Abstract
The aesthetic success of implant-supported restorations on anterior teeth is largely influenced by the surrounding soft tissue. In considering soft tissue aesthetics, several criteria may be evaluated: gingival volume, contour, color, and consistency. The ideal soft tissue qualities around implants are the same expectations as the soft tissue around natural teeth. As with natural teeth, the gingiva should be dense and firm. The color is often described as “coral pink.” The contour is ideally knife edged with a gingival scallop that is consistent with the adjacent teeth. In order to establish these soft tissue goals, several treatment options are available. Commonly, soft tissue grafting is considered. A summary of various grafting sources, surgical design, and timing of procedures is reviewed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Gingival grafting
- Allografts
- Connective tissue
- Dental implants
- Gingival aesthetics
- Oral plastic surgery
- Palatal grafting
- Acellular dermal graft
The science of implant dentistry often includes discussions of survival rates and success rates. However, with the predictability of dental implants being established through a vast volume of research, the spotlight of attention is now on dental implant aesthetics.
While aesthetics may be dependent on subjective criteria, there are general objective guidelines that may be used as a reference. Common objective criteria for gingival aesthetics include gingival health, interdental closure, zenith of the gingival contour, and balance of the gingival levels (Magne and Belser 2003).
The aesthetic success of implant-supported restorations is largely influenced by the surrounding soft tissue. However, because aesthetics is the product of both gingival and dental aesthetics working together, it is important to note that defects in the quality of the dental prosthesis and/or the implant placement and position cannot be corrected by periodontal procedures (Magne and Belser 2003). The ideal soft tissue qualities around implants are evaluated by the same criteria as the soft tissue around natural teeth. In regard to tissue quality, the gingiva should be dense and firm. The color is often described as “coral pink.” The contour is ideally knife edged with a gingival scallop that is consistent with the adjacent teeth and fills the interdental spaces. In order to establish these soft tissue goals, several treatment options are available. This chapter will provide an overview of many of the more documented procedures and also provide a discussion as to the timing and sequencing of these surgical options relative to implant therapy.
The aesthetic standard by which any dental implant is measured is often how it compares to the adjacent teeth. While implant aesthetics may be influenced by factors such as implant position and platform, abutment selection, and dental materials, the framing of all of these components is the soft tissue. Perhaps the most important soft tissue topic regarding dental implants is the gingival biotype (Jia-Hui et al. 2011; Linkevicius et al. 2009).
1 The Significance of the Gingival Biotype
The gingival biotype is often separated into two categories: thick and thin. Each of these gingival biotypes has observable clinical qualities that can be used to differentiate the two.
Generally, a thin tissue biotype is often associated with a highly scalloped gingival architecture. The surrounding band of keratinized and attached gingiva may be narrow, while the marginal gingiva is often thin and delicate. The underlying bone is also thin and commonly associated with dehiscences or fenestrations in the labial plate. In contrast, a thick gingival biotype is commonly characterized with a wide flat gingival architecture with minimal scalloping. Here, a wider band of keratinized and attached gingiva is often found, while the marginal gingiva is thick and resilient. Thicker underlying bone generally lies beneath a thicker gingival biotype. The clinical significance of these two gingival biotypes is that the thin biotype typically responds to insult or injury with recession of the gingiva, whereas the thicker biotype is much more resistant to gingival recession (Jung et al. 2007; Kao et al. 2008; Kois and Kan 2001).
2 The Gingival Biotype and Dental Implants
Much research has been devoted to the significance of the gingival biotype surrounding dental implants. While early studies suggest that the type of tissue surrounding dental implants was unrelated to implant survival and retention, there are a number of papers recognizing the importance of a thick gingival biotype in regenerative procedures as well as preservation of the crestal hard and soft tissues. In dental implant cases where bone grafting is indicated prior to implant placement, a thicker biotype aids in the primary passive closure of the surgical site as well as improved vascularity. Passive primary closure and graft stability are important keys to successful bone grafting. When it comes to dental implant aesthetics, thicker tissue types are important for masking any potential graying areas in the cervical portion of the restoration from either the implant platform or the abutment. Thicker gingival biotypes have also been shown to minimize the amount of crestal bone loss as well as gingival recession once the implant has been restored (Fu et al. 2011).
