Abstract
Patients with multifocal branch-duct intraductal papillary mucinous neoplasm (BD-IPMN) present unique challenges for clinicians. These include determining the true risk of malignancy in patients with diffuse disease, the need for surgical resection, and the extent of resection if indicated. The literature does not demonstrate an intrinsically elevated risk of invasive disease in patients with multifocal relative to unifocal BD-IPMN. In absence of symptoms or recurrent pancreatitis, management is dictated by the lesion with the highest risk of malignancy, as determined by international consensus guidelines. Total pancreatectomy provides complete surgical clearance of BD-IPMN in patients with multifocal disease, and is increasingly associated with low morbidity and an acceptable quality of life. However, partial pancreatectomy may be safely performed even if gross disease is left in the remnant pancreas. Extended surveillance without resection is an acceptable strategy for patients with multifocal disease in which no cyst demonstrates high-risk stigmata or worrisome features. All strategies demonstrate low rates of development of invasive disease during follow-up.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Intraductal papillary mucinous neoplasm
- Branch-duct intraductal papillary mucinous neoplasm
- BD-IPMN
- Multifocal
- Pancreas
- Total pancreatectomy
- Observation
- Surveillance
- Pancreatic cancer
Case Presentation
A 68-year-old female in good health was noted to have numerous small pancreatic cysts during the work-up and subsequent operation for an abdominal liposarcoma. She had no personal history of pancreatic disease, nor any pertinent family history. In addition to the aforementioned liposarcoma, a preoperative CT scan of the abdomen demonstrated innumerable small cysts throughout the pancreatic parenchyma with apparent communication with the pancreatic duct, the greatest of which measured 0.9 cm in diameter. The pancreatic duct was noted to be 4 mm in maximum diameter. Postoperatively, the patient recovered uneventfully and received a dedicated work-up of these pancreatic cysts, including endoscopic ultrasound and fine needle aspiration (EUS-FNA). Definitive communication with the pancreatic duct was not identified. However, the largest cyst measuring 1.3 × 0.9 cm was found to have a mural nodule; cyst fluid aspirate was consistent with a mucinous lesion. A presumptive diagnosis of multifocal branch-duct type intraductal papillary mucinous neoplasm (BD-IPMN) was made, and a total pancreatectomy was recommended, given the extent of parenchymal involvement. The patient was referred for a second opinion regarding the management of these cysts.
Overview of Multifocal Bd-IPMN
Pancreatic cysts are increasingly identified on cross-sectional imaging, occurring in approximately 2.4–19.6% percent of CT and MRI examinations [1, 2]. Of these, lesions with malignant potential—in particular mucinous cystic neoplasm, main-duct intraductal papillary neoplasm (MD-IPMN), and BD-IPMN—require prompt identification. BD-IPMN is generally believed to have the lowest malignant potential of the three. Invasive carcinoma or high-grade dysplasia (referred to as “malignant” disease) is reportedly found in 12–47% of resected BD-IPMN specimens [3,4,5,6,7]. This range narrows to 16.1–29.5% when looking only at series with n > 100 [8]. In contrast, the reported risk of malignant disease in patients with MD-IPMN ranges between 38 and 68% [4, 6, 8,9,10,11,12,13]. It should be noted that these numbers rely heavily on retrospective data subject to selection bias, particularly as they represent the risk of malignancy in series of resected specimens. Data regarding the true risk of malignancy in BD-IPMN remains elusive, as increasing numbers of patients with presumed BD-IPMN are observed rather than offered resection.
Guidelines have been issued to aid the clinician in identifying patients with presumed BD-IPMN at risk for malignancy. The 2012 International Association of Pancreatology Consensus Guidelines (ICG) identify high-risk stigmata (obstructive jaundice, enhancing solid component, main pancreatic duct >10 mm) and worrisome features (pancreatitis, cyst size ≥3 cm, main pancreatic duct 5–9 mm, non-enhancing mural nodule, and abrupt change in pancreatic duct diameter with distal pancreatic atrophy) of and for malignant BD-IPMN [8]. Cysts with high-risk stigmata should be considered for resection straight away; those with worrisome features require further investigation with EUS-FNA to better characterize the lesion. In 2015, the American Gastroenterological Association (AGA) issued evidence-based guidelines for the management of incidentally discovered pancreatic cysts [14, 15]. Neither the 2012 ICG nor the 2015 AGA guidelines directly address multifocal disease.
