Abstract
The effect of titanium sulfide particles on the grain size characteristic of low carbon steel was analyzed. Optical microscope (OM), scanning electron microscope (SEM) were used to characterize the grain size and particles. The result showed that grain size increased from 19.95 to 60.56 μm after heat treatment . The particles were mainly titanium sulfide in the size range of 0.2–0.8 μm and the volume fraction decreased significantly from 0.0084 to 0.0023%. The thermodynamic calculation resulted that these particles were dissolved during heat treatment. The pinning force of grain boundary and the driving force of grain growth were calculated. Based on experimental results and theoretical calculations, titanium sulfide particles with diameter from 0.2 to 0.8 μm and volume fraction of 0.0084% would be sufficient to inhibit the ferrite grain growth.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
It is well known that controlling grain size has been recognized as a viable approach to obtain desired properties [1]. Moreover, inclusions are known to play an important role in grain size because they can pin dislocations and grain boundaries [2, 3]. Additionally, the type and the size of the precipitates are related to the pinning force, and the grain growth is determined by the balance of the driving force and the pinning force. When the driving force exceeds the pinning force, grain growth can take place [4].
The element titanium acts with sulfur in steel and forms various kinds of precipitates such as Ti4C2S2, TiS, TiS2, Ti2S, etc. Many studies have been carried out on Ti-added ultra-low carbon steels and found that small amount of alloying elements Ti can retarding the grain growth through the precipitation of second phases [5]. Many researchers have also studied the effect of hundreds of nanometers particles on the grain size during heat treatment . Zhou et al. [6] established that the particles in the size range of 0.3–1.2 μm could effectively pinned the grain boundary and strongly hindered the grain growth. Nakayama et al. [7] found that AlN in the size of less than 0.5 μm in semi-processed non-oriented electrical steel inhibited the grain growth at the final annealing. Ti is commonly used as alloying element to improve the properties and it has strong chemical affinity for sulfide in steels [8]. However, the pinning effect of titanium sulfides in the size range of 0.1–1 μm on grain size in low carbon steel is rarely reported.
The aim of the present study, therefore, was to investigate the effect of titanium sulfides in the size range of 0.1–1 μm on grain size in low carbon steel during heat treatment. The results indicate that the dissolution of titanium sulfides particles in the size range of 0.2–0.8 μm during heat treatment will be effective in facilitating grain growth.
Experiment Procedure
The sample S1 was obtained after stress relief annealing of low carbon steel sheet. The stress relief annealed steel sheet was then annealed in 1023 K for about several hours, cooled in the furnace to room temperature. The sample taken from this procedure was named as sample S2. The carbon content of steel is ultra-low, it also contains Si, S, P, N, Al, Ti and other elements.
For grain analysis and particle characterization, samples were cut from original steel. Then the samples (10 mm × 10 mm × 0.35 mm) were prepared by grinding and polishing. The sample was etched by 3% Nital for microstructure observation by using LEICA DM6000 M light microscope. For volume fraction calculation of particles in the size of 0.1–1 μm, 75 micrographs were continuously taken at 3000× magnification using Hitachi SU1510 scanning electron microscope (SEM). For the purpose of determining the types of the particles in the size of 0.1–1 μm, the particles were chemical extracted with nonaqueous solvent and then collected on a filter with pore size of 3, 1 and 0.1 μm successively. The 0.1 μm film were examined with JSM-6700F cold field emission scanning electron microscope (FE-SEM) equipped with energy dispersive spectrometry (EDS) to analyze the chemistry composition of the particles.
Results
Grain Growth Behavior
Both steel samples, S1 and S2, exhibite a ferrite microstructure as shown in Fig. 1. Sample S1 has a finer grain than sample S2, and sample S2 has a more uniform distribution than sample S1. The mean grain sizes of samples S1 and S2 measured by linear intercept method are 19.95 and 60.56 μm, respectively.
