Abstract
Cytokinesis in E. coli is organized by a cytoskeletal element designated the Z ring. The Z ring is formed at midcell by the coalescence of FtsZ filaments tethered to the membrane by interaction of FtsZ’s conserved C-terminal peptide (CCTP) with two membrane-associated proteins, FtsA and ZipA. Although interaction between an FtsZ monomer and either of these proteins is of low affinity, high affinity is achieved through avidity – polymerization linked CCTPs interacting with the membrane tethers. The placement of the Z ring at midcell is ensured by antagonists of FtsZ polymerization that are positioned within the cell and target FtsZ filaments through the CCTP. The placement of the ring is reinforced by a protein network that extends from the terminus (Ter) region of the chromosome to the Z ring. Once the Z ring is established, additional proteins are recruited through interaction with FtsA, to form the divisome. The assembled divisome is then activated by FtsN to carry out septal peptidoglycan synthesis, with a dynamic Z ring serving as a guide for septum formation. As the septum forms, the cell wall is split by spatially regulated hydrolases and the outer membrane invaginates in step with the aid of a transenvelope complex to yield progeny cells.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- E. coli
- FtsZ
- Z ring
- FtsA
- ZipA
- Zap proteins
- Cytokinetic machinery
- Divisome
- Polymerization driven avidity
- Min system
- Oscillatíon
- Nucleoid occlusion
- Ter linkage
- Septal PG synthesis
- FtsEX
- FtsN
Overview of Cell Cycle Regulation – Two Key Proteins
The bacterial cell cycle is primarily regulated at the initiation of two major events, DNA replication and cytokinesis (septation). Studies in E. coli indicate that the regulatory inputs that control these two events converge on just two proteins, DnaA for DNA replication and FtsZ for cytokinesis (Fig. 2.1). DnaA , which assembles into an oligomer on oriC, is required to unwind the DNA so that DnaB, the replicative helicase, can be loaded and the replication forks established (Bramhill and Kornberg 1988; Erzberger et al. 2006). FtsZ assembles into the Z ring, a cytoskeletal element that determines the site of cytokinesis and functions as a scaffold to recruit additional division proteins to synthesize septal peptidoglycan (PG) (Bi and Lutkenhaus 1991). These two major events are not obligatorily coupled, since DNA replication and segregation can continue in the absence of cytokinesis. This chapter is focused on E. coli, with occasional references to results from other organisms as indicated.
Regulatory inputs for cell cycle control converge on two key proteins, DnaA and FtsZ. DnaA-ATP assembles on oriC to initiate DNA replication. FtsZ assembles into a Z ring that determines the division plane by organizing the machinery to synthesize the septum. Whereas DnaA-ATP assembles on the oriC template, the Z ring does not have a landmark and is a self organizing organelle that assembles where conditions are favorable
DnaA and Initiation of Replication
Much of the control of the cell cycle operates at the level of initiation of DNA replication. Donachie found that the ratio of cell mass to DNA origins was a constant, independent of growth rate, and proposed that replication was initiated when cells double their critical size, with cytokinesis occurring a fixed time later (Donachie 1968). The consequences can be readily observed upon a shift up in growth rate. When a culture is shifted from a slow to a fast growth rate, the rate of mass increase immediately shifts to the new growth rate and new rounds of DNA replication initiate as the mass doubles and before the previous round is finished (Cooper and Helmstetter 1968). The first cytokinetic event, however, is delayed because the time required to replicate the chromosome and to divide are constant and longer than the fast doubling time. As a consequence, the cells are larger at the first division and then reach a new steady state. Under these conditions, cytokinesis follows a fixed time after DNA replication is initiated.
Initiation occurs when DnaA-ATP binds to sites located within oriC, a unique 245 base pair region on the chromosome. oriC has three high affinity sites, as well as many low affinity sites, for DnaA-ATP (Leonard and Grimwade 2015). Adjacent to these sites is a region designated DUE, the DNA unwinding element, which is converted to a single-stranded region when all DnaA binding sites are occupied and where DnaB is loaded (Bramhill and Kornberg 1988). Although the high affinity sites are usually occupied, the loading of the low affinity sites is highly regulated, as their occupation results in the triggering of initiation (Mott and Berger 2007). Occupancy of the low affinity sites each cell cycle requires the synthesis of DnaA to generate DnaA-ATP since DnaA-ADP, which is generated following an initiation event, does not readily exchange with ATP (Kato and Katayama 2001). Thus, DnaA must be synthesized each cell cycle to obtain the ATP-bound form, consistent with the old observation that each new round of initiation of replication requires protein synthesis (Helmstetter 1974). It has been suggested that it is the ratio of DnaA-ATP to DnaA-ADP that is critical for the firing of oriC but this is controversial (Donachie and Blakely 2003; Vadia and Levin 2015). The reader is referred to several excellent reviews on the regulation of replication, as this article will focus on cytokinesis (Mott and Berger 2007; Leonard and Grimwade 2015; Katayama et al. 2010).
FtsZ and the Z Ring
Assembly of the Z ring at the division site is the first step in bacterial cytokinesis (Bi and Lutkenhaus 1991). The Z ring was the first cytoskeletal element to be described in bacteria and is assembled from FtsZ filaments formed by the polymerization of FtsZ (Ma and Margolin 1999), the ancestral homologue of eukaryotic tubulin (Lowe and Amos 1998). It is a very dynamic structure (T1/2 < 10 s) (Chen and Erickson 2005), formed by the coalescence of FtsZ filaments attached to the membrane. This process is under spatial regulation to ensure that the Z ring is assembled at midcell, between segregated chromosomes (Lutkenhaus 2007).
The mechanisms of spatial regulation appear quite different among diverse bacteria and can include negative as well as positive systems (Lutkenhaus 2007; Monahan et al. 2014; Mannik and Bailey 2015). In addition, multiple systems can exist within the same organism and each contribute to spatial regulation. These systems are usually not essential but their absence often leads to altered morphology due to misplacement of the septum. In E. coli the negative regulatory systems include Min (minicell) and NO (nucleoid occlusion). The Min system is highly conserved and widely distributed across diverse bacterial species (Rothfield et al. 2005). Loss of spatial regulation in Min mutants leads to assembly of the Z ring near the cell poles, resulting in the formation of anucleate minicells, thus emphasizing that the position of the Z ring dictates the site of cytokinesis (Bi and Lutkenhaus 1993). NO, which prevents Z ring assembly over the nucleoid, is present in many bacteria and loss of this system leads to Z rings forming over chromosomes delayed for segregation (Bernhardt and De Boer 2005). In addition, a positive regulatory system in E. coli involves linkage between the Ter macrodomain of the chromosome and the Z ring (Bailey et al. 2014). This latter system is revealed when the other two are removed.
In contrast to DnaA, which binds to defined sequences in oriC to produce a helical filament on the DNA that can initiate replication (Mott and Berger 2007), the Z ring does not have a template. It is a dynamic self-organizing structure that is positioned by spatial regulation and, once established, determines the division site (Lutkenhaus 2007). Importantly, FtsZ is expressed at a constant rate and the major control of Z ring assembly is due to the spatial regulation that positions the Z ring to midcell (Weart and Levin 2003).
Components of the Cytokinetic Machinery – Cell Division Genes
The identification of cell division genes in E. coli started in the 1960s with the isolation of mutants with a filamenting temperature sensitive phenotype (fts) (Van De Putte et al. 1964; Hirota et al. 1968). Such mutants continue to replicate and segregate their DNA at the nonpermissive temperature but grow as long nonseptate filaments that are unable to form colonies. This conditional lethality allowed the cloning of the respective genes, which in turn led to the characterization of their gene products. Although mutations in many non cell division genes can lead to a filamentous phenotype, these were eventually ruled out as their effect on cell division was indirect.
Key steps in the characterization of a cell division gene are the demonstration that it is essential and that the gene product localizes to the division site. This has led to the realization that the complex process of cytokinesis is carried out by only 12 essential genes (ftsZ, ftsA, zipA, ftsE, ftsX, ftsK, ftsQ, ftsL, ftsB, ftsW, ftsI and ftsN) (De Boer 2010; Lutkenhaus et al. 2012). FtsE/FtsX are essential even though loss of these genes can be suppressed by increased osmolarity (Reddy 2007). Many other proteins localize to the division site but their genes are not essential because their function is redundant (for example, genes for Zap proteins and amidases) or they only contribute to the efficiency of division (Tol-Pal complex). Other genes are essential, but are not specific for cell division, because they are also involved in cell elongation (genes for PG synthesis).
Additional insight into the process of cell division came from investigating the phenotypic effect of penicillin derivatives (Spratt 1975). Among the derivatives are some (cephalexin and piperacillin) that selectively block cytokinesis without affecting cell elongation. These antibiotics specifically target penicillin-binding protein 3 (PBP3), the product of ftsI, supporting its role in cytokinesis. Among the 12 or so penicillin binding proteins in E. coli, only PBP3 is used exclusively in cytokinesis (Young 2001). Less specific penicillins, which target multiple PBPs, lead to lysis at the division site, as this is a major site of cell wall synthesis (Spratt 1975).
Assembly of the Z Rings
The assembly of the divisome occurs in two temporally distinct steps (Aarsman et al. 2005). In the first step, the Z ring (also referred to as the proto-ring (Rico et al. 2013)) is formed at midcell (Bi and Lutkenhaus 1991). Proteins that interact with FtsZ coassemble with FtsZ to form the ring. Key among these are ZipA and FtsA, which function as membrane tethers for FtsZ filaments (Hale and De Boer 1997; Pichoff and Lutkenhaus 2002). In addition, various Zap proteins (ZapA, ZapC and ZapD) interact with FtsZ and can crosslink FtsZ filaments (Gueiros-Filho and Losick 2002; Hale et al. 2011; Durand-Heredia et al. 2011; Huang et al. 2013). FtsEX also assembles early and FtsE is reported to interact with FtsZ (Corbin et al. 2007). The formation of the Z ring is under spatial regulation, ensuring that it is formed at midcell (Lutkenhaus 2007). After a delay that can be up to 1/3 of a cell cycle, the remaining proteins are recruited to form a complete divisome (Aarsman et al. 2005).
FtsZ and Tubulin Form Dynamic Structures
The globular domains of FtsZ and tubulin are remarkably similar (Lowe and Amos 1998; Nogales et al. 1998). They belong to a distinct family of GTPases that undergo dynamic polymerization dependent upon GTP hydrolysis (Nogales et al. 1998; Mukherjee and Lutkenhaus 1998). Whereas tubulin assembles into a 13-stranded microtubule, FtsZ assembles into a linear filament, equivalent to one strand of a microtubule, and undergoes treadmilling driven by GTP hydrolysis (Mukherjee and Lutkenhaus 1994, 1998; Chen and Erickson 2005; Loose and Mitchison 2014). Despite FtsZ forming a linear filament, assembly, like that of microtubules, is cooperative, with a critical concentration near 1 μM (Mukherjee and Lutkenhaus 1998; Chen et al. 2005). Importantly, this family of proteins can use the dynamic capability provided by GTP hydrolysis to explore intracellular space. Microtubules use the assembly dynamics to search for kinetochores, in the process of forming a spindle (Kirschner and Mitchison 1986; Heald and Khodjakov 2015), whereas FtsZ uses dynamics to search for conditions that are favorable for forming the Z ring (Lutkenhaus 2007). These conditions are where the concentration of factors that antagonize FtsZ polymerization is the lowest.
CCTP – High Affinity Through Polymerization Driven Avidity
Both FtsZ and tubulin have long C-terminal segments which, although quite different, mediate interaction with a number of interacting proteins (Erickson et al. 2010; Roll-Mecak 2015). The FtsZ C-terminal segment is composed of a long non-conserved linker (~50 amino acids in E. coli) that connects the globular (GTP-polymerizing) domain to a very conserved short region of about 14 residues (Fig. 2.2a). This short region has been termed the C-terminal tail (CTT) or CCTP (conserved C-terminal peptide) (Du et al. 2015). It mediates interaction between FtsZ and many of its partners, including the membrane anchors, ZipA and FtsA, and the spatial regulators, MinC/MinD and SlmA (Ma and Margolin 1999; Haney et al. 2001). The CCTP is an example of a short linear peptide embedded within a region of intrinsic disorder (the linker) that drives the interaction of a protein with a variety of unrelated proteins; something more common among eukaryotic proteins (Uversky 2013).
FtsZ interacts with many partners through the CCTP. (a) FtsZ contains a globular tubulin domain attached to the CCTP by a long linker (50 amino acids in E. coli). It is a rare example in prokaryotes of a short linear peptide embedded within a region of intrinsic disorder (the linker) that drives the interaction of a protein with variety of unrelated proteins. (b) Image and cartoon of an FtsZ filament in vivo. Electron cryotomogram of an E. coli cell in which FtsZ (D212N) was expressed shows an FtsZ filament 16 nm from the cytoplasmic membrane (Szwedziak et al. 2014). Similar filaments are observed in WT cells. The accompanying cartoon depicts the FtsZ filament tethered to the cytoplasmic membrane by ZipA and FtsA. High affinity of the filaments for the membrane is due to polymerization driven avidity as multiple CCTPs contact multiple membrane partners
The binding sites for the CCTP on ZipA and FtsA have little in common and there are quite different side chain contacts between residues in the CCTP and these partners (Szwedziak et al. 2012; Mosyak et al. 2000; Schumacher and Zeng 2016). The CCTP has relatively weak affinity for these partners (with a KD in the 30–50 μM range), indicating that an FtsZ monomer is unlikely to localize to the membrane. However, an FtsZ filament has high affinity for the membrane anchors due to avidity (Du et al. 2015). Thus, FtsZ filaments are at the membrane (Fig. 2.2b) and GTP-hydrolysis releases FtsZ monomers into the cytoplasm.
