Abstract
A patient with iatrogenic acute pericardial tamponade presents in the interventional cardiology suite during pacemaker placement. This chapter reviews diagnosis and treatment of pericardial tamponade. Cardiac tamponade is marked by the restriction of cardiac filling by increased pericardial pressure secondary to accumulation of fluid in the pericardial space. Its etiology can be infectious, noninfectious as well as autoimmune. While acute idiopathic pericarditis is the leading cause of tamponade worldwide, it is proportionately much more common in patients with tubercular, neoplastic or purulent pericarditis. The classic physical exam findings of a patient with pericardial tamponade are sinus tachycardia, jugular venous distension, and pulsus paradoxus. Secondary signs of pericarditis include muffled heart sounds or rub. It is generally diagnosed when both a pericardial effusion and hemodynamic compromise are present. ECG and transthoracic echocardiography are the most useful noninvasive methods of diagnosing pericardial tamponade. The hemodynamic goals of treating tamponade are to maintain preload, high heart rate, systemic vascular resistance, and contractility. Anesthetic agents, such as midazolam, fentanyl, ketamine and etomidate, and vasopressors, such as norepinephrine, vasopressin, and epinephrine, support the patient’s physiological parameters while allowing the interventionalist or surgeon to relieve the tamponade. For patients with cardiac tamponade and hemodynamic instability, drainage by percutaneous pericardiocentesis or pericardial window is necessary. The main complication of pericardiocentesis is related to re-bleeding into the pericardial sac, which is avoided by leaving a drain in place. Other complications include acute left ventricular failure with pulmonary edema caused by sudden increases in systemic venous return or acute right ventricular dilation from pulmonary hypertension if pericardial fluid is removed too rapidly.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Cardiac tamponade
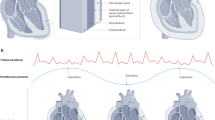
- Pulsus paradoxus
- Hemodynamic goals for tamponade
- Anesthetic agents for tamponade
- Indications for pericardiocentesis and pericardial window
Patient Scenario
A 37-year old man presents to his primary care physician concerned about multiple episodes of dizziness associated with palpitations. He is unable to exert himself in his activities of daily living. His past medical history is significant for hyperlipidemia, anxiety, gastroesophageal reflux disease, occasional migraines, lower back pain, and constipation. He had an appendectomy at age 12, but otherwise, no surgical history. He has an allergy to bananas and latex. His medications include simvastatin, lorazepam, esomeprazole, naproxen, and colace. He smokes one pack of cigarettes per day. He does not consume alcohol and has never tried taking recreational drugs. On physical exam he is found to be bradycardic at 42 beats per minute. His blood pressure is 110/65, respiratory rate 16 breaths per minute, and oxygen saturation 98% on room air. His lungs exam is remarkable for mild rales at the bases bilaterally and he has 2+ edema in his lower extremities. His ECG shows complete heart block. After medical optimization of his heart failure symptoms, he presents to an interventional cardiologist for pacemaker placement. During the pacemaker placement, which was performed under sedation, the patient moves on the procedure table as the first lead was being placed. He then becomes suddenly dyspneic and increasingly hypotensive. His blood pressure is 80/45, heart rate 110 beats per minute, respiratory rate 25 breaths per minute and pulse oximetry 98%. Pericardial tamponade is suspected.
Questions
-
What is the definition of pericardial tamponade?
Cardiac tamponade is defined as pathologic fluid in the pericardial space leading to a restriction in ventricular filling and subsequently a decrease in cardiac output. Normally, the pericardial sac contains 20–50 mL of serous fluid that mitigates the force of friction on the epicardium. Cardiac tamponade occurs when the reserve volume of the pericardial sac is exceeded by filling with blood, clot, gas, fluid, or pus [1]. The physiology of cardiac tamponade is less a function of the effusion’s composition and more a function of the rate at which it accumulates. There are four generally accepted categories of cardiac tamponade. Acute cardiac tamponade usually occurs within minutes and is most often secondary to hemopericardium from trauma. It occurs when blood accumulates more rapidly than the pericardial sac can accommodate. In severe cases, this can lead to complete collapse of the right atrium and/or the right ventricle. Subacute cardiac tamponade most often occurs over days to weeks due to large effusions, which accumulate more slowly, often from idiopathic or neoplastic causes. Low pressure cardiac tamponade is a special case when pericardial effusion and hypovolemic shock coexist. Due to the fact that ventricular pressures are low, the pressure gradient across the myocardium may be large even with relatively low pericardial pressures [2]. Finally, regional tamponade develops when a loculated effusion or hematoma exerts pressure across the myocardium. This is more difficult to diagnose on physical exam because typical findings are absent due to a focal source of compression [3].
