Abstract
Advances in computer technology have made it possible to perform prolonged digital continuous video EEG monitoring of many critically ill patients simultaneously. Studies using continuous EEG monitoring (cEEG) have found that these patients often have nonconvulsive seizures (NCSzs) that may contribute to secondary brain injury. Improving quantitative EEG (qEEG) software speeds data review to allow screening of multiple prolonged recordings to detect NCSz and provides continuous information about changes in brain function in real time at the bedside and remotely. Optimal sensitivity and specificity of qEEG tools is obtained with full electrode montages and careful maintenance of scalp electrodes. Standardized nomenclature for EEG patterns seen in critically ill patients has been tested, published, and utilized for multicenter research on these patterns. Guidelines on when and how to perform cEEG have been published. New electrode technologies, such as MRI-compatible electrodes, help reduce the burden on EEG technologists while limiting interruptions in recordings. In addition to detecting NCSz, cEEG can also be used for dynamic detection of other changes in brain function such as ischemia and can be coupled with other modalities of monitoring brain physiology such as microdialysis, tissue oximetry, and intracranial electrophysiology. Together, these tools can allow for early detection of brain at risk for injury and alert the physician to intervene before the damage becomes irreversible.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Nonconvulsive seizures (NCSzs) and nonconvulsive status epilepticus (NCSE) are increasingly recognized as a common occurrence in the ICU, where 6–59% of patients undergoing continuous EEG monitoring (cEEG) may have NCSz, depending on the study population [1–5] (Fig. 3.1). NCSz, as the term is used in this chapter, refers to electrographic seizures with little or no overt clinical manifestations. NCSE occurs when NCSzs are prolonged; a common definition is continuous or near-continuous electrographic seizures lasting at least 30 min [6–8]. Some experts included recurrent electrographic seizures occupying more than 30 min in any 1 h [9]. The Neurocritical Care Society guidelines on SE defined NCSE as any continuous electrographic seizure activity for ≥5 min [10]. More recently, ILAE taskforce defined SE as “a condition resulting from either the failure of mechanisms responsible for termination of seizures or from the initiation of mechanisms, which lead to abnormally prolonged seizures, after time point t1. It is a condition, which can have long term consequences (after time point t2) including neuronal death, neuronal injury….” [11] For focal SE with impaired consciousness, the proposed t1 (after which seizures need to be acutely treated) is estimated to be 10 min, while the proposed t2 (after which more aggressive therapy may be justified) is >60 min [11]. Most patients with NCSz (about 75% averaging many studies) have purely electrographic seizures [1] (Fig. 3.2), but NCSz can be associated with other subtle signs such as face and limb twitching, nystagmus, eye deviation, pupillary abnormalities (including hippus), and autonomic instability [12–14]. None of these signs are highly specific for NCSz and are often seen under other circumstances in the critically ill patient; thus, cEEG is necessary to diagnose NCSz. In this chapter, we will discuss the implementation of cEEG in the critically ill and how to review the data, including available quantitative EEG (qEEG) tools that enable efficient review of the vast amount of raw EEG generated by prolonged monitoring. We will also review which patients are appropriate candidates for cEEG as well and the numerous EEG patterns that may be encountered. Finally, we will discuss future directions for cEEG and neurophysiological monitoring in the ICU.