Tissue thickness is important for the optimal health and aesthetics of a dental implant, but it is important to note that tissue thickness is also affected by implant design, implant position, as well as prosthesis design. Generally, implant-supported crowns and abutments with a more flat or even concave profile allow for thicker tissue than their convex-shaped counterparts (Linkevicius et al. 2009; Rompen et al. 2007). Narrow-diameter implants also afford more gingival tissue than wider-diameter implants (Small et al. 2001). Implant position is important, and more facially positioned implants are associated with thinner tissues and generally more apical crown/abutment margins. The significance of implant position cannot be ignored, as it is a relatively irreversible procedure once the implant has osseointegrated (not absolutely irreversible, implants can be backed out at 300 ncm). Therefore, it is important to understand that grafting procedures are intended to complement and enhance proper implant position; they are not meant to correct deficiencies in implant position (or size) (Lazzara and Porter 2006; Nispakultorn et al. 2010).
3 Identifying Gingival Biotype
A number of techniques and methods have been proposed to help identify gingival biotypes (Fu et al. 2011). Unfortunately, many of these methods involve subjective criteria and observations. For example, one common method is simple visual inspection/observation utilizing the common characteristics of a thick or thin biotype: the gingival architecture, the amount of keratinized/attached gingiva, the morphology of the teeth, etc. Another technique is transgingival probing, where the thickness of the tissue can be directly measured by inserting a probe horizontally through the gingiva. While highly accurate, this technique requires the use of local anesthesia which may be considered a somewhat invasive diagnostic procedure. The use of cone beam computed tomography (CBCT) has also been proposed. While not invasive, this adds additional costs to the patient. Perhaps the most reliable method that is both noninvasive and cost-efficient is to simply probe the sulcus around the teeth with a periodontal probe. If the outline of the probe is visible through the tissue, then the gingival biotype is considered thin. If it is not visible, then the biotype is considered thick. Another quantifiable measure relative to edentulous areas (i.e., potential implant sites) is that a thick biotype has tissue thickness equal to or greater than 2.5 mm (Abrahamsson et al. 1996).
4 Indications for Gingival Grafting
4.1 Correcting Ridge Defects with Gingival Grafting
A common classification system for identifying ridge defects was described by Seibert et al. where three types of ridge deficiencies were identified (Seibert and Salama 1996).
-
Class I. Horizontal defect exists only. While this may occur on either the facial or lingual aspects of the ridge, Seibert Class I defects typically describe labial/buccal side defects.
-
Class II. Vertical defect exists only. In Seibert Class II defects, the horizontal dimensions of the edentulous ridge have been preserved, but there is loss of vertical height. These types of defects may also be associated with the loss of the interproximal height of the bone on the adjacent teeth, which is a critical determinant of the final aesthetic outcome.
-
Class III. Both a horizontal and a vertical defect exist.
A number of soft tissue grafting techniques are particularly useful in the treatment of Seibert Class I, II, and III ridges by restoring lost ridge volume. Additionally, gingival augmentation, bone augmentation, or a combination of both surgeries (performed either simultaneously or performed in sequence) may be used to correct edentulous ridge defects for the purpose of improving aesthetics or to prepare the ridge for implant surgery.
5 Converting a Thin Gingival Biotype to a Thick Gingival Biotype
There are several advantages previously mentioned to converting a thin gingival biotype to a thicker gingival biotype (Chung et al. 2006; Kennedy 1974; Kois and Kan 2001; Linkevicius et al. 2009; Warrer et al. 1995).
-
Pre-prosthetically, thicker biotypes are better suited to resist gingival recession.
-
Thicker biotypes are less prone to inflammation.
-
Prior to implant surgery, thicker biotypes aid in primary closure.
-
During bone augmentation procedures, a thick biotype offers improved vascularity and graft stability.
-
Dental implant-supported fixed prostheses have superior aesthetics when a thicker biotype is present.
There are a number of clinical scenarios where gingival grafting may be indicated.
Gingival grafts are frequently used to convert the existing gingival biotype to aid additional bone grafting or augmenting the size/volume of the tissues for aesthetics (Fu et al. 2011). Gingival grafting may also be used to eliminate unsightly scars such as amalgam tattoos. The “graying” occasionally seen from implant components through thin tissue may also be minimized or removed. Also, inflamed marginal tissue may be restored to health if traditional periodontal procedures aimed at reducing inflammation are unsuccessful. In the event of exposed restoration margins or dental implant threads, gingival grafting is indicated but is limited by several factors. First of all, just as in the case of gingival recession around natural teeth, the amount of root coverage or thread coverage is limited by the height of the interproximal bone (Salama et al. 1998; Seibert and Salama 1996). Secondly, as mentioned earlier, gingival position is influenced by implant position, diameter, and design (Rompen et al. 2007; Small et al. 2001). These are factors that obviously cannot be changed with gingival grafting once the dental implant has been placed.