In addition to FtsA and ZipA, FtsZ filaments interact with several regulatory proteins (MinC/MinD and SlmA) and at least one Zap protein, ZapD, through the CCTP (Lutkenhaus et al. 2012; Durand-Heredia et al. 2012; Schumacher and Zeng 2016). MinC/MinD and SlmA are antagonists of FtsZ assembly that become positioned in the cell by interacting with the membrane and DNA respectively. Once in position, they use a two-pronged mechanism to disrupt FtsZ filaments. Both antagonists bind to an FtsZ filament through interaction with the CCTP and, in a second step, sever the filament (Cho et al. 2011; Shen and Lutkenhaus 2010; Du and Lutkenhaus 2014). The interaction between the CCTP and SlmA follows the same principle as for ZipA; monomers bind weakly and filaments bind strongly due to avidity (Du et al. 2015). These differential affinities for monomers and filaments, along with a putative severing mechanism, enable MinC/MinD and SlmA to effectively disrupt FtsZ filaments, even though they are present in the cell at much lower concentrations than FtsZ.
Membrane-Tethered FtsZ Filaments Coalesce at Midcell with the Aid of Zap Proteins
The two membrane associated proteins that tether FtsZ filaments to the membrane are quite different, although both bind to the CCTP of FtsZ (Pichoff and Lutkenhaus 2002; Ma and Margolin 1999; Haney et al. 2001). ZipA is a Type 1b transmembrane protein with a long linker connecting the transmembrane domain to a globular domain that binds the CCTP of FtsZ (Hale and De Boer 1997). In contrast, FtsA is a peripheral membrane protein that binds to the membrane through a C-terminal amphipathic helix (Pichoff and Lutkenhaus 2005). It is an actin related protein, with an unusual domain structure but assembles into actin-like filaments on a lipid bilayer in vitro (Szwedziak et al. 2012). So far, no ATPase activity has been associated with assembly and the polymers are not dynamic (Lara et al. 2005; Szwedziak et al. 2012). FtsA polymers have also been observed in vivo at the membrane when overexpressed (Szwedziak et al. 2012). In one model, the C-terminal amphipathic helix obstructs polymerization and binding to the membrane relieves this inhibition (Krupka et al. 2014). In the absence of the C-terminal amphipathic helix, polymerization occurs in the cytoplasm but is less efficient, suggesting that the membrane enhances assembly, which is consistent with the model (Pichoff and Lutkenhaus 2005).
FtsZ filaments are formed at the membrane throughout the cell and even oscillate between the ends of the cell under the influence of the Min system (Thanedar and Margolin 2004; Bisicchia et al. 2013a). Eventually these membrane bound filaments coalesce into a Z ring at midcell with the aid of the Zap proteins. Three of the Zap proteins (ZapA, C and D) crosslink FtsZ filaments (Hale et al. 2011; Durand-Heredia et al. 2011, 2012; Gueiros-Filho and Losick 2002; Mohammadi et al. 2009; Dajkovic et al. 2010), whereas a fourth, ZapB, does not interact directly with FtsZ but interacts with ZapA (Galli and Gerdes 2010). The loss of all three Zaps that interact directly with FtsZ results in increased cell length and poor viability, whereas loss of any one has less effect, indicating some functional overlap (Durand-Heredia et al. 2012).
However, the three Zap proteins crosslink FtsZ filaments through different mechanisms. ZapA forms dimers and tetramers and is believed to crosslink filaments by interacting with the lateral sides of filaments (Pacheco-Gomez et al. 2013), whereas ZapD is a dimer and binds to CCTPs on adjacent filaments (Durand-Heredia et al. 2012). In contrast, ZapC is a monomer that binds to the FtsZ globular domain, but can crosslink FtsZ filaments since each monomer has two unique FtsZ-binding sites (Schumacher et al. 2015).
Although crosslinking filaments by Zap proteins facilitates Z ring formation, it is not clear if direct lateral interaction between FtsZ filaments also has a role. FtsZ filaments are readily bundled in vitro, depending upon cations and pH, but the bundles lack repetitive protein-protein interactions (Erickson et al. 2010). One FtsZ mutant displays many interesting phenotypes, similar to FtsA* (see later). This mutant displays increased bundling in vitro and it is likely that the bundling contributes to the phenotypes but how is not clear (Haeusser et al. 2015).
Structure of the Z Ring
One of the most intriguing questions is the structure of the Z ring, or more specifically the arrangement of filaments in the ring. Two approaches have been used to try and resolve this, super resolution fluorescence microscopy and electron cryotomography. By super high-resolution fluorescence microscopy the Z ring appears patchy, which has been interpreted as a discontinuous ring (Fu et al. 2010; Strauss et al. 2012). In nonconstricting cells, the ring appears to be 115 nm in width and ~700 nm in diameter. Interestingly, increasing the FtsZ concentration does not affect the dimensions of the midcell ring but leads to formation of additional misplaced rings, suggesting a defined structure (Fu et al. 2010). About 500 molecules of FtsZ are required to form a filament (with a 4.3 nm intersubunit distance) sufficient in length to encircle the division site in a nonconstricting cell. Since E. coli contains ~6500 molecules of FtsZ per cell (fast growth rate) (Li et al. 2014) and ~30% of FtsZ is in the ring (Stricker et al. 2002), there is sufficient FtsZ assembled to encircle the septum about four times.
FtsZ filaments are difficult to detect by electron microscopy due to the density of the cytoplasm, their low numbers (lack of a regular lateral array) and their proximity to the membrane. However, FtsZ filaments have been clearly observed by electron cryotomography, approximately 16 nm from the membrane (Li et al. 2007; Szwedziak et al. 2014) (Fig. 2.2b). Furthermore, varying the length of the linker changes the distance of the filaments from the membrane, confirming that the observed filaments are FtsZ (Szwedziak et al. 2014). In addition, when FtsA was overproduced along with a hydrolysis-deficient mutant of FtsZ (D212N), an FtsA filament was observed half way between the membrane and the FtsZ filament. Although this lends support to the idea that FtsA polymers can form in vivo, FtsA filaments are not observed in wild type cells (Fig. 2.2b). Presumably, this is because they are very short and less abundant than FtsZ filaments, consistent with a ratio of FtsZ to FtsA that is ~7 to 1 (Li et al. 2014). When overproduced, FtsZ (D212N) filaments were observed as doublets with an interfilament spacing (center-to-center distance) of 6.8 nm, too large to arise from lateral interactions (Szwedziak et al. 2014). Whether these doublets can also occur under wild type conditions is not clear but, even under these overproduction conditions, bundles of FtsZ filaments are not observed, indicating bundling due to lateral interactions is unlikely.
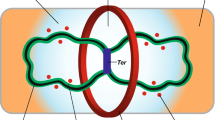
The Ter region of the chromosome is connected to the Z ring by a protein network (ZapA, ZapB and MatP), designated the Ter linkage. Conventional fluorescence microscopy revealed that ZapB is located interior to the Z ring, whereas ZapA colocalized with FtsZ (Galli and Gerdes 2010). Since MatP condenses the Ter region into a macrodomain and ZapB links MatP to the Z ring through ZapA, a model emerged in which there was a continuous link between the Ter region and the Z ring (Mercier et al. 2008; Espeli et al. 2012). Higher resolution imaging revealed the dimensions of ZapA rings were similar to Z rings whereas ZapB rings are thicker and have a smaller diameter, with MatP interior to ZapB,which supports the linkage model (Buss et al. 2015). This linkage is not essential for division, as ZapA or ZapB mutants are viable, although Z rings appear to have difficulty in forming and are sometimes askew. Once formed, however, Z rings lacking ZapA or ZapB appear similar to rings in wild type cells (Buss et al. 2013).
In Vitro Reconstruction
One of the goals in biology is in vitro reconstruction of a system to demonstrate that the identified components reconstitute the behavior observed in vivo. Although this is a big challenge in the field of cytokinesis, especially considering the important role of the cell wall, attempts have been made to study the interaction of division components with a lipid bilayer. As an initial approach, the CCTP of FtsZ was replaced with an amphipathic helix to bypass the need for membrane anchors. Such an FtsZ construct was able to bind to a lipid bilayer and, when placed inside vesicles, caused constriction (Osawa et al. 2008). Although GTP was necessary, its hydrolysis was not, indicating that just formation of the filaments on the membrane was responsible for the observed deformation of the vesicles.
Second generation reconstitution experiments used FtsZ and one of the natural membrane anchors, ZipA or FtsA. Reconstitution on a flat lipid bilayer showed recruitment of FtsZ filaments to the bilayer, with the proteins self-organizing into complex patterns (Loose and Mitchison 2014). Whereas ZipA led to FtsZ forming seemingly static but dynamic bundles, FtsA caused FtsZ to form dynamic vortices that underwent rapid reorganization; the dynamics of the system were due to treadmilling of FtsZ filaments. Although GTP was required (for FtsZ), surprisingly ATP was not (ADP being sufficient for FtsA). Reconstitution experiments have also been done with the proteins inside vesicles. By using an FtsA hyperactive mutant (FtsA*), which appears to behave better in vitro than the wild type protein, Z rings were occasionally observed within unilamellar vesicles and observed to constrict in an ATP and GTP dependent manner (Osawa and Erickson 2013). ZipA-containing vesicles have also been observed to shrink when FtsZ and GTP were included (Cabre et al. 2013). Together, these experiments demonstrated the remarkable ability of FtsA, ZipA and FtsZ to self-organize and raised the possibility that, together, they could provide a constrictive force in vivo.
Additional Roles of the Z Ring
Since the E. coli cell is under ~3 atmospheres of pressure, there has been speculation as to the force that drives septation. It is clear that peptidoglycan synthesis is required, as any block to PG synthesis halts septation (Spratt 1975). It was suggested that, in addition to a well-characterized scaffolding function, the Z ring provides a constrictive force at the leading edge of the septum (Erickson et al. 2010). This suggestion arose from the presence of FtsZ in mycoplasma, which lack a cell wall, and also from the ability of FtsZ to assemble both curved and straight filaments. This possible activity is supported by in vitro reconstitution studies, where FtsZ containing a membrane tether was observed to invaginate lipid vesicles (Osawa et al. 2008). However, the lack of an effect of varying FtsZ’s GTPase activity on the rate of constriction in vivo has been used to argue against this proposal (Coltharp et al. 2016) but this is still controversial. Even if the Z ring provides force in vivo, the constriction would be limited by the rate of PG synthesis. In any event, the Z ring is not the only contributor, as FtsZ leaves the invaginating septum before cytokinesis is complete (Soderstrom et al. 2014). Another role for the Z ring is as a guide for the invaginating septum. FtsZ mutants deficient in GTPase activity result in a twisted septum (Bi and Lutkenhaus 1992). Such mutants assemble what appears to be a normal ring but the stable FtsZ filaments become twisted into a spiral induced by cell wall growth. This observation has led to the idea that rapidly reorganizing FtsZ filaments are necessary to guide formation of a symmetrical septum (Dajkovic and Lutkenhaus 2006).
Spatial Regulation of the Z Ring
The Z ring is placed at midcell with great precision without the use of any known landmarks. So far two negative systems (Min and NO [nucleoid occlusion, mediated by SlmA]), and one positive system (Ter linkage) have been identified that contribute to the placement of the Z ring in E. coli (Lutkenhaus 2007; Mannik and Bailey 2015). The Min system prevents formation of the Z ring away from midcell while SlmA, responsible at least in part for NO, prevents formation of the Z ring over the nucleoid (Lutkenhaus 2007). In addition to this negative regulation, the Ter linkage promotes Z ring assembly in the vicinity of the Ter region on the nucleoids, near midcell (Bailey et al. 2014). The contribution of each system is revealed following its inactivation alone or in combination (Fig. 2.3). In some cases, the effect is growth rate dependent (Min– SlmA–) and at slow growth rates all three systems can be deleted and the Z ring still has a preference for midcell, indicating at least one additional mechanism must exist (Bailey et al. 2014).
Spatial regulation of the Z ring. Three systems contribute to the spatial regulation of the Z ring – Min, NO and Ter linkage and their influence is revealed following inactivation of a system alone or in combination. Under normal growth conditions inactivation of Min results in a dramatic phenotype with minicells and nucleoid containing cells of heterogeneous cell length, whereas a more subtle phenotype is observed with loss of the Ter linkage (ΔzapB) and none with loss of NO (ΔslmA). The phenotype due to deleting two or more of the systems depends upon growth rate. At fast growth rates loss of Min and SlmA leads to lethal filamentation whereas at slow growth rates cells survive but with central and polar rings. Loss of all three systems leads to additional polar rings
Min System
The Min system was recognized early-on as having an important role in spatial regulation of cytokinesis, since its absence (Δmin mutants) results in cytokinesis also occurring at the poles of the cell and, consequently, minicell formation (Adler et al. 1967) (Fig. 2.3). This regulation of cytokinesis operates at the level of Z ring formation (Bi and Lutkenhaus 1993; Pichoff and Lutkenhaus 2001). Consistent with this, the Min system consists of an antagonist of FtsZ assembly, MinC, which is recruited to the membrane by MinD and directed away from midcell by MinE (De Boer et al. 1989; Hu et al. 1999; Raskin and De Boer 1999a). This system in not static and the Min proteins rapidly oscillate between the ends of the cell, with the Z ring forming at midcell where their influence is at a minimum (Meinhardt and De Boer 2001). Two elements of the Min system have been under intense scrutiny. One is the mechanism of the oscillation and the other is the mechanism by which the Min system antagonizes FtsZ assembly.
Min Oscillation
The Min oscillator is a geometry sensing system with a preferred wavelength (on the order of 3–5 microns) (Varma et al. 2008; Wu et al. 2015). The important features of the oscillation were largely determined by manipulating the expression of the Min proteins in vivo and the mechanism was derived from studying their biochemistry, including in vitro reconstitution and computer simulations. The in vivo expression demonstrated that MinD and MinE were sufficient to establish the oscillation, that the ratio between them was critical, and that MinC was the division inhibitor and a passenger in the oscillation (De Boer et al. 1989; Raskin and De Boer 1999b; Hu and Lutkenhaus 1999). During the oscillation, MinD and MinC accumulate at one pole and are flanked by a MinE ring. As this ring moves closer to the pole, MinC and MinD are released and they re-assemble at the other pole, flanked again by a MinE ring (Fu et al. 2001; Hale et al. 2001) (Fig. 2.4a).
