatment of knee osteoarthritis. Arthritis 2011:454873. doi:10.1155/2011/454873
Sahin AO, Buitenhuis M (2012) Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adh Migr 6(1):39–48. doi:10.4161/cam.18975
Sandell LJ, Aigner T (2001) Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis. Arthritis Res 3(2):107–113
Schminke B, Miosge N (2014) Cartilage repair in vivo: the role of migratory progenitor cells. Curr Rheumatol Rep 16(11):461. doi:10.1007/s11926-014-0461-4
Schminke B, Frese J, Bode C, Goldring MB, Miosge N (2016a) Laminins and nidogens in the pericellular matrix of chondrocytes: their role in osteoarthritis and chondrogenic differentiation. Am J Pathol 186(2):410–418. doi:10.1016/j.ajpath.2015.10.014
Schminke B, Trautmann S, Mai B, Miosge N, Blaschke S (2016b) Interleukin 17 inhibits progenitor cells in rheumatoid arthritis cartilage. Eur J Immunol 46(2):440–445. doi:10.1002/eji.201545910
Sohni A, Verfaillie CM (2013) Mesenchymal stem cells migration homing and tracking. Stem Cells Int 2013:130763. doi:10.1155/2013/130763
Steadman JR, Rodkey WG, Briggs KK (2002) Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg 15(3):170–176
Stein GS, Lian JB, van Wijnen AJ, Stein JL, Montecino M, Javed A, Zaidi SK, Young DW, Choi JY, Pockwinse SM (2004) Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 23(24):4315–4329. doi:10.1038/sj.onc.1207676
Steinwachs MR, Guggi T, Kreuz PC (2008) Marrow stimulation techniques. Injury 39(Suppl 1):S26–S31. doi:10.1016/j.injury.2008.01.042
Tesche F, Miosge N (2005) New aspects of the pathogenesis of osteoarthritis: the role of fibroblast-like chondrocytes in late stages of the disease. Histol Histopathol 20(1):329–337
Tew S, Redman S, Kwan A, Walker E, Khan I, Dowthwaite G, Thomson B, Archer CW (2001) Differences in repair responses between immature and mature cartilage. Clin Orthop Relat Res (391 Suppl):S142–S152
Theveneau E, Mayor R (2013) Collective cell migration of epithelial and mesenchymal cells. Cell Mol Life Sci 70(19):3481–3492. doi:10.1007/s00018-012-1251-7
Toh ML, Miossec P (2007) The role of T cells in rheumatoid arthritis: new subsets and new targets. Curr Opin Rheumatol 19(3):284–288. doi:10.1097/BOR.0b013e32805e87e0
Vasiliadis HS, Danielson B, Ljungberg M, McKeon B, Lindahl A, Peterson L (2010) Autologous chondrocyte implantation in cartilage lesions of the knee: long-term evaluation with magnetic resonance imaging and delayed gadolinium-enhanced magnetic resonance imaging technique. Am J Sports Med 38(5):943–949. doi:10.1177/0363546509358266
Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM (1994) Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am 76(4):579–592
Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M (2002) Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage 10(3):199–206. doi:10.1053/joca.2001.0504
Wang L, Wang L, Cong X, Liu G, Zhou J, Bai B, Li Y, Bai W, Li M, Ji H, Zhu D, Wu M, Liu Y (2013) Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy. Stem Cells Dev 22(24):3192–3202. doi:10.1089/scd.2013.0023
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Oellerich, D., Miosge, N. (2017). Chondrogenic Progenitor Cells and Cartilage Repair. In: Grässel, S., Aszódi, A. (eds) Cartilage. Springer, Cham. https://doi.org/10.1007/978-3-319-53316-2_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-53316-2_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53314-8
Online ISBN: 978-3-319-53316-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)