Abstract
After extracting rare earths minerals, iron minerals and fluorite from Bayan Obo ore, niobium and scandium which are riched in the mineral processing tailings coexist with the remaining rare earth. In order to recovery these valuable elements, the tailings was disposed with the method of activating roasting-acid leaching. The tailings was roasted with NaCl–Ca(OH) 2 under a temperature of 900 °C for 1.5 h. Weight percentages of NaCl-to-tailings and Ca(OH)2-to-tailings are 10% and 20% respectively. Then the roasted ore experienced two leaching stages from “hydrochloric acid pre-leaching at 90 °C for 1.5 h” to “intensified sulfuric acid leaching at 300 °C for 1 h”. The results show that, the leaching rates of niobium, scandium and rare earth in roasted ore could reach 86.80, 97.42 and 97.94% respectively under the conditions above. Moreover, the radioactivity per unit mass of leaching residue was reduced to 745 Bq/kg and environmentally friendly.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Bayan Obo ore is a deposit which mainly contains iron, rare earths, niobium and other valuable elements [1,2,3,4]. A series of problems has been faced and solved by technical personnel in Baotou Steel. At present, mineral separation technologies about iron minerals and rare earths mineral are very mature [5,6,7,8,9]. However, niobium concentration with niobium grade 4.20% and recovery 28.25% can be obtained by conventional flotation process [10, 11]. Due to that unsatisfactory result, much work of niobium flotation was made continuously. Li et al. recycled rare earths and niobium from mineral processing tailings by the method of flotation-magnetic separation-gravity separation. They obtained niobium concentrate with a grade of 1.66% and a recovery of 35.58% [12]. An ore dressing experiment on extracting niobium from eschynite was made by Beijing General Research Institute of Mining and Metallurgy. Grade and recovery of niobium concentrate in this process are 15.12 and 57.46% respectively [13]. In view of low grades, various types and small sizes, it is very difficult to extract niobium from Chinese niobium minerals with flotation. Niobium minerals are soluble in hydrofluoric acid and heating sulfuric acid under suitable conditions. As a result hydrometallurgical is used for extracting niobium by more and more people. They also may be decomposed by sodium hydroxide and chlorine decomposition. Wu et al. leached niobium from tantalum-niobium bearing minerals with sulfuric acid and hydrogen peroxide. 81% of the niobium leaching rate is achieved [14]. The scandium has no independent minerals in Bayan Obo ore and exists as isomorphism in other minerals. Method of beneficiation is not suitable for the effective extraction of scandium. Li et al. confirmed that rare earth elements including scandium can be transformed into solutions by roasting the target minerals in concentrated sulfuric acid at 250–300 °C and leaching with water [15]. In addition, low-content niobium and scandium existing in Bayan Obo tailings are hardly to be separated out by flotation. So hydrometallurgy is a good selection for extracting the valuable elements from Bayan Obo tailings. In this paper, the process of activating roasting- acid leaching will be studied to extract niobium, scandium and rare earth from tailings.
Experimental
Materials
Niobium and scandium are concentrated in tailings after iron minerals, rare earth minerals and fluorite are separated out from Bayan Obo ore. The tailings above are known simply as “Bayan Obo tailings” and selected as materials in this study. Chemical composition of tailings is listed in Table 1. Table 2 is the specific mineral composition of tailings.
Comparing with Bayan Obo ore, contents of niobium and scandium have tripled in Bayan Obo tailings. Percentage of niobium and scandium in tailings reach 0.36% and 0.03%. Furthermore, rare earth which is not separated off takes a percentage of 2.14% in tailings. It is worthy extracting these valuable elements from the tailings. Tables 3 and 4 are distributions of Nb2O5 and Sc2O3 in tailings respectively.
Distributions of niobium and scandium in tailings show that, more than 70% of the niobium exists in the form of niobite, ilmenorutile, eschynite and pyrochlore. Scandium which distributes in pyroxene and amphibole accounts for 55.3% of the total content. According to characteristic of Bayan Obo ore, existing forms of rare earth are mainly bastnasite and monazite. So the minerals mentioned above are determined to be target minerals for extracting niobium, scandium and rare earth.
