Abstract
During the steel scrap melting process in an electric arc furnaces, the generated dust may contain a significant amount of zinc. The content of zinc in case of melting galvanized steel scrap is variable and ranges from 15 to 40 wt%. This dust cannot be re-used for the steelmaking process. On the other hand the zinc content is too low to treat the steel dust as a feedstock in pyrometallurgy of zinc. When they are disposed in landfills they have adversely effect the environment. This work presents selected results of research and development of new technology, which was developed in order to utilization steelmaking dust in the sintering process. One of the products (zinc-rich) can be used as a high-quality raw material for production of zinc by pyro- or hydrometallurgical processes. The second one product from the shaft furnace can be used as a feedstock for the steelmaking process.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Protection of the steel surface with zinc coating is a commonly used anti-corrosive technique. About 50% of the world production of zinc is used for the galvanizing treatment of steel parts, which prolongs the service life 3–4 times. Hence, a large amount of the galvanized steel scrap is formed, which is melted in electric arc furnaces (EAF). In this process, zinc is vaporized and oxidized. Due to the high melting point of ZnO (1957 °C), it is transferred to the particulate matter in the form of fine dust. On the average, in the EAF process of steel production, per one tonne of the product falls 15–25 kg of dust formed in this process; the final amount of dust depends on the feedstock type and the technology used [1]. This dust contains 15–40% Zn and approximately 20% Fe as well as other elements such as cadmium, nickel, chromium, manganese, coal, tin, antimony and copper. In the natural environment, all these metals are transferred to the surface and ground water, degrading the environment [2]. Moreover, accumulating in the bodies of plants and animals end up in human bodies. Because they do not decompose, once absorbed have the ability to bioaccumulate [3]. Therefore their storage creates a serious threat to the natural environment.
Waste generated during the metallurgical process should be returned to the same process or to other processes. Owing to this, the waste-free technology can become a reality. During re-melting of galvanized steel scrap, the dust generated can not be recycled and returned to the steelmaking process because of the previously mentioned high zinc content. On the other hand, this content is not sufficiently high to treat the dust as a raw material applicable in the processes of zinc metallurgy (it can be used only as an additive).
Current processing technologies for metallurgical dust are focused on the pyro- and hydrometallurgical methods. Pyrometallurgical processes rely mainly on high-temperature reduction of zinc contained in the dust and its re-oxidation in gaseous phase. One of the most known and used processes is currently the Waelz kiln process [4, 5]. There are more than 14 plants in the world using this process [6]. Other, very similar process, both in commercial and pilot plant operation process are: ALLMET, AUSMELT, METWOOL, PRIMUS, ZINCOX, OXYCUP, CONTOP, ENVIROPLAST, TECNORED, OXICUP, REDSMELT [7,8,9,10,11,12]. Hydrometallurgical processes rely on leaching of zinc waste and separation of zinc in the process of electrolysis, by extraction through ion exchange, precipitating the insoluble zinc compounds or forcing their crystallization [13]. Currently, developed industrial technologies include: ZINCEX, EZINEX, RECUPAC [14, 15]. There are still many studies in the laboratory stage [16,17,18,19,20,21].
Despite the availability of so many processes for the recovery of zinc from metallurgical dust, none of them can solve the problem comprehensively, i.e. combine in a single process the recovery of zinc from waste and the manufacture of an iron-carrying feedstock for the blast furnaces. The development and implementation of such a method would bring many ecological and economical advantages. It would also mean a step forward in the global trends of environmental protection by pointing out the possibility of using a waste-free technology. The challenge related with this issue has been taken by the staff of researchers from the AGH—University of Science and Technology within the framework of a national project implemented in cooperation with industrial partners.
Experiments and Results
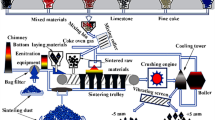
This paper presents the selected laboratory tests for high-temperature reduction of ZnO during sintering of briquettes containing steelmaking dust. The studies constitute one of the stages in the development of a new, waste-free technology for the management of metallurgical dust with high zinc content, originating from electric arc furnaces. Figure 1 shows the flow of materials and agents in the devices used for the proposed technological process.
