Abstract
In view of the steady depletion of primary sources of copper and the increased global demand for refined copper, it becomes necessary to explore some secondary sources for possible extraction of copper. The waste copper smelter dust (CSD) is a rich secondary resource for copper as shown by the chemical composition of the South African Palabora coppers smelter plant CSD that assayed 18.02, 13.36, and 3.4 wt% copper, iron and sulphur; respectively. Studies on CSD have focused majorly on either dust characterization or treatment, while hydrometallurgical extraction without pretreatment and with pretreatment using techniques such as oxidative roasting are also considered quite attractive. The challenge of iron dissolution during the leaching stage in these processes necessitates adequate purification of the leach liquor before the extraction of the metal as nano-particles. Hence, this review examined the theories relating to the characterization and treatment of CSD for copper recovery as nanoparticles; with factors having a bearing on the treatment process such as kinetics considered with the aim of providing scientific basis for the research.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
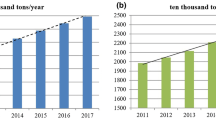
The United States of America (U.S.A) was the leading copper consumer and is credited with roughly 16% of the total consumption of world refined copper, which approximates to 2.4 million tons as at 1980 [1]. In 2002, the U.S.A was surpassed by China as the world’s leading consumer of refined copper. The thriving economy in China further added to its increase in annual refined copper consumption during the 8 years from 1999 to 2007 as illustrated on Fig. 1 [2].
Global consumption of copper from 1980 to 2008 (adapted from [2])
Consequently, it is predicted that by 2025, the global demand for refined copper will increase to 29.5 million metric tons from the 22.4 million metric tons predicted for 2015 [3]. This increasing global demand for refined copper has impacted negatively on the primary sources of copper, thus making secondary copper processing an ever-increasing importance within the global copper industry [4].
Recently, the copper industry has witnessed significant growth in the recovery of copper and other metal values from secondary materials [5,6,7] such as:
-
(a)
Metallurgical wastes—copper smelter dust, low grade slags, residues, anode slimes, oxide residues etc.
-
(b)
Industrial wastes—bars, pipes, wire, copper sheeting, ship screws, etc.
-
(c)
Consumer wastes—bronze and brass applications.
-
(d)
Electrical and electronic waste (e-waste)—domestic electrical, computer, audio-visual, and
-
(e)
Telecommunication appliances
At present, the disposal of metallurgical wastes such as copper smelter dust (CSD) is becoming costly owing to increasingly stringent environmental regulations [8]. Furthermore, the physical and chemical nature of CSD (such as the content of heavy metals) causes it to be classified as hazardous waste [9]. While the South African National Standard (SANS) annual target level of dust is 300 mg/m2/day and the industrial action level of dust is 1200 mg/m2/day [10]. Table 1 presents a breakdown of the CSD deposition rates around the Palabora mining company’s (PMC) smelter and in its vermiculite operations plant also in Phalaborwa Town [11]. In view of the above, there has been an increasing interest in developing processes for the recovery of metals from these wastes [12].
Often, pyrometallurgical and hydrometallurgical processes are employed for treating such residues [12]. But the pyrometallurgical processes require investment in expensive equipment, results in high energy consumption and thus, leads to the production of worthless residues [13,14,15,16]. As a result, much focus has been on hydrometallurgical processes [12].
The leach-solvent extraction-electrowinning process option has been the traditional hydrometallurgical route of recovering copper from its source as copper cathodes [17, 18]. But recovering the copper value as nano-particles is more attractive, because of its vast applications in areas such as heat transfer and microelectronics and coupled with the fact that it is significantly less expensive than silver and gold; thus making it a worthy alternative to noble metals in some applications [19, 20]. Currently, a novel approach at laboratory scale was reported by Darezereshki and Bakhtiari [21]; it combines hydrometallurgical techniques with nano-technology to recover the oxide of copper as precipitate of nano-particles from leach solution of CSD. Yet; additional work is required to enhance the process to be able to produce metallic copper nano-particles with acceptable grade and recovery from this metallurgical waste.
Hence, in the light of the above mentioned, this review will be looking at the theoretical background, the characterization and treatment of CSD , the recovery of copper as nano-particles from leach solution of CSD.
Theoretical Background
Copper Smelter Dust (CSD)
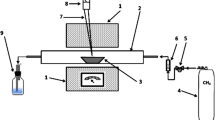
Copper smelter dust is the product consisting of SO2, N2, O2, water vapor, heavy metals and other impurities recovered from exhaust gas streams found in furnaces, flues and settling chambers as a result of roasting, smelting and converting operations from copper refining processes as illustrated on Fig. 2 [17, 22, 23]. The basic elements and their concentrations, are a function of the following: the characteristics of the sulphureted ores that are processed, the species and their concentration, the reactor type used in the different stages of the process together with the conditions under which these stages operate [24]. All these factors add up to influence the chemical and mineralogical characteristics of the copper smelter dusts (Tables 2, 3, 7 and 8), thus giving a reason for their variability and as a result, the processes chosen for their treatment, are based on the recovery of valuable elements and the stabilization of undesirable elements present in the copper smelter dusts [25].