Incidence of nonconvulsive seizures in different populations of critically ill children and adults. The confidence intervals were not reported by the studies, but were calculated based on the number of subjects in the study and the proportion of patients in whom nonconvulsive seizures were detected. Data is derived from (a) Abend NS, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology 2013; 81:383–391. (b) Abend NS, et al. Electroencephalographic monitoring during hypothermia after pediatric cardiac arrest. Neurology 2009; 72:1931–1940. (c) Arndt DH, et al. Subclinical early posttraumatic seizures detected by continuous EEG monitoring in a consecutive pediatric cohort. Epilepsia 2013; 54(10):1780–1788. (d) Carrera E, et al. Continuous electroencephalographic monitoring in critically ill patients with CNS infections. Arch Neurol 2008; 65 (12):1612–1618. (e) Claassen J, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients: Neurology 2004; 62:1743–1748. (f) Claassen J, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology 2007; 69:1356–1365. (g) Crepeau AZ, et al. Value analysis of continuous EEG in patients during therapeutic hypothermia after cardiac arrest. Resuscitation 2014 (85):785–789. (h) Gilmore EJ, et al. Acute brain failure in severe sepsis: A prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med 2015; 41(4):686–694. (i) Mani R, et al. The frequency and timing of epileptiform activity on continuous electroencephalography in comatose post-cardiac- arrest syndrome patients treated with therapeutic hypothermia. Resuscitation 2012 (83):840–847. (j) O’connor KL, et al. High risk for seizures following subarachnoid hemorrhage regardless of referral bias. Neurocrit Care 2014; 21:476–482. (k) O’neill BR, et al. Incidence of seizures on continuous EEG monitoring following traumatic brain injury in children. J Neurosurg Pediatr 2015; 16: 167–176. (l) Oddo M, et al. Continuous Electroencephalography in the medical intensive care unit. Crit Care Med 2009; 37 (6): 2051–2056. (m) Payne ET, et al. Seizure Burden is independently associated with short-term outcome in critically ill children. Brain 2014; 137: 1429–1438. (n) Ronne-Engstrom E , Winkler T. Continuous EEG monitoring in patients with traumatic brain injury reveals high incidence of epileptiform activity. Arch Neurol Scand 2006; 114: 47–53. (o) Schreiber JM, et al. Continuous video EEG monitoring for patients with acute encephalopathy in a pediatric intensive care unit. Neurocrit Care 2012; 17:31–38. (p) Topjian AA, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med 2013; 41 (1):210–213. (q) Vespa PM, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology 2010; 75 (9):792–798. (r) Vespa PM, et al. Acute seizures after intracerebral hemorrhage: A factor in progressive midline shift and outcome. Neurology 2003; 60:1441–1446. (s) Westover B, et al. The probability of seizures during EEG monitoring in critically ill adults. Clinical Neurophysiology 2015; 126:463–471 (Published with kind permission from © Lawrence J. Hirsch, MD 2016. All Rights Reserved)
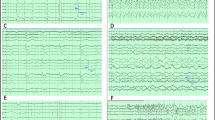
Nonconvulsive status epilepticus. This is the EEG from a 29-year-old man with a history of liver transplantation and chronic immunosuppression who presented with convulsive status epilepticus due to encephalitis. He was treated with intravenous anticonvulsants and movements ceased, but he remained comatose. His EEG demonstrated electrographic seizure activity without clinical correlate. Low-frequency filter (LFF) = 1 Hz, high-frequency filter (HFF) = 70 Hz, notch off (Published with kind permission from © Lawrence J. Hirsch, MD 2013. All Rights Reserved)
How to Monitor
Obtaining high-quality cEEG recordings in the ICU is a challenge. Adequate technologist coverage is necessary to connect patients promptly, including off hours, and maintain those connections 24 h/day. Critically ill patients are frequently repositioned and transported to tests, which makes maintaining electrode integrity difficult. In both of our centers, we often employ collodion to secure disk electrodes and check the electrodes twice daily, usually supplemented by keeping the live recordings visible remotely to see which patients require electrode maintenance. Newer electrodes, such as subdermal wires, which may be more secure and lead to less skin breakdown, may be appropriate for comatose patients who are expected to undergo cEEG for many days to weeks [15]. While these electrodes may take more time to apply, they require less maintenance and are MRI and CT compatible (both safe and not affecting image interpretation), thereby saving substantial technologist time. Concerns for image artifacts and patient safety make it necessary to remove and then reapply standard disk electrodes when patients undergo brain MRIs, but there has been some progress in creating practical MRI- and CT-compatible electrodes [16], including conductive plastic electrodes. Figure 3.3 shows CT and MRI images taken with these electrodes in place displaying minimal image artifact and CT images in a different patient with considerable image artifact caused by the electrodes. MRI-compatible disposable plastic cup electrodes are now commercially available and can be used in the ICU setting to minimize risk of transmitting infections [17].