Experiment Theory
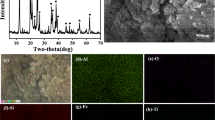
The method of activating roasting-acid leaching is applied in this study. Calcium hydroxide is chosen as decomposer and activator in roasting process. Figure 1 is XRD diagram of the roasted products of niobium concentrate and Ca(OH)2. The calcium hydroxide could transform niobium minerals into acid-soluble minerals. Calcium hydroxide is conductive to the decomposition of silicate minerals such as pyroxene and amphibole at high temperature. Bastnasite and monazite could be decomposed into rare earth oxide effectively under the action of Ca(OH)2 [16]. On the face of it, activating roasting is extremely advantageous for leaching niobium and scandium further. Furthermore, sodium chloride could improve chemical reaction rate through accelerating mass transfer depending on its low-melting point. So the added sodium chloride will reduce required temperature of decomposition reaction. That practice not only saves energy consumption, but also prevents rare earth from losing activity under a high roasting temperature. Then roasted ore experiences hydrochloric acid leaching and sulfuric acid leaching successively. Hydrochloric acid is used for leaching rare earth and scandium to prevent rare earth forming deposits with Na+ and SO4 2−. In order to guarantee leaching rate of niobium , hydrochloric acid leaching residue is leached in sulfuric acid for a intensified leaching process.
The chemical reactions mentioned in Eqs. 1–4 describe chemical reactions between rare earth minerals and added Ca(OH)2. Figure 1 is XRD diagram of roasted products of niobium concentrate with Ca(OH)2. It is clearly that the roasted products are mainly many Ca-containing compounds, such as Ca((Ti0.8Fe0.1Nb0.1)O3) and Ca(Ti0.4Fe0.3Nb0.3)O3.
Experiment Method
The tailings are mixed with NaCl–Ca(OH) 2 according to a certain percentage. Then the mixture is disposed with the method of activating roasting in furnace. The roasted ore is grinded and leached by 6 mol/L hydrochloric acid at 90 °C for 1.5 h. The leaching residue and liquid are separated through filtering. After this step, the leaching residue is leached by 18 mol/L sulfuric at 300 °C for 1 h. A new leaching residue and liquid are obtained. At last, contents of valuable elements in leaching residue and leaching liquid are determined with ICP.
Effect of Adding Amount of NaCl on Leaching Rates of Valuable Elements
As a low-melting compound, sodium chloride is added into reactants to intensity mass transfer at low temperature. It is showed in Fig. 2 that, leaching rates of rare earth and scandium rise as adding amount of sodium chloride increases. The more amount of sodium chloride is added in tailings, the stronger effect of mass transfer is. Then the decomposition effect of Ca(OH)2 on Sc-containing silicate (pyroxene, amphibole) intensifies accordingly. Decomposition reactions between rare earth minerals and Ca(OH)2 become severe, too. However, the rule is different for the leaching process of niobium minerals. Adding excessive sodium chloride at the roasting process is detrimental to leaching of niobium. When the percentage of NaCl-to-tailings is 10%, the sodium chloride is beneficial for the leaching of all valuable elements. In that case, leaching rates of niobium, scandium and rare earth are 83.7, 89.94 and 96.50% respectively.
Effect of Adding Amount of Ca(OH)2 on Leaching Rates of Valuable Elements
This experiment explored variation trends of objective elements leaching rates as the adding amount of Ca(OH)2 increases. It can be known from Fig. 3 that a small amount of Ca(OH)2 could play roles of decomposer, activator. When the weight percentage of Ca(OH)2-to-tailings is 20%, leaching rates of niobium , scandium and rare earth are 86.53, 95.47 and 98.61% respectively. Increasing additive amount of Ca(OH)2 continuously cannot improve leaching rates of valuable elements obviously. Overmuch Ca(OH)2 not only increases cost, but also absorbs much heat with the decomposition of Ca(OH)2. The best additive amount of Ca(OH)2 is 20%.