Schematic drawing of the locations of the gas cleaning devices in a copper-plant (adapted from [22])
Mechanism of Dust Formation
There are two kinds of dust that forms in metallurgical smelting processes, namely mechanically formed dust which consists of small particles of the charging material that get carried away with the process gas and the chemically formed dust, which consists of vaporized components that condense into particles as the process off-gas temperature cools down. Mechanical dust forms as a result of the entrainment of small solid and/or liquid particles from charged material into process off-gas. Mechanical entrainment involves the elutriation by the process gas of small solid and liquid particles that travel at a relatively high velocity through the process [26, 27]. Process reactions can cause the charging material to collapse or fragment into smaller dust particle.
Chemical dust formation is caused by the vaporization of components from the process, followed by the condensation of the components from the process gas at lower temperatures in the off-gas system [27]. Materials with low melting points such as As, Sb, their oxides and sulphide, often have high vapour pressure and tend to vaporise during smelting, forming chemical dust. Chemical dust has a very fine particle size because of the nucleation and growth processes occurring as the process off-gas cools down. In copper smelting processes, easily volatile components include Ag, As, Pb, Sb, and Zn. Copper can also vaporize in the hot combustion zone of the furnaces. Oxygen enrichment increases the combustion temperature in the flash furnace and as a result, more copper is vaporized. Vaporized copper particles are very fine and easily drift out of the furnace with the off-gas.
Uses of Copper Smelter Dust (CSD)
Although there is no report in literature of application of copper nano-particles obtained from leach solution of CSD ; except for its recovery from this waste [21]. Yet, copper nano-particles, due to their excellent physical and chemical properties and cheap production method, have been of great interest. Copper nano-particles have extensive applications as heat transfer systems [28], antimicrobial materials [29, 30], super strong materials [31, 32], sensors [33, 34] and catalysts [35, 36].
Studies on CSD can be divided into two groups; those focused on dust characterization [22, 37] and those focused on dust treatment [8, 15,16,17, 38,39,40,41], even though the two are often considered together.
Characterization of Copper Smelter Dust
Chemical Composition of Copper Smelter Dust
There are about six analytical techniques reported in literatures that have been used to determine the chemical composition (CC) of CSDs and these has been summarized into a table of analytical methods and relevant research findings (Table 2).
Therefore, the findings from the use of these analytical techniques showed that overall, this metallurgical waste often contains contaminants (Tables 2 and 3) such as antimony (Sb), bismuth (Bi), arsenic (As), lead (Pb), zinc (Zn), cadmium (Cd), selenium (Se), tellurium (Te) and compounds thereof [8, 12, 42, 43]. But it often contains significant amount of copper (Tables 2 and 3), titanium and alumina as in the case of PMC’s CSD (Table 3).
Particle Size of Copper Smelter Dust
The major techniques reportedly adopted to determine the particle size distribution (PSD) of this metallurgical waste has been summarized on Table 4. As can be seen on Table 4, the marlven particle size analyzer (MPA) was used to determine the PSD of a CSD from a smelter in South Korea; nevertheless, results using this techniques tend to appear coarser than those of other methods. Austin and Shah [44] proposed a method for inter-conversion of laser diffraction (MPA) and sieve-size distributions (screening), and a simple conversion can be developed by regression for materials of constant characteristics. Additionally, the outcomes can depend on the relative refractive indices of the solid particles and liquid medium (usually, though not necessarily, water), and even particle shape. Furthermore, on one hand the most commonly used method for PSD determination of CSDs is the wet screening method (Table 4), probably because of its simplicity. While on the other hand the cyclosizer is often used to classify the residues produced after the dissolution studies of CSDs, into coarse and fine fractions (Table 4).
Hence, the average size of this CSD as determined by these aforementioned methods fall within the size range of 5–50 µm (Tables 4 and 5) as have been reported in literature [39, 43]. Furthermore, the CSD is said to be an ultrafine material requiring the use of the wet aspect (Table 6) of these techniques for its PSD analysis. This is in order to minimize losses through clogging and prevent dusting issues that could lead to environmental pollution [45].
Mineralogical Composition of Copper Smelter Dust
Depending on where this dust was collected (Fig. 2), often the main minerals of the CSD are magnetite, cuprospinel, chalcopyrite, chalcanthite, delafosite, chalcocynite, zincosite and claudetite (Tables 7 and 8). The major technique used to determine the minerals in this dust is the XRD (Oriol et al. 2011; [39, 46]); although seldom used SEM/EDS and TEM/EDS for this purpose [40, 41, 47]. Below are the brief descriptions of the techniques used in the mineralogical analysis of CSD’s , as reported in literature; also including the earlier discussed SEM, under particle size determination.