In this technology, a mixture comprising adequate quantities of dust, mill scale, coke breeze, and a binder is moistened in the mixing process and then subjected to briquetting. Briquettes are next undergoing the reduction in a shaft furnace, where in the temperature range of 1100–1200 °C the zinc oxide is reduced with carbon contained in the briquettes and CO which is formed by the Boudouard reaction. Zinc reduced to the form of gas is discharged together with other process gases and undergoes re-oxidation outside the sintering furnace. Then it is removed from the furnace in the form of dust. The dust can be used as a high-quality commercial product and a raw material for the zinc production. In the processes conducted in a shaft furnace, the reaction of reduction also includes the lead oxide, but because of its low content in the processed steelmaking dust, it should assume the form of fine droplets in the briquette rather than occur as a separate phase. A small portion of lead can move to the gaseous phase. The process of reduction should also cover iron oxides, reduced to FeO and Fe3O4. The exhaust gases are directed to the heat exchanger and then to the furnace stack. To carry out the process it is necessary to supply the air and exhaust gases with proper oxygen content, such that will ensure in the sintering process the required amount of heat resulting from the combustion of carbon contained in the briquettes and a reducing atmosphere in the reducing zone. The residue from the process in the form of sintered briquettes will be discharged by means of a screw conveyor and used in the iron and steel metallurgy.
The selection of guidelines for the project and preliminary studies were carried out on the metallurgical dust from one of the national steel mills. The mixture for the briquetting process was selected in such a way as to provide:
-
the required integration of briquettes (the selection of binder),
-
the required strength before and after sintering,
-
the required amount of reducing agent to obtain a high rate of the ZnO reduction,
-
the required amount of fuel capable of producing the required temperature.
In accordance with the objectives of the process, to make the material suitable for re-use in the steel industry, the content of Fe after sintering and reduction should be minimum 50% and that of zinc maximum 2%. To meet the requirement of adequate iron content after sintering, in addition to the metallurgical dust, the mixture for briquetting should also contain mill scale with approx. 70% Fe. As a binder, a two-component mixture of Ca(OH)2 and molasses was used. Its task was to ensure full integration of grains during pressing and high mechanical strength of briquettes. Additionally, the selected ingredients were not acting as a ballast in later metallurgical processes. Two types of reducing agents were tested in the studies, i.e. coke and coal dust. Carbon in the sintering process acts as a reductant and a source of heat that is needed to ensure proper temperature of the process. The amount of carbon was chosen in such a way as to provide adequate progress in the reaction of the reduction of metal oxides, to make up for the negative energy balance of chemical reactions and provide heating of the substrate to an adequate sintering temperature. The compositions of mixtures prepared for briquetting are shown in Table 1. The steelmaking dust contained 33.2 wt% Zn, 22.6 wt% Fe, 9 wt% Cl, 3 wt% Pb, 2.5 wt% Ca, 2 wt% Si, 1.5 wt% Mn and 1 wt% C.
Mixtures were pressed into the form of briquettes without parting plane and marked in further part of the study as briquettes B1, B2 and B3 formed from mixtures M1, M2 and M3, respectively. Their compressive strength, understood as a force causing their destruction, was 300, 340 and 470 for briquettes B1, B2 and B3, respectively. Figure 2 shows a model of the roll forming briquettes without parting plane and the shape of a single briquette.
In order to determine the removal ratio of zinc, the briquettes were subjected to the process of sintering in a laboratory chamber furnace. For this purpose, they were placed in a graphite crucible and covered with a cover also made of graphite. Thus a reducing atmosphere was created in the crucible, necessary for the reduction of zinc oxides. Zinc reduced to the gaseous form, after leaving the space of the crucible was oxidized with oxygen present in the furnace chamber and deposited on the ceramic lining of the furnace as a yellow solid matter. Tests were conducted at 1000, 1100 and 1200 °C and at each of those temperatures, three different times of 15, 30 and 45 min were additionally applied. Figure 3 shows the appearance of briquettes before and after the sintering at 1200 °C for 30 min.
After the experiment, the briquettes were cooled, weighed, and then ground to a form of particulate and analyzed for the zinc content on an ARL QUANT’X X-ray spectrometer. The results of the analysis allowed for the determination of zinc removal ratio (η) from the briquettes (Eq. 1), to establish next optimum conditions of the sintering process.
where:
- m(0) :
-
initial content of zinc in the sample (before reduction), g
- m(t) :
-
final content of the zinc in the sample (after reduction), g
The measured values of mass changes expressed as a relative weight loss in samples and the calculated values of the zinc removal ratio are summarized in Table 2.