Effect of Calcination Time on Leaching Rates of Valuable Elements
Sufficient calcination time is a requisite for high conversion rate of reactant. The mixed ore was roasted in muffle furnace at 800 °C for 0.5, 1, 1.5, 2 and 2.5 h. Weight percentages of Ca(OH)2-to-tailings and NaCl-to-tailings are still 20% and 10% respectively. Leaching rates of each element under different roasting time are showed in Fig. 4. When the roasting time is less than 1.5 h, leaching rates of scandium stay in low values which are lower than 85%. If roasting time is extended to 1.5 h or more, the index increased by ten percent and reached above 97.42%. Roasting time is equally important to leaching rates of niobium, too. Niobium minerals cannot be transformed into acid soluble compounds completely in a short calcination time. According to the change trend of niobium minerals leaching rate in Fig. 4, the shortest transformation time is determined as 1.5 h. However, calcination time has little effect on the leaching rates of rare earth. Its leaching rate has reached 98.14% when roasting time is 0.5 h. In consideration of the leaching rates of niobium, scandium and rare earth, 1.5 h is thought to be the best calcination time.
Effects of Calcination Temperature on Leaching Rates of Valuable Elements
Certain amount of tailings is mixed with NaCl–Ca(OH) 2 . Weight percentages of NaCl-to-tailing and Ca(OH)2-to-tailngs are 10% and 20% respectively. The mixture was calcined in muffle furnace at different temperatures for 1.5 h. The leaching rates of niobium , scandium and rare earth are showed in Fig. 5. It is obvious that the leaching rate of niobium is less than 80% when calcination temperature is lower than 800 °C. In contrast, leaching rates of rare earth is higher than 90%, which is relatively high. When calcination temperature is higher than 800 °C, leaching rate of niobium is close to 90%. This phenomenon is related to the transmission rate of the reactant. The transmission rate of the substance in solid is slower than that in liquid. The added sodium chloride plays a role of liquid mass transfer when the calcination temperature approaches melting point of NaCl (801 °C). The same change rule emerges at the leaching process of scandium. The leaching rates of scandium increased over 90% only when the calcination temperature exceeded 800 °C. In the experimental temperature range, leaching rates of the rare earth is almost constant. A better calcination temperature is determined to be 900 °C.
Radio activity per unit mass of acid-leached residue was tested because of the presence of thorium in Bayan Obo tailings. The test results showed that per unit mass of acid-leached residue had been reduced to 745 Bq/kg.
Conclusions
-
(1)
Elements analysis of Bayan Obo tailings shows that niobium and scandium contents all triple comparing that in Bayan Obo ore. In the tailings, niobium mainly exists in niobite, ilmenorutile, eschynite and pyrochlore. More than half of the scandium which has no independent minerals exists in pyroxene and amphibole.
-
(2)
Under the action of Ca(OH)2, niobium minerals are transformed into Ca-containing compounds. The niobium in new generated compounds could be leached out by sulfuric acid effectively.
-
(3)
As a decomposer, activator and fluxing agent, NaCl–Ca(OH) 2 was mixed with Bayan Obo tailings. Percentages of Ca(OH)2-to-tailings and NaCl-to-tailings were 20% and 10%. The mixture was roasted in muffle furnace at 900 °C for 1.5 h. Then the roasted ore was leached by hydrochloric acid and sulfuric acid for 1.5 and 1 h step by step. Leaching temperatures of hydrochloric acid process and sulfuric acid process are 90 and 300 °C. At this condition, leaching rates of niobium , scandium and rare earth reach 86.80, 97.42 and 97.94%, respectively.
-
(4)
Because there is 0.021% thorium existing in the Bayan Obo tailings, it is necessary to evaluate radioactivity of the leaching residue. The test result showed that per unit mass of acid-leached residue had been reduced to 745 Bq/kg. The acid-leached residue is environmentally friendly.
References
Yu, L., Liu, J., & Wang, Z. C. (2007). New progress in comprehensive utilization of tailings in Bao Steel’s concentrator. Multipurpose Utilization of Mineral Resources, 3, 32–34.