-
What are the causes of tamponade?
Cardiac tamponade arises from a number of causes including infectious, noninfectious and autoimmune origins. While acute idiopathic pericarditis is the leading cause of tamponade worldwide, it is proportionately much more common in patients with tuberculous, neoplastic, or purulent pericarditis [4]. A large prospective series evaluated the etiologies of primary acute pericarditis [5]. Nearly half had acute idiopathic pericarditis, and the remaining patients commonly had metastatic disease and tuberculous pericarditis. Infectious causes of tamponade can be divided into viral, bacterial, fungal and parasitic causes. Coxsackie, echovirus and adenovirus are common viral causes, while Staphylococcus, Streptococcus, pneumococcus, and tuberculosis are the most common bacterial causes [6]. Fungal causes include Histoplasmosis, aspergillosis, and coccidioidomycosis, and the most common parasitic causes are echinococcus and toxoplasmosis. Metastatic lung and breast cancer, Hodgkin’s lymphoma and melanoma are the most common sources of malignant effusions. Primary cardiac causes are comprised of early infarction pericarditis, post-pericardiotomy syndrome, dissecting aneurysms and myocarditis. Tamponade can be induced by blunt and penetrating trauma like, and it can occur as a complication of cardiac catheterization and pacemaker placement. Rheumatologic causes, include SLE and vasculitis, and occur secondary to inflammation. Uremia is a common metabolic cause. Any process which leads to an effusion, particularly if large or rapidly accumulating, can induce tamponade physiology.
-
What are the physical exam findings of a patient who has pericardial tamponade?
Physical exam findings of a patient with pericardial tamponade are sinus tachycardia, jugular venous distension, and pulsus paradoxus. Secondary signs of pericarditis include muffled heart sounds and rub. Dyspnea (sensitivity 87–89%), tachycardia (sensitivity 77%), pulsus paradoxus (sensitivity 82%), and elevated JVP (sensitivity 76%) are most useful in their negative predictive value [7]. That is, they are useful for ruling out tamponade when absent. If a patient has pulsus paradoxus, the odds of having tamponade increase threefold, and if absent, the odds of having tamponade are reduced 30-fold.
-
What is pulsus paradoxus?
Pulsus paradoxus is an exaggeration of a normal decrease in systolic blood pressure, which occurs during inspiration [8]. Under conditions of increased pericardial pressures, changes in right and left ventricular volume inevitably affect each other, as there is no additional reserve volume in the pericardial sac. At least three mechanisms have been proposed to explain this phenomenon [9]. With inspiration, increased negative pressure in the intrathoracic cavity increases venous return to the right side of the heart. The increased preload, in turn, causes bulging of the septal wall into the left ventricle, decreasing end-diastolic left ventricular volume. This exemplifies ventricular interdependence. Increased compliance of pulmonary vasculature also occurs during inspiration, leading to pooling in the pulmonary circulation. Finally, the increase in negative intrathoracic pressure opposes contraction of the left side of the heart, leading to increased afterload. These three forces conspire to decrease systolic blood pressure. Kussmaul’s sign describes the disappearance of the peripheral pulse with inspiration, which is caused by the drop in blood pressure described above.
-
What are the diagnostic criteria for pericardial tamponade?
The diagnostic criteria for cardiac tamponade are not well defined. It is generally diagnosed when both a pericardial effusion and hemodynamic compromise are present. Another commonly used definition is one which can only be made retrospectively: the presence of a pericardial effusion as seen by either transthoracic or transesophageal echocardiography and hemodynamic compromise that is relieved by pericardial fluid drainage [10].
-
What noninvasive and invasive testing will help establish a diagnosis of tamponade?
While many techniques may be helpful in diagnosing pericardial effusion, establishing the diagnosis of tamponade physiology on the basis of noninvasive testing is challenging. EKG is the first test often performed. PR segment depression, low voltage QRS complex, and electrical alternans are EKG findings that are specific but not sensitive for pericardial effusion [11]. Overall, EKG has a low sensitivity for diagnosing pericardial effusion and cardiac tamponade.
