Abstract
Cardiac tamponade is a cardiovascular emergency that can potentially be fatal if left untreated. It consists of a rapidly developing pericardial effusion, accumulating so fast that it doesn’t allow the heart to distend properly during diastole, essentially “compressing” the heart. This results in a markedly diminished left ventricle diastolic volume, and therefore stroke volume and cardiac output are also compromised. The end results are signs and symptoms of low cardiac output and systemic congestion. The most common cause is secondary to large pericardial effusions due to malignancies, iatrogenic (peri-procedure) and post-cardiac surgery. The diagnosis is made clinically, supported by hypotension, tachycardia, muffled heart sounds, jugular venous distention, and the presence of pulses paradoxus. Echocardiography should confirm the diagnosis, not make it. It is important to keep in mind that several echocardiographic parameters should be assessed before reaching a conclusion and one single echocardiographic abnormality by itself is not definitive. Other diagnostic tools include chest radiograph, electrocardiogram, and computed tomography, but echocardiogram remains the most useful paraclinical assessment. Treatment of cardiac tamponade generally involves drainage either by pericardiocentesis or surgical drainage (these procedures are covered in another chapter). The timing of the intervention depends on the hemodynamic stability of the patient and the etiology of the effusion.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
7.1 The Scope of the Problem
Cardiac tamponade is a condition caused by several clinical conditions which can eventually lead to cardiovascular collapse as a final event. High-clinical suspicion is paramount in order to identify these patients and provide timely treatment. In current times, with percutaneous cardiovascular interventions becoming more readily available and with early discharges being emphasized, we may see more of these patients presenting to the emergency room (ER).
7.2 Prevalence
The prevalence of cardiac tamponade varies depending on the etiology and degree of pericardial effusion. Table 7.1 lists the main etiologies of cardiac tamponade and their prevalence. In a study including 322 patients with moderate to severe pericardial effusion, the prevalence of cardiac tamponade was of 37% [1]. An older study in patients undergoing pericardiocentesis documented cardiac tamponade in 48% of the cohort; however, the sample size was small [2]. Although it varies through studies, the most common causes of an effusion resulting in tamponade are malignancy 26–44%, idiopathic 8–27%, iatrogenic 14–21%, and post-cardiac surgery 9–28%. During atrial fibrillation, a study with more than 5000 patients found an incidence close to 1%, being more frequent in patients who underwent ablation with radiofrequency when compared to cryoballoon [3].
7.3 High-Clinical Suspicion in the ER
There should be a high index of suspicion of cardiac tamponade in patients who have had a recent cardiac procedure, history of malignancy, chest trauma, or other clinical conditions (Table 7.1) and have elevated jugular venous pressure (JVP) on clinical examination. Suspicion should be even higher if they present with hypotension or borderline blood pressure measurements. A quick bedside echocardiogram in the ER can determine if further detailed testing is needed. If these patients are not identified promptly early on their presentation, they can decompensate quickly. We recommend early thoracentesis in the setting of atrial or ventricular collapse by echocardiography , even in the case of clinical stability patients with severe pericardial effusion.
7.4 Risk Factors
The likelihood of a pericardial effusion developing cardiac tamponade depends on several factors, for which understanding the underlying pathophysiology is of critical importance. In a normal heart, intrapericardial pressures (IPP) are lower than intracardiac pressures at baseline and have physiologic changes in response to intrathoracic and intracardiac pressures. The presence of greater than the normal fluid in the pericardial space increases the IPP , the extent to which it will do so depends on the pressure-volume relationship, which itself depends on the time frame in which the fluid accumulated. In acute effusions resulting from cardiac injury, for example, the pericardial reserve volume is low, and small amount of fluid as low as 100–200 cc may increase IPP significantly.