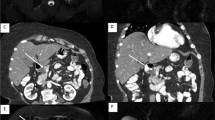
However, multifocal disease is common in patients with BD-IPMN, many of whom have innumerable lesions (Fig. 28.1). The reported incidence of multifocality varies widely from 0 to 83%, but a more conservative estimate of 25–41% is generally accepted [6, 16,17,18]. The pronounced incidence of multifocal disease has given rise to the notion that IPMN is a manifestation of a field defect of genetic susceptibility for the entire gland. Data demonstrating the risk of recurrence and malignancy in the remnant pancreas despite margin-negative resection of IPMN gives credence to such a theory [19, 20]. On the other hand, tissue analyses performed on patients with multifocal BD-IPMN have revealed striking genetic heterogeneity between synchronous lesions, indicating that each may result from an independent genetic event, even in the setting of diffuse disease [16].
a, b, c—a Axial and b coronal T2 weighted magnetic resonance imaging of the abdomen in a patient with multifocal BD-IPMN demonstrating innumerable pancreatic cysts. No mural nodules or solid masses are appreciated; no cyst measures >3 cm in diameter. c Coronal magnetic resonance cholangiopancreatography (MRCP) image of the same patient. The common bile duct is normal in caliber (white arrow). The main pancreatic duct is incompletely visualized; the visible portion is normal in caliber (black arrow). In this patient, EUS was subsequently performed to confirm communication between these cysts and the main pancreatic duct. EUS was also performed to fully evaluate the diameter of the main pancreatic duct throughout the entire gland
The management of patients with suspected multifocal BD-IPMN presents the clinician with a number of additional challenges in addition to those inherent in the management of solitary BD-IPMN (see box below). Among these include the ability to confidently confirm the diagnosis of multifocal BD-IPMN. Disturbingly, in one series a significant percentage of multifocal lesions preoperatively classified as BD-IPMN were found to have main pancreatic duct involvement, indicating a true diagnosis of mixed-IPMN [9]. Estimating the risk of malignancy is another challenge; even if no individual lesion displays high risk or worrisome features, is there reason to believe that multifocal disease itself is a marker of increased risk of malignancy? [21]. Fortunately, most series have not found an intrinsically higher rate of malignancy in patients with multifocal disease relative to unifocal disease (Table 28.1). In patients with intermediate-risk solitary lesions, further characterization relies heavily on EUS-FNA. This is logistically problematic in patients with innumerable lesions, all of which cannot be reasonably sampled. Finally, if surgery is to be recommended, the extent of resection is often difficult to determine on an anatomic basis. Between 17 and 52% of patients with multifocal BD-IPMN have disease that is either diffuse in nature or extends beyond the boundaries of segmental resections such as pancreaticoduodenectomy, extended pancreaticoduodenectomy, central pancreatectomy, and distal pancreatectomy [6, 22]. The surgeon therefore must often choose to perform a total pancreatectomy or choose to leave gross disease behind.
Challenges in the management of multifocal BD-IPMN
-
Confirmation of the diagnosis of multifocal BD-IPMN and excluding main pancreatic duct involvement.
-
Assessment of malignancy risk in patients with innumerable cysts.
-
Total pancreatectomy versus segmental resection of dominant cyst(s) with postoperative surveillance of residual disease.
-
Determination of surveillance method, interval, and duration in patients undergoing observation.
Clinical Management of Multifocal BD-IPMN
The management of patients with multifocal BD-IPMN ranges from complete surgical clearance of all disease via total pancreatectomy, to segmental resection of the dominant cyst with subsequent surveillance of the remnant pancreas (with or without residual disease present), to prolonged surveillance of the entire gland without surgical resection. As is the case for all patients with pancreatic cysts, surgery is recommended for those with symptomatic disease on the basis of a well-established relationship between the presence of symptoms and the risk of high-grade dysplasia and invasive disease [23].
Similarly, surgery is recommended for patients suffering from recurrent episodes of pancreatitis in association with multifocal BD-IPMN—believed to result from obstruction of the main pancreatic duct by viscous mucin. Pancreatitis is typically mild when associated with IPMN but frequently recurrent and sometimes refractory in absence of surgical intervention. The majority of the literature does not indicate an increased risk of malignancy for IPMN-associated with pancreatitis, but this is an association that has been poorly studied [24, 25]. In patients with asymptomatic disease, selection of the appropriate course of management will depend on the characteristics of the dominant cyst(s) and the location of lesions with high-risk stigmata or worrisome features. Clinicians will also have to weigh the patient’s fitness to undergo a major pancreatectomy, discuss the relative risks and benefits of surgery against the risks of prolonged surveillance, and take patient preference into account.