Hence, for an improvement on the recovery of copper from this metallurgical waste, a comprehensive physical-chemical-mineralogical characterization of the CSD is needed [37]. Since most characterization requirements are mainly based on current operating practices, there is the need to use different analytical techniques to appropriately characterize the CSD in order to generate more robust data for decision making.
Treatment of Copper Smelter Dust for Copper Recovery
Enrichment of Copper Smelter Dust
Gravity Separation Method
The gravity methods of separation are used to treat a great variety of materials ranging from heavy metals such as lead sulphide (specific gravity of 7.5) to coal (specific gravity of 1.3) at particle sizes in some cases below 50 µm (Wills 2003). So therefore, for an effective separation, there must be a marked density difference between the mineral(s) of interest and the gangue(s) minerals; thus obeying the concentration criterion, which is ≥ ±2.5 (wills 2003). Also bearing in mind that the motion of a particle in a fluid is not just dependent on the specific gravity alone; but also on its particle size and shape [48].
Going through Table 9, one would clearly observe the marked difference in specific gravity (S.G.) of some of the minerals contained in the CSD—Chalcopyrite (S.G. = 4.30) and Bismuthinite (S.G. = 7.20), Tenorite (S.G. = 6.50) and Arsenolite (S.G. = 3.20) and Gunningite (S.G. = 3.87) for which separation is possible (i.e. ≥ ±2.5) with this method; but with a particle sizes range from 10 to 38 µm (Fig. 3; Tables 4 and 5); only the modern gravity techniques (Knelson and Falcon concentrators) have proven to be efficient for beneficiation of materials in this particle size range of up to 50 µm(Wills 2003).
Magnetic Separation Method
Here, the main physical property of note is the magnetic susceptibility; separation using this method is possible, for the characteristic mineralogical composition of most CSDs −0.70–1.30 amps for Chalcopyrite, 0.50–1.00 dc amps for cuprospinel, 0.01 amps Magnetite and 0.10–0.30 amps for Hematite (Table 10). Yet, the particle size remains an important factor to consider, if this method is to be used to separate the contained minerals from its gangue; considering the fine-ultrafine nature of the CSD, dusting will definitely be an issue to deal with should this method be used to upgrade it. Otherwise, the wet approach will be better suited for upgrading this metallurgical waste, like the wet high intensity magnetic separator (WHIMS).
Electrostatic Separation Method
This is another minerals processing technique that depends on the difference in electrical conductivity (Table 11). Here, the difference between the electrical conductivity of copper and the gangue minerals in the CSD—595.800 Cu, 9.363 Bi, 102.987 Fe, 48.431 Pb and 25.641 Sb is significant [49]; and separation is possible on this basis; but what is of note is that the sample must be very dry for a good result, while bearing in mind that it is also particle size sensitive.
Froth Flotation Method
Froth flotation is a highly versatile method for physically separating particles based on differences in the ability of air bubbles to selectively adhere to specific mineral surfaces in mineral/water slurry. Froth flotation can be adapted to a broad range of mineral separations, as it is possible to use chemical treatments (Table 12) to selectively alter mineral surfaces so that they have the necessary properties for the separation. It is particularly useful for processing fine-grained ores that are not amenable to conventional gravity concentration [50]. The mineralogical composition of the tailings obtained from the froth flotation of PMC’s CSD is presented on Table 13; it reveals significant amounts of titanium compounds (25.20 wt%) on one hand, silicon and aluminum oxide as mullite (45.50 wt%) on the other; thus, suggesting a further beneficiation, for an efficient processing of the CSD.
Roasting Pretreatment
The decreasing rate of copper in ores now requires pre-treatment of ores (Fig. 4). Thus suggesting that direct leaching and the associated challenge of iron dissolution in the process presents the need for thorough purification and controlled methodology prior to recovery of copper in the subsequent stage [51].
Hence, the hydrometallurgical extraction of copper from its sulphide sources, often involves the use of the roast-leach process option. This process option is carried out by first subjecting the copper source to either low temperatures (partial roasting) or high temperatures (dead roasting) with the hope of producing dissolvable calcine for copper recovery [52].
Kinetics and Mechanism of Roasting CSD
The essence of chemical kinetics is to study the rates of chemical reactions. The kinetics of oxidative and sulphation roasting is a function of the transport of reactant gas to the unreacted solid and the rate of reaction between the solid and gas. This in turn is a function of the following parameters:
-
(i)
Temperature of the system,
-
(ii)
The particle size,
-
(iii)
The porosity of the condensed product phase,
-
(iv)
The shape of the concentrate particles, and the
-
(v)
The chemical composition and mineralogy.