Transthoracic echocardiogram (TTE) clearly demonstrates the presence or absence of effusion. A study by Merce et al. prospectively assessed patients with moderate to large pericardial effusions over a 2-year period for tamponade [12]. They found that collapse of one or more right cardiac chambers was highly sensitive for tamponade, while abnormal venous flow pattern by Doppler was more specific—systolic over diastolic predominance, respiratory accentuation of this difference and expiration inversion of the diastolic component. Other TTE signs of tamponade include exaggerated inspiratory variation of the right and left ventricle, collapse of any chamber, IVC plethora and abnormal reduction in flow across the mitral and aortic valves during inspiration [13].
Invasive measurements, such as Swan Ganz catheter measurements generally reveal pulmonary hypertension in tamponade with pulmonary artery systolic pressures ranging between 35 and 50 mmHg. Cardiac catheterization, though not necessary for diagnosis, may reveal equilibration of average intracardiac diastolic pressures. An arterial line tracing will show a narrow pulse pressure and a low mean arterial pressure.
-
What vascular access would you obtain prior to treating pericardial tamponade?
If pericardial tamponade is present, or highly suspected, prior to the induction of anesthesia, placing an arterial line and a central venous line (CVL) are of great use. An arterial line will offer beat-to-beat blood pressure readings, while the CVL will permit reliable titration of pressors prior to drainage of the effusion and provide central venous pressure readings. Further, after the tamponade is relieved, there can sometimes be a catecholamine surge marked by increased blood pressure and heart rate that will be quickly recognized with an arterial line. Given, the critical nature of this condition, with compromised cardiac output and the possibility of PEA arrest, decompression of the tamponade should not be delayed by placing invasive monitors. Due to the risk of significant bleeding during or after evacuation of the pericardial collection, large bore intravenous access is also necessary for resuscitation.
-
What are the hemodynamic goals for patients with tamponade?
For patients with cardiac tamponade, the hemodynamic goals are to maintain preload, chronotropy, afterload, and inotropy. Colloquially speaking, the goals are to keep a patient “full, fast and tight.” Patients with acute tamponade are preload dependent. Increased venous return can overcome the pericardial pressure and restore the gradient between the chambers. So, it is important to administer intravenous fluids and avoid hypovolemia. Given that stroke volume is relatively fixed, cardiac output is dependent upon heart rate. Bradycardia is rarely tolerated. Patients with tamponade physiology require both inotropic support and an increase in systemic vascular resistance (SVR). Given that the heart is constricted externally from filling, a decrease in contractility is poorly tolerated. Increasing the SVR is the best way to ensure coronary perfusion. Norepinepherine and vasopressin, therefore, are the preferred pressors for patients with tamponade physiology. Epinephrine also addresses each of the hemodynamic goals [14].
Another obstacle in caring for a patient with tamponade is ventilation. The effect of positive pressure ventilation is detrimental to cardiac filling. This is generally overcome by maintaining low peak pressures, low PEEP as well as low tidal volumes. Minute ventilation is maintained by increasing the respiratory rate. Given these restrictions, ventilation may not be adequate, and PaCO2 may rise. Permissive hypercapnia may be tolerated on an individual basis but can lead to RV failure.
-
What are the medical treatments for pericardial effusion?
Patients with stable pericardial effusions may be managed medically by treating the underlying cause of the effusion. Inflammatory causes of pericarditis may be treated with NSAIDs. Aspirin, indomethacin and ibuprofen are the first-line therapy. Ibuprofen, has the lowest complication rate and has a favorable effect on coronary blood flow [15]. However, in post-infarction pericarditis, aspirin is favored. Colchicine may also be added for its impact on acute pericarditis and efficacy in preventing recurrences. Systemic steroids are reserved for patients with pericarditis caused by autoimmune disease or uremia. For long-term prevention of pericarditis, intracardiac steroids have shown some efficacy with almost 80% symptom-free remission at one year [16].
-
Which anesthetic agents would you choose for sedation and general anesthesia in a patient with tamponade physiology?
In hemodynamically unstable patients, the use of local anesthetics and a subhypnotic doses of midazolam, ketamine or fentanyl are appropriate in preparation for a subxiphoid pericardial drainage procedure [17, 18]. These agents have minimal effect on heart rate, contractility, and SVR, making them ideal for a patient with tamponade. For critically compromised patients ultimately requiring a pericardial window, the anesthetic may begin with sedation until a bridging pericardiocentesis is completed. After the tamponade is relieved, general anesthesia may be induced. The heart is then able to fill and contract normally and the negative inotropic effects of propofol and inhaled anesthetics will be less detrimental to the patient’s hemodynamics.