Min system. (a) The Min proteins oscillate between the poles of the cell as described in the text. (b) The dynamic interaction of MinD and MinE with the membrane is fueled by MinE stimulated ATP hydrolysis by MinD. (c) An FtsZ filament tethered to the membrane is attacked by MinCD and SlmA using a two-pronged mechanism (lightning bolts). These antagonists are recruited to FtsZ filaments through interaction with the CCTP resulting in a second interaction to break the filament
Biochemical and genetic studies revealed MinC acted directly on FtsZ filaments and that the dynamic interplay of the Min proteins with the membrane that underlies the oscillation is driven by MinD’s ATPase cycle (Hu et al. 1999; Hu and Lutkenhaus 2001). MinD dimerizes in the presence of ATP and binds to the membrane through a C-terminal amphipathic helix (Fig. 2.4b)(Szeto et al. 2002; Hu and Lutkenhaus 2003). Dimerization of MinD is necessary to provide sufficient membrane affinity and to generate binding sites for MinE and MinC, which overlap at the dimer interface (Wu et al. 2011). MinE binding to MinD stimulates the ATPase activity causing the release of MinD from the membrane (Hu et al. 2003; Lackner et al. 2003). The interaction of MinE with MinD is quite complex as MinE is in a latent form (6-beta strands) that must convert to the active form (4 –beta strands) to bind MinD (Park et al. 2011). The conversion of MinE to the active form also releases N-terminal amphipathic helices that allows MinE to bind the membrane as it binds MinD (Fig. 2.4b). Although MinE is a dimer and has two MinD binding sites the geometry of the complex only allows MinE to bind one side of a MinD dimer. After stimulating MinD’s ATPase and causing the release of MinD from the membrane, MinE can linger transiently on the membrane before returning to the latent form in the cytoplasm. This step gives MinE a chance to search for another membrane bound MinD to cooperatively remove MinD from the membrane before dissociating. Although genetic and biochemical studies indicate that MinE binding to one side of a MinD dimer is sufficient to stimulate the ATPase activity (Park et al. 2012), a recent model invokes a MinE dimer binding on each side of MinD to stimulate ATP hydrolysis (Vecchiarelli et al. 2016). This 2:1 ratio may explain the existence of the MinE ring.
The recruitment of MinC to the membrane by MinD generates a complex that can antagonize FtsZ assembly (De Boer et al. 1989; Pichoff and Lutkenhaus 2001). Since MinD forms a symmetric dimer, it has a binding site for MinC on each side (Fig. 2.4c). Also, since MinC is a dimer it has the potential to bridge MinD dimers to form an alternating copolymer attached to the membrane. In fact, such unusual copolymers form in vitro and in vivo when overexpressed (Ghosal et al. 2014; Conti et al. 2015). However, mutations that separate copolymer formation from MinC-MinD interaction do not affect Min function indicating that such copolymers are not necessary for Min function (Park et al. 2015). Also, the excess of MinD over MinC in vivo (7 to 1) does not favor copolymers (Li et al. 2014).
Due to the relative simplicity the Min oscillation, it was modeled to determine how the oscillatory behavior could be achieved. Many models were developed that generate the oscillatory behavior in silico (Meinhardt and De Boer 2001; Huang et al. 2003; Kruse et al. 2007; Fange and Elf 2006). A general feature of the models is that MinD displays cooperative attachment to the membrane and recruits MinE, which stimulates MinD’s ATPase causing the release of MinD. The MinD released from a polar region undergoes nucleotide exchange before it can rebind, which gives it a chance to escape the pole. If it does not diffuse away, it can readily rebind due to the presence of membrane bound MinD, but if it diffuses away it takes a higher concentration to bind to the other pole, as it has to overcome the absence of membrane-bound MinD (Fig. 2.4a). This property of MinD, along with the sequestration of MinE in a MinE ring at the edge of the MinD polar zone, drives the oscillation (Huang et al. 2003; Halatek and Frey 2012).
Importantly, pattern formation by the Min system has been reconstituted in vitro on planar lipid bilayers as well as in 3-dimensional objects (Loose et al. 2008; Ivanov and Mizuuchi 2010; Schweizer et al. 2012). The reconstitution confirmed that pattern formation by the Min system only required MinD, MinE, a lipid bilayer and ATP. On a planar bilayer MinD and MinE form waves that migrate across the surface with the concentration of MinE highest at the rear of the wave. For reasons that are not clear the wavelength is much larger than observed in vivo. MinC also oscillates when it was included and these reconstituted Min patterns alternating with FtsZ assemblies (using FtsZ with a membrane tether) on the lipid bilayers (Arumugam et al. 2014). These patterns form even though the densities of the Min proteins on the membrane are quite high and Min proteins are not depleted from the overlying solution. Modeling, however, indicates that depletion of the Min proteins from the cytoplasm plays an important role (Huang et al. 2003). In a more recent reconstitution system, conditions were found in which pattern formation was accompanied by Min protein depletion from the overlying solution. Under these conditions radially enlarging disks of MinD were formed that were surrounded by a MinE ring, which then rapidly decreased in diameter (Vecchiarelli et al. 2016). These disks oscillated with neighboring disks and are likely to better reflect the in vivo situation.
MinC/MinD Antagonism of Z Ring Formation
MinC has two functional domains, an N-terminal domain (MinCN) that interacts directly with the globular domain of FtsZ and antagonizes polymerization in vitro and a C- terminal domain (MinCC) that binds MinD and the CCTP of FtsZ (Hu and Lutkenhaus 2000; Cordell et al. 2001; Dajkovic et al. 2008). Based upon the ability of MinCN to antagonize assembly in vitro and the isolation of FtsZ mutants resistant to the two domains of MinC, a model emerged for MinC antagonism (Shen and Lutkenhaus 2009, 2010). In this model the interaction of MinCC/MinD with the CCTP and the membrane positions MinCN near the FtsZ filament, leading to breakage of the filament (Fig. 2.4c). Based upon the location of residues altered by resistance mutations, the target of MinCN is helix 10 of FtsZ, which is near the junction of two subunits in an FtsZ filament.
MinCC/MinD binds to the CCTP of FtsZ and can compete with FtsA and ZipA (Shen and Lutkenhaus 2009). Thus, it is possible that the oscillation of the Min proteins delays formation of the Z ring through competition for the CCTP. In smaller cells there is likely to be less of an area at midcell free of the oscillating MinC/MinD than in longer cells. Interestingly, FtsA* has higher affinity for the CCTP, which may explain why it has some resistance to MinC/MinD (Geissler et al. 2007; Pichoff et al. 2012). This model may also explain why FtsA* cells are able to assemble a Z ring at a smaller cell size than wild type cells (Geissler et al. 2007).
Min and DNA Segregation
There are reports that the inactivation of the Min system affects DNA segregation (Akerlund et al. 2002; Di Ventura et al. 2013). One effect is due to the lack of resolution of chromosome dimers in a fraction of cells in a Min mutant.
The main phenotype of Min mutants is the formation of minicells and the heterogeneous length of nucleoid containing cells. This is due to minicell divisions occurring at the expense of medial divisions resulting in an increased average cell length of the nucleated cells. Chromosome dimers form in approximately 10% of cells per cell-cycle, due to recombination between newly formed daughter chromosomes (Steiner et al. 1999). Separation of chromosome dimers is effected by the divisome protein FtsK (see section “FtsK”) and therefore occurs during division at midcell. However, a chromosome dimer present in a nucleated cell that produces a minicell and skips division at midcell cannot be rescued by the dimer resolution system; this results in a dramatic segregation defect in that cell. Other reports indicate that loss of Min causes a slight delay in bulk chromosome segregation in all cells in the population (Akerlund et al. 2002; Di Ventura et al. 2013). The basis for such an effect is unclear, although it was recently reported that MinD binds DNA and that the Min oscillation may serve to assist DNA segregation. However, the minD mutation studied affected membrane binding as well as DNA binding, questioning such a possibility (Di Ventura et al. 2013).
Nucleoid Occlusion and SlmA
A long-standing observation in bacterial cell biology is that cytokinesis over the nucleoid, which would result in guillotining of the chromosome, is rarely observed. This led to the concept of nucleoid occlusion – that, somehow, the nucleoid prevented cytokinesis in its vicinity (Woldringh et al. 1990). Later it was shown that the Z ring does not form over the nucleoid in cells where division was blocked, resulting in the hypothesis that the nucleoid exerted a negative effect on Z ring assembly (Yu and Margolin 1999). Factors responsible were identified in both E. coli and B. subtilis. In both cases a protein was identified that bound to sites (~50) that were present in the oriC proximal two third of the chromosome but absent in the Ter region (Wu et al. 2009; Bernhardt and De Boer 2005; Cho et al. 2011).
In E. coli the SlmA protein, a member of the TetR repressor family, was found to mediate NO and interact directly with FtsZ (Cho et al. 2011). An unrelated protein in B. subtilis (Noc) is functionally analogous but does not interact directly with FtsZ (Adams et al. 2015).
In the absence of DNA, SlmA weakly antagonizes FtsZ assembly, but this activity is dramatically enhanced upon binding to DNA containing a SlmA binding site (SBS) (Cho et al. 2011; Cho and Bernhardt 2013). The structure of free SlmA and SlmA bound to an SBS revealed that SlmA undergoes a conformational change upon DNA binding that is typical of TetR family members, leading to increased affinity for FtsZ (Tonthat et al. 2011; Tonthat et al. 2013). Residues affected by the conformational change are required for SlmA function and the recent structure of DNA/SlmA/CCTP peptide complex confirms these residues are involved in binding to the CCTP of FtsZ (Schumacher and Zeng 2016). SlmA is a dimer and binds cooperatively to an SBS site as a dimer of dimers, with the possibility that additional dimers are also recruited. A cell contains about 5 SlmA dimers per SBS binding site on the chromosome (Li et al. 2014).
The ability of SlmA bound to an SBS to depolymerize FtsZ requires the CCTP (Du and Lutkenhaus 2014). Furthermore, the interaction of SlmA with a monomer of FtsZ is of low affinity, similar to the interaction of the CCTP with ZipA and FtsA (Du et al. 2015). However, as observed with ZipA, the affinity is dramatically enhanced by avidity, that is, by a multimer of FtsZ binding to SlmA dimers. Thus, SlmA bound to an SBS preferentially binds to polymers of FtsZ. Once SlmA is bound to a polymer of FtsZ it is thought to break the polymer, although the mechanism is not clear (Cho and Bernhardt 2013; Cho et al. 2011). A mutation altering a residue in the long helix 7, which connects the two globular domains of FtsZ, is resistant to the action of SlmA (Du and Lutkenhaus 2014). This suggests that SlmA bound to an FtsZ filament through interaction with the CCTP makes an additional contact to stimulate GTP hydrolysis (Fig. 2.4c). Thus, SlmA, like MinC/MinD, uses a two-pronged mechanism to attack FtsZ filaments (Du and Lutkenhaus 2014).
During the cell cycle SlmA bound to the chromosome is transported away from midcell by segregation of the chromosome (Fig. 2.3, center). This movement creates a void at midcell making it permissive for FtsZ filaments to persist at the membrane, whereas delays in segregation, for example, caused by disruption of DNA replication or segregation, prevent this movement and formation of the Z ring at midcell (Bernhardt and De Boer 2005). Although SlmA can clearly prevent assembly of the Z ring in the vicinity of an unsegregated nucleoid, its role in the normal placement of the Z ring is not clear. Deletion of SlmA does not affect cell morphology, due to the dominance of the Min system, however, when both Min and SlmA are deleted cell division is prevented at fast growth rates (Bernhardt and De Boer 2005). Under these conditions FtsZ filaments are distributed throughout the cell and sufficient filaments are unable to accumulate at any one position in the cell to form a complete Z ring (Fig. 2.3). Consistent with this, raising the level of FtsZ rescues such cells, and results in Z rings at midcell and also at the poles. Cells lacking Min and SlmA are also rescued by growth at high temperature or in minimal medium (Bernhardt and De Boer 2005; Shen and Lutkenhaus 2010). Under this latter condition, cytokinesis occurs mostly at midcell and few minicells are produced, indicating additional mechanisms of directing Z rings to midcell (Fig. 2.3). This led to the discovery of the Ter linkage and its role in Z ring formation.
Ter Linkage
The impetus for looking for positive Z ring regulation in addition to the two negative systems came from work in both E. coli and B. subtilis. Work in B. subtilis revealed that in the absence of the two negative regulatory systems (Min and NO) the Z ring is placed at midcell in outgrowing spores with good precision but with reduced efficiency (Rodrigues and Harry 2012). Examination of E. coli forced to grow at reduced diameter revealed misshapen cells, but with Z rings placed between the nucleoids, even in the absence of Min and SlmA, suggesting the chromosome itself may provide positional information (Mannik et al. 2012). This result, combined with the observation that Z rings still form at midcell (as well as the poles) in the absence of Min and SlmA at slow growth rates, led to a search for a possible mechanism (Fig. 2.3).
Time-lapse microscopy of a mutant lacking both Min and SlmA growing in minimal medium (which suppresses the otherwise lethal effect of deleting both of these systems) revealed that the position of Z rings correlated more precisely with the center of segregating chromosomes than with the middle of the cell (Bailey et al. 2014). In these cells, Z ring formation over the nucleoid was preceded by the arrival of the Ter region. In newborn cells, the Ter region is located at the poles but rapidly snaps to the cell center as the Ter region is replicated (Espeli et al. 2012). Since the Ter region is organized into a distinct macrodomain by MatP (Mercier et al. 2008), this led to a model in which the MatP-Ter region is linked to the Z ring through ZapA and ZapB, a model supported by super resolution microscopy (Buss et al. 2015) (Fig. 2.3).