Total Pancreatectomy
Total pancreatectomy is the most definitive treatment for multifocal BD-IPMN, and the only curative procedure for patients with diffuse gland involvement. Total pancreatectomy has historically been avoided secondary to prohibitive perioperative morbidity and mortality, obligate pancreatic endocrine and exocrine insufficiency, and attendant poor quality of life. However, elective total pancreatectomy is being increasingly performed, and IPMN is an increasingly common indication [26,27,28]. A recent single-center review of 100 patients undergoing total pancreatectomy for pancreatic adenocarcinoma demonstrated a significant decrease in perioperative morbidity and mortality over the past four decades [27]. Perioperative morbidity remains common (between 19 and 66%) but is typically minor; perioperative mortality is now reported to be 2% [27, 29]. Once considered “brittle diabetes,” it has been demonstrated that postoperative glycemic control after total pancreatectomy for IPMN can be managed with similar success as in patients with type 1 diabetes mellitus. Diabetes-related quality of life is also similar between type 1 diabetics and patients post-total pancreatectomy [30, 31]. Finally, patient-reported overall quality of life after total pancreatectomy approximates or is similar to patients undergoing partial pancreatic resection [32, 33]. Nonetheless, the postoperative management of these patients remains a challenge requiring diligent management; as many as one-half of patients may require readmission within 12 months of total pancreatectomy [34].
Data regarding the outcomes of total pancreatectomy for IPMN—let alone multifocal BD-IPMN—are extremely limited. Two small cohort studies describe individual patient outcomes after total pancreatectomy for IPMN (n = 5) [35, 36]. One series of 39 patients with IPMN who underwent elective total pancreatectomy demonstrated an overall five-year survival rate of 43%; five-year survival for noninvasive and invasive disease was 90 and 22%, respectively [34]. Unfortunately, these results were not stratified based on IPMN type (main-duct versus branch-duct). For patients with noninvasive IPMN (multifocality not specified), another series demonstrated a recurrence rate of 0% (0/13) after total pancreatectomy compared to 8% (5/60) after partial pancreatectomy [37].
Which patients with multifocal BD-IPMN should be considered for total pancreatectomy? Those patients with multiple high-risk lesions meeting ICG criteria for resection who are not amenable to segmental resection on an anatomic basis may require total pancreatectomy. An argument for total pancreatectomy can also be made for those with multifocal BD-IPMN and a strong family history of pancreatic cancer. When compared to patients without a family history of pancreatic cancer, those with familial pancreatic cancer are more likely to develop an IPMN-associated malignancy in the setting of multifocal disease. These patients are also more likely to harbor high-grade dysplasia in sub-centimeter lesions [38].
Partial Pancreatectomy and Postoperative Surveillance
For the majority of patients with multifocal disease and one or more cysts that meet ICG criteria for resection, strong consideration should be given for partial pancreatectomy with postoperative surveillance. The argument for this management strategy is predicated on two facts which are supported by the literature: [1] leaving behind gross residual BD-IPMN does not increase the risk of developing subsequent malignancy in the remnant pancreas (provided no lesion with high-risk stigmata or worrisome features is missed), and [2] leaving gross residual disease does create a need for extended postoperative surveillance of the remnant pancreas, as such is required even after R0 resection of unifocal BD-IPMN. Unfortunately, supportive data is limited by the fact that the majority of series compile MD-, mixed-, and BD-IPMN together. However, it is known that the risk of recurrence and subsequent malignancy is higher after resection of MD- and mixed-IPMN compared to BD-IPMN [39]. In interpreting these studies for the purpose of managing patients with multifocal BD-IPMN, it is likely that the risk of recurrence and malignancy in the remnant pancreas is even lower than will be discussed.