In patients who require general anesthesia for a pericardial window, it is essential to choose an induction agent, which has minimal impact on inotropy and vasomotor tone, such as etomidate or ketamine [19]. Aggressive fluid resuscitation is important prior to the induction of anesthesia. Additionally, the use of infusions or boluses of norepinephrine, vasopressin, or epinephrine may be necessary during induction.
-
When would you choose to do a pericardiocentesis versus a pericardial window with a drain?
For patients with cardiac tamponade and hemodynamic instability, quick drainage is essential. The indications for either pericardiocentesis or surgical drainage are (1) overt clinical tamponade in patients with purulent pericarditis or large, idiopathic or chronic pericardial effusion and (2) either unresolved or relapsing tamponade after pericadiocentesis and persistent active illness three weeks after hospital admission [20]. Both percutaneous and surgical drainage are highly effective at alleviating symptoms and hypotension. During percutaneous drainage, a catheter is placed under echocardiographic guidance into the pericardial sac and left in place until there is no further drainage from the site. Surgical drainage is preferred for loculated effusions, re-accumulation of fluid, when pericardial biopsy is required, or in cases of coagulopathy—for ultimate control of the surgical field in the event of severe bleeding. In nearly every situation, coagulation studies should be obtained before the procedure, and correction of coagulopathy should be achieved prior to the intervention. It is important to drain fluid in no larger than 1 L increments, as rapid removal can cause acute right ventricular dilation [15]. Major complications from pericardiocentesis occur in only about 1% of cases [21]. Surgical drainage bares a higher risk mainly because it requires general anesthesia, which can induce hypotension when the effusion has not first been drained. Percutaneous pericardiocentesis carries fewer complications when compared to surgical drainage [22]. In cases of traumatic hemopericardium or a dissecting aortic aneurysm, surgical intervention is generally preferred because relief of tamponade does not address the cause of the effusion and may allow further bleeding.
-
What are the complications of percutaneous pericardiocentesis and the creation of a pericardial window?
The main complications of pericardiocentesis are related to bleeding into the pericardial sac, however, when a drain is left in place, this is unlikely to present as a problem. Other complications include acute left ventricular failure with pulmonary edema because of sudden increases in systemic venous return. Cardiac perforations are rare events, but may be life-threatening. Arrhythmias, arterial perforation, pneumothorax, vagal response, pleuropericardial fistulas, and infection have all been reported [23]. In past studies of surgical drainage, mortality has been as high as 20%, although these findings could have been confounded by underlying disease [24]. Recent data reveal reported lower rates [25]. In a large study of patients undergoing echo-guided pericardiocentesis, the effusion recurrence rate for simple pericardiocentesis was 27%, and for those who underwent surgical drainage 14% [21]. The main predictors of recurrence were the lack of extended drainage, malignancy, positive cytology, large effusion, and renal failure.
References
Spodick D. Acute cardiac tamponade. N Engl J Med. 2003. http://www.nejm.org/doi/full/10.1056/NEJMra022643. Accessed 23 July 2015.
Sagrista-Sauleda J, Angel J, Sambola A, Alguersuari J, Permanyer-Miralda G, Soler-Soler J. Low-pressure cardiac tamponade: clinical and hemodynamic profile. Circulation. 2006;114(9):945–52. doi:10.1161/CIRCULATIONAHA.106.634584.
Chuttani K, Pandian N, Mohanty P. Left ventricular diastolic collapse. An echocardiographic sign of regional cardiac tamponade. Circulation. 1991. http://circ.ahajournals.org/content/83/6/1999.short. Accessed 23 July 2015.
Permanyer-Miralda G. Acute pericardial disease: approach to the aetiologic diagnosis. Heart. 2004. http://heart.bmj.com/content/90/3/252.short. Accessed 23 July 2015.
Permanyer-Miralda G. Primary acute pericardial disease: a prospective series of 231 consecutive patients. Am J …. 1985. http://www.sciencedirect.com/science/article/pii/0002914985910239. Accessed 23 July 2015.
Shabetai R. Diseases of the pericardium. In: Schlant RC AR, editor. Hurst’s the heart. Vol 8th ed.; 1994.
Roy CL, Minor MA, Brookhart MA, Choudhry NK. Does this patient with a pericardial effusion have cardiac tamponade? JAMA. 2007;297(16):1810–8. doi:10.1001/jama.297.16.1810.