Particle Characterization
Figure 2 represents the number of particles per unit area (NA) in detected size range of 0.1–1 μm in samples S1 and S2, the results were grouped with interval of 0.1 μm. The particles detected are nearly spherical, therefore the size represents the diameter of the particles. It was shown that fewer particles in this size range were detected in sample S2. As for particles in size range of 0.2–0.8 μm, the value of NA decrease significantly from 397 to 55. The volume fraction (f v ) of the particles can also be calculated by using Dehoff equations [9] based on the date obtained from 75 SEM micrographs captured continuously at a magnification of 3000×. The f v was calculated to be 0.0084 and 0.0023% for sample S1 and S2, respectively.
Figures 3 and 4 represents the typical particles extracted by electrolysis from samples. It can be found that the particles in sample S1 were mainly titanium sulfide with a nearly spherical shape as shown in Fig. 3a. Figure 3b shows a typical EDS spectrum from such particles, the Ti/S atomic ratio is close to one. In sample S2, FE-SEM analysis shows that few titanium sulfide particles were found as shown in Fig. 4a. Figures 3b and 4b show the typical EDS spectrum of titanium sulfide particles from Figs. 3a and 4a, respectively. And the Ti/S atomic ratio is close to one. The C, O and Pt peaks result from the filter membrane.
The atomic ratios of Ti and S were investigated in order to deduce the type of titanium sulfide particles in samples. The result were represented in Fig. 5. It shows obviously that the atomic ratios are between 0.7 and 1.1, and most of them are close to 1.0.
Discussions
Thermodynamics Calculation
Based on the analysis of the atomic ratios of Ti and S, it can be deduced that titanium sulfide particles observed in sample S1 are TiS particles . The assumption for the thermodynamics calculation is that titanium sulfide particles in this study are TiS particles . And then the effect of heat treatment temperature on TiS particles was analyzed. The corresponding equilibrium solubility product formula of TiS is [10]:
where T is the temperature (K), the mass percent of Ti and S in the steel are 0.00108 and 0.0008% respectively.
The relationship between the amount of precipitation of sulfur and the titanium is expressed in Eq. 2. The concentrations of titanium and sulfur at lower temperature were expressed in Eqs. 3 and 4.
where \( {\Delta [}\% {\text{S}}\text{]} \) and \( \Delta [\% {\text{Ti]}} \) are the amount of sulfur and the titanium respectively, S1 and Ti1 are the concentration of sulfur and the titanium when the temperature is T1, and S2, Ti2 are the concentration of sulfur and the titanium when the temperature is dropped to T2.
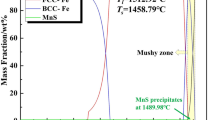
Equation 5 is derived from the Eqs. 2–5. According to Eq. 5, the amount of TiS particle and the value of [%Ti], [%S] can be calculated at different temperatures, as shown in Fig. 6. On the one hand, based on the chemical composition of present experimental steel, the TiS particles are precipitated in the temperature ranged from 964 to 1216 K. On the other hand, soaking time is long enough for the dissolution of TiS particles . It can be deduced that the TiS particles may dissolve during the heat treatment in the condition of 1023 K in this study. This also could explain the fact that there were few titanium sulfide particles detected in sample S2. Therefore, heat treatment can cause dissolution of titanium sulfide particles, which could be responsible for the grain growth.
Pinning Force and Driving Force
In order to further analyze the influence of titanium sulfide particles on the grain growth during heat treatment, the pinning force of particles and the driving force of grain growth were discussed. The assumption for the theoretical calculation is that all particles in size range from 0.2 to 0.8 μm are titanium sulfide particles. For calculation of the pinning force of particles, the following type of equation [11] has often been used:
where Fp is the pinning force, γ is grain boundary energy, taking 0.8 J/m2 [12, 14], r is mean radius of particles, f is the volume fraction of particles in the size of 0.2–0.8 μm. According to Eq. 6, the larger the f, the greater the pinning force. Fp was calculated to be 6.3 × 104 Pa in this study, where r is 0.50 μm and f is 0.0084%. The driving force of grain growth is expressed as the following type of equation [13]:
where Fd is driving force, D is the average radius of the grains and Z is the size advantage (the ratio of the maximum grain size to the average grain size), taken as 2 [14]. The Fd was calculated to be 4 × 104 Pa, where D is 9.98 μm of sample S1, the value of the γ is the same as the above. It should be noted that the pinning force caused by titanium sulfide particles was larger than the driving force of grain growth. That is to say, the titanium sulfide particles in the size of 0.1–1 μm played a role in hindering the grain growth.