The CSD of PMC is composed of the Cu–Fe–S–O system (Table 7); it can be upgraded first by any of the appropriate mineral processing methods and/or pre-treated (either the concentrate (e.g. from froth flotation) or the CSD) via oxidative roasting to make it amenable to leaching for the recovery of its contained copper value. The thermal analysis of the CSD of PMC was carried out using both the thermogravimetric and differential thermogravimetric (DSC-TGA) to determine the thermal behavior of this CSD (Figs. 5 and 6), they reveal vaporization of water of crystallization (100–120 °C) and series of decomposition (500, 750 °C) marked by weight losses and gain; probably as a result of the formation of sulphates and oxides of copper, iron, aluminum, titanium etc.
Dissolution and Extraction of Copper from Copper Smelter Dust
Dissolution of Copper from Copper Smelter Dust
The treatment of CSD has been studied in detail by several authors ([23, 43, 17]; Wu et al. 2014). Based on the processes put forward at the pilot and/or industrial level, in line with the reasons specified, the processes can be classified as:
- (A)
- (B)
- (C)
Hence, a good number of the studies concentrate on hydrometallurgical treatments of the dusts, using acid or alkaline reagents [61,62,63,64,65,66,67,68].
Solvent Extraction of Copper from Copper Smelter Dust
For metallurgical application esters and ketones of phosphoric acid with straight or branched chain alcohols are found particularly suitable. Also alkyl amines are used, in all cases dissolved in a suitable solvent such as kerosene. The extraction may occur either by neutral ionic pairs being absorbed by solvent and forming a loose chemical bond with the organic molecule, or by a cation or an anion being bonded to the molecule in exchange for a hydrogen cation or a chloride or nitrate anion. Solvent extraction has found particular application in the extraction of copper from copper smelter dust [21]; thus resulting in a 50% purity improvement of leach solution (Tables 14 and 15).where possible (mineral processing), this option can be avoided, but for the effect of the toxic chemicals to the environment (cliskov et al. 2013).
Electrowinning of Copper from CSD
The electrowinning of copper ions derived from dissolution, or solvent extraction is an important contributor to the global copper product supply. The process of electrolysis for copper was first advanced in the late 19th century and notwithstanding much advancement in technology, the principles and rudimentary equipment remain the same [69].
Kinetics of Dissolution of Copper from CSD
On the basis of thermodynamics, the final equilibrium state for a reaction may be predicted, but thermodynamics gives no information about the rate at which this equilibrium is approached [48]. Hence, the yield of such a reaction is a function of firstly, the position of the equilibrium between the reacting substances and secondly, the rate at which equilibrium is attained [70]. Bakhtiari et al. [71] studied the bio-leaching kinetics of copper from CSD . Based on the data obtained, it was deduced that at pH 1.8 and the pulp density less than 7%, the dissolution of copper followed shrinking core kinetic model and the process was limited by diffusion of lixiviant. However with a pulp density of 7%, the process was reported to be reaction limited.
Recovery of Copper as Nano-particles from Purified Leach Solution of CSD
Chemical Reduction Method
The chemical reduction of copper salts is the easiest, simplest and the most commonly used production method for copper nano-particles. During the production of metallic copper nanoparticles good control of shapes and sizes is achievable using chemical reduction of copper precursors [72, 73]. Conventionally, in the chemical reduction techniques, a copper salt is reduced by a reducing agent such as sodium borohydride [74], hydrazine [75], ascorbate [76], glucose [77], polyol [78] as well as isopropyl alcohol with cetyltrimethylammonium bromide [79].
The Solvothermal Method (Thermal Decomposition Method)
This method involves the selection of a proper precursor and localized heating. There are several reports of copper nano-particles being produced using this method. Zhang et al. [80] produced copper nano-particles with the average diameter of 10 nm from the thermal decomposition of copper oxalate precursor. CuO nano-particles were also synthesized from the thermal decomposition of the precursors: brochantite Cu4(SO4)(OH)6, and posnjakite Cu4(SO4)(OH)6.H2O [71, 81].
Hence, the production of metallic copper nano-particles with good control of shapes and sizes is achievable using chemical reduction method [72, 73]. The grade of the copper nano-particles is important, so much that a method that guarantees simplicity, very low grain size, the presence of a single phase and production of high purity nano-crystals, together with high crystallinity and ecofriendliness attributes, such as the thermal decomposition method will be better suited for this purpose [21, 82].
Thermochemistry
Computational Thermochemistry
Thermochemical data are a vital factor in the safe and successful upgrade of chemical processes in the chemical industry. Despite collection of experimental thermochemical data for many molecules, there are quite a number of species for which there are no data. Furthermore, the data in the collections are sometimes incorrect. Experimental measurements of thermochemical processes are often expensive and difficult, so it is highly desirable to have computational methods that can make reliable predictions (Curtiss and Pople 1999). Howbeit, the FactSage 7.0 computer package, which consists of a series of information, calculation and manipulation modules that enable one to access and manipulate compound and solution databases. With the various modules running under Microsoft Windows one can perform a wide variety of thermochemical calculations and generate tables, graphs and figures of interest to chemical and physical metallurgists [83].