CT- and MRI-compatible EEG electrodes. (a) A CT “scout” image demonstrating the placement of a full montage of conductive plastic electrodes (white arrows) on the scalp of a patient undergoing cEEG monitoring. (b) An axial image from the CT scan from the same patient demonstrates minimal artifact due to the electrodes (white arrows). Note there is no evidence of “streaking” common to head CT with conventional electrodes. (c) Axial FLAIR MRI performed 3 days later with electrodes in place. Note only minimal image artifact near the scalp (white arrows). (d) Axial CT in a different patient with multicompartmental intracranial hemorrhage showing beam hardening CT artifact caused by intact standard metal electrodes (blue arrows) (Modified with kind permission from © Lawrence J. Hirsch, MD 2013. All Rights Reserved)
There are numerous sources of artifact in the ICU environment that make cEEG challenging. Some are easily identified and filtered out such as 60 Hz (or 50 Hz in Europe) line noise from nearby electrical equipment. Others, however, such as pacemaker artifact, chest percussion, vibrating beds, ventilator activity, and intravenous drips, may be difficult to distinguish from seizures or other rhythmic or periodic cerebral activities [18–20] (Fig. 3.4). Simultaneous video recording is useful for distinguishing brain signals from artifact, especially rhythmic patterns such as those seen with chest percussion. In addition, video recording helps correlate EEG patterns with patient behaviors. In some cases, periodic EEG patterns can be determined to be ictal if they are time-locked to subtle patient movements [21]. In addition, some significant EEG patterns in the critically ill appear after the patient is stimulated, which is easily determined by reviewing the video [22, 23] (see below).
Common ICU EEG artifacts. (a) Rhythmic bitemporal artifact due to chest compression in a medical ICU patient (arrow). (b) Respirator artifact due to fluid collecting in the tubing (arrow). These patterns are easily recognized on simultaneous video recording as they are synchronized with respirations. (c) Left temporal rhythmic waveforms (arrow) due to patting in an infant. This pattern is sometimes easy to confuse with seizures without video as it often shows a physiological field with evolution in frequency and amplitude. (d) Semirhythmic right temporal artifact (black arrow) due to chest percussion mimicking right LPDs or potentially ictal activity in a patient with true left hemisphere LPDs (white arrows). (e) Right occipital 6 Hz rhythmic artifact (arrow) due to automatic bed oscillation. (f) Rhythmic 1–1.5 Hz artifact (arrow) due to chewing in an edentulous patient. LFF = 1 Hz, HFF = 70 Hz, notch off (Published with kind permission from © Lawrence J. Hirsch, MD 2013. All Rights Reserved)
The number of electrodes used in cEEG studies varies considerably. In both our centers, we typically perform “full electrode” recordings using 16 or more active electrodes in addition to one or two reference electrodes and cardiac leads. Other authors have used reduced electrode configurations [24]. The advantage of a reduced electrode system is that it is faster to apply and easier to maintain. It is also easier to work around other neuro-monitoring devices, surgical wounds, or ventricular drains common in neuro-ICU patients. However, a full electrode configuration improves the ability to distinguish brain signals from artifact, aids in spatial localization of pathological activity, and provides a safety factor in case one or more leads fail, including allowing qEEG calculations and alarms to continue to function adequately [25]. In addition, reduced electrode methods, especially when coupled to qEEG tools, may miss clinically significant events. For instance, Shellhaas et al. [26] found that neonatologists evaluating amplitude-integrated EEG (aEEG) using only two electrodes for seizure detection, a technique employed in purpose-built devices common in neonatal ICUs, detected only 12–38% of seizures identified using conventional electrode arrangements. Although emergent below-the-hairline EEG recordings have only moderate sensitivities and specificities [27], they are almost certainly better than no EEG at all; a full EEG should be done when possible to confirm or refute the results. Several disposable headpieces including pre-gelled electrodes are now available and can be utilized, allowing fast application by ICU nurses, house staff, and other staff not fully trained in EEG electrode application [28].