6 Gingival Grafting Sources
A brief summary of various graft types/materials along with the basic technique for their use has been included below (Fig. 14.1).
There are several gingival grafting techniques available to correct various soft tissue deficiencies. When discussing gingival grafts, the donor tissue is often classified into two categories: autografts and allografts.
In reviewing the literature, autografts are the more popular choice for augmenting gingival tissue (Seibert and Salama 1996). Common donor sites include the hard palate, maxillary tuberosity, and edentulous ridges.
Another popular graft material which can be used in a similar manner to autogenous gingival tissue is human acellular dermal graft tissue (Park 2005). This graft material is derived from natural tissue, which is processed to remove the cells that are associated with tissue rejection and graft failure. Its use in dentistry has been thoroughly documented and has been cited several times in both dental and medical literature.
7 Common Gingival Grafting Techniques
7.1 Free Gingival Graft
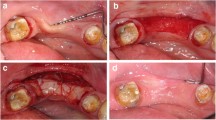
The free gingival graft was popularly described by Sullivan and Atkins in 1968. This autogenous graft is typically harvested from the palate (Sullivan and Atkins 1968) but may be harvested at any intraoral location where attached keratinized tissue is present, such as the maxillary tuberosity or an edentulous ridge. When treating the natural dentition or dental implants, free gingival grafts are an effective technique for increasing the band of keratinized and attached gingiva as well as the transgingival thickness of the tissue (Miller 1985). In treating the edentulous ridge, free gingival onlay grafts are effective in treating Seibert Class I, II, or III deficiencies (Seibert and Salama 1996). Once established, the free gingival onlay graft may aid in wound closure, stabilization, and vascularity to future hard tissue ridge augmentations in the area (Fu et al. 2011). Additionally, free gingival grafts may also be used to improve the contours and aesthetics of the final prosthesis (Fig. 14.2a–g).
(a, b) Preoperative view of the vertical and horizontal ridge deficiency related to the edentulous ridge. Soft tissue augmentation with a free gingival graft was prescribed prior to any hard tissue grafting or implant placement. (c) The free gingival graft has been harvested from the palate and is approximately 4 mm thick in the center of the graft. (d) The graft is sutured to the recipient bed, which is prepared by denuding the existing loose mucosa from the underlying bony ridge. (e) Two-week postoperative visit. Sutures will be removed at this visit, but revascularization and maturation of the graft are occurring at this time. (f) Radiograph revealing the placement of two dental implants to support a fixed prosthesis. (g) Two-year photograph showing the final fixed prosthesis. Note the excellent health and quality of the marginal tissue, the improvement in the gingival biotype, and the architecture of the prosthetic-gingival interface
One limitation of a free gingival onlay graft is its anatomic variability relative to size and thickness (Reiser et al. 1996). For example, patients may have a limited amount of useful available palatal tissue, which is bound by the height of the palatal vault, the location of vital structures, and the pre-existing thickness of the palatal tissue. Depending on the anatomical limitations as well as the size of the ridge defect, multiple procedures may be required to correct certain edentulous ridge defects. Another limitation is color matching. Free gingival onlay grafts often heal in a way that the margins of the graft are visible and the grafts itself may be a lighter shade than the surrounding gingiva.
8 Subepithelial Connective Tissue Graft
The subepithelial connective tissue (SECT) graft is another type of autogenous graft that has several indications for grafting around dental implants. One of its first introductions described its use for correcting gingival recession defects on natural teeth as a predictable method for root coverage (Langer and Langer 1985). The SECT graft is also most commonly harvested from the palate with the same limitations as mentioned for the free gingival graft. The difference between the two graft types, as the name would suggest, is that the SECT does not have a layer of epithelium on its surface (Levine 1991).