Analyzing the events possibly taking place during sintering, it was found that the observed changes in the weight of briquettes before and after the sintering might be due to several, simultaneously occurring processes, such as:
-
the reduction of oxides of zinc, lead and iron,
-
the evaporation of gaseous zinc and partly of Pb,
-
the evaporation of H2O,
-
partial decomposition and evaporation of binder,
-
the combustion of fuel (coke or coal dust depending on the type of mixture).
Detailed analysis of the obtained results listed above leads to the conclusion that the weight loss in briquettes is adequate to the zinc removal ratio. However, considering the occurrence of other above mentioned processes, running in parallel with the reduction of ZnO, it is impossible to estimate process effectiveness based on the weight loss in briquettes only (hence results also the need to repeat the analysis after each experiment). The calculated values of the zinc removal ratio are illustrated in Fig. 4 for each of the conducted experiments.
It was found that briquettes sintered at 1100 and 1200 °C were compact and sintered within their whole volume; they retained their shape and had adequate mechanical strength. Although they were not tested for strength properties, their disintegration in a steel mortar during the preparation for analysis after sintering has proved to be very difficult. Examining the obtained results, it can be concluded that the zinc removal ratio during sintering depends on both temperature and time of the sintering process. Ensuring adequate residence time of briquettes (at least 30 min) in the zone of a temperature close to 1200 °C should provide, in the case of using coke breeze as a reducing agent, the zinc removal ratio equal to 95%. This means that the required content of Zn < 2% and Fe > 50% will be satisfied, and the sinter can be used as a feedstock for blast furnaces.
Conclusions
Summing up the results of studies of the high-temperature sintering process, it can be concluded that the zinc removal ratio depends on the following factors:
-
Type of reducing agent. It has been assumed that the reactions of the reduction of metal oxides inside the briquettes occur by both direct reduction with carbon (2) and indirect reduction with gaseous CO (3) formed in the Boudouard reaction (4).
$$ {\text{MeO}} + {\text{C}} = {\text{Me}} + {\text{CO}} $$(2)$$ {\text{MeO}} + {\text{CO}} = {\text{Me}} + {\text{CO}}_{ 2} $$(3)$$ {\text{CO}}_{2} + {\text{C}} = 2{\text{CO}} $$(4)The results have clearly indicated the stronger effect of coke breeze as a reducing agent. Coke breeze was used in the briquettes B1 and B3. This effect is associated with a higher content of carbon in this agent, higher reactivity and lower level of impurities (tar, dust and sulphur).
-
Temperature. The strongest effect of temperature was visible after 15 min of the experiment duration for each of the tested mixtures. After this time, the temperature increase from 1000 to 1200 °C has increased the zinc removal ratio by approx. 30%. However, after 30 and 45 min, the effect has weakened considerably and, depending on the mixture composition, has yielded the result of only 15–25%.
-
Time of reduction. At lower temperatures, obtaining high zinc removal ratio takes longer time. Even after 45 min this level did not exceed 80% in the case of briquettes comprising coke breeze as a reducing agent. At a temperature of 1200 °C, practically after 15 min, the reduction of zinc oxides has assumed a high yield of up to 85–90%. This applied to briquettes containing in their composition coke breeze as a reducing agent.
-
Contact of reactants. Proper and effective contact of the reactants is important in the case when the reactions of reduction also occur in the solid phase (direct reduction). From this point of view, the applied briquetting process seems to be the best solution.
-
The content of H 2 O. Higher moisture content in the mixture can have a beneficial effect on the sintering process, due to the presence of pores generated by moisture evaporation, which facilitate transport of reactants to the reaction zone (CO gas) and the discharge of products, i.e. of the gaseous zinc, from the reaction zone. In this case, moisture content in briquettes was observed to have no major effect on the zinc removal ratio. The main purpose of the additional moistening of the mixture was to obtain briquettes of an adequate strength, and this goal was achieved.