Present and Future Challenges of CSD
The known resource recovery process options involves capital and operating costs greater than can be right by the revenue of the recovered metal. Thus, the copper smelting industry shows increasing interest in developing processes for the recovery of the copper value from this metallurgical waste. As a result, it would be beneficial to have a processing method by which impurities could be removed from the CSD , while sustaining an acceptable level of revenue metals in the resulting concentrate. It would also be gainful if this could be achieved in an economic manner in order to justify the treatment of stockpiled dust.
Conclusion
The deduction from discussions leading to this point, can be summarized as this; that the waste copper smelter dust plays host to substantial amount of copper value, majorly in the form of oxides and often in small but appreciable amount as sulphides as indicated by XRD results particularly with respect to that from PMC. The bulk of this metallurgical waste occurs in the size range below 53 μm as can be seen from the SEM and wet screening results. The recovery of the copper value contained in this metallurgical dusts can either be by leaching directly or using the roast-leach process options, in which case, the waste material will be pre-treated by roasting to transform the copper sulphides to sulphates or oxides after which leaching with either water or dilute acid to take the contained copper into solution will be carried out. Solvent extraction treatment of the leached solution will also be considered to purify the solution before recovery of the copper value as nanoparticles; bearing in mind the desirability of copper nanoparticles as good substitutes to noble metals; so that recovering the CSD’s contained copper value as nano-particles is believed a better approach of converting this metallurgical waste to product. Furthermore, all of these can be achieved in an economical manner by reducing the time, cost and manpower required, through effective pre-experimental searching for optimal conditions, composition etc. by thermodynamic calculations.
Recommendation
In order to ensure an efficient processing of the waste CSD, it is strongly recommended that an intermediate mineral processing stage be introduced to the current hydrometallurgical processing of CSD, where possible; so as to reduce/remove the contained impurities and consequently enhance the chances of obtaining substantial amount of copper as nanoparticles with desired grade.
References
A.A. Baba, K.I. Ayinla, F.A. Adekola, M.K. Ghosh, O.S. Ayanda, R.B. Bale, A.R. Sheik, S.R. Pradhan, A review on novel techniques for chalcopyrite ore processing. Int. J. Min. Eng. Mineral Process. 1(1), 1–16 (2012)
USGS, Facts about Copper: copper uses, resources, supply, demand and production information 2009, http://eology.comusgsuses-of-copper/. Accessed 15/12/2010
R.T. Jones, P.J. Mackey, An overview of copper smelting in Southern Africa (2015)
J. Wood, S. Creedy, R. Matusewicz, M. Reuter, Secondary copper processing using Outotec Ausmelt TSL technology, in Proceedings of MetPlant (2011), pp. 460–467
M. Reuter, A. van Schaik, Thermodynamic metrics for measuring the “sustainability” of design for recycling. JOM 60(8), 39–46 (2008)
European Commission, European Dioxin Inventory—Secondary Copper Production [online] (2009). Available from http://ec.europa.eu/environment/dioxin/pdf/stage1/seccopper.pdf. Accessed 23 Feb 2011. A. Umer et al., Selection of a suitable method for the synthesis of copper nanoparticles. Nano 7(05), 1230005 (2012)
J.B. Wang, C.H. Hung, C.H. Hung, G.P. Chang-Chien, Polychlorinated dibenzo-p-dioxin and dibenzofuran emissions from an industrial park clustered with metallurgical industries. J. Hazard. Mater. 161(2), 800–807 (2009)
V. Montenegro, H. Sano, T. Fujisawa, Recirculation of Chilean copper smelting dust with high arsenic content to the smelting process. Mater. Trans. 49(9), 2112–2118 (2008)
Regulation EC, No 1907/2006 of the European Parliament and of the Council of 18 December 2006, concerning the Registration. Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive, vol. 45 (1999), pp. 1–849
SANS, South African National Standard: Ambient Air Quality—Limits for Common Pollutants (2005)