Unlike the free gingival graft, a SECT graft can be utilized as either an onlay graft or an inlay graft. Both techniques have an array of clinical applications. As an inlay graft, in which the SECT graft is placed beneath a full- or partial-thickness flap, the gingival biotype may be thickened. Considering how thick and thin biotypes react to local irritants such as bacterial plaque, calculus, retained cement, food impaction, etc. (Kennedy 1974), the SECT graft is an excellent way to treat chronically inflamed gingiva once these local factors are removed (Fig. 14.5a–g). Similarly, the gray shadowing that may transfer through the gingiva from a dental implant may be masked if the SECT graft achieves the necessary thickness of approximately 2 mm (Fu et al. 2011). Please note, however, that this technique does not correct gingival discoloration caused by improper implant positioning and/or size selection. For situations where a modest amount of tissue is desired to augment existing gingiva, the SECT inlay graft may also be a practical, predictable choice of treatment (Fig. 14.3a–f).
(a) Preoperative view of the marginal inflammation that exists around the implant-supported crown #8. The treatment performed was a subepithelial connective tissue (SECT) graft. (b) The initial tunnel flap design is started with sulcular incisions using a #15 scalpel. (c) The SECT graft is removed from the palate with the desired thickness of approximately 1.5–2.0 mm. (d) Once the SECT graft is trimmed to ensure that it covers the entire implant surface and adjacent bony structures, it is inserted underneath the tunnel flap through the facial sulcus. It is recommended that any thread exposure noted be debrided and contaminated as thoroughly as possible. Examples of agents used for implant surface decontamination include a tetracycline slurry, citric acid, or EDTA. (e) The graft is secured beneath the flap with firm pressure with or without the addition of sutures. (f) A gingivoplasty is performed 12 weeks later to remove any undesired contours or scars that may be present. This was performed with a round diamond bur. (g) The final result at 6 years following SECT grafting surgery
When a SECT graft is utilized as an onlay graft, its applications are very similar to those of a free gingival onlay graft. There are a couple of differences that should be pointed out, however. First of all, the SECT graft has a much better color match to the surrounding tissue at the recipient site since the graft does not include the surface epithelium. For aesthetic considerations alone, the SECT graft is often preferred over the free gingival graft, especially in the maxillary arch where gingival display is more prominent (Levine 1991). A SECT onlay graft is effective at both thickening the gingival biotype and correcting small ridge deficiencies and discolorations such as amalgam tattoos (Figs. 14.4a–f and 14.5).
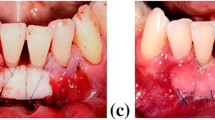
(a) For missing teeth #s 6–7, a single dental implant was placed in the #6 position with the final restoration being an implant-supported crown on #6 with a cantilevered pontic to replace #7. (b) A close-up view of the gingival deficiency in the papilla between the implant crown #6 and the pontic #7. (c) A SECT graft harvested from the maxillary tuberosity was utilized to augment the gingival defect. The recipient bed consists of a straight-line incision apical to the mucogingival junction that extends from #6 to 7. The graft is introduced through the incision and placed in the area of the defect. (d) The graft is then secured to the recipient site with resorbable sutures. Primary closure is obtained with non-resorbable sutures. (e) Two-week follow-up. Sutures will be removed at this time. Note the significant improvement in the papillary tissue. (f) Final photograph 1 year later
(a) Tooth #9 was extracted after a failed root canal treatment as well as a failed attempt at an apicoectomy. A successful implant and crown was eventually placed to satisfactorily restore #9, but the patient is unhappy with the existing amalgam tattoo. (b) In preparation for a subepithelial connective tissue (SECT) onlay graft, a recipient bed was prepared over the area of the amalgam tattoo by removing the overlying loose mucosa. (c) SECT graft removed from the palate. (d) The graft is secured to the bed with non-resorbable sutures. (e) At 12 weeks, the SECT onlay graft has healed, eliminating the amalgam tattoo. However, the gingiva is overly thick and unevenly contoured. (f) Using a round diamond bur, a gingivoplasty is performed. (g) Final result at 5 years. Note the thickness, contour, and quality of the gingiva as well as the stability of the crown/gingiva interface
Another variation of the subepithelial connective tissue graft is the vascularized interpositional periosteal connective tissue (VIP-CT) graft (Sclar 2003). This type of subepithelial connective tissue graft is a pedicle graft, where the base of the graft is left attached as it is simply slid or folded over toward the recipient site (Fig. 14.6a–j). The advantage of this type of graft is that the blood supply to the graft is maintained and never severed. Like the free gingival graft or SECT graft, soft tissue volume may be augmented with this technique. Because this technique involves the connective tissue only, it also provides an excellent tissue color match. This procedure may also be combined with regenerative procedures.