Neither the presence of microscopic nor macroscopic disease in the remnant pancreas appears to increase the risk of developing IPMN-associated malignancy. In a study of 191 patients who underwent segmental resection for noninvasive IPMN (subtype not specified), 38 patients were left with disease in the remnant. One patient (1/38, 2.6%) developed invasive disease in the remnant during a mean follow-up of 41 months. Of the 153 patients with complete operative clearance of IPMN, 31 recurred in the remnant, and three developed invasive disease (3/153, 2.0%, mean follow-up 73 months). The authors therefore concluded that in comparison to those with complete operative clearance of IPMN, those with residual disease were not at increased risk for the development of malignancy [40]. Additional evidence is derived from numerous small studies demonstrating a benign course for residual BD-IPMN without high-risk stigmata or worrisome features. In a review of 37 patients with multifocal BD-IPMN, 22 patients had gross disease in the remnant that was observed with serial CT scans. Over a mean follow-up of 40 months, no clinically significant disease progression was identified [41]. Another small study demonstrated similar results, with no morphologic changes in ICG-negative BD-IPMN left in the remnant pancreas after 84 months mean follow-up (n = 16) [19]. Most recently, 33 patients with gross residual BD-IPMN after partial pancreatectomy were observed for a mean of 61 months; mean cyst size increased from 10 mm to 13 mm, and no lesion developed high-risk stigmata or worrisome features [42].
Furthermore, performing a partial pancreatectomy to remove a dominant cyst in a patient with multifocal BD-IPMN does not alter the need for postoperative surveillance. That is to say, nearly all patients undergoing partial pancreatectomy—even those with complete operative clearance of BD-IPMN—require extended surveillance. It is known that the risk of recurrence is related to the type of IPMN, presence of invasive disease, and status of the surgical margin. The largest study to date demonstrates a 17% overall recurrence rate after resection, inclusive of all subtypes of IPMN [37, 39, 42, 43]. A positive surgical margin impacts the timing and risk of recurrence, but even in the setting of negative margins the reported recurrence rate for all IPMN subtypes is 13–14% [20, 42]. One series of 210 confirmed BD-IPMNs found the overall recurrence risk to be 15%; 85% of recurrences occurred in the remnant pancreas, and 32% were invasive [42]. Thus while segmental resection is not contraindicated in patients with multifocal BD-IPMN, many authors strongly recommend postoperative surveillance of the remnant pancreas. This recommendation applies to patients in whom there is complete operative clearance of BD-IPMN as well as those with residual BD-IPMN in the remnant pancreas [19, 20, 39, 44].
The ideal duration of surveillance is unclear. Some authors have identified recurrence up to 8 years after resection, and therefore recommend indefinite surveillance[43, 44]. As our understanding of the natural history of benign-appearing BD-IPMN evolves, the recommendations for surveillance may change. One study found that in the subset of patients that underwent resection for noninvasive BD-IPMN, recurrence was almost uniformly benign (95%), prompting the authors to suggest that surveillance in that population may be unnecessary [42]. Indeed, the AGA guidelines recommend MRI surveillance of the remnant pancreas every 2 years only if the resection specimen contained high-grade dysplasia or invasive disease; the guidelines recommend against routine surveillance of the remnant when no high-grade dysplasia or invasive disease was identified in the specimen. This is justified by the low risk of malignant recurrence after resection of noninvasive BD-IPMN [15].
Case Continued
Review of the patient’s prior work-up revealed discordant findings between the CT and EUS performed at the outside institution. EUS did not confirm the communication between the main pancreatic duct and the many pancreatic cysts identified on CT. More importantly, however, was the EUS-identified mural nodule in the dominant cyst that was not seen on CT. Both findings—but in particular the latter—influence management; therefore a repeat EUS-FNA was performed out our institution.
Repeat EUS identified numerous small pancreatic cysts with clear communication with the main pancreatic duct. A dominant cyst measuring 1.5 × 0.8 cm was identified in the body of the pancreas. No mural nodule was identified in any cyst. The diameter of the main pancreatic duct was measured at 4 mm in the neck, tapering to 2–3 mm in the head and 1–2 mm in the tail. Cyst aspirate was consistent with a mucinous lesion. The results of the repeat EUS-FNA thus confirmed the diagnosis of multifocal BD-IPMN without main pancreatic ductal involvement.