In chronic effusions the pericardium has time to adapt and become more elastic, increasing the pericardial reserve volume and allowing a greater amount of fluid to accumulate before the IPP exceeds intracardiac pressures. Increased IPP results in underfilling of the right atrium, producing a decrease in ventricular dimensions and cardiac output. Progressive increases in IPP eventually lead to equalization of intrapericardial and intracardiac pressures, impairing cardiac output and resulting in cardiovascular collapse as a final event [6]. Cardiac tamponade has a progressive course which can be classified into several stages. Table 7.2 describes the events that occur in each of these four stages.
Some conditions causing pericardial effusion are more likely to evolve into cardiac tamponade than others. Table 7.3 compares conditions that are likely, less likely, and very unlikely to progress to pericardial effusion.
7.5 Clinical Presentation
7.5.1 Main Clinical Characteristics
-
Dyspnea
-
Tachypnea
-
Syncope
-
Tachycardia
-
Hypotension
-
Pulsus paradoxus
-
Jugular venous distension
-
Lung fields clear to auscultation
-
Muffled heart sounds
7.5.2 Physical Exam
A physical exam is of great importance, given the diagnosis of cardiac tamponade is a clinical one, and imaging studies should be confirmatory. A systematic review calculated a pooled sensitivity for physical exam findings in cardiac tamponade: pulsus paradoxus 82%, tachycardia 77%, hypotension 26%, diminished heart sounds 28%, and JVD 76% [9].
In cardiac tamponade, inspiration results in an increased filling of the right atrium and ventricle, producing septal shifting toward the left ventricle (LV) and decreasing its filling. These changes result in a decreased cardiac output, producing a drop of systolic blood pressure during inspiration manifested as pulsus paradoxus. Pulsus paradoxus is usually measured with a sphygmomanometer by inflating the brachial cuff above the systolic pressure and deflating it slowly. A note is taken when the first Korotkoff sound is heard, and as the cuff continues to deflate, the Korotkoff sounds will only be present during expiration initially; careful attention must be paid to when the Korotkoff sounds are heard both during inspiration and expiration, marking the second and final measurement.
Pulsus paradoxus is present when the difference between the first and second measurements is greater than 10 mmHg. A physiologic measurement should produce a difference of less than 6 mmHg. A value greater than 10 mmHg has a sensitivity of 98% and a specificity of 70%; if the cutoff is increased to 12 mmHg, the specificity increases to 83% [9]. Pulsus paradoxus can also be present in different pathologies listed in Table 7.4. The absence of pulsus paradoxus in the presence of cardiac tamponade may be due to several cardiac conditions in which the intracardiac pressure and flows are modified. These conditions include left ventricular (LV) or right ventricular (RV) dysfunction, LV hypertrophy, aortic regurgitation, atrial septal defect, extreme hypotension, local cardiac adhesions, and acute LV myocardial infarction [10, 11].
The acute cardiac compression triad, also known as Becks triad, consists in hypotension, JVD, and diminished heart sounds. While it is frequently mentioned when entertaining a diagnosis of cardiac tamponade , clinicians must be aware that the population initially studied included surgical patients with intrapericardial hemorrhage from cardiac trauma, myocardial rupture from myocardial infarction, and aortic or coronary rupture [12]. Medical patients commonly present with a slower accumulating effusion evolving into cardiac tamponade, in which the absence of Becks triad should not rule out the diagnosis [13].
7.5.3 Chest X-ray
Chest X-ray can provide initial clues to the presence of a large pericardial effusion, but it is not diagnostic and does not provide information as to whether cardiac tamponade is present. Findings include an enlarged heart in a “water bottle” shape (Fig. 7.1) compared to previous (sensitivity 71%, specificity 41%), a pericardial fat stripe (sensitivity 12%, specificity 94%), and a predominant left-sided pleural effusion (sensitivity 20%, specificity 100%) [14]. A systematic review found a pooled sensitivity of 89% for cardiomegaly in the diagnosis of cardiac tamponade [9].
7.5.4 Electrocardiogram
The presence of low voltage in the ECG should raise suspicion for cardiac tamponade , as seen in a study where 61% of the patients with cardiac tamponade had low voltage but was not present in stable patients with large pericardial effusions [15]. However, other studies have shown lower sensitivities with low QRS voltage; a systematic review found a pooled sensitivity of only 42% [9].