At slow growth, a ∆min ∆slmA double mutant is rescued and rings form at midcell and the poles but the polar rings infrequently lead to minicells (Bailey et al. 2014) (Fig. 2.3). However, disruption of the Ter linkage by deleting one of the components disrupts the precision of the placement of the Z ring at midcell and dramatically increases the frequency by which polar rings form and constrict. This observation indicates that the Ter linkage aids the positioning of the Z ring and promotes its usage at midcell.
Overlap in Spatial Regulation
As described above, at least three mechanisms have been identified that contribute to the positioning of the Z ring in E. coli (Fig. 2.3). Together, these systems ensure that the Z ring is formed precisely at midcell. Several observations indicate the Min system is the major determinant of Z ring positioning under normal growth conditions. First, deletion of the Min system has a dramatic morphological effect as polar rings form, minicells are produced and the average cell length is increased due to minicell divisions occurring at the expense of medial divisions (Teather et al. 1974; Bi and Lutkenhaus 1990). Second, in wild type cells the Z ring can occasionally form before Ter relocates from the pole to midcell (Bailey et al. 2014). Third, in anucleate cells (therefore lacking SlmA and Ter) the Z ring is positioned at midcell (presumably by Min) with only slightly less precision than in WT cells (Sun et al. 1998). In contrast, deletion of just SlmA has no obvious effect on Z ring assembly or cell morphology during normal growth (Bernhardt and De Boer 2005) and disrupting the Ter linkage (deletion of ZapA or ZapB) only results in a 15% increase in the average cell length and some skewed Z rings (Fig. 2.3) (Hale et al. 2011).
From Z Ring to Divisome, Recruitment and Activation
Once the Z ring is established, additional essential proteins are recruited to form the divisome (Fig. 2.5a). These proteins must carry out septal PG synthesis. How these proteins are recruited to the Z ring and activated is an active area of investigation.
The Z ring organizes the divisome. (a) FtsZ, FtsA and ZipA form the Z ring and FtsEX is added. After a delay the remaining proteins are added to form the divisome. Although all are essential under standard growth conditions they have been divided into core (black) and non-core (red). (b) The non-core proteins can be bypassed by FtsA* mutations (green). Under these conditions (for example, loss of ZipA) the interaction of FtsN with FtsA may be responsible for back recruiting the other division proteins
Downstream Proteins and Septal PG Synthesis
The Z ring functions as a scaffold to organize the septal PG synthesis, analogous to MreB functioning as an organizer of a PG machine for cell elongation (elongasome) (Egan et al. 2015, Szwedziak and Lowe 2013). Whereas the recruitment pathway for the elongasome is difficult to assess (MreB and components are dispersed throughout the length of the cell), the recruitment pathway for the divisome is accessible to study due to its unique occurrence in the cell. A summary of these divisome proteins and their function precedes a discussion of their recruitment.
FtsEX
FtsEX is a member of the ABC transporter family, although FtsEX is not thought to transport small molecules (Schmidt et al. 2004). The closest homologue to FtsEX is the LolCDE system, which extracts lipidated proteins from the cytoplasmic membrane for transfer to the outer membrane (Narita and Tokuda 2006). FtsEX localizes relatively early to the Z ring and appears to play three roles in cell division (Corbin et al. 2007). Two roles are essential: (1) FtsEX recruits downstream proteins and (2) FtsEX’s ATPase activity is required for the initiation of constriction (Arends et al. 2009). The requirement for FtsEX in these two roles can be suppressed by high osmolarity, overexpression of FtsN or by mutations in ftsA (ftsA*) (Reddy 2007). FtsE mutants unable to bind or hydrolyze ATP are able to carry out the recruitment function, but are unable to promote the start of constriction (Arends et al. 2009). Recent evidence indicates FtsEX carries out these two functions by modulating the activity of FtsA, possibly by altering its polymerization state (Du et al. 2016).
In addition to these two essential roles, FtsEX also recruits the periplasmic protein EnvC and together they activate two periplasmic amidases, in a step requiring ATP hydrolysis by FtsE (Yang et al. 2011). Thus, FtsEX couples ATP hydrolysis in the cytoplasm to the start of constriction and to the splitting of the bonds cleaved by the amidases. High osmolarity does not suppress the requirement for FtsEX for activation of the two amidases and cells have a mild chaining defect. A third amidase can carry out the cleavage step, but does so less efficiently. In the absence of all three amidases, cell chaining is quite dramatic but is not lethal, indicating other hydrolytic enzymes can do this inefficiently (Heidrich et al. 2001).
FtsK
FtsK is a DNA translocase that has 4 N-terminal transmembrane segments connected to a translocase domain through a long linker (Begg et al. 1995). The only essential role of FtsK is its role in the recruitment pathway, which only requires the N-terminal membrane region (Wang and Lutkenhaus 1998). Two separate domains within the long linker interact with the Z ring, possibly by interacting directly with FtsZ (Dubarry et al. 2010). FtsK is able to translocate DNA and this C-terminal domain is required for the resolution of chromosome dimers (formed in ~10% of cells by homologous recombination between daughter chromosomes) by stimulating the XerCD recombinase. The localization of FtsK to the septum spatially restricts dimer resolution to the septal region (Steiner et al. 1999).
FtsQ, FtsL and FtsB
The FtsQLB proteins form a complex that has been extensively characterized by genetic and biochemical approaches (Buddelmeijer and Beckwith 2004). All three proteins are single-pass membrane proteins. Although the proteins show poor conservation at the amino acid level, a careful phylogenetic analysis revealed they are highly conserved throughout bacteria with a cell wall (Gonzalez et al. 2010). FtsL and FtsB form coiled-coil proteins with the transmembrane region contributing to the formation of the alpha helical region in the periplasm (Gonzalez and Beckwith 2009; Gonzalez et al. 2010; Glas et al. 2015). The three proteins interact in the periplasm through their extreme C-terminal regions. Mutations in ftsL and ftsB that reduce or eliminate the requirement for FtsN map to a region in the middle of the coiled-coil domain of each protein (possibly a kink in the coil) and these regions were designated CCD, for control of cell division (Tsang and Bernhardt 2015; Liu et al. 2015).
FtsW
FtsW is a multipass transmembrane protein (10 transmembrane segments) that is highly conserved and a member of the SEDS (shape, elongation, division and sporulation) family (Boyle et al. 1997; Pastoret et al. 2004). Other family members include RodA, involved in cell elongation, and SpoVE, involved in spore formation in B. subtilis (not present in E. coli). Some biochemical evidence suggests that FtsW may serve as a flippase for lipid II (required for peptidoglycan synthesis) (Mohammadi et al. 2011); however, genetic evidence indicates that the flippase is MurJ (Meeske et al. 2015). Members of the SEDS family are each associated with a specific penicillin-binding protein (FtsW with FtsI [PBP3] and RodA with PBP2) (Typas et al. 2012). Recent biochemical and genetic evidence indicates RodA is a transglycosylace suggesting that FBW is also a transglycosylace (Meeske et al. 2016; Cho et al. 2016). It so then syptal PG synthesis is carried out by FBW and FBI(PBP3) acting out together to carry out the two necessary enzymatic activities(transglycosylace and transpeptidation. The role of the higher molecular weight PBPs (PBP la and PBP lb), long thought to be the primary synthesis is unclear, but might be needed to remade and strengthen PG made by FBW and FBI.
FtsI
FtsI encodes penicillin-binding protein 3 (PBP3), which is dedicated to cytokinesis (Spratt 1975). It is a single pass integral membrane protein with the periplasmic domain composed of two domains, a C-terminal transpeptidase domain preceded by a domain of unknown function. FtsI depends upon FtsW for localization to the division site (Mercer and Weiss 2002). The transmembrane domain is responsible for recruitment to the septum and mutations in the domain of unknown function prevent FtsN recruitment (Wissel and Weiss 2004). Biochemical studies indicate FtsI interacts with a number of proteins involved in cell wall synthesis, including PBP1b and FtsN (Muller et al. 2007).
FtsN
FtsN is a single pass integral membrane protein and is the last essential protein to arrive at the division site (Dai et al. 1993, 1996; Addinall et al. 1997). The cytoplasmic N-terminal domain interacts with FtsA (Busiek et al. 2012; Pichoff et al. 2015; Liu et al. 2015) and the C-terminal domain constitutes a SPOR domain that binds to denuded glycan chains (Gerding et al. 2009; Yahashiri et al. 2015). These chains are formed transiently at the septum by amidases that cleave the peptide side chains during processing of septal PG. Connecting these two domains is a region that is predicted to contain 3 small helical regions (Yang et al. 2004). One of these constitutes the essential region of FtsN (E domain) that activates septal PG synthesis (Gerding et al. 2009). This region is the only absolutely essential region of FtsN, since it can complement an FtsN depletion strain when it is overexpressed and exported to the periplasm. However, at the physiological level the N-terminal cytoplasmic and C-terminal SPOR domains are essential in order to efficiently localize the E domain to the septum (Busiek and Margolin 2014; Pichoff et al. 2015).
Septal PG Machine
Estimates of protein copy numbers indicate there are ~150 molecules per cell of FtsQ, FtsL, FtsB, FtsW, FtsI, FtsN at slow growth rates (Li et al. 2014). As mentioned, FtsQLB form a well-documented complex that persists even outside of the Z ring. The similar copy numbers of these divisome proteins and the web of interactions among them suggest they are in a stoichiometric complex, which would constitute a septal PG biosynthetic machine (Li et al. 2014). In fact, a large complex containing many of these proteins was detected by native gel electrophoresis (Trip and Scheffers 2015). E. coli has 3 class A PBPs – 1a, 1b and 1c (have both transglycosylase and transpeptidase activities). Either PBP1a or PBP1b is sufficient for survival, suggesting overlap in the function of these two PBPs (Yousif et al. 1985). However, cells growing with only PBP1a are less robust, indicating PBP1b is the more important synthetic enzyme. PBP1b, but not PBP1a, is known to interact with FtsN and FtsI and is also present in a similar copy number (Muller et al. 2007). Since PBP1b appears to be a dimer it was suggested that the septal PG machine exists as a dimeric complex, with PBP1b at the core and two copies of each of the Fts proteins (FtsQ-N) (Egan and Vollmer 2015). This means that there are only ~75 of these dimeric septal PG machines present in slow growing cells. The level of FtsK is about the same as the others, but it is known to form a hexamer and, thus, may not be a stoichiometric part of the machine (Bisicchia et al. 2013b). Consistent with this, FtsK can be bypassed with little cost to the cell by mutations in ftsA (Geissler and Margolin 2005).
Recruitment of Divisome Components to the Z Ring
Once the Z ring is established, the remaining proteins are recruited. The initial approaches used to examine the recruitment pathway resulted in a mostly linear dependency pathway; however, subsequent studies have found a web of interactions that can contribute to localization.
Depletion Studies Leading to a Linear Dependency Pathway
A mostly linear dependency pathway for the recruitment of downstream division proteins to the Z ring arose from depleting individual components and seeing which proteins are still recruited (Fig. 2.5a). For example, following depletion of FtsL the Z ring forms and FtsK and FtsQ localize but the other essential proteins do not (Ghigo et al. 1999). This sort of analysis resulted in the elaboration of the linear sequential pathway. With the realization that FtsQLB form a complex outside of the Z ring, it is clear that depletion of FtsL disrupts this complex (Buddelmeijer and Beckwith 2004). Nonetheless, FtsQ localizes in the absence of FtsL demonstrating that FtsQ can localize to the Z ring in the absence of its partners, and thus contains the information for recruitment (Gonzalez et al. 2010). Targeting FtsL directly to the Z ring (by fusion to ZapA, see below) revealed that it was able to recruit FtsB and downstream proteins even in the absence of FtsQ (Goehring et al. 2006). Thus, the FtsLB complex can recruit the downstream proteins in the absence of FtsQ, indicating that the information for recruiting downstream proteins lies within FtsL and FtsB, whereas FtsQ is required to bring FtsLB to the Z ring.
Lessons from Fusions Leading to Forced Localization
Another approach used to study protein recruitment to the division site is to fuse a protein in the middle of the pathway to a protein that binds directly to FtsZ, such as ZapA (Goehring et al. 2005). Such a fusion can recruit proteins downstream, but can also back-recruit upstream proteins. This recruitment occurred even if FtsA, which interacts with several downstream proteins, is depleted. Thus, for example, ZapA-FtsQ can recruit both the upstream protein FtsK and downstream proteins like FtsI to the Z ring in the absence of FtsA, suggesting FtsA’s role is indirect. One notable exception among the downstream proteins was FtsN, which is not recruited by ZapA fusions if FtsA was removed. Also, FtsN is not recruited by ZapA fusions to other proteins (FtsL, FtsW) if FtsQ was removed (Goehring et al. 2006). Thus, the recruitment of FtsN is complex and dependent upon FtsI, as shown in the linear recruitment assay, but is also dependent upon FtsQ and FtsA, as revealed by the ZapA fusions.
In another approach, the long linker of FtsK (that connects the membrane region to the DNA translocase domain) was fused to a number of integral membrane division proteins (Dubarry et al. 2010). Fusion of just the linker region to FtsW resulted in normal cellular morphology (in the absence of FtsK), indicating that the other regions of FtsK are dispensable and that the only essential function of FtsK is participation in the recruitment pathway. This analysis also revealed that the linker region had two separable regions that interacted with the Z ring and was capable of bringing FtsW, and all other proteins, to the Z ring.