Cai, Z. L., Cao, M. L., Che, L. P., Yu, Y. F., & Hong, H. Y. (2009). Study on the beneficiation process for recovering rare-earth from the LIMS tailings of HLMS rougher concentrate after magnetizing roasting in Baogang concentrator. Metal Mine, 7, 155–157.
Wu, B., Shang, H., & Wen, J. K. (2013). Leaching of niobium from low-grade refractory tantalum-niobium bearing minerals. Chinese Journal of Rare Metals, 37(5), 791–797.
EI-Hussaini, O. M., & Mahdy, M. A. (2002). Sulfuric acid leaching of Kab Amiri niobium-tantalum bearing minerals, Central Eastern Desertm. Egypt. Hydrometallurgy, 64, 219–229.
Wang, W. W., Pranlol, Y., & Cheng, C. Y. (2011). Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy, 108, 100–108.
Makanyire, T., Jha, A., & Sutcliffe, S. (2016). Kinetics of hydrochloric acid leaching of niobium from TiO2 residues. International Journal of Mineral Processing, 64, 219–229.
Rodriguez, M. H., Rosales, G. D., Pinna, E. G., & Suarez, D. S. (2015). Extraction of niobium and tantalum from ferrocolumbite by hydrofluoric acid pressure leaching. Hydrometallurgy, 156, 17–20.
Liao, C. S., Jia, J. T., Zhang, Y., & Xu, G. (2011). Extraction of scandium from ion-adsorptive rare earth deposit by naphthenic acid. Journal of Alloys and Compound, 323–324, 833–837.
Zhang, B., Liu, C. J., Li, C. L., & Jiang, M. F. (2014). A novel approach for recovery of rare earth and niobium from Bayan Obo tailings. Minerals Engineering, 65, 17–23.
Sun, X. Q., Ji, Y., Guo, L., & Chen, J. (2011). A novel ammonium ionic liquid based extraction strategy for separating scandium from yttrium and lanthanides. Separation and Purification Technology, 81, 25–30.
Xu, Y. H., Liu, H. J., Meng, Z. J., Cui, J. G., Zhao, W. Y., & Li, L. C. (2012). Decomposition of bastnasite and monazite mixed rare earth minerals calcined by alkali liquid. Rare Earths, 30(2), 155–158.
Gibson, C. E., Kelebek, S., & Ghamirian, M. A. (2015). Niobium oxide mineral flotation: A review of relevant literature and the current state of industrial operations. International Journal of Mineral Processing, 137, 82–97.
Xu, Y. H., Liu, H. J., Cui, J. G., Meng, Z. J., Zhao, W. Y., & Li, L. C. (2012). Techniques for clean smelting and resource comprehensive recycle of Baotou rare earth concentrates. Journal of Chinese Social Rare Earths, 30(5), 632–635.
Kuzmin, V. I., Pashkov, G. L., Lomaev, V. G., Voskresenskaya, E. N., Kuzmina, V. N. (2012). Combined approaches for comprehensive processing of rare earth metal ores. Hydrometallurgy, 129–130, 1–6.
Li, L. C., et al. (2007). Extraction and separation of rare earth (p. 1). Chi Feng: Inner Mongolia Science and Technology Press.
Wu, W. Y., Bian, X., Wu, Z. Y., Sun, S. C., & Tu, G. F. (2007). Reaction process of monazite and bastnaesite mixed rare earth minerals calcined by CaO-NaCl-CaCl2. Transactions of Nonferrous Metals Society of China, 17, 864–868.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 The Minerals, Metals & Materials Society
About this paper
Cite this paper
Zhang, B., Xue, X., Huang, X., Yang, H., Han, J. (2017). Study on Leaching Valuable Elements from Bayan Obo Tailings. In: Meyers, M., et al. Proceedings of the 3rd Pan American Materials Congress. The Minerals, Metals & Materials Series. Springer, Cham. https://doi.org/10.1007/978-3-319-52132-9_63
Download citation
DOI: https://doi.org/10.1007/978-3-319-52132-9_63
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-52131-2
Online ISBN: 978-3-319-52132-9
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)