7.5.4.1 Transthoracic Echocardiogram
The findings seen on echocardiography reflect the effects increased intrapericardial pressure has on intracardiac pressures (Fig. 7.2). They are summarized in Table 7.5.
Inferior vena cava dilatation measured during M mode greater than 2.1 cm with less 50% collapse in diameter with inspiration can be found in up to 92% of patients. The same study showed a sensitivity of 97% with a specificity of 40%, as it can be present in other conditions that increase systemic venous pressure [16]. On M mode, an increase in RV dimensions with a subsequent decrease in LV dimensions during inspiration can be observed [17].
Chamber collapse observed on 2D echocardiography is usually the result of the intrapericardial pressure being higher than the intracardiac pressure and lasts until the pressures are reversed again . Diastolic right atrial (RA) collapse is assessed in cardiac tamponade starting at the peak for the R wave. When the RA collapse lasts more than one-third of the cardiac cycle (right atrial time index >0.34; right atrial time index = # of frames with RA collapse/# of frames in duration of cardiac cycle) , it has a sensitivity of up to 94% and a specificity and positive predictive value of 100% [18, 19]. Another study showed a much lower sensitivity of 68%, a specificity of 66%, and a positive predictive value of 52% [20].
RV diastolic collapse (Fig. 7.3) is usually observed at the end of the T wave and is present when there has been a decrease of 20% in cardiac output without hypotension [21]. It can have a sensitivity of 92% and specificity and positive predictive value of 100% [22]. The severity of the cardiac tamponade is related to the length of RV collapse during the cardiac cycle [23]. Another study showed a much lower sensitivity of 60%, specificity of 90%, and positive predictive value of 77%. The presence of collapse of both chambers had a sensitivity of 45%, specificity of 92%, and positive predictive value of 74% [20].
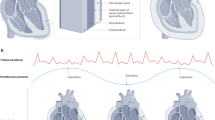
Hemodynamic changes to respiratory patterns can be observed in Doppler echocardiography. As a result of decreased cardiac output, there are decreased flow velocities through the left ventricular outflow tract, as well as a decrease in velocities with inspiration. Reflecting decreased LV filling, there is a decrease in mitral peak E inflow velocity during inspiration, with the lowest value being on the initial beat of inspiration (mitral peak E velocity is highest during expiration). A decrease in mitral peak E inflow velocity during inspiration of more than 30% is usually considered diagnostic (Fig. 7.4).
In the tricuspid valve, the exact opposite changes occur given the increased flow; with expiration there is a decrease of tricuspid peak E inflow velocity, with the lowest being the initial beat during expiration (tricuspid peak E velocity is highest during inspiration). A decrease in tricuspid peak E inflow velocity during expiration of >60% (it will be a negative value) is suggestive of cardiac tamponade. Both values are calculated: (expiration-inspiration)/expiration. It is important to take into consideration that these findings by themselves do not confirm the diagnosis of cardiac tamponade and additional parameters need to be analyzed [19, 20, 24, 25].
Hepatic vein Doppler velocities also provide clues suggesting the presence of cardiac tamponade; velocities are lower in the 20–40 cm/s range. There is a predominance of systolic flow over diastolic flow; with expiration there can be the absence of diastolic flow, diastolic flow reversal, or no forward flow in advanced tamponade. The presence of hepatic flow changes has a sensitivity of 75%, specificity of 91%, and positive predictive value of 82% [19, 20, 26].
7.5.5 Computed Tomography
Computed tomography is not the initial diagnostic tool to evaluate pericardial tamponade. However it’s a commonly performed test which can provide valuable information. Real-time cine cardiac CT can show similar findings as echocardiography, such as chamber collapse and septal bounce. Additionally, it can help delineate the anatomy in pericardiocentesis, identify loculated effusions, and assess the presence of compressive hematoma or calcified pericardium [27]. Findings on non-cardiac CT that can be seen in cardiac tamponade include increased diameter of the vena cava compared to the aorta and reflux of contrast into the inferior vena cava and hepatic veins [28]. However, these findings can be seen in pathologies that increase the right ventricular pressure and are not specific for cardiac tamponade.