Bypass Mutations Suggest Some Components Are Core Whereas Others Are Non-core
In yet another approach to explore divisome assembly, conditions were explored that bypass one or more of the normally essential genes. The initial approach showed that ZipA could be bypassed by a mutation in ftsA, designated ftsA* (Geissler et al. 2003) (Fig. 2.5b). This mutation could also bypass FtsEX and FtsK without much of an effect on cell morphology (Geissler and Margolin 2005; Reddy 2007). Subsequent work showed that many ftsA mutations could bypass ZipA and that almost all of these mutations reduced the ability of FtsA to self-interact (Pichoff et al. 2012). A reasonable possibility is that these mutations in ftsA decrease its oligomerization status and increase the affinity between FtsA and some downstream component, making ZipA, FtsK or FtsEX (normally part of the linear recruitment pathway) dispensable.
The relative ease at which ZipA, FtsK and FtsEX can be bypassed suggests that their addition to the core divisome (FtsZ, FtsA, FtsQLB, FtsI, FtsW, FtsN) improves coordination of chromosome segregation with cytokinesis (FtsK) or enhances coordination of septal PG synthesis and hydrolysis (FtsEX). ZipA may have been added to link additional (nonessential) proteins to the Z ring. FtsN can also be bypassed by some mutations in other components but there is more cost associated with the bypass, as cells are elongated (Tsang and Bernhardt 2015; Liu et al. 2015). Such mutations map to ftsL, ftsB and ftsA and lead to a more activated divisome that is less dependent upon FtsN. Most of the genes for the core components map to a large cluster of genes at the 2 min region (all except for ftsB and ftsN) and encode the machinery for the minimal Z ring (FtsZ and FtsA), septal PG synthesis (FtsW and FtsI), the connector (FtsQLB) and the activator (FtsN).
Role of FtsA and ZipA in Recruitment of Downstream Proteins
Although a Z ring can form with either FtsA or ZipA providing the membrane connection, both are required for downstream proteins to be recruited (Pichoff and Lutkenhaus 2002). Of the two, FtsA is the more important player in divisome assembly, since mutations in ftsA can bypass ZipA (Geissler et al. 2003) and FtsA, but not ZipA, is known to interact with a number of downstream proteins (Karimova et al. 2005). Since the ftsA mutations that bypass ZipA were found to reduce the ability of FtsA to self-interact, it led to a model in which FtsA monomers are the form of FtsA active in the recruitment pathway (Pichoff et al. 2012). In this model ZipA’s essential role is to reduce FtsA’s oligomerization state, possibly by competing for the CCTP of FtsZ (Fig. 2.6). Also, in this model FtsA’s self-interaction competes with the interaction of downstream proteins for FtsA; domain 1C of FtsA is involved in both self- interaction and interaction with FtsN, but the two interactions are proposed to be mutually exclusive. Overproduction of FtsN also bypasses ZipA and the FtsA-FtsN interaction is required (Pichoff et al. 2015). It is likely that the overproduced FtsN promotes the FtsA interaction. Overproduction of FtsN also bypasses FtsEX and this bypass also requires the FtsA-FtsN interaction. Since loss of FtsEX disrupts the normal recruitment pathway, it raises the possibility that the FtsA-FtsN interaction is responsible for back recruiting all divisome components to the ring under these conditions (Fig. 2.5b). Back recruitment by FtsN may also explain why FtsA*, impaired for self-interaction, can bypass the need for FtsEX (Reddy 2007), as it may favor the FtsA-FtsN interaction.
Assembly of the divisome and activation of septal PG synthesis. (a) Divisome assembly. FtsZ filaments are tethered to the membrane by FtsA and ZipA. The arrival of FtsEX promotes monomeric FtsA at the Z ring, which results in recruitment of downstream proteins. These proteins are held in an inactive state by FtsQLB, FtsA and FtsW. (b) Divisome activation. The arrival of FtsN at the septum activates the divisome. The N-terminus of FtsN interacts with nonpolymerized FtsA in the cytoplasm and the E domain acts in the periplasm to overcome the inhibition of PG synthesis by FtsQLB and W. FtsN at the septum is reinforced by the SPOR domain interacting with denuded glycan chains formed by the action of amidases controlled by FtsEX. FtsEX acts on FtsA and continuous ATP hydrolysis by FtsEX is required for both PG synthesis and hydrolysis
The Role of FtsN in Activation of the Divisome
The arrival of FtsN to the divisome signals that the divisome is complete and septal PG biosynthesis can be initiated (Gerding et al. 2009; Lutkenhaus 2009; Weiss 2015). Recruitment of FtsN to the septum is quite complex and requires FtsA, FtsQ and FtsI (section “Lessons from fusions leading to forced localization”) (Addinall et al. 1997; Goehring et al. 2006). If FtsN interacts with FtsA, why does it depend upon FtsI and FtsQ and why is it not recruited to the Z ring as soon as it is formed? Overexpression of the N-terminal domain of FtsN (cyto and transmembrane domains) leads to relatively early recruitment to the ring (Busiek and Margolin 2014). This recruitment is dependent upon FtsA but, in contrast to full length FtsN, is independent of FtsQ and FtsI. Possibly, FtsN is in a complex with other proteins (it is known to interact with PBP1b and FtsI) that impedes its recruitment to the septum, constraints which don’t apply to the N-terminal domain.
In the current self-enhancing model (Gerding et al. 2009), the recruitment of FtsN to the septum is initiated by the cytoplasmic N-terminus interacting with an FtsA monomer in order to bring the E domain to the divisome to activate septal PG synthesis (Liu et al. 2015; Gerding et al. 2009). The activation of PG synthesis ultimately leads to amidase activation through FtsEX and EnvC (Yang et al. 2011). This is turn reinforces the recruitment of FtsN to the septal region, by the SPOR domain binding to PG strands processed by the amidases (Yahashiri et al. 2015). Many additional proteins are then recruited to the septum in an FtsN-dependent fashion, although it is likely to be indirect, since it is the activation of septal PG synthesis that is required (Gerding et al. 2009).
How PG synthesis is activated by the E domain is not clear; however, the isolation of mutations that bypass the E domain provides some possible clues and implicates FtsQLB and FtsA in the regulatory pathway (Tsang and Bernhardt 2015; Liu et al. 2015). In this model, FtsQLB and FtsA are recruited to the septum in the off state and the arrival of FtsN pushes the system to the on state with the E domain acting in the periplasm and the N-terminus of FtsN acting in the cytoplasm (Fig. 2.6). In this model FtsN acting in the cytoplasm interacts with FtsA that has a reduced oligomerization state and the E domain in the periplasm activates septal PG synthesis. Originally it was thought that the E domain activated PG synthesis directly (possibly by acting on PBP3); however, the isolation of the E bypass mutations in ftsL and ftsB suggests that the E domain relieves inhibition of PG synthesis imposed by FtsQLB. FtsN tethered to FtsA in the cytoplasm may also act as a link to tether septal PG synthesis to the Z ring.
A recent investigation into the essential roles of FtsEX indicate that FtsEX acts on FtsA to promote recruitment of downstream proteins and appears to do this by promoting FtsA monomers (Du et al. 2016). It was also shown that continual ATP hydrolysis by FtsEX was required for septal PG synthesis to occur (Yang et al. 2011). Since the role of FtsEX in regulating septal PG hydrolysis is well established, these results indicate that FtsEX coordinates septal PG synthesis with septal PG hydrolysis.
One of the striking phenotypes of a strong mutation in ftsB or ftsL (that bypasses the E domain of FtsN) is the smaller cell size associated with these mutations. Although they bypass FtsN, FtsN is still required for the small cell phenotype (Tsang and Bernhardt 2015; Liu et al. 2015). Cell cycle analysis suggests that the smaller cell size is due to FtsQLB (with one of the strong mutations) overcoming the delay normally observed between formation of the Z ring and arrival of the late divisome components (such as FtsQLB). It is not clear why there is a delay between the establishment of the Z ring and the formation of the mature divisome in wild type cells, but understanding how these mutations work should illuminate the mechanism behind this delay.
In Caulobacter crescentus, cytokinesis is inhibited following DNA damage, by the production of small integral membrane peptides that bind to and inhibit FtsN or FtsW (Modell et al. 2011, 2014). A smaller cell size is observed with mutations in ftsW that provide resistance to these inhibitors. In E. coli, FtsW is also implicated in the regulation of the onset of cytokinesis (Du et al. 2016). This suggests that the whole divisome (FtsQLBWI and PBP1b) is held in an inactive state and needs to be activated by FtsN.
Enigmatic Role of MreB and the Cell Elongation System in Cytokinesis
MreB is more closely related to actin than FtsA and is necessary for the maintenance of rod shape in many bacteria (Van Den Ent et al. 2001). MreB assembles into filaments at the membrane that organize the machinery for synthesizing peptidoglycan around the cylinder of the cell, necessary for elongation (elongasome) (Typas et al. 2012). This system has some components that are functionally analogous to some components of the cell division machinery. For example, RodA is related to FtsW and PBP2 is related to PBP3 and presumably they carry out analogous biochemical activities (Typas et al. 2012). A role for MreB in cytokinesis is not obvious, as genes of the elongation machinery can be deleted and cells grow and divide with a spherical morphology. Deletion of RodA, PBP2 or MreB is well tolerated at slow growth rates; however, at faster growth rates intracellular vesicles accumulate due to excess membrane synthesis and growth is impaired (Bendezu and De Boer 2008).
The fact that the elongation genes can be deleted indicates they are not essential for cytokinesis; however, a role is suggested by several results. MreB and PBP2 are recruited relatively early to the Z ring, suggesting some role in division (Van Der Ploeg et al. 2013). In at least some bacteria the Z ring can drive cell elongation before switching to a constriction mode. However, in Caulobacter crescentus, where this step is quite extensive, MreB is not required (Aaron et al. 2007). Notably, MreB interacts directly with FtsZ and mutants that fail to interact fail to divide (Fenton and Gerdes 2013). This raises the possibility that MreB is required for the division apparatus to make the transition from a cylindrical wall to initiate constriction.
Splitting the Septum
In E. coli the cell wall is split by amidases as the septum forms, resulting in a constriction. Deletion of the three amidase genes (amiA, amiB and amiC), but not just two, results in a severe chaining phenotype, but is not lethal (Heidrich et al. 2001). This result indicates that the amidases are primarily responsible for splitting the septum but other enzymes can do so inefficiently. The amidases remove the peptide stems from peptidoglycan, resulting in denuded glycan chains that exist transiently at the septum. The existence of denuded glycan chains was deduced from the biochemical binding properties of SPOR domains (Ursinus et al. 2004) and the amidase dependent localization of SPOR domain-containing proteins, of which, E. coli has four (FtsN, RplA, DamX and DedD) (Gerding et al. 2009; Arends et al. 2010). This was further supported by demonstrating the loss of SPOR-binding after treating PG ghosts with an enzyme that is specific to denuded glycan chains (Yahashiri et al. 2015).
Importantly, the amidases require activators; AmiA and AmiB require EnvC and AmiC requires NlpD (Uehara et al. 2010). EnvC is recruited to the septum rather early by FtsEX, whereas the amidases depend on FtsN to activate the divisome before they localize (Peters et al. 2011). The recruitment of EnvC by FtsEX occurs in the absence of ATP, whereas the activation of the amidase requires ATP hydrolysis. These results suggest that hydrolysis of ATP by FtsEX modulates EnvC so that the autoinhibition of AmiB is relieved (Yang et al. 2012). Mutants defective in the Tol-Pal complex display defects in outer membrane integrity and in cell division. Subsequent studies revealed that these proteins (TolQARB and Pal) localize to the septum and have an important role in ensuring that the outer membrane follows the growth of the invaginating cell wall (Gerding et al. 2007).
Cell Size Regulation
Cell size in E. coli increases with growth rate, increasing in both width and length (Pierucci 1978). This increase in cell size accommodates the increased DNA content of fast growing cells due to multifork replication (Cooper and Helmstetter 1968). The doubling time of fast growing cells is less than the time to replicate the chromosome (~40 min); however, this problem is solved by initiation of another round of replication before the previous round is finished.
Metabolic Regulation
How is the increase in cell size as a function to growth rate regulated? In B. subtilis it was found that division is transiently delayed in fast growing cells to achieve an increased cell size and that the level of UDP-glucose provided a metabolic signal (Weart et al. 2007). The UDP-glucose effect is mediated by an enzyme (UgtP) in a pathway for synthesis of a cell wall sugar. At higher growth rates the increased level of UDP-glucose stimulates the interaction between UgtP and FtsZ, delaying assembly of the Z ring. Consistent with this, inactivation of the UgtP pathway does not affect the growth rate but results in cells that are ~25% smaller. A similar nutrient-sensing pathway also exists in E. coli and also involves UDP-glucose but in a pathway for a periplasmic polysaccharide (Hill et al. 2013). The enzyme (OpgH) in this case is unrelated to UgtP but also acts on FtsZ in a UDP-glucose dependent fashion. Knocking out the relevant genes in the pathway also results in a size reduction. Thus, these two evolutionarily distant organisms both use UDP-glucose utilizing enzymes to couple growth rate to cell size.
Mutations Reducing Cell Size
In addition to knocking out the UDP-glucose sensing pathway, several mutations in essential cell division genes have been isolated that unexpectedly cause E. coli to grow at reduced cell size without affecting the growth rate. The first of these is the ftsA* mutation, which reduces cell size at fast growth rates by 25% (Geissler et al. 2007). More recently, mutations in ftsB and ftsL have also been shown to reduce cell size by a similar amount (Tsang and Bernhardt 2015; Liu et al. 2015). The mechanisms responsible for the size reduction by the various fts mutations could be different. For ftsB and ftsL the mutations appear to eliminate the delay between the establishment of the Z ring and the formation and activation of the complete divisome. Thus, newborn cells increase in cell size before assembling a Z ring but, as soon as it appears, it immediately matures into an active divisome. These mutations appear to activate the divisome complex but why this eliminates the delay in recruitment of the downstream division proteins is not clear. In contrast, the ftsA* mutation appears to lead to the assembly of Z rings at a smaller cell size (Geissler et al. 2007). The reason the Z ring assembles at a smaller cell size is not known but may be due to the higher affinity that FtsA* displays for the CCTP of FtsZ (Pichoff et al. 2012). This higher affinity may promote Z ring assembly by competing better with negative regulators (MinC/MinD and SlmA) that also bind the CCTP.