Data Analysis
Several days of cEEG generates gigabytes of data that, in its raw form, is time consuming for a neurophysiologist to review, especially if many patients are being monitored simultaneously. Furthermore, the raw EEG may be difficult for non-experts, such as ICU physicians and nurses, to interpret at the bedside. Therefore, concerning electrographic events may not be noticed until several hours later, when the neurophysiologist reviews the file, unless real-time remote monitoring is performed continuously (currently available only in a minority of academic centers). Computing advances have enabled the use of qEEG algorithms to reduce the data and provide graphical representation of significant patterns and trends to speed review. Some of the commonly employed qEEG methods are discussed below (see [29] for a detailed review).
Many qEEG data reduction and trending tools are based on transforming the raw cEEG into a time-frequency series using algorithms such as short-time Fourier transform or continuous wavelet transform. Several hours of cEEG recordings can be reduced to a single screen of time-frequency values using a compressed spectral array or density spectral array. The time-frequency data can be averaged over scalp regions or hemispheres to further reduce the data. Using these techniques, the abrupt changes in cEEG spectral power in a relatively narrow frequency range during seizures are highlighted, allowing quick assessment of seizure frequency and duration (Fig. 3.5). Time-frequency transformation of the cEEG can be further manipulated to provide a single scalar value for each epoch of time. For instance, Claassen et al. [30] showed that the ratio of total hemispheric power in the alpha-frequency band (8–13 Hz) to the total power in the delta-frequency band (1–4 Hz) after maximal alerting, or poststimulation alpha-delta ratio (ADR), was the most useful qEEG parameter for detecting delayed cerebral ischemia in patients with high-grade subarachnoid hemorrhage (SAH) (see later). Hemispheric asymmetries in spectral power, computed as ratio of left and right total power for all EEG frequencies or as relative differences at each frequency, can be used to quickly identify focal seizures (e.g., Fig. 3.5). The greatest utility of reducing the cEEG to single scalar values is that these values can easily be displayed and interpreted on bedside monitors like heart rate and blood pressure. This could allow for early identification of neurophysiological events by the ICU staff and alarms to trigger patient examination and could lead to more responsive treatment. Another measure used as part of quantitative EEG trending analysis is the rhythmicity spectrogram. This measure highlights the rhythmic or periodic component of different frequencies, thus facilitating seizure identification. A diagonal pattern in particular is characteristic of seizures as it shows rhythmicity rapidly and consistently changing in frequency, a typical pattern of ictal evolution [29].