Conclusions
-
(1)
The particles in the size range of 0.2–0.8 μm are mainly spherical titanium sulfide. Thermal calculation indicated that titanium sulfide could dissolve during the heat treatment.
-
(2)
The driving force for grain growth is 4 × 104 Pa. It is less than the pinning force, which was calculated to be 6.3 × 104 Pa. Titanium sulfide particles with diameter from 0.2 to 0.8 μm and volume fraction of 0.0084% would be sufficient to inhibit the ferrite grain growth.
-
(3)
The grain size is significantly increased from 19.95 to 60.56 μm after the heat treatment , and this phenomenon is related to the dissolution of titanium sulfide particles during heat treatment.
References
Estrin, Y., & Vinogradov, A. (2013). Extreme grain refinement by severe plastic deformation: a wealth of challenging science. Acta Materialia, 61(3), 782–817.
Langdon, T. G. (2013). Twenty-five years of ultrafine-grained materials: Achieving exceptional properties through grain refinement. Acta Materialia, 61(19), 7035–7059.
Apps, P. J., Bowen, J. R., & Prangnell, P. B. (2003). The effect of coarse second-phase particles on the rate of grain refinement during severe deformation processing. Acta Materialia, 51(10), 2811–2822.
Gladman, T. (2004). Grain size control (p. 183). London: Maney.
Tao, X., Gu, J., & Han, L. (2014). Carbonitride dissolution and austenite grain growth in a high Cr ferritic heat-resistant steel. ISIJ International, 54(7), 1705–1714.
Zhou, B., et al. (2016). In-situ observation of grain refinement in the simulated heat-affected zone of high-strength low-alloy steel by Zr-Ti combined deoxidation. Metal and Material International, 22, 267–275.
Nakayama, T., & Honjou, N. (2000). Effect of aluminum and nitrogen on the magnetic properties of non-oriented semi-processed electrical steel sheet. Journal of Magnetism and Magnetic Materials, 213(1), 87–94.
Mitsui, H., et al. (2009). Phase equilibria in FeS-XS and MnS-XS (X = Ti, Nb and V) systems. ISIJ International, 49(7), 936–941.
Dehoff, R. T., & Rhines, F. N. (1968). Quantitative microscopy (p. 128). New York: McGraw-Hill Book Company.
Yang, X., et al. (1996). Solubility products of titanium sulphide and carbosulphide in ultra-low carbon steels. ISIJ International, 36(10), 1286–1294.
DeArdo, A. J., Ratz, G. A., & Wray, P. J. (1982). Thermomechanical processing of microalloyed austenite: Proceedings of the International Conference on the Thermomechanical Processing of Microalloyed Austenite. Metallurgical Society of AIME.
Chapa, M., et al. (2002). Influence of Al and Nb on optimum Ti/N ratio in controlling austenite grain growth at reheating temperatures. ISIJ International, 42(11), 1288–1296.
Gladman, T. (1966). Proceedings of the Royal Society of London, A294, 298.
Anna, K., et al. (2015). Effect of Nb microalloying on reversion and grain growth in a high-Mn 204Cu austenitic stainless steel. ISIJ International, 55(10), 2217–2224.
Acknowledgements
This work was supported by 973 Project (No.2011CB012902) and National Natural Science Foundation of China (No. U1460103). The authors also wish to express their grateful thanks to the Instrumental Analysis & Research Center of Shanghai University for the instrument supports.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Wu, Y., Peng, B., Li, F., Zheng, S., Li, H. (2017). Effect of Titanium Sulfide Particles on Grain Size in Low Carbon Steel. In: Meyers, M., et al. Proceedings of the 3rd Pan American Materials Congress. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-52132-9_70
Download citation
DOI: https://doi.org/10.1007/978-3-319-52132-9_70
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52131-2
Online ISBN: 978-3-319-52132-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)