Size Control and the Cell Cycle
Under steady state growth, E. coli cells fall within a relatively narrow size range. Recent work from several labs revealed that this homeostasis is achieved by a relatively simple mechanism. By monitoring the growth of individual cells it was revealed that cells grow a constant amount (incremental or adder rule) between divisions, regardless of cell size at birth (Campos et al. 2014; Taheri-Araghi et al. 2015). How this is achieved is not clear but constant extension between divisions, along with division at midcell, is sufficient for a population of cells to maintain a narrow cell length distribution. Interestingly, it appears that it is division itself that resets the mechanism, as a polar division that generates a minicell in a MinC mutant is sufficient to do so (Campos et al. 2014).
Summary
The discovery of the Z ring initiated the field of the bacterial cytoskeleton and led to a simple model in which the Z ring orchestrated the synthesis of septal PG, resulting in cytokinesis (Bi and Lutkenhaus 1991). Subsequent studies revealed that FtsZ is the ancestral homologue of tubulin and FtsA is an actin related protein that tethers FtsZ filaments to the membrane. FtsZ and FtsA, along with ZipA and various Zap proteins, coassemble into the Z ring. This step is spatially regulated by at least 3 systems to ensure the Z ring is assembled at midcell. After a delay additional proteins are recruited to the Z ring to form a functional divisome. These additional proteins constitute the septal PG machine, which is activated by FtsN through integration of signals in the cytoplasm and the periplasm. The divisome is a complex and sophisticated machine that is highly regulated and we are only beginning to understand how it is regulated.
References
Aaron M, Charbon G, Lam H, Schwarz H, Vollmer W, Jacobs-Wagner C (2007) The tubulin homologue FtsZ contributes to cell elongation by guiding cell wall precursor synthesis in Caulobacter crescentus. Mol Microbiol 64:938–952
Aarsman ME, Piette A, Fraipont C, Vinkenvleugel TM, Nguyen-Disteche M, den Blaauwen T (2005) Maturation of the Escherichia coli divisome occurs in two steps. Mol Microbiol 55:1631–1645
Adams DW, Wu LJ, Errington J (2015) Nucleoid occlusion protein Noc recruits DNA to the bacterial cell membrane. EMBO J 34:491–501
Addinall SG, Cao C, Lutkenhaus J (1997) FtsN, a late recruit to the septum in Escherichia coli. Mol Microbiol 25:303–309
Adler HI, Fisher WD, Cohen A, Hardigree AA (1967) Miniature Escherichia coli cells deficient in DNA. Proc Natl Acad Sci U S A 57:321–326
Akerlund T, Gullbrand B, Nordstrom K (2002) Effects of the Min system on nucleoid segregation in Escherichia coli. Microbiology 148:3213–3222
Arends SJ, Kustusch RJ, Weiss DS (2009) ATP-binding site lesions in FtsE impair cell division. J Bacteriol 191:3772–3784
Arends SJ, Williams K, Scott RJ, Rolong S, Popham DL, Weiss DS (2010) Discovery and characterization of three new Escherichia coli septal ring proteins that contain a SPOR domain: DamX, DedD, and RlpA. J Bacteriol 192:242–255
Arumugam S, Petrasek Z, Schwille P (2014) MinCDE exploits the dynamic nature of FtsZ filaments for its spatial regulation. Proc Natl Acad Sci U S A 111:E1192–E1200
Bailey MW, Bisicchia P, Warren BT, Sherratt DJ, Mannik J (2014) Evidence for divisome localization mechanisms independent of the Min system and SlmA in Escherichia coli. PLoS Genet 10:e1004504
Begg KJ, Dewar SJ, Donachie WD (1995) A new Escherichia coli cell division gene, ftsK. J Bacteriol 177:6211–6222
Bendezu FO, De Boer PA (2008) Conditional lethality, division defects, membrane involution, and endocytosis in mre and mrd shape mutants of Escherichia coli. J Bacteriol 190:1792–1811
Bernhardt TG, De Boer PA (2005) SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over Chromosomes in E. coli. Mol Cell 18:555–564
Bi E, Lutkenhaus J (1990) FtsZ regulates frequency of cell division in Escherichia coli. J Bacteriol 172:2765–2768
Bi EF, Lutkenhaus J (1991) FtsZ ring structure associated with division in Escherichia coli. Nature 354:161–164
Bi E, Lutkenhaus J (1992) Isolation and characterization of ftsZ alleles that affect septal morphology. J Bacteriol 174:5414–5423
Bi E, Lutkenhaus J (1993) Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol 175:1118–1125
Bisicchia P, Arumugam S, Schwille P, Sherratt D (2013a) MinC, MinD, and MinE drive counter-oscillation of early-cell-division proteins prior to Escherichia coli septum formation. MBio 4:e00856–e00813
Bisicchia P, Steel B, Mariam Debela MH, Lowe J, Sherratt D (2013b) The N-terminal membrane-spanning domain of the Escherichia coli DNA translocase FtsK hexamerizes at midcell. MBio 4:e00800–e00813
Boyle DS, Khattar MM, Addinall SG, Lutkenhaus J, Donachie WD (1997) ftsW is an essential cell-division gene in Escherichia coli. Mol Microbiol 24:1263–1273
Bramhill D, Kornberg A (1988) Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell 52:743–755
Buddelmeijer N, Beckwith J (2004) A complex of the Escherichia coli cell division proteins FtsL, FtsB and FtsQ forms independently of its localization to the septal region. Mol Microbiol 52:1315–1327
Busiek KK, Margolin W (2014) A role for FtsA in SPOR-independent localization of the essential Escherichia coli cell division protein FtsN. Mol Microbiol 92:1212–1226
Busiek KK, Eraso JM, Wang Y, Margolin W (2012) The early divisome protein FtsA interacts directly through its 1c subdomain with the cytoplasmic domain of the late divisome protein FtsN. J Bacteriol 194:1989–2000
Buss J, Coltharp C, Huang T, Pohlmeyer C, Wang SC, Hatem C, Xiao J (2013) In vivo organization of the FtsZ-ring by ZapA and ZapB revealed by quantitative super-resolution microscopy. Mol Microbiol 89:1099–1120
Buss J, Coltharp C, Shtengel G, Yang X, Hess H, Xiao J (2015) A multi-layered protein network stabilizes the Escherichia coli FtsZ-ring and modulates constriction dynamics. PLoS Genet 11:e1005128
Cabre EJ, Sanchez-Gorostiaga A, Carrara P, Ropero N, Casanova M, Palacios P, Stano P, Jimenez M, Rivas G, Vicente M (2013) Bacterial division proteins FtsZ and ZipA induce vesicle shrinkage and cell membrane invagination. J Biol Chem 288:26625–26634
Campos M, Surovtsev IV, Kato S, Paintdakhi A, Beltran B, Ebmeier SE, Jacobs-Wagner C (2014) A constant size extension drives bacterial cell size homeostasis. Cell 159:1433–1446
Chen Y, Erickson HP (2005) Rapid in vitro assembly dynamics and subunit turnover of FtsZ demonstrated by fluorescence resonance energy transfer. J Biol Chem 280:22549–22554
Chen Y, Bjornson K, Redick SD, Erickson HP (2005) A rapid fluorescence assay for FtsZ assembly indicates cooperative assembly with a dimer nucleus. Biophys J 88:505–514
Cho H, Bernhardt TG (2013) Identification of the SlmA active site responsible for blocking bacterial cytokinetic ring assembly over the chromosome. PLoS Genet 9:e1003304
Cho H, Mcmanus HR, Dove SL, Bernhardt TG (2011) Nucleoid occlusion factor SlmA is a DNA-activated FtsZ polymerization antagonist. Proc Natl Acad Sci U S A 108:3773–3778
Cho H, Wivagg CW, Kapoor M, Barry Z, Rohs PD, Shu M, Marto JA, Garner TC, Bernhardt TG (2016) Bacterial cell wall biogenesis is mediated by SEDS and PBP polymerase families functioning semi – autonomously. Nat Microbiol 1:16172
Coltharp C, Buss J, Plumer TM, Xiao J (2016) Defining the rate-limiting processes of bacterial cytokinesis. Proc Natl Acad Sci U S A 113:E1044–E1053
Conti J, Viola MG, Camberg JL (2015) The bacterial cell division regulators MinD and MinC form polymers in the presence of nucleotide. FEBS Lett 589:201–206
Cooper S, Helmstetter CE (1968) Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol 31:519–540
Corbin BD, Wang Y, Beuria TK, Margolin W (2007) Interaction between cell division proteins FtsE and FtsZ. J Bacteriol 189:3026–3035
Cordell SC, Anderson RE, Lowe J (2001) Crystal structure of the bacterial cell division inhibitor MinC. EMBO J 20:2454–2461
Dai K, Xu Y, Lutkenhaus J (1993) Cloning and characterization of ftsN, an essential cell division gene in Escherichia coli isolated as a multicopy suppressor of ftsA12(Ts). J Bacteriol 175:3790–3797
Dai K, Xu Y, Lutkenhaus J (1996) Topological characterization of the essential Escherichia coli cell division protein FtsN. J Bacteriol 178:1328–1334
Dajkovic A, Lutkenhaus J (2006) Z ring as executor of bacterial cell division. J Mol Microbial Biotechnol 11:140–151
Dajkovic A, Lan G, Sun SX, Wirtz D, Lutkenhaus J (2008) MinC spatially controls bacterial cytokinesis by antagonizing the scaffolding function of FtsZ. Curr Biol 18:235–244
Dajkovic A, Pichoff S, Lutkenhaus J, Wirtz D (2010) Cross-linking FtsZ polymers into coherent Z rings. Mol Microbiol 78:651–668
De Boer PA (2010) Advances in understanding E. coli cell fission. Curr Opin Microbiol 13:730–737
De Boer PA, Crossley RE, Rothfield LI (1989) A division inhibitor and a topological specificity factor coded for by the minicell locus determine proper placement of the division septum in E. coli. Cell 56:641–649
Di Ventura B, Knecht B, Andreas H, Godinez WJ, Fritsche M, Rohr K, Nickel W, Heermann DW, Sourjik V (2013) Chromosome segregation by the Escherichia coli Min system. Mol Syst Biol 9:686
Donachie WD (1968) Relationship between cell size and time of initiation of DNA replication. Nature 219:1077–1079
Donachie WD, Blakely GW (2003) Coupling the initiation of chromosome replication to cell size in Escherichia coli. Curr Opin Microbiol 6:146–150
Du S, Lutkenhaus J (2014) SlmA antagonism of FtsZ assembly employs a two-pronged mechanism like MinCD. PLoS Genet 10:e1004460
Du S, Park KT, Lutkenhaus J (2015) Oligomerization of FtsZ converts the FtsZ tail motif (conserved carboxy-terminal peptide) into a multivalent ligand with high avidity for partners ZipA and SlmA. Mol Microbiol 95:173–188
Du S, Pichoff S, Lutkenhaus J (2016) FtsEX acts on FtsA to regulate divisome assembly and activity. Proc Natl Acad Sci U S A 113. doi:10.1073/pnas.1606656113
Dubarry N, Possoz C, Barre FX (2010) Multiple regions along the Escherichia coli FtsK protein are implicated in cell division. Mol Microbiol 78:1088–1100
Durand-Heredia JM, Yu HH, de Carlo S, Lesser CF, Janakiraman A (2011) Identification and characterization of ZapC, a stabilizer of the FtsZ ring in Escherichia coli. J Bacteriol 193:1405–1413
Durand-Heredia J, Rivkin E, Fan G, Morales J, Janakiraman A (2012) Identification of ZapD as a cell division factor that promotes the assembly of FtsZ in Escherichia coli. J Bacteriol 194:3189–3198
Egan AJ, Vollmer W (2015) The stoichiometric divisome: a hypothesis. Front Microbiol 6:455
Egan AJ, Biboy J, Van’t Veer I, Breukink E, Vollmer W (2015) Activities and regulation of peptidoglycan synthases. Philos Trans R Soc Lond Ser B Biol Sci 370
Erickson HP, Anderson DE, Osawa M (2010) FtsZ in bacterial cytokinesis: cytoskeleton and force generator all in one. Microbiol Mol Biol Rev 74:504–528
Erzberger JP, Mott ML, Berger JM (2006) Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat Struct Mol Biol 13:676–683
Espeli O, Borne R, Dupaigne P, Thiel A, Gigant E, Mercier R, Boccard F (2012) A MatP-divisome interaction coordinates chromosome segregation with cell division in E. coli. EMBO J 31:3198–3211
Fange D, Elf J (2006) Noise-induced Min phenotypes in E coli. PLoS Comput Biol 2:e80
Fenton AK, Gerdes K (2013) Direct interaction of FtsZ and MreB is required for septum synthesis and cell division in Escherichia coli. EMBO J 32:1953–1965
Fu X, Shih YL, Zhang Y, Rothfield LI (2001) The MinE ring required for proper placement of the division site is a mobile structure that changes its cellular location during the Escherichia coli division cycle. Proc Natl Acad Sci U S A 98:980–985
Fu G, Huang T, Buss J, Coltharp C, Hensel Z, Xiao J (2010) In vivo structure of the E. coli FtsZ-ring revealed by photoactivated localization microscopy (PALM). PLoS One 5:e12682
Galli E, Gerdes K (2010) Spatial resolution of two bacterial cell division proteins: ZapA recruits ZapB to the inner face of the Z-ring. Mol Microbiol 76:1514–1526
Geissler B, Margolin W (2005) Evidence for functional overlap among multiple bacterial cell division proteins: compensating for the loss of FtsK. Mol Microbiol 58:596–612