U. Neveling, Palabora Mining Company Annual report on ambient air quality monitoring (2011)
L. Qiang, I.S. Pinto, Z. Youcai, Sequential stepwise recovery of selected metals from flue dusts of secondary copper smelting. J. Clean. Prod. 84, 663–670 (2014)
I.S. Pinto, H.M. Soares, Selective leaching of molybdenum from spent hydrodesulphurisation catalysts using ultrasound and microwave methods. Hydrometallurgy 129, 19–25 (2012)
I.S. Pinto, H.M. Soares, Recovery of molybdates from an alkaline leachate of spent hydrodesulphurisation catalyst–proposal of a nearly-closed process. J. Clean. Prod. 52, 481–487 (2013)
F. Bakhtiari, H. Atashi, M. Zivdar, S.S. Bagheri, Continuous copper recovery from a smelter’s dust in stirred tank reactors. Int. J. Miner. Process. 86(1), 50–57 (2008)
F. Bakhtiari, M. Zivdar, H. Atashi, S.S. Bagheri, Bioleaching of copper from smelter dust in a series of airlift bioreactors. Hydrometallurgy 90(1), 40–45 (2008)
F.J. Alguacil, I. Garcia-Diaz, F. Lopez, O. Rodriguez, Recycling of copper flue dust via leaching-solvent extraction processing. Desalin. Water Treat. 56(5), 1202–1207 (2015)
G.A. Kordosky, Copper recovery using leach/solvent extraction/electrowinning technology: forty years of innovation, 2.2 million tonnes of copper annually. J. S. Afr. Inst. Min. Metall. 102(8), 445–450 (2002)
N.N. Hoover, B.J. Auten, B.D. Chandler, Tuning supported catalyst reactivity with dendrimer-templated Pt-Cu nanoparticles. J. Phys. Chem. B 110(17), 8606–8612 (2006)
Y. Niu, R.M. Crooks, Preparation of dendrimer-encapsulated metal nanoparticles using organic solvents. Chem. Mater. 15(18), 3463–3467 (2003)
E. Darezereshki, F. Bakhtiari, Synthesis and characterization of tenorite (CuO) nanoparticles from smelting furnace dust (SFD). J. Min. Metall. B: Metall. 49(1), 21–26 (2013)
C. Samuelsson, G. Carlsson, Characterization of copper smelter dusts. CIM Bull. 94(1051), 111–115 (2001)
Y. Chen, T. Liao, G. Li, B. Chen, X. Shi, Recovery of bismuth and arsenic from copper smelter flue dusts after copper and zinc extraction. Miner. Eng. 39, 23–28 (2012)
D.K. Steele, K.S. Gritton, S.B. Odedirk, Treatment of Copper Smelting and Refining Wastes (US Department of the Interior, Bureau of Mines, 1994)
A.K. Biswas, W.G. Davenport, Extractive Metallurgy of Copper: International Series on Materials Science and Technology, vol. 20. Elsevier (2013)
D.R. Swinbourne, E. Simak, A. Yazawa, V. Melbourne, Accretion and dust formation in copper smelting-thermodynamic considerations, in Sulfide Smelting (2002), pp. 247–259
E. Miettinen, Thermal conductivity and characteristics of copper flash smelting flue dust accretions. Teknillinen korkeakoulu (2008)
J.A. Eastman, S.U.S. Choi, S. Li, W. Yu, L.J. Thompson, Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 78(6), 718–720 (2001)
R.K. Guduru, K.L. Murty, K.M. Youssef, R.O. Scattergood, C.C. Koch, Mechanical behavior of nanocrystalline copper. Mater. Sci. Eng., A 463(1), 14–21 (2007)
Y. Wang, M. Chen, F. Zhou, E. Ma, High tensile ductility in a nanostructured metal. Nature 419(6910), 912–915 (2002)
X. Kang, Z. Mai, X. Zou, P. Cai, J. Mo, A sensitive nonenzymatic glucose sensor in alkaline media with a copper nanocluster/multiwall carbon nanotube-modified glassy carbon electrode. Anal. Biochem. 363(1), 143–150 (2007)
K.B. Male, S. Hrapovic, Y. Liu, D. Wang, J.H. Luong, Electrochemical detection of carbohydrates using copper nanoparticles and carbon nanotubes. Anal. Chim. Acta 516(1), 35–41 (2004)
Y. Guo, W. Meyer-Zaika, M. Muhler, S. Vukojević, M. Epple, Cu/Zn/Al xerogels and aerogels prepared by a sol–gel reaction as catalysts for methanol synthesis. Eur. J. Inorg. Chem. 2006(23), 4774–4781 (2006)
M.L. Kantam, V.S. Jaya, M.J. Lakshmi, B.R. Reddy, B.M. Choudary, S.K. Bhargava, Alumina supported copper nanoparticles for aziridination and cyclopropanation reactions. Catal. Commun. 8(12), 1963–1968 (2007)
J.A. Rodriguez, P. Liu, J. Hrbek, J. Evans, M. Perez, Water gas shift reaction on Cu and Au nanoparticles supported on CeO2 (111) and ZnO (000$ bar 1$): intrinsic activity and importance of support interactions. Angew. Chem. 119(8), 1351–1354 (2007)
C. Pecharromán, A. Esteban-Cubillo, I. Montero, J.S. Moya, E. Aguilar, J. Santarén, A. Alvarez, Monodisperse and corrosion-resistant metallic nanoparticles embedded into sepiolite particles for optical and magnetic applications. J. Am. Ceram. Soc. 89(10), 3043–3049 (2006)