7.5.6 Differential Diagnosis
During the initial presentation in the ER, the differential diagnosis can be wide; it includes conditions that increase right-sided pressures resulting in elevated JVP and may produce hypotension conditions which should include heart failure, pulmonary embolism, constrictive pericarditis, large pleural effusions impairing cardiac filling, mediastinal hematomas impairing cardiac filling, and advanced cirrhosis.
7.6 Treatment
7.6.1 Medical Management
If there is evidence of cardiac tamponade, medical management has a limited role in the treatment of cardiac tamponade. While a patient is awaiting pericardiocentesis, intravenous fluids are commonly given to expanding right-side chambers. However, there is evidence that overhydration in these patients may be harmful. Fluid resuscitation with 250 ml up to 500 ml produced an increase in cardiac output, cardiac index, and systolic blood pressure; with higher intracardiac pressures, elevated heart rates, lower systolic blood pressure, and cardiac index as predictors of a >15% increase in CI as a response to fluid administration. After the 500 ml cutoff, pulmonary capillary wedge pressure , pulmonary pressure, right atrial pressure, and intrapericardial pressure continue to rise, decreasing cardiac output and resulting in pulmonary edema [29]. Therefore, if fluid administration is planned, it should be performed in a judicious manner.
7.6.2 Pericardiocentesis
The presence of pericardial effusion producing cardiac tamponade physiology usually warrants drainage of the pericardial effusion via pericardiocentesis or a surgical approach. Pericardiocentesis may be a better option in emergent cases, as it can be performed with fewer delays and can also be performed at bedside if needed. An exception is a hemopericardium secondary to myocardial rupture from myocardial infarction and secondary to type A aortic dissection, where pericardiocentesis should not delay surgical treatment. In these cases, there is a concern that performing pericardiocentesis can worsen the patient’s condition by elevating blood pressure, worsening aortic tear, and increasing the gradient between the false lumen and pericardial effusion, thus increasing the size of the effusion [30]. However, in patients with type A aortic dissection, it has been shown that removal of a small amount of fluid (average fluid removal 40 cc) can help stabilize patients awaiting surgery [31]. In patients with cardiogenic shock, pericardiocentesis should be performed urgently. Patients with chest trauma , purulent pericarditis, loculated effusions, and iatrogenic effusions with uncontrollable bleeding should also generally be drained via a surgical approach. In stable patients with a diagnosis of cardiac tamponade, timing of drainage may be an element of the debate. There are scoring systems to help guide decision-making of when to perform pericardiocentesis. The European Society of Cardiology published a triage strategy to aid the timing of pericardiocentesis, where a score ≥ 6 warrants urgent pericardiocentesis and a score < 6 pericardiocentesis can be delayed for up to 12/48 hours (Fig. 7.5).
There are several approaches to perform pericardiocentesis , and the decision on which one to use should be made depending on the location of the effusion as seen on echocardiography or computed tomography. The three main approaches used are left apical, subxiphoid, and left parasternal. In general, it is recommended to perform pericardiocentesis guided by echocardiography , as it has been shown to be successful with a relatively low risk of complications [32]. Fluoroscopy has been used for many years to guide pericardiocentesis, with the subxiphoid approach being more common during this modality. It is commonly used when pericardial tamponade develops during cardiac procedures. Although not readily available in all hospitals, CT-guided pericardiocentesis can be a useful tool, especially when echocardiographic windows are suboptimal [33]. The technical aspects of pericardiocentesis are detailed in the procedures chapter.
Table 7.6 summarizes the most important recommendations on the diagnosis and treatment of cardiac tamponade, according to the ESC.
7.7 Additional Clinical Practice Takeaways
-
The diagnosis of cardiac tamponade is a clinical one.
-
The most common causes are malignancy, iatrogenic, and post-cardiac surgery.