Geissler B, Elraheb D, Margolin W (2003) A gain-of-function mutation in ftsA bypasses the requirement for the essential cell division gene zipA in Escherichia coli. Proc Natl Acad Sci U S A 100:4197–4202
Geissler B, Shiomi D, Margolin W (2007) The ftsA* gain-of-function allele of Escherichia coli and its effects on the stability and dynamics of the Z ring. Microbiology 153:814–825
Gerding MA, Ogata Y, Pecora ND, Niki H, De Boer PA (2007) The trans-envelope Tol-Pal complex is part of the cell division machinery and required for proper outer-membrane invagination during cell constriction in E. coli. Mol Microbiol 63:1008–1025
Gerding MA, Liu B, Bendezu FO, Hale CA, Bernhardt TG, De Boer PA (2009) Self-enhanced accumulation of FtsN at division sites and roles for other proteins with a SPOR domain (DamX, DedD, and RlpA) in Escherichia coli cell constriction. J Bacteriol 191:7383–7401
Ghigo JM, Weiss DS, Chen JC, Yarrow JC, Beckwith J (1999) Localization of FtsL to the Escherichia coli septal ring. Mol Microbiol 31:725–737
Ghosal D, Trambaiolo D, Amos LA, Lowe J (2014) MinCD cell division proteins form alternating copolymeric cytomotive filaments. Nat Commun 5:5341
Glas M, van den Berg Van Saparoea HB, Mclaughlin SH, Roseboom W, Liu F, Koningstein GM, Fish A, den Blaauwen T, Heck AJ, de Jong L, Bitter W, de Esch IJ, Luirink J (2015) The soluble periplasmic domains of Escherichia coli cell division proteins FtsQ/FtsB/FtsL form a trimeric complex with submicromolar affinity. J Biol Chem 290:21498–21509
Goehring NW, Gueiros-Filho F, Beckwith J (2005) Premature targeting of a cell division protein to midcell allows dissection of divisome assembly in Escherichia coli. Genes Dev 19:127–137
Goehring NW, Gonzalez MD, Beckwith J (2006) Premature targeting of cell division proteins to midcell reveals hierarchies of protein interactions involved in divisome assembly. Mol Microbiol 61:33–45
Gonzalez MD, Beckwith J (2009) Divisome under construction: distinct domains of the small membrane protein FtsB are necessary for interaction with multiple cell division proteins. J Bacteriol 191:2815–2825
Gonzalez MD, Akbay EA, Boyd D, Beckwith J (2010) Multiple interaction domains in FtsL, a protein component of the widely conserved bacterial FtsLBQ cell division complex. J Bacteriol 192:2757–2768
Gueiros-Filho FJ, Losick R (2002) A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev 16:2544–2556
Haeusser DP, Rowlett VW, Margolin W (2015) A mutation in Escherichia coli ftsZ bypasses the requirement for the essential division gene zipA and confers resistance to FtsZ assembly inhibitors by stabilizing protofilament bundling. Mol Microbiol 97:988–1005
Halatek J, Frey E (2012) Highly canalized MinD transfer and MinE sequestration explain the origin of robust MinCDE-protein dynamics. Cell Rep 1:741–752
Hale CA, De Boer PA (1997) Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli. Cell 88:175–185
Hale CA, Meinhardt H, De Boer PA (2001) Dynamic localization cycle of the cell division regulator MinE in Escherichia coli. EMBO J 20:1563–1572
Hale CA, Shiomi D, Liu B, Bernhardt TG, Margolin W, Niki H, De Boer PA (2011) Identification of Escherichia coli ZapC (YcbW) as a component of the division apparatus that binds and bundles FtsZ polymers. J Bacteriol 193:1393–1404
Haney SA, Glasfeld E, Hale C, Keeney D, He Z, de Boer P (2001) Genetic analysis of the Escherichia coli FtsZ.ZipA interaction in the yeast two-hybrid system. Characterization of FtsZ residues essential for the interactions with ZipA and with FtsA. J Biol Chem 276:11980–11987
Heald R, Khodjakov A (2015) Thirty years of search and capture: the complex simplicity of mitotic spindle assembly. J Cell Biol 211:1103–1111
Heidrich C, Templin MF, Ursinus A, Merdanovic M, Berger J, Schwarz H, De Pedro MA, Holtje JV (2001) Involvement of N-acetylmuramyl-L-alanine amidases in cell separation and antibiotic-induced autolysis of Escherichia coli. Mol Microbiol 41:167–178
Helmstetter CE (1974) Initiation of chromosome replication in Escherichia coli. I. Requirements for RNA and protein synthesis at different growth rates. J Mol Biol 84:1–19
Hill NS, Buske PJ, Shi Y, Levin PA (2013) A moonlighting enzyme links Escherichia coli cell size with central metabolism. PLoS Genet 9:e1003663
Hirota Y, Jacob F, Ryter A, Buttin G, Nakai T (1968) On the process of cellular division in Escherichia coli. I. Asymmetrical cell division and production of deoxyribonucleic acid-less bacteria. J Mol Biol 35:175–192
Hu Z, Lutkenhaus J (1999) Topological regulation of cell division in Escherichia coli involves rapid pole to pole oscillation of the division inhibitor MinC under the control of MinD and MinE. Mol Microbiol 34:82–90
Hu Z, Lutkenhaus J (2000) Analysis of MinC reveals two independent domains involved in interaction with MinD and FtsZ. J Bacteriol 182:3965–3971
Hu Z, Lutkenhaus J (2001) Topological regulation of cell division in E. coli. spatiotemporal oscillation of MinD requires stimulation of its ATPase by MinE and phospholipid. Mol Cell 7:1337–1343
Hu Z, Lutkenhaus J (2003) A conserved sequence at the C-terminus of MinD is required for binding to the membrane and targeting MinC to the septum. Mol Microbiol 47:345–355
Hu Z, Mukherjee A, Pichoff S, Lutkenhaus J (1999) The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization. Proc Natl Acad Sci U S A 96:14819–14824
Hu Z, Saez C, Lutkenhaus J (2003) Recruitment of MinC, an inhibitor of Z-ring formation, to the membrane in Escherichia coli: role of MinD and MinE. J Bacteriol 185:196–203
Huang KC, Meir Y, Wingreen NS (2003) Dynamic structures in Escherichia coli: spontaneous formation of MinE rings and MinD polar zones. Proc Natl Acad Sci U S A 100:12724–12728
Huang KH, Durand-Heredia J, Janakiraman A (2013) FtsZ ring stability: of bundles, tubules, crosslinks, and curves. J Bacteriol 195:1859–1868
Ivanov V, Mizuuchi K (2010) Multiple modes of interconverting dynamic pattern formation by bacterial cell division proteins. Proc Natl Acad Sci U S A 107:8071–8078
Karimova G, Dautin N, Ladant D (2005) Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J Bacteriol 187:2233–2243
Katayama T, Ozaki S, Keyamura K, Fujimitsu K (2010) Regulation of the replication cycle: conserved and diverse regulatory systems for DnaA and oriC. Nat Rev Microbiol 8:163–170
Kato J, Katayama T (2001) Hda, a novel DnaA- related protein, regulates the replication cycle in Escherichia coli. EMBO J 20:4253–4262
Kirschner M, Mitchison T (1986) Beyond self- assembly: from microtubules to morphogenesis. Cell 45:329–342
Krupka M, Cabre EJ, Jimenez M, Rivas G, Rico AI, Vicente M (2014) Role of the FtsA C terminus as a switch for polymerization and membrane association. MBio 5:e02221
Kruse K, Howard M, Margolin W (2007) An experimentalist’s guide to computational modelling of the Min system. Mol Microbiol 63:1279–1284
Lackner LL, Raskin DM, De Boer PA (2003) ATP-dependent interactions between Escherichia coli Min proteins and the phospholipid membrane in vitro. J Bacteriol 185:735–749
Lara B, Rico AI, Petruzzelli S, Santona A, Dumas J, Biton J, Vicente M, Mingorance J, Massidda O (2005) Cell division in cocci: localization and properties of the Streptococcus pneumoniae FtsA protein. Mol Microbiol 55:699–711
Leonard AC, Grimwade JE (2015) The orisome: structure and function. Front Microbiol 6:545
Li Z, Trimble MJ, Brun YV, Jensen GJ (2007) The structure of FtsZ filaments in vivo suggests a force-generating role in cell division. EMBO J 26:4694–4708
Li GW, Burkhardt D, Gross C, Weissman JS (2014) Quantifying absolute protein synthesis rates reveals principles underlying allocation of cellular resources. Cell 157:624–635
Liu B, Persons L, Lee L, De Boer PA (2015) Roles for both FtsA and the FtsBLQ subcomplex in FtsN-stimulated cell constriction in Escherichia coli. Mol Microbiol 95:945–970
Loose M, Mitchison TJ (2014) The bacterial cell division proteins FtsA and FtsZ self-organize into dynamic cytoskeletal patterns. Nat Cell Biol 16:38–46
Loose M, Fischer-Friedrich E, Ries J, Kruse K, Schwille P (2008) Spatial regulators for bacterial cell division self-organize into surface waves in vitro. Science 320:789–792
Lowe J, Amos LA (1998) Crystal structure of the bacterial cell-division protein FtsZ. Nature 391:203–206
Lutkenhaus J (2007) Assembly dynamics of the bacterial MinCDE system and spatial regulation of the Z ring. Annu Rev Biochem 76:539–562
Lutkenhaus J (2009) FtsN–trigger for septation. J Bacteriol 191:7381–7382
Lutkenhaus J, Pichoff S, Du S (2012) Bacterial cytokinesis: from Z ring to divisome. Cytoskeleton (Hoboken) 69:778–790
Ma X, Margolin W (1999) Genetic and functional analyses of the conserved C-terminal core domain of Escherichia coli FtsZ. J Bacteriol 181:7531–7544
Mannik J, Bailey MW (2015) Spatial coordination between chromosomes and cell division proteins in Escherichia coli. Front Microbiol 6:306
Mannik J, Wu F, Hol FJ, Bisicchia P, Sherratt DJ, Keymer JE, Dekker C (2012) Robustness and accuracy of cell division in Escherichia coli in diverse cell shapes. Proc Natl Acad Sci U S A 109:6957–6962
Meeske AJ, Sham LT, Kimsey H, Koo BM, Gross CA, Bernhardt TG, Rudner DZ (2015) MurJ and a novel lipid II flippase are required for cell wall biogenesis in Bacillus subtilis. Proc Natl Acad Sci U S A 112:6437–6442
Meeske AJ, Riley EP, Robins WP, Uehara T, Mekalanos JJ, Kahne D, Walker S, Kruse AC, Bernhardt TG, Rudner D (2016) SEDS proteins are a widespread family at bacterial cell wall polymerases. Nature 357:634–6384
Meinhardt H, De Boer PA (2001) Pattern formation in Escherichia coli: a model for the pole-to- pole oscillations of Min proteins and the localization pole oscillations of Min proteins and the localization of the division site. Proc Natl Acad Sci U S A 98:14202–14207
Mercer KL, Weiss DS (2002) The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site. J Bacteriol 184:904–912
Mercier R, Petit MA, Schbath S, Robin S, El Karoui M, Boccard F, Espeli O (2008) The MatP/matS site-specific system organizes the terminus region of the E. coli chromosome into a macrodomain. Cell 135:475–485
Modell JW, Hopkins AC, Laub MT (2011) A DNA damage Checkpoint in Caulobacter crescentus inhibits cell division through a direct interaction with FtsW. Genes Dev 25:1328–1343
Modell JW, Kambara TK, Perchuk BS, Laub MT (2014) A DNA damage-induced, SOS-independent checkpoint regulates cell division in Caulobacter crescentus. PLoS Biol 12:e1001977
Mohammadi T, Ploeger GE, Verheul J, Comvalius AD, Martos A, Alfonso C, Van Marle J, Rivas G, Den Blaauwen T (2009) The GTPase activity of Escherichia coli FtsZ determines the magnitude of the FtsZ polymer bundling by ZapA in vitro. Biochemistry 48:11056–11066
Mohammadi T, Van Dam V, Sijbrandi R, Vernet T, Zapun A, Bouhss A, Diepeveen-De Bruin M, Nguyen-Disteche M, de Kruijff B, Breukink E (2011) Identification of FtsW as a transporter of lipid-linked cell wall precursors across the membrane. EMBO J 30:1425–1432
Monahan LG, Liew AT, Bottomley AL, Harry EJ (2014) Division site positioning in bacteria: one size does not fit all. Front Microbiol 5:19
Mosyak L, Zhang Y, Glasfeld E, Haney S, Stahl M, Seehra J, Somers WS (2000) The bacterial cell-division protein ZipA and its interaction with an FtsZ fragment revealed by X-ray crystallography. EMBO J 19:3179–3191
Mott ML, Berger JM (2007) DNA replication initiation: mechanisms and regulation in bacteria. Nat Rev Microbiol 5:343–354
Mukherjee A, Lutkenhaus J (1994) Guanine nucleotide-dependent assembly of FtsZ into filaments. J Bacteriol 176:2754–2758
Mukherjee A, Lutkenhaus J (1998) Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J 17:462–469
Muller P, Ewers C, Bertsche U, Anstett M, Kallis T, Breukink E, Fraipont C, Terrak M, Nguyen-Disteche M, Vollmer W (2007) The essential cell division protein FtsN interacts with the murein (peptidoglycan) synthase PBP1B in Escherichia coli. J Biol Chem 282:36394–36402
Narita S, Tokuda H (2006) An ABC transporter mediating the membrane detachment of bacterial lipoproteins depending on their sorting signals. FEBS Lett 580:1164–1170
Nogales E, Downing KH, Amos LA, Lowe J (1998) Tubulin and FtsZ form a distinct family of GTPases. Nat Struct Biol 5:451–458
Osawa M, Erickson HP (2013) Liposome division by a simple bacterial division machinery. Proc Natl Acad Sci U S A 110:11000–11004
Osawa M, Anderson DE, Erickson HP (2008) Reconstitution of contractile FtsZ rings in liposomes. Science 320:792–794