E. Balladares, U. Kelm, S. Helle, R. Parra, E. Araneda, Chemical-mineralogical characterization of copper smelting flue dust. Dyna 81(186), 11–18 (2014)
M. Vítková, V. Ettler, J. Hyks, T. Astrup, B. Kříbek, Leaching of metals from copper smelter flue dust (Mufulira, Zambian Copperbelt). Appl. Geochem. 26, S263–S266 (2011)
A. Morales, M. Cruells, A. Roca, R. Bergó, Treatment of copper flash smelter flue dusts for copper and zinc extraction and arsenic stabilization. Hydrometallurgy 105(1), 148–154 (2010)
A.B. Vakylabad, A comparison of bioleaching ability of mesophilic and moderately thermophilic culture on copper bioleaching from flotation concentrate and smelter dust. Int. J. Miner. Process. 101(1), 94–99 (2011)
A.B. Vakylabad, M. Schaffie, M. Ranjbar, Z. Manafi, E. Darezereshki, Bio-processing of copper from combined smelter dust and flotation concentrate: A comparative study on the stirred tank and airlift reactors. J. Hazard. Mater. 241, 197–206 (2012)
J.Y. Wu, F.C. Chang, H.P. Wang, M.J. Tsai, C.H. Ko, C.C. Chen, Selective leaching process for the recovery of copper and zinc oxide from copper-containing dust. Environ. Technol. 36(23), 2952–2958 (2015)
T.K. Ha, B.H. Kwon, K.S. Park, D. Mohapatra, Selective leaching and recovery of bismuth as Bi2O3 from copper smelter converter dust. Sep. Purif. Technol. 142, 116–122 (2015)
L.G. Austin, I. Shah, A method for inter-conversion of Microtrac and sieve size distributions. Powder Technol. 35(2), 271–278 (1983)
B.A. Wills, T. Napier-Munn, Wills’ mineral processing technology: an introduction to the practical aspects of ore treatment and mineral recovery. Butterworth-Heinemann (2015)
Z.F. Xu, L.I. Qiang, H.P. Nie, Pressure leaching technique of smelter dust with high-copper and high-arsenic. Trans. Nonferrous Met. Soc. China 20, s176–s181 (2010)
V. Ettler, M. Vítková, M. Mihaljevič, O. Šebek, M. Klementová, F. Veselovský, P. Vybíral, B. Kříbek, Dust from Zambian smelters: mineralogy and contaminant bioaccessibility. Environ. Geochem. Health 36(5), 919–933 (2014)
T. Rosenqvist, Principles of Extractive Metallurgy. Tapir Academic Press (2004)
The encyclopedia of earth, 2015: https://en.wikipedia.org/wiki/Encyclopedia_of_Earth
S.K. Kawatra, T.C. Eisele, Depression of Pyrite Flotation by Yeast and Bacteria. Mineral Biotechnology: Microbial Aspects of Mineral Beneficiation, Metal Extraction, and Environmental Control (2001), p. 3
S. Prasad, B.D. Pandey, Alternative processes for treatment of chalcopyrite—a review. Miner. Eng. 11(8), 763–781 (1998)
F. Magagula, High temperature roasting of sulphide concentrate and its effect on the type of precipitate formed. Doctoral dissertation, 2012
Z.B. Yin, E. Caba, L. Barron, D. Belin, W. Morris, M. Vosika, R. Bartlett, Copper extraction from smelter flue dust by lime-roast/ammoniacal heap leaching. Residues and Effluents: Processing and Environmental Considerations (1992), pp. 255–267
B. Gorai, R.K. Jana, Z.H. Khan, Electrorefining electrolyte from copper plant dust. Mater. Trans. 43(3), 532–536 (2002)
M.P. Smirnov, V.T. Khvan, G.A. Bibenina, R.P. Kefilyan, N.I. Il’yasov, Complex treatment of lead and rhenium containing sulfate dusts from copper plants. Tsvetn. Met. 6, 3–6 (1984)
Z.W. Zhang, W. Lu, F. Zheng, Separation and recovery of copper and zinc from flue dust. Huanjing Kexue 11(6), 1012–1016 (1992)
M. Carter, E.R. Vance, L.P. Aldridge, M. Zaw, G. Khoe, Immobilization of arsenic trioxide in cementitious materials, in Australasian Institute of Mining and Metallurgy (1994), pp. 275–280
Y. Fu, L. Jiang, D. Wang, Removal of arsenic from copper smelter flue dust by calcinations. Yelian Bufen 6, 14–16 (2000)
Z. Yu, Process for bismuth recovery from the flue dust of copper smelting. Huaxue Shijie 28(10), 465–468 (1987)
H. Mochida, O. Iida, Copper smelter flue dust treatment (Kokai Tokkyo Koho, Jap, 1988), p. 3
H.H. Law, M.P. Bohrer, P. O’Hara, Recovery of metals from copper smelter furnace flue dust. Residues and Effluents: Processing and Environmental Considerations (1992), pp. 295–310
R.S. Kunter, W.E. Bedal, Chloride-process treatment of smelter flue dusts. JOM 44(12), 35–38 (1992)