(a) Following a traumatic accident, this patient lost teeth #s 9 and 10. Following numerous attempts at regeneration, two implants were eventually placed in the #9 and #10 positions. The patient’s goal is to establish a more symmetrical gingival height between #8 and 9 as well as the creation of a papilla between the two implants. Prior to surgery, a provisional prosthesis was placed. (b) The provisional prosthesis is removed. The recommended treatment in this instance is the vascularized interpositional connective tissue (VIP-CT) graft. (c) The connective tissue graft is taken from the left side of the palate, extending from tooth #14 in the posterior to #8 anteriorly. Please note that the VIP-CT graft calls from the graft to maintain fixed to the palate, preserving the blood supply of the graft. (d) Once freed from the palate, the VIP-CT graft is then flipped or rotated and placed over the ridge defect. The graft is still secured to the palate near the apex of implant #9. (e) Facial view of the VIP-CT graft in place. (f) Once the overlying flap is primarily closed over the VIP-CT graft, the increase in anticipated volume can be observed. (g) Following final closure of the flaps, the provisional prosthesis is reshaped to ensure light to no contact occurs over the graft, and it is temporarily luted to place. (h) Twelve-week follow-up of the patient reveals that excellent healing of the VIP-CT graft has occurred. The majority of the desired volume has been maintained. (i) Two-year follow-up. The final prosthesis has been delivered, and the periodontal condition of the crowns is excellent. The gingival zeniths of #8 and #9 are even, and a gingival papilla between #9 and #10 has formed. (j) Radiograph at 3 years demonstrates the position of the implants and the relationship of the crestal bone relative to the bone of the adjacent teeth. Note the distance of the crown margins relative to the implant platforms
9 Acellular Dermal Grafts
One disadvantage of autogenous soft tissue grafts is the need for a second surgical site. This is associated with additional potential complications for the patient including increased bleeding, swelling, pain, and discomfort. In addition, there is a limited amount of tissue available at common intraoral sites such as the palate or maxillary tuberosity.
Acellular dermal grafts are typically allografts derived from human cadavers. They are then processed commonly via proprietary methods to eliminate the cellular component of the graft.
Clinically, an acellular dermal graft (ADG) is often considered in lieu of the SECT graft (Park 2005). It is recommended that ADGs be completely submerged under passive primary closure. This is in contrast to the SECT graft where the graft may be left partially exposed without compromising the final result. ADGs are not commonly utilized as onlay grafts. These are typically used as inlay grafts and have a number of clinical applications. Like the SECT graft, it is used in the natural dentition to treat and correct gingival recession. It may also be utilized as a membrane during guided tissue regenerations (GTR) and guided bone regeneration (GBR) procedures. In implant-related dentistry, ADGs may be utilized as SECT grafts to establish a thicker biotype and augment ridge defects (Fig. 14.7a–e).
(a) Preoperative photo of tooth #8, which is given a hopeless prognosis due to a horizontal root fracture. An extraction is planned along with simultaneous socket grafting. (b) Once the tooth is extracted, it is apparent that a large dehiscence in the labial plate exists. (c) Prior to placing a socket graft, an acellular dermal graft (ADG) is hydrated in sterile water for 20 min. (d) The ADG is used over the bony dehiscence to serve as a membrane. (e) Following the placement of a bone replacement graft (mineralized cortical bone allograft in this case), the ADG is folded over the coronal portion of the socket and sutured with non-resorbable sutures. (f) This photograph demonstrates the soft tissue contours around the implant healing abutment approximately 4 months after the dental implant has been placed. Please note the gingival symmetry and contour around the healing abutment
Conclusion
During the course of dental implant therapy, the management and development of soft tissues is often vital to the creation of proper gingival architecture for the aesthetics, form, function, and longevity of the final prosthesis. While many techniques and materials are available for purposes such as the transformation of the gingival biotype, the augmentation of soft tissues, or the creation of the desired prosthesis-soft tissue interface, the large majority of procedures involve the use of autografts and allografts. While there are basic indications for each material and technique, the most appropriate decision should be discussed between the surgeon and the patient, taking morbidity, costs, time, practicality, and predictability into consideration.