Pacheco-Gomez R, Cheng X, Hicks MR, Smith CJ, Roper DI, Addinall S, Rodger A, Dafforn TR (2013) Tetramerization of ZapA is required for FtsZ bundling. Biochem J 449:795–802
Park KT, Wu W, Battaile KP, Lovell S, Holyoak T, Lutkenhaus J (2011) The Min oscillator uses MinD-dependent conformational changes in MinE to spatially regulate cytokinesis. Cell 146:396–407
Park KT, Wu W, Lovell S, Lutkenhaus J (2012) Mechanism of the asymmetric activation of the MinD ATPase by MinE. Mol Microbiol 85:271–281
Park KT, Du S, Lutkenhaus J (2015) MinC/MinD copolymers are not required for Min function. Mol Microbiol 98(5):895–909
Pastoret S, Fraipont C, den Blaauwen T, Wolf B, Aarsman ME, Piette A, Thomas A, Brasseur R, Nguyen-Disteche M (2004) Functional analysis of the cell division protein FtsW of Escherichia coli. J Bacteriol 186:8370–8379
Peters NT, Dinh T, Bernhardt TG (2011) A fail-safe mechanism in the septal ring assembly pathway generated by the sequential recruitment of cell separation amidases and their activators. J Bacteriol 193:4973–4983
Pichoff S, Lutkenhaus J (2001) Escherichia coli division inhibitor MinCD blocks septation by preventing Z-ring formation. J Bacteriol 183:6630–6635
Pichoff S, Lutkenhaus J (2002) Unique and overlapping roles for ZipA and FtsA in septal ring assembly in Escherichia coli. EMBO J 21:685–693
Pichoff S, Lutkenhaus J (2005) Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Mol Microbiol 55:1722–1734
Pichoff S, Shen B, Sullivan B, Lutkenhaus J (2012) FtsA mutants impaired for self-interaction bypass ZipA suggesting a model in which FtsA’s self-interaction competes with its ability to recruit downstream division proteins. Mol Microbiol 83:151–167
Pichoff S, Du S, Lutkenhaus J (2015) The bypass of ZipA by overexpression of FtsN requires a previously unknown conserved FtsN motif essential for FtsA-FtsN interaction supporting a model in which FtsA monomers recruit late cell division proteins to the Z ring. Mol Microbiol 95:971–987
Pierucci O (1978) Dimensions of Escherichia coli at various growth rates: model for envelope growth. J Bacteriol 135:559–574
Raskin DM, De Boer PA (1999a) MinDE-dependent pole-to-pole oscillation of division inhibitor MinC in Escherichia coli. J Bacteriol 181:6419–6424
Raskin DM, De Boer PA (1999b) Rapid pole-to- pole oscillation of a protein required for directing division to the middle of Escherichia coli. Proc Natl Acad Sci U S A 96:4971–4976
Reddy M (2007) Role of FtsEX in cell division of Escherichia coli: viability of ftsEX mutants is dependent on functional SufI or high osmotic strength. J Bacteriol 189:98–108
Rico AI, Krupka M, Vicente M (2013) In the beginning, Escherichia coli assembled the proto-ring: an initial phase of division. J Biol Chem 288:20830–20836
Rodrigues CD, Harry EJ (2012) The Min system and nucleoid occlusion are not required for identifying the division site in Bacillus subtilis but ensure its efficient utilization. PLoS Genet 8:e1002561
Roll-Mecak A (2015) Intrinsically disordered tubulin tails: complex tuners of microtubule functions? Semin Cell Dev Biol 37:11–19
Rothfield L, Taghbalout A, Shih YL (2005) Spatial control of bacterial division-site placement. Nat Rev Microbiol 3:959–968
Schmidt KL, Peterson ND, Kustusch RJ, Wissel MC, Graham B, Phillips GJ, Weiss DS (2004) A predicted ABC transporter, FtsEX, is needed for cell division in Escherichia coli. J Bacteriol 186:785–793
Schumacher MA, Zeng W (2016) Structures of the nucleoid occlusion protein SlmA bound to DNA and the C-terminal domain of the cytoskeletal protein FtsZ. Proc Natl Acad Sci U S A 113:4988–4993
Schumacher MA, Zeng W, Huang KH, Tchorzewski L, Janakiraman A (2015) Structural and functional analyses reveal insights into the molecular properties of the E. coli Z ring stabilizing protein, ZapC. J Biol Chem 291:2485
Schweizer J, Loose M, Bonny M, Kruse K, Monch I, Schwille P (2012) Geometry sensing by self-organized protein patterns. Proc Natl Acad Sci U S A 109:15283–15288
Shen B, Lutkenhaus J (2009) The conserved C- terminal tail of FtsZ is required for the septal localization and division inhibitory activity of MinC(C)/MinD. Mol Microbiol 72:410–424
Shen B, Lutkenhaus J (2010) Examination of the interaction between FtsZ and MinCN in E. coli suggests how MinC disrupts Z rings. Mol Microbiol 75:1285–1298
Soderstrom B, Skoog K, Blom H, Weiss DS, Von Heijne G, Daley DO (2014) Disassembly of the divisome in Escherichia coli: evidence that FtsZ dissociates before compartmentalization. Mol Microbiol 92:1–9
Spratt BG (1975) Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A 72:2999–3003
Steiner W, Liu G, Donachie WD, Kuempel P (1999) The cytoplasmic domain of FtsK protein is required for resolution of chromosome dimers. Mol Microbiol 31:579–583
Strauss MP, Liew AT, Turnbull L, Whitchurch CB, Monahan LG, Harry EJ (2012) 3D-SIM super resolution microscopy reveals a bead-like arrangement for FtsZ and the division machinery: implications for triggering cytokinesis. PLoS Biol 10:e1001389
Stricker J, Maddox P, Salmon ED, Erickson HP (2002) Rapid assembly dynamics of the Escherichia coli FtsZ-ring demonstrated by fluorescence recovery after photobleaching. Proc Natl Acad Sci U S A 99:3171–3175
Sun Q, Yu XC, Margolin W (1998) Assembly of the FtsZ ring at the central division site in the absence of the chromosome. Mol Microbiol 29:491–503
Szeto TH, Rowland SL, Rothfield LI, King GF (2002) Membrane localization of MinD is mediated by a C-terminal motif that is conserved across eubacteria, archaea, and chloroplasts. Proc Natl Acad Sci U S A 99:15693–15698
Szwedziak P, Lowe J (2013) Do the divisome and elongasome share a common evolutionary past? Curr Opin Microbiol 16:745–751
Szwedziak P, Wang Q, Freund SM, Lowe J (2012) FtsA forms actin-like protofilaments. EMBO J 31:2249–2260
Szwedziak P, Wang Q, Bharat TA, Tsim M, Lowe J (2014) Architecture of the ring formed by the tubulin homologue FtsZ in bacterial cell division. Elife 3:e04601
Taheri-Araghi S, Bradde S, Sauls JT, Hill NS, Levin PA, Paulsson J, Vergassola M, Jun S (2015) Cell-size control and homeostasis in bacteria. Curr Biol 25:385–391
Teather RM, Collins JF, Donachie WD (1974) Quantal behavior of a diffusible factor which initiates septum formation at potential division sites in Escherichia coli. J Bacteriol 118:407–413
Thanedar S, Margolin W (2004) FtsZ exhibits rapid movement and oscillation waves in helix-like patterns in Escherichia coli. Curr Biol 14:1167–1173
Tonthat NK, Arold ST, Pickering BF, Van Dyke MW, Liang S, Lu Y, Beuria TK, Margolin W, Schumacher MA (2011) Molecular mechanism by which the nucleoid occlusion factor, SlmA, keeps cytokinesis in check. EMBO J 30:154–164
Tonthat NK, Milam SL, Chinnam N, Whitfill T, Margolin W, Schumacher MA (2013) SlmA forms a higher-order structure on DNA that inhibits cytokinetic Z-ring formation over the nucleoid. Proc Natl Acad Sci U S A 110:10586–10591
Trip EN, Scheffers DJ (2015) A 1 MDa protein complex containing critical components of the Escherichia coli divisome. Sci Rep 5:18190
Tsang MJ, Bernhardt TG (2015) A role for the FtsQLB complex in cytokinetic ring activation revealed by an ftsL allele that accelerates division. Mol Microbiol 95:925–944
Typas A, Banzhaf M, Gross CA, Vollmer W (2012) From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat Rev Microbiol 10:123–136
Uehara T, Parzych KR, Dinh T, Bernhardt TG (2010) Daughter cell separation is controlled by cytokinetic ring-activated cell wall hydrolysis. EMBO J 29:1412–1422
Ursinus A, Van Den Ent F, Brechtel S, De Pedro M, Holtje JV, Lowe J, Vollmer W (2004) Murein (peptidoglycan) binding property of the essential cell division protein FtsN from Escherichia coli. J Bacteriol 186:6728–6737
Uversky VN (2013) The most important thing is the tail: multitudinous functionalities of intrinsically disordered protein termini. FEBS Lett 587:1891–1901
Vadia S, Levin PA (2015) Growth rate and cell size: a re-examination of the growth law. Curr Opin Microbiol 24:96–103
Van De Putte P, Van D, Roersch A (1964) The selection of mutants of Escherichia coli with impaired cell division at elevated temperature. Mutat Res 106:121–128
Van Den Ent F, Amos LA, Lowe J (2001) Prokaryotic origin of the actin cytoskeleton. Nature 413:39–44
Van Der Ploeg R, Verheul J, Vischer NO, Alexeeva S, Hoogendoorn E, Postma M, Banzhaf M, Vollmer W, Den Blaauwen T (2013) Colocalization and interaction between elongasome and divisome during a preparative cell division phase in Escherichia coli. Mol Microbiol 87:1074–1087
Varma A, Huang KC, Young KD (2008) The Min system as a general cell geometry detection mechanism: branch lengths in Y-shaped Escherichia coli cells affect Min oscillation patterns and division dynamics. J Bacteriol 190:2106–2117
Vecchiarelli AG, Li M, Mizuuchi M, Hwang LC, Seol Y, Neuman KC, Mizuuchi K (2016) Membrane-bound MinDE complex acts as a toggle switch that drives Min oscillation coupled to cytoplasmic depletion of MinD. Proc Natl Acad Sci U S A 113:E1479–E1488
Wang L, Lutkenhaus J (1998) FtsK is an essential cell division protein that is localized to the septum and induced as part of the SOS response. Mol Microbiol 29:731–740
Weart RB, Levin PA (2003) Growth rate- dependent regulation of medial FtsZ ring formation. J Bacteriol 185:2826–2834
Weart RB, Lee AH, Chien AC, Haeusser DP, Hill NS, Levin PA (2007) A metabolic sensor governing cell size in bacteria. Cell 130:335–347
Weiss DS (2015) Last but not least: new insights into how FtsN triggers constriction during Escherichia coli cell division. Mol Microbiol 95:903–909
Wissel MC, Weiss DS (2004) Genetic analysis of the cell division protein FtsI (PBP3): amino acid substitutions that impair septal localization of FtsI and recruitment of FtsN. J Bacteriol 186:490–502
Woldringh CL, Mulder E, Valkenburg JA, Wientjes FB, Zaritsky A, Nanninga N (1990) Role of the nucleoid in the toporegulation of division. Res Microbiol 141:39–49
Wu LJ, Ishikawa S, Kawai Y, Oshima T, Ogasawara N, Errington J (2009) Noc protein binds to specific DNA sequences to coordinate cell division with chromosome segregation. EMBO J 28:1940–1952
Wu W, Park KT, Holyoak T, Lutkenhaus J (2011) Determination of the structure of the MinD- ATP complex reveals the orientation of MinD on the membrane and the relative location of the binding sites for MinE and MinC. Mol Microbiol 79:1515–1528
Wu F, van Schie BG, Keymer JE, Dekker C (2015) Symmetry and scale orient Min protein patterns in shaped bacterial sculptures. Nat Nanotechnol 10:719–726
Yahashiri A, Jorgenson MA, Weiss DS (2015) Bacterial SPOR domains are recruited to septal peptidoglycan by binding to glycan strands that lack stem peptides. Proc Natl Acad Sci U S A 112:11347–11352
Yang JC, Van Den Ent F, Neuhaus D, Brevier J, Lowe J (2004) Solution structure and domain architecture of the divisome protein FtsN. Mol Microbiol 52:651–660
Yang DC, Peters NT, Parzych KR, Uehara T, Markovski M, Bernhardt TG (2011) An ATP-binding cassette transporter-like complex governs cell-wall hydrolysis at the bacterial cytokinetic ring. Proc Natl Acad Sci U S A 108:E1052–E1060
Yang DC, Tan K, Joachimiak A, Bernhardt TG (2012) A conformational switch controls cell wall-remodelling enzymes required for bacterial cell division. Mol Microbiol 85:768–781
Young KD (2001) Approaching the physiological functions of penicillin-binding proteins in Escherichia coli. Biochimie 83:99–102
Yousif SY, Broome-Smith JK, Spratt BG (1985) Lysis of Escherichia coli by beta-lactam antibiotics: deletion analysis of the role of penicillin- binding proteins 1A and 1B. J Gen Microbiol 131:2839–2845
Yu XC, Margolin W (1999) FtsZ ring clusters in min and partition mutants: role of both the Min system and the nucleoid in regulating FtsZ ring localization. Mol Microbiol 32:315–326
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Lutkenhaus, J., Du, S. (2017). E. coli Cell Cycle Machinery. In: Löwe, J., Amos, L. (eds) Prokaryotic Cytoskeletons. Subcellular Biochemistry, vol 84. Springer, Cham. https://doi.org/10.1007/978-3-319-53047-5_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-53047-5_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-53045-1
Online ISBN: 978-3-319-53047-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)