Surveillance Alone
The ICG recommend treatment for patients with multifocal BD-IPMN based on the characteristics of the cyst with the highest risk of malignancy; if no lesion demonstrates high-risk stigmata or worrisome features, then a period of observation may be pursued [8]. This recommendation hinges on the presumption that multifocality is not itself an indicator of high risk. A detailed clinicopathologic review found a majority of multifocal BD-IPMN to be of gastric-foveolar epithelial subtype (less aggressive) with low to intermediate dysplasia, indicating multifocality itself is unlikely to be a manifestation of underlying aggressive tumor biology [16]. Observational data has demonstrated that multifocal disease is found in the same percentage of patients with and without invasive disease; additionally the percentage of patients who develop invasive disease during follow-up does not differ between patients with multifocal versus unifocal BD-IPMN [5]. In a retrospective review of a large cohort of 131 patients with a radiologic and/or pathologic diagnosis of multifocal BD-IPMN, 121 were managed conservatively and 10 underwent surgery. Of the 121 managed conservatively, all were alive and asymptomatic and none required surgery during a mean follow-up of 40 months (range 12–127 months) [22]. Another study directly compared a cohort of multifocal IPMN undergoing surveillance to a similar cohort of unifocal IPMN; cysts meeting ICG high-risk stigmata or worrisome features were excluded (n = 77 vs. n = 54). During follow-up, there was no difference in the progression—cyst growth, development of high-risk stigmata or worrisome features—of the dominant cyst in patients with multifocal disease as compared to the index lesion in patients with unifocal disease [45].
The true risk of developing malignancy while undergoing surveillance for multifocal BD-IPMN remains unknown; few studies address this question directly. However, extrapolating from data regarding unifocal BD-IPMN gives reason to believe this risk is low. A recent large meta-analysis and systematic review of patients with solitary BD-IPMN (20 studies included, n = 2177) found the risk of developing pancreatic malignancy to be 3.7% during follow-up (mean follow-up range 29.3–76.7 months). The rate of death related to pancreatic malignancy was 0.9% [46]. Another study of 211 patients with “low-risk” BD-IPMN found the cumulative risk of cancer at 7 years by Kaplan–Meier estimate to be 1.2% [47].
Prior to beginning a period of observation for what is thought to be low-risk multifocal BD-IPMN, one caveat requires careful consideration by the patient and clinician. Successful nonoperative management of low-risk BD-IPMN is contingent upon the accuracy with which this diagnosis can be clinically made. It has been recently demonstrated that main pancreatic duct involvement is frequently missed by preoperative imaging alone; in 233 patients with suspected isolated BD-IPMN, final pathologic diagnosis revealed main pancreatic duct involvement in 29% of patients [9]. Another study demonstrated that the diagnosis was confirmed in only 64% of suspected patients, and main pancreatic duct involvement was identified in 20% of patients [48]. Confidence in the diagnosis and a thorough investigation of the main pancreatic duct—with EUS if necessary—is therefore a critical component in the successful nonoperative management of these patients.
Finally, the duration of surveillance for low-risk multifocal BD-IPMN also remains unknown. As slow growth in cyst size and steady increase in the number of cysts have been documented over time, some clinicians recommend extended surveillance [49]. One study demonstrated a low but persistent risk of malignancy in low-risk BD-IPMN after 1 year of surveillance [47]. Although the evidence backing any decision regarding duration of surveillance is limited, the AGA guidelines recommend discontinuing surveillance of pancreatic cysts if no change has been noticed over 5 years [15].
Case Conclusion
As the patient’s disease was confirmed to be multifocal BD-IPMN without any lesion demonstrating high-risk stigmata or worrisome features for malignancy, surveillance was recommended over resection as a primary management strategy. If the patient develops symptoms, suffers from recurrent pancreatitis, or develops a lesion meeting ICG criteria for resection during follow-up, a discussion of the relative risks and benefits of partial pancreatectomy versus total pancreatectomy will inform further management.
Conclusion
Multifocal disease is common in patients with BD-IPMN. Appropriate management hinges on a patient-by-patient appraisal of the risk of malignancy in the dominant lesion (if present) as well as the remainder of the gland. Currently, data upon which the clinician can make this appraisal is limited; understanding these limitations is of critical importance. As in the management of any patient with suspected BD-IPMN, the first step in management is the identification of the presence or absence of lesions containing high-risk stigmata or worrisome features for malignancy. ICG and AGA guidelines inform the decision of whether or not to operate. Patients with multifocal disease may be at increased risk for occult main pancreatic duct involvement entailing a higher risk of malignancy; observation may be safely pursued in patients with low-risk multifocal disease after a thorough investigation of the main pancreatic duct with MRCP and/or EUS. If an operation is required, then total pancreatectomy or partial pancreatectomy with postoperative surveillance is required.