References
A. Guézenneca, J. Huber, F. Patisson, P. Sessiecq, J. Birat, D. Ablitzer, Dust formation in electric arc furnace: birth of the particles. Powder Technol. 157, 2–11 (2005). doi:10.1016/j.powtec.2005.05.006
P. Ostrowska, K. Mierzwa, Recovery of zinc from selected metallurgical waste. Hutnik-WH 64, 369–373 (2007)
Z. Wozniacki, T. Telejko, R. Kenig, Sintering as the method of utilization of steelmaking dusts with a high content of zinc oxides. Hutnik-WH 81, 166–171 (2014)
K. Mager, U. Meurer, B. Garcia-Egocheaga, N. Goicoechea, Recovery of zinc oxide from secondary raw materials: New developments of Waelz process, in Recycling of Metals and Engineered Materials. (The Minerals, Metals and Materials Society, 2000), pp. 329–344
Steel Dust Recycling, Waelz Kiln Technology. Available: http://www.globalsteeldust.com/waelz_kiln_technology [06/10 2016]
N.G. Gandiaga, B.G.E. Vergara, Proceedings of the “Rewas” 99: Global symposium on recycling, waste treatment and clean technology, TMS, INASMET, San Sebastian, (1999) pp. 1511–152
Zunkel A D Recovering zinc and lead from electric arc furnace dust: a technology status Report, in Recycling of Metals and Engineered Materials, ed. by D.L. Stewart, J.C. Daley, R.L. Stephens (Wiley, Hoboken, NJ, USA, 2000) doi: 10.1002/9781118788073.ch21
J.L. Roth, R. Frieden, T. Hansmann, J. Monai, M. Solvi, PRIMUS, a new process for recycling by-products and producing virgin iron. Rev. Met. Paris 98, 987–996 (2001)
M. Holtzer, A. Kmita, A. Roczniak, The recycling of materials containing iron and zinc in the oxycup process. Arch. Foundry Eng. 15, 126–130 (2015)
N.A. Barcza, D.G.C Robertson, A.F.S Schoukens, Enviroplas technology for the recovery of lead and zinc from lead blast furnace slags, in Proceedings of International lead and zinc study group 6th International Conference “Recycling lead and zinc into the 21th century, Madrid, Spain (1995)
M.A.V. Abdel-Latif, Fundamentals of zinc recovery from metallurgical wastes in the enviroplast process. Miner. Eng. 15, 945–952 (2002)
W. Lu, D. Huang, The evolution of ironmaking process based on coal containing iron ore agglomerates. ISIJ Int. 41, 807–812 (2001)
M. Jha, V. Kumar, R. Singh, Review of hydrometallurgical recovery of zinc from industrial waste. Resour. Conserv. Recycl. 33, 1–22 (2001). doi:10.1016/S0921-(00)00095-1
G. Diaz, C. Martin, C. Frias, F. Sanchez, Emerging application of ZINCEX and PLACID technologies. JOM 85–98 (2001)
M. Olper, The EZINEX process—a new way and advanced way zinc from a for electrowinning zinc from a chloride solution, in World Zinc, ’93 ed. by I.G. Matthew (Australian Institute of Mining and Metallurgy, Victoria, Australia 1993), pp. 491–494
T. Nakamura, E. Shibata, U.T. Takasu, H. Itou, Basic consideration on EAF dust treatment using hydrometallurgical processes. Resour. Process. 55, 144–148 (2008)
Z. Youcai, R. Stanforth, Integrated hydrometallurgical process for production zinc from electric arc furnace dust in alkaline medium. J. Hazard. Mater. B80, 223–240 (2000)
N. Leclerc, E. Meux, J.-M. Lecuire, Hydrometallurgical recovery of zinc and lead from electric arc furnace dust using monotrilotriacetate anion and hexahydrated ferric chloride. J. Hazard. Mater. B91, 257–270 (2002)
D.M. Lenz, F.B. Mmartins, Lead and zinc selective precipitation from leach electric arc furnace dust solutions. Revista Materia 12, 503–509 (2007)
M. Cruells, A. Roca, C. Nunez, Electric arc furnace flue dusts: characterization and leaching with sulphuric acid. Hydrometallurgy 31, 213–231 (1992)
P. OustadakisS, P.E. Takiridis, A. Katsiapi, S. Agatzinileonardou, Hydrometallurgical process for zinc recovery from electric arc furnace dust (EAFD), Part I: characterization and leaching by diluted sulphuric acid. J. Hazard. Mater. 179, 1–7 (2010)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Palimaka, P., Pietrzyk, S., Stepien, M. (2017). Recycling of Zinc from the Steelmaking Dust in the Sintering Process. In: Zhang, L., et al. Energy Technology 2017. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-52192-3_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-52192-3_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52191-6
Online ISBN: 978-3-319-52192-3
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)