References
Abrahamsson I, Berglundh T, Wennstrom J, Lindhe J (1996) The periimplant hard and soft tissues at different implant systems. A comparative study in the dog. Clin Oral Implants Res 7(3):212–219
Chung DM, Oh TJ, Shotwell JL, Misch CE, Wang H (2006) Significance of keratinized mucosa in maintenance of dental implants with different surfaces. J Periodontol 77:1410–1420
Fu J, Lee A, Wang HL (2011) Influence of tissue biotype on implant esthetics. Int J Oral Maxillofac Implants 26:499–508
Jia-Hui F, Lee A, Wang HL (2011) Influence of Tissue Biotype on Implant Esthetics. Int J Oral Maxillofac Implants 26:499–508
Jung RE, Sailer I, Hammerle CH, Attin T, Schmidlin P (2007) In vitro color changes of soft tissue caused by restorative materials. Int J Periodont Rest Dent 27:251–257
Kao RT, Fagan MC, Conte GJ (2008) Thick vs thin gingival biotypes: A key determinant in treatment planning for dental implants. J Calif Dent Assoc 36:193–198
Kennedy JE (1974) Effect of inflammation on collateral circulation of the gingiva. J Periodontal Res 9:147–152
Kois JC, Kan JY (2001) Predictable peri-implant gingival aesthetics: surgical and prosthodontics rationales. Pract Proced Aesthet Dent 13:691–698
Langer B, Langer L (1985) Subepithelial connective tissue graft technique for root coverage. J Periodontol 56:715–772
Lazzara RJ, Porter SS (2006) Platform switching: A new concept in implant dentistry for controlling postrestorative crestal bone levels. Int J Perio Rest Dent 26:9–17
Levine RA (1991) Covering denuded root surface with the subepithelial connective tissue graft. Compend Contin Educ Dent 12:568
Linkevicius T, Apse P, Med H, Grybauskas S, Puisys A (2009) The influence of soft tissue thickness on crestal bone changes around implants: a 1-year prospective controlled clinical trial. Int J Oral Maxillofac Implants 24:712–719
Magne P, Belser U (2003) Bonded porcelain restorations in the anterior dentition. A biomimetic approach. Quintessance, Chicago
Miller PD Jr (1985) A classification of marginal tissue recession. Int J Periodont Restor Dent 5:9
Nisapakultorn K, Suphanantachat S, Silkosessak O, Rattanamongkolgul S (2010) Factors affecting soft tissue level around anterior maxillary singe-tooth implants. Clin Oral Implants Res 21:662–670
Park JB (2005) Increasing the width of keratinized mucosa around endosseous implant using acellular dermal matrix allograft. Implant Dent 15:275–281
Reiser G, Bruno J, Mahan P, Larkin L (1996) The subepithelial connective tissue graft palatal donor site: anatomic considerations for surgeons. Intl J Periodontics Restor Dent 6(2):130–137
Rompen E, Raepsaet N, Domken O, Touati B, Van Dooren E (2007) Soft tissue stability at the facial aspect of gingivally converging abutment in the esthetic zone: a pilot clinical study. J Prosthet Dent 97:5119–5125
Salama H, Salama MA, Garber D, Pinhas A (1998) The interproximal height of bone: a guidepost to predictable aesthetic strategies and soft tissue contours in anterior tooth replacement. Pract Periodont Aesthet Dent 10(9):1131–1141
Sclar A (2003) Soft tissue and esthetic considerations in implant therapy. Quintessance, Chicago
Seibert JS, Salama H (1996) Alveolar ridge preservation and reconstruction. Periodontol 2000 11:69–84
Small PN, Tarnow DP, Cho SC (2001) Gingival recession around wide-diameter versus standard-diameter implants: a 3- to 5-year longitudinal prospective study. Pract Proced Aesthet Dent 13:143–146
Sullivan HC, Atkins JC (1968) Free autogenous gingival grafts. III. Utilization of grafts in the treatment of gingival recession. Periodontics 6:152
Warrer K, Buser D, Lang NP, Karring T (1995) Plaque-induced peri-implantitis in the presence or absence of keratinized mucosa. An experimental study in monkeys. Clin Oral Implants Res 6:131–138
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Wong, D.H. (2017). Development of the Soft Tissue with Gingival Grafting. In: Karateew, E. (eds) Implant Aesthetics. Springer, Cham. https://doi.org/10.1007/978-3-319-50706-4_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-50706-4_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-50704-0
Online ISBN: 978-3-319-50706-4
eBook Packages: MedicineMedicine (R0)