Take-away Points for the Successful Management of Multifocal BD-IPMN
-
Careful evaluation of the main pancreatic duct is necessary to rule out mixed-IPMN. Concordant MRCP and EUS findings are sought.
-
The risk of malignancy is related to the features of the highest risk cyst.
-
Total pancreatectomy no longer carries prohibitive morbidity and unacceptable quality of life in patients for whom it is indicated or preferred.
-
Segmental pancreatectomy is acceptable even if gross residual disease is left behind, but only if none of the remaining lesions have high-risk stigmata or worrisome features for malignancy.
-
Observation is a safe management strategy for patients confirmed to have multifocal BD-IPMN without any lesion meeting ICG criteria for resection.
References
Zhang XM, Mitchell DG, Dohke M, Holland GA, Parker L. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology. 2002;223(2):547–53.
de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8(9):806–11.
Correa-Gallego C, Do R, Lafemina J, Gonen M, D’Angelica MI, DeMatteo RP, et al. Predicting dysplasia and invasive carcinoma in intraductal papillary mucinous neoplasms of the pancreas: development of a preoperative nomogram. Ann Surg Oncol. 2013;20(13):4348–55.
Shimizu Y, Yamaue H, Maguchi H, Yamao K, Hirono S, Osanai M, et al. Predictors of malignancy in intraductal papillary mucinous neoplasm of the pancreas: analysis of 310 pancreatic resection patients at multiple high-volume centers. Pancreas. 2013;42(5):883–8.
Pelaez-Luna M, Chari ST, Smyrk TC, Takahashi N, Clain JE, Levy MJ, et al. Do consensus indications for resection in branch duct intraductal papillary mucinous neoplasm predict malignancy? a study of 147 patients. Am J Gastroenterol. 2007;102(8):1759–64.
Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246(4):644–51; discussion 51–4.
Fritz S, Klauss M, Bergmann F, Hackert T, Hartwig W, Strobel O, et al. Small (Sendai negative) branch-duct IPMNs: not harmless. Ann Surg. 2012;256(2):313–20.
Tanaka M, Fernandez-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12(3):183–97.
Fritz S, Schirren M, Klauss M, Bergmann F, Hackert T, Hartwig W, et al. Clinicopathologic characteristics of patients with resected multifocal intraductal papillary mucinous neoplasm of the pancreas. Surgery. 2012;152(3 Suppl 1):S74–80.
Salvia R, Fernandez-del Castillo C, Bassi C, Thayer SP, Falconi M, Mantovani W, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239(5):678–85; discussion 85–7.
Moriya T, Hashimoto Y, Traverso LW. The duration of symptoms predicts the presence of malignancy in 210 resected cases of pancreatic intraductal papillary mucinous neoplasms. J Gastrointest Surg. 2011;15(5):762–70; discussion 70–1.
Ferrone CR, Correa-Gallego C, Warshaw AL, Brugge WR, Forcione DG, Thayer SP, et al. Current trends in pancreatic cystic neoplasms. Arch Surg. 2009;144(5):448–54.
Hackert T, Fritz S, Klauss M, Bergmann F, Hinz U, Strobel O, et al. Main-duct intraductal papillary mucinous neoplasm: high cancer risk in duct diameter of 5 to 9 mm. Ann Surg. 2015;262(5):875–80; discussion 80–1.
Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148(4):824–48, e22.
Vege SS, Ziring B, Jain R, Moayyedi P. Clinical Guidelines C. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148(4):819–22.
Matthaei H, Norris AL, Tsiatis AC, Olino K, Hong SM, dal Molin M, et al. Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2012;255(2):326–33.
Waters JA, Schmidt CM, Pinchot JW, White PB, Cummings OW, Pitt HA, et al. CT vs MRCP: optimal classification of IPMN type and extent. J Gastrointest Surg. 2008;12(1):101–9.
Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, et al. Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 2007;133(1):72–9; quiz 309–10.
Ohtsuka T, Kono H, Tanabe R, Nagayoshi Y, Mori Y, Sadakari Y, et al. Follow-up study after resection of intraductal papillary mucinous neoplasm of the pancreas; special references to the multifocal lesions and development of ductal carcinoma in the remnant pancreas. Am J Surg. 2012;204(1):44–8.