Guntheroth W, Morgan B. Effect of Respiration on Venous Return and Stroke Volume in Cardiac Tamponade Mechanism Of Pulsus Paradoxus. Circ …. 1967. http://circres.ahajournals.org/content/20/4/381.short. Accessed 23 July 2015.
Reddy P, Curtiss E, Uretsky B. Spectrum of hemodynamic changes in cardiac tamponade. Am J Cardiol. 1990. http://www.sciencedirect.com/science/article/pii/000291499090540H. Accessed 23 July 2015.
Fowler N. Cardiac tamponade. A clinical or an echocardiographic diagnosis? Circulation. 1993. http://circ.ahajournals.org/content/87/5/1738.short. Accessed 23 July 2015.
Eisenberg M. The diagnosis of pericardial effusion and cardiac tamponade by 12-lead ECG: a technology assessment. CHEST …. 1996. http://journal.publications.chestnet.org/article.aspx?articleid=1069876. Accessed 23 July 2015.
Mercé J, Sagristà-Sauleda J. Between clinical and Doppler echocardiographic findings in patients with moderate and large pericardial effusion: implications for the diagnosis of cardiac tamponade. Am Hear …. 1999. http://www.sciencedirect.com/science/article/pii/S0002870399701936. Accessed 23 July 2015.
Pepi M, Muratori M. Echocardiography in the diagnosis and management of pericardial disease. J Cardiovasc Med (Hagerstown). 2006;7(7):533–44. doi:10.2459/01.JCM.0000234772.73454.57.
Grocott HP, Gulati H, Srinathan S, Mackensen GB. Anesthesia and the patient with pericardial disease. Can J Anesth. 2011;58(10):952–66. doi:10.1007/s12630-011-9557-8.
Maisch B, Seferović PM, Ristić AD, et al. Guidelines on the diagnosis and management of pericardial diseases executive summary; The Task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J. 2004;25(7):587–610. doi:10.1016/j.ehj.2004.02.002.
Maisch B, Ristic D, Pankuweit S. Intrapericardial treatment of autoreactive pericardial effusion with triamcinolone. Eur Hear J. 2002. http://eurheartj.oxfordjournals.org/content/ehj/23/19/1503.full.pdf. Accessed 26 July 2015.
Trigt P Van, Douglas J, Smith P. A prospective trial of subxiphoid pericardiotomy in the diagnosis and treatment of large pericardial effusion. A follow-up report. Ann …. 1993. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1243074/. Accessed 27 July 2015.
Webster JA, Self DD. Anesthesia for pericardial window in a pregnant patient with cardiac tamponade and mediastinal mass. Can J Anaesth. 2003;50(8):815–8. doi:10.1007/BF03019378.
O’Connor CJ, Tuman KJ. The intraoperative management of patients with pericardial tamponade. Anesthesiol Clin. 2010;28(1):87–96. doi:10.1016/j.anclin.2010.01.011.
Cardiology G. Management of pericardial e V usion. 2001:235–240.
Tsang T, Enriquez-Sarano M. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin …. 2002. http://www.sciencedirect.com/science/article/pii/S0025619611622118. Accessed 26 July 2015.
Gumrukcuoglu H, Odabasi D. Management of cardiac tamponade: a comperative study between echo-guided pericardiocentesis and surgery—a report of 100 patients. Cardiol Res …. 2011. http://www.hindawi.com/journals/crp/2011/197838/abs/. Accessed 26 July 2015.
Duvernoy O, Borowiec J, Helmius G, Erikson U. Complications of percutaneous pericardiocentesis under fluoroscopic guidance. Acta Radiol. 1992;33(4):309–13. doi:10.1177/028418519203300405.
Piehler J, Pluth J. Surgical management of effusive pericardial disease. Influence of extent of pericardial resection on clinical course. J …. 1985. http://europepmc.org/abstract/med/4046619. Accessed 27 July 2015.
Andrade-Alegre R, Mon L. Subxiphoid pericardial window in the diagnosis of penetrating cardiac trauma. Ann Thorac Surg. 1994;58(4):1139–41. doi:10.1016/0003-4975(94)90473-1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Liberman, K.M. (2017). Pericardial Tamponade. In: Aglio, L., Urman, R. (eds) Anesthesiology. Springer, Cham. https://doi.org/10.1007/978-3-319-50141-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-50141-3_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-50139-0
Online ISBN: 978-3-319-50141-3
eBook Packages: MedicineMedicine (R0)