G.J. Roman-Moguel, G. Plascencia, J. Pérez, A. García, Copper recycling from waste pickling solutions. JOM 47(10), 18–19 (1995)
P.J. Gabb, J.P. Evans, Kennecott Utah Copper Corporation, Hydrometallurgical processing of impurity streams generated during the pyrometallurgy of copper. U.S. Patent 5,616,168, 1997
A. Robles, A.E. Serna, M. Sandez, Alkaline arsenic leaching from smelter flue dust and leaching solution regeneration, in Copper99-Cobre99, International Conference on TMS-AIME, Warrendale, PA (1999), pp. 261–272
E. Vircikova, M. Havlik, Removing as from converter dust by a hydrometallurgical method. JOM 51(9), 20–23 (1999)
J.J. Ke, R.Y. Qin, Arsenic removal and bismuth recovery from copper smelter flue dust (2000)
M.I. Martín, A. López-Delgado, F.A. López, A.G. Coedo, M.T. Dorado, F.J. Alguacil, Treatment of copper converter flue dust for the separation of metallic/non-metallic copper by hydrometallurgical processing. J. Chem. Eng. Jpn. 36(12), 1498–1502 (2003)
N.T. Beukes, J. Badenhorst, Copper electrowinning: theoretical and practical design. J. South Afr. Inst. Min. Metall. 109(6), 343–356 (2009)
O. Kubaschewski, C.B. Alcock, P.J. Spencer, Materials Thermodynamics (1993)
F. Bakhtiari, E. Darezereshki, One-step synthesis of tenorite (CuO) nano-particles from Cu4(SO4)(OH)6 by direct thermal-decomposition method. Mater. Lett. 65(2), 171–174 (2011)
X. Song, S. Sun, W. Zhang, Z. Yin, A method for the synthesis of spherical copper nanoparticles in the organic phase. J. Colloid Interface Sci. 273(2), 463–469 (2004)
S. Kapoor, T. Mukherjee, Photochemical formation of copper nanoparticles in poly (N-vinylpyrrolidone). Chem. Phys. Lett. 370(1), 83–87 (2003)
M. Aslam, G. Gopakumar, T.L. Shoba, I.S. Mulla, K. Vijayamohanan, S.K. Kulkarni, J. Urban, W. Vogel, Formation of Cu and Cu2O nanoparticles by variation of the surface ligand: preparation, structure, and insulating-to-metallic transition. J. Colloid Interface Sci. 255(1), 79–90 (2002)
H. Zhu, C. Zhang, Y. Yin, Novel synthesis of copper nanoparticles: influence of the synthesis conditions on the particle size. Nanotechnology 16(12), 3079 (2005)
Y. Wang, P. Chen, M. Liu, Synthesis of well-defined copper nanocubes by a one-pot solution process. Nanotechnology 17(24), 6000 (2006)
S. Panigrahi, S. Kundu, S.K. Ghosh, S. Nath, S. Praharaj, S. Basu, T. Pal, Selective one-pot synthesis of copper nanorods under surfactantless condition. Polyhedron 25(5), 1263–1269 (2006)
B.K. Park, S. Jeong, D. Kim, J. Moon, S. Lim, J.S. Kim, Synthesis and size control of monodisperse copper nanoparticles by polyol method. J. Colloid Interface Sci. 311(2), 417–424 (2007)
A.A. Athawale, P.P. Katre, M. Kumar, M.B. Majumdar, Synthesis of CTAB–IPA reduced copper nanoparticles. Mater. Chem. Phys. 91(2), 507–512 (2005)
X. Zhang, D. Zhang, X. Ni, J. Song, H. Zheng, Synthesis and electrochemical properties of different sizes of the CuO particles. J. Nanopart. Res. 10(5), 839–844 (2008)
E. Darezereshki, F. Bakhtiari, A novel technique to synthesis of tenorite (CuO) nanoparticles from low concentration CuSO4 solution. J. Min. Metall. B 47(1), 73–78 (2011)
K. Byrappa, Hydrothermal processing, in Kirk-Othmer Encyclopedia of Chemical Technology (2005)
C.W. Bale, P. Chartrand, S.A. Degterov, G. Eriksson, K. Hack, R.B. Mahfoud, J. Melançon, A.D. Pelton, S. Petersen, FactSage thermochemical software and databases. Calphad 26(2), 189–228 (2002)
Wikipedia 2014 https://en.wikipedia.org/wiki
S. Rosenblum, I.K. Brownfield, Magnetic Susceptibilities of Minerals. US Department of the Interior, US Geological Survey (2000)
Z.A. Zanetell, Penalty element separation from copper concentrates utilizing froth flotation (2007)
O. Font, N. Moreno, G. Aixa, X. Querol, R. Navia, Copper Smelting Flue Dust: A Potential Source of Germanium
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Okanigbe, D.O., Popoola, A.P.I., Adeleke, A.A. (2017). Hydrometallurgical Processing of Copper Smelter Dust for Copper Recovery as Nano-particles: A Review. In: Zhang, L., et al. Energy Technology 2017. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-52192-3_21
Download citation
DOI: https://doi.org/10.1007/978-3-319-52192-3_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52191-6
Online ISBN: 978-3-319-52192-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)