Winner M, Epelboym I, Remotti H, Lee JL, Schrope BA, Chabot JA, et al. Predictors of recurrence in intraductal papillary mucinous neoplasm: experience with 183 pancreatic resections. J Gastrointest Surg. 2013;17(9):1618–26.
Raman SP, Kawamoto S, Blackford A, Hruban RH, Lennon AM, Wolfgang CL, et al. Histopathologic findings of multifocal pancreatic intraductal papillary mucinous neoplasms on CT. Am J Roentgenol. 2013;200(3):563–9.
Salvia R, Partelli S, Crippa S, Landoni L, Capelli P, Manfredi R, et al. Intraductal papillary mucinous neoplasms of the pancreas with multifocal involvement of branch ducts. Am J Surg. 2009;198(5):709–14.
Stark A, Donahue TR, Reber HA, Hines OJ. Pancreatic cyst disease: a review. JAMA. 2016;315(17):1882–93.
Morales-Oyarvide V, Mino-Kenudson M, Ferrone CR, Gonzalez-Gonzalez LA, Warshaw AL, Lillemoe KD, et al. Acute pancreatitis in intraductal papillary mucinous neoplasms: a common predictor of malignant intestinal subtype. Surgery. 2015;158(5):1219–25.
Venkatesh PG, Navaneethan U, Vege SS. Intraductal papillary mucinous neoplasm and acute pancreatitis. J Clin Gastroenterol. 2011;45(9):755–8.
Almond M, Roberts KJ, Hodson J, Sutcliffe R, Marudanayagam R, Isaac J, et al. Changing indications for a total pancreatectomy: perspectives over a quarter of a century. HPB (Oxford). 2015;17(5):416–21.
Reddy S, Wolfgang CL, Cameron JL, Eckhauser F, Choti MA, Schulick RD, et al. Total pancreatectomy for pancreatic adenocarcinoma: evaluation of morbidity and long-term survival. Ann Surg. 2009;250(2):282–7.
Murphy MM, Knaus WJ 2nd, Ng SC, Hill JS, McPhee JT, Shah SA, et al. Total pancreatectomy: a national study. HPB (Oxford). 2009;11(6):476–82.
Stauffer JA, Nguyen JH, Heckman MG, Grewal MS, Dougherty M, Gill KR, et al. Patient outcomes after total pancreatectomy: a single centre contemporary experience. HPB (Oxford). 2009;11(6):483–92.
Jamil LH, Chindris AM, Gill KR, Scimeca D, Stauffer JA, Heckman MG, et al. Glycemic control after total pancreatectomy for intraductal papillary mucinous neoplasm: an exploratory study. HPB Surg. 2012;2012:381328.
Billings BJ, Christein JD, Harmsen WS, Harrington JR, Chari ST, Que FG, et al. Quality-of-life after total pancreatectomy: is it really that bad on long-term follow-up? J Gastrointest Surg. 2005;9(8):1059–66; discussion 66–7.
Epelboym I, Winner M, DiNorcia J, Lee MK, Lee JA, Schrope B, et al. Quality of life in patients after total pancreatectomy is comparable with quality of life in patients who undergo a partial pancreatic resection. J Surg Res. 2014;187(1):189–96.
Muller MW, Friess H, Kleeff J, Dahmen R, Wagner M, Hinz U, et al. Is there still a role for total pancreatectomy? Ann Surg. 2007;246(6):966–74; discussion 74–5.
Barbier L, Jamal W, Dokmak S, Aussilhou B, Corcos O, Ruszniewski P, et al. Impact of total pancreatectomy: short- and long-term assessment. HPB (Oxford). 2013;15(11):882–92.
Inagaki M, Obara M, Kino S, Goto J, Suzuki S, Ishizaki A, et al. Pylorus-preserving total pancreatectomy for an intraductal papillary-mucinous neoplasm of the pancreas. J Hepatobiliarypancreat Surg. 2007;14(3):264–9.
Yamaguchi K, Konomi H, Kobayashi K, Ogura Y, Sonoda Y, Kawamoto M, et al. Total pancreatectomy for intraductal papillary-mucinous tumor of the pancreas: reappraisal of total pancreatectomy. Hepatogastroenterology. 2005;52(65):1585–90.
Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123(5):1500–7.