-
The presence of a pulsus paradoxus >10 mmHg is suggestive of cardiac tamponade.
-
Echocardiographic finding suggestive of cardiac tamponade include dilated and not collapsible IVC, right chamber collapse, mitral peak E velocity during inspiration with a >30% decrease when compared to expiration, tricuspid peak E velocity during expiration with a >60% decrease when compared to inspiration, hepatic systolic flow reversal, septal flattening, and swinging of the heart.
-
Pericardial drainage either by pericardiocentesis or surgical is generally the treatment; hemodynamic status and etiology guide the urgency of the procedure.
References
Sagristà-Sauleda J, Mercé J, Permanyer-Miralda G, Soler-Soler J. Clinical clues to the causes of large pericardial effusions. Am J Med. 2000;109:95–101.
Colombo A, Olson HG, Egan J, Gardin JM. Etiology and prognostic implications of a large pericardial effusion in men. Clin Cardiol. 1988;11:389–94.
Hamaya R, Miyazaki S, Taniguchi H, Kusa S, Nakamura H, Hachiya H, et al. Management of cardiac tamponade in catheter ablation of atrial fibrillation: single-centre 15 year experience on 5222 procedures. Europace. 2018;20:1776–82.
Tsang TSM, Enriquez-Sarano M, Freeman WK, Barnes ME, Sinak LJ, Gersh BJ, et al. Consecutive 1127 therapeutic echocardiographically guided pericardiocenteses: clinical profile, practice patterns, and outcomes spanning 21 years. Mayo Clin Proc. 2002;77:429–36.
Corey GR, Campbell PT, Van Trigt P, Kenney RT, O’Connor CM, Sheikh KH, Kisslo JA, Wall TC. Etiology of large pericardial effusions. Am J Med. 1993;95(2):209–13.
Appleton C, Gillam L, Koulogiannis K. Cardiac Tamponade. Cardiol Clin. 2017;35:525–37.
Risti AD, Imazio M, Adler Y, Anastasakis A, Badano LP, Brucato A, et al. Triage strategy for urgent management of cardiac tamponade: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2014;35:2279–84.
Goldstein JA. Cardiac tamponade, constrictive pericarditis, and restrictive cardiomyopathy. Curr Probl Cardiol. 2004;29:503–67.
Roy CL, Minor MA, Brookhart MA, Choudhry NK. Does This Patient With a Pericardial Effusion Have Cardiac Tamponade? JAMA. 2007;297(16):1810–8.
Swami A, Spodick DH. Pulsus paradoxus in cardiac tamponade: a pathophysiologic continuum. Clin Cardiol. 2003;26:215–7.
Naeije R, Chemla D, Dinh-Xuan AT, Vonk Noordegraaf A. Physiology in respiratory medicine. Eur Respir J. 2013;41:7–7.
Beck CS. Two cardiac compression triads. JAMA. 1935;104:714–6.
Guberman BA, Fowler NO, Engel PJ, Gueron M, Allen JM. Cardiac tamponade in medical patients. Circulation. 1981;64:633–40.
Eisenberg MJ, Dunn MM, Kanth N, Gamsu G, Schiller NB. Diagnostic value of chest radiography for pericardial effusion. J Am Coll Cardiol. 1993;22:588–93.
Bruch C, Schmermund A, Dagres N, Bartel T, Caspari G, Sack S, et al. Changes in QRS voltage in cardiac tamponade and pericardial effusion: reversibility after pericardiocentesis and after anti-inflammatory drug treatment. J Am Coll Cardiol. 2001;38:219–26.
Himelman RB, Kircher B, Rockey DC, Schiller NB. Inferior vena cava plethora with blunted respiratory response: a sensitive echocardiography sign of cardiac tamponade. J Am Coll Cardiol. 1988;12:1470–7.
Settle HP, Adolph RJ, Fowler NO, Engel P, Agruss NS, Levenson NI. Echocardiographic study of cardiac tamponade. Circulation. 1977;56:951–9.