Shi C, Klein AP, Goggins M, Maitra A, Canto M, Ali S, et al. Increased prevalence of precursor lesions in familial pancreatic cancer patients. Clin Cancer Res. 2009;15(24):7737–43.
Passot G, Lebeau R, Hervieu V, Ponchon T, Pilleul F, Adham M. Recurrences after surgical resection of intraductal papillary mucinous neoplasm of the pancreas: a single-center study of recurrence predictive factors. Pancreas. 2012;41(1):137–41.
Miller JR, Meyer JE, Waters JA, Al-Haddad M, Dewitt J, Sherman S, et al. Outcome of the pancreatic remnant following segmental pancreatectomy for non-invasive intraductal papillary mucinous neoplasm. HPB (Oxford). 2011;13(11):759–66.
Kwon JH, Kim SC, Song KB, Lee JH, Hwang DW, Park KM, et al. Surgical outcomes of multifocal branch duct intraductal papillary mucinous neoplasms of pancreas. Korean J Hepatobiliary Pancreat Surg. 2014;18(4):152–8.
Marchegiani G, Mino-Kenudson M, Ferrone CR, Morales-Oyarvide V, Warshaw AL, Lillemoe KD, et al. Patterns of recurrence after resection of IPMN: who, when, and how? Ann Surg. 2015;262(6):1108–14.
Ridtitid W, DeWitt JM, Schmidt CM, Roch A, Stuart JS, Sherman S, et al. Management of branch-duct intraductal papillary mucinous neoplasms: a large single-center study to assess predictors of malignancy and long-term outcomes. Gastrointest Endosc. 2016;84(3):436–45.
White R, D’Angelica M, Katabi N, Tang L, Klimstra D, Fong Y, et al. Fate of the remnant pancreas after resection of noninvasive intraductal papillary mucinous neoplasm. J Am Coll Surg. 2007;204(5):987–93; discussion 993–5.
Rosenblatt R, Dorfman V, Epelboym I, Poneros JM, Sethi A, Lightdale C, et al. Demographic features and natural history of intermediate-risk multifocal versus unifocal intraductal papillary mucinous neoplasms. Pancreas. 2015;44(3):478–83.
Crippa S, Capurso G, Camma C, Delle Fave G, Castillo CF, Falconi M. Risk of pancreatic malignancy and mortality in branch-duct IPMNs undergoing surveillance: a systematic review and meta-analysis. Digest Liver Dis. 2016;48(5):473–9.
Lawson RD, Hunt GC, Giap AQ, Krinsky ML, Slezak J, Tang RS, et al. Pancreatic cysts suspected to be branch duct intraductal papillary mucinous neoplasm without concerning features have low risk for development of pancreatic cancer. Ann Gastroenterol. 2015;28(4):487–94.
Correa-Gallego C, Ferrone CR, Thayer SP, Wargo JA, Warshaw AL. Fernandez-Del Castillo C. Incidental pancreatic cysts: do we really know what we are watching? Pancreatology. 2010;10(2–3):144–50.
Castelli F, Bosetti D, Negrelli R, Di Paola V, Zantedeschi L, Ventriglia A, et al. Multifocal branch-duct intraductal papillary mucinous neoplasms (IPMNs) of the pancreas: magnetic resonance (MR) imaging pattern and evolution over time. Radiol Med. 2013;118(6):917–29.
Mori Y, Ohtsuka T, Kono H, Ideno N, Aso T, Nagayoshi Y, et al. Management strategy for multifocal branch duct intraductal papillary mucinous neoplasms of the pancreas. Pancreas. 2012;41(7):1008–12.
Fritz S, Klauss M, Bergmann F, Strobel O, Schneider L, Werner J, et al. Pancreatic main-duct involvement in branch-duct IPMNs: an underestimated risk. Ann Surg. 2014;260(5):848–55; discussion 855–6.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Stark, A.P., Hines, O.J. (2017). Multifocal Branch-Duct Intraductal Papillary Mucinous Neoplasm. In: Pawlik, T., Weber, S., Gamblin, T. (eds) Case-Based Lessons in the Management of Complex Hepato-Pancreato-Biliary Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-50868-9_28
Download citation
DOI: https://doi.org/10.1007/978-3-319-50868-9_28
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-50867-2
Online ISBN: 978-3-319-50868-9
eBook Packages: MedicineMedicine (R0)