Gillam LD, Guyer DE, Gibson TC, King ME, Marshall JE, Weyman AE. Hydrodynamic compression of the right atrium: a new echocardiographic sign of cardiac tamponade. Circulation. 1983;68:294–301.
Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, et al. American Society of Echocardiography Clinical Recommendations for Multimodality Cardiovascular Imaging of Patients with Pericardial Disease. J Am Soc Echocardiogr. 2013;26:965–1012.e15.
Mercé J, Sagristà-Sauleda J, Permanyer-Miralda G, Evangelista A, Soler-Soler J. Correlation between clinical and Doppler echocardiographic findings in patients with moderate and large pericardial effusion: implications for the diagnosis of cardiac tamponade. Am Heart J. 1999;138:759–64.
Schiller NB, Botvinick EH. Right ventricular compression as a sign of cardiac tamponade: an analysis of echocardiographic ventricular dimensions and their clinical implications. Circulation. 1977;56:774–9.
Armstrong WF, Schilt BF, Helper DJ, Dillon JC, Feigenbaum H. Diastolic collapse of the right ventricle with cardiac tamponade: an echocardiographic study. Circulation. 1982;65:1491–6.
Leimgruber PP, Klopfenstein HS, Wann LS, Brooks HL. The hemodynamic derangement associated with right ventricular diastolic collapse in cardiac tamponade: an experimental echocardiographic study. Circulation. 1983;68:612–20.
Appleton CP, Hatle LK, Popp RL. Cardiac tamponade and pericardial effusion: respiratory variation in transvalvular flow velocities studied by Doppler echocardiography. J Am Coll Cardiol. 1988;11:1020–30.
Leeman DE, Levine MJ, Come PC. Doppler echocardiography in cardiac tamponade: exaggerated respiratory variation in transvalvular blood flow velocity integrals. J Am Coll Cardiol. 1988;11:572–8.
Burstow DJ, Oh JK, Bailey KR, Seward JB, Tajik AJ. Cardiac tamponade: characteristic Doppler observations. Mayo Clin Proc. 1989;64:312–24.
Yared K, Baggish AL, Picard MH, Hoffmann U, Hung J. Multimodality imaging of pericardial diseases. JACC Cardiovasc Imaging. 2010;3:650–60.
Restrepo CS, Lemos DF, Lemos JA, Velasquez E, Diethelm L, Ovella TA, et al. Imaging findings in cardiac tamponade with emphasis on CT. Radiographics. 2007;27:1595–610.
Singh V, Dwivedi SK, Chandra S, Sanguri R, Sethi R, Puri A, et al. Optimal fluid amount for haemodynamic benefit in cardiac tamponade. Eur Heart J Acute Cardiovasc Care. 2014;3:158–64.
Isselbacher EM, Cigarroa JE, Eagle KA. Cardiac tamponade complicating proximal aortic dissection. Is pericardiocentesis harmful? Circulation. 1994;90:2375–8.
Hayashi T, Tsukube T, Yamashita T, Haraguchi T, Matsukawa R, Kozawa S, et al. Impact of controlled pericardial drainage on critical cardiac tamponade with acute type a aortic dissection. Circulation. 2012;126:S97–101.
Maggiolini S, Gentile G, Farina A, De Carlini CC, Lenatti L, Meles E, et al. Safety, efficacy, and complications of pericardiocentesis by real-time echo-monitored procedure. Am J Cardiol. 2016;117:1369–74.
Neves D, Silva G, Morais G, Ferreira N, Carvalho M, Gama Ribeiro V, et al. Computed tomography-guided pericardiocentesis – a single-center experience. Rev Port Cardiol. 2016;35:285–90.
Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36:2921–64.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Jerjes-Sánchez, C., Trevino, A. (2019). Cardiac Tamponade in the ER. In: Cardiology in the ER. Springer, Cham. https://doi.org/10.1007/978-3-030-13679-6_7
Download citation
DOI: https://doi.org/10.1007/978-3-030-13679-6_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-13678-9
Online ISBN: 978-3-030-13679-6
eBook Packages: MedicineMedicine (R0)