qEEG trends (comprehensive panel view) from Persyst 12™ (Persyst. Inc.; San Diego, California) in a 24-year-old woman with SE. (a) 2 h qEEG page showing long-term trends: Artifact intensity (first from top) displays the amount of muscle artifact, vertical and lateral eye movement present. The intensity of these artifacts may help determine the state of the patient. Seizure probability (2nd from top): Red bars display seizure probability on a scale from 0 to 1, as determined by Persyst seizure detection algorithm. Rhythmicity spectrogram for left and right hemispheres (3rd and 4th from top, respectively) illustrates rhythmic components of different frequencies, darker colors being more rhythmic. FFT spectrogram for left and right hemispheres (5th and 6th from top, respectively) demonstrates power of different frequencies at different time periods. Time is displayed on x-axis, frequencies on y-axis, and amplitude of power of different frequencies as different colors on z-axis (see color scale). Relative asymmetry spectrogram (7th from top): illustrates comparison of power of different frequencies at homologous electrodes in each hemisphere (blue if higher power on left, red if on right). Suppression percentage (8th from top) displays the percent of the EEG record that is below a determined threshold amplitude (e.g., 10 μV). No EEG suppression is seen in this panel. aEEG (9th from top; combined left and right hemispheres; left, blue; right, red; overlap, pink): displays mean filtered and smoothed EEG amplitude (y-axis) across time (x-axis). FFT power ratio (last from top) illustrates alpha/delta ratio across time in both left (blue) and right (red) hemisphere. 26 seizures were detected in this 2 h page (black arrow heads), evidenced by surges in FFT power and aEEG, as well as evolving rhythmicity on rhythmicity spectral analysis. All of these seizures were also detected by seizure probability index (red bars on seizure probability index; 2nd panel from top). (b) 6 h qEEG page for the same patient shows significant decline in the number of seizures in the second half of the page. Using longer time windows allows for greater appreciation of long-term trends and assists in monitoring response to therapy. (c) Raw EEG for one of the detected seizures. LFF = 1 Hz, HFF = 70 Hz, notch off (Published with kind permission from © Lawrence J. Hirsch, MD 2016. All Rights Reserved)
Other trending algorithms highlight amplitude measures, which can also be used to detect seizures. Amplitude-integrated EEG (aEEG) displays compressed, smoothed, and full-wave rectified EEG signal (Fig. 3.5). It is particularly useful for assessing background amplitude and burst suppression. aEEG is commonly used in commercial devices in neonatal ICUs [31] to assess the background EEG and occasionally as an initial screening tool for detecting seizures, although aEEG may be inadequately sensitive and is probably not specific enough for detecting seizures [26]. Envelope trend displays median amplitude of raw EEG background activity within a specified frequency range in a chosen time period, thus minimizing the effect of transient change in EEG signal created by artifacts, which are common in the ICU environment [32]. Multiple seizure detection algorithms are now available and can be utilized for automated seizure detection in the ICU setting [33]. Nonetheless, reliance on qEEG tools without the ability to review the raw EEG for non-cerebral signals can lead to false-positive seizure detections; thus, qEEG should only be interpreted in conjunction with the raw EEG wave forms and in conjunction with skilled electroencephalographers with special training in ICU EEG whenever possible [34].
Quantitative EEG tools can also calculate the degree of burst suppression of the EEG background to allow for easy titration of medications to induce coma, a common treatment of status epilepticus or refractory elevated intracranial pressure [35]. EEG-based monitors such as bispectral index [36], patient state index [37], Narcotrend [38] and entropy systems [38] have been in use in operating rooms and ICUs for nearly two decades to monitor depth of sedation. While these single-purpose devices use proprietary algorithms, evaluation of the raw cEEG or qEEG measures can also provide information about arousal in the paralyzed patient [39]. Data on the utility of these algorithms in those with underlying neurological issues is limited, as is the use of these devices to detect seizures; this should not be done without confirmation via review with expert review of raw EEG [40].
Several studies have evaluated the utility of qEEG trends analysis for seizure identification in critically ill individuals. Stewart et al. [41] investigated the sensitivity of qEEG for seizure identification in critically ill children by qEEG-naïve neurophysiologists and found that the median sensitivity of compressed spectral array (CSA) analysis was 83.3%, while that of aEEG was 81.5%. Missed seizures were more likely to be strictly focal, of low amplitude, or short duration [41]. In another larger study involving critically ill adults, 89% of seizures were identified utilizing CSA-guided review by qEEG-naïve neurology residents after receiving 2 h of qEEG training [42]. Another recent study evaluated the use of multiple qEEG panels for seizure identification and found that the overall sensitivity for seizure identification using these panels across all reviewer types was 84%, while the overall specificity was 69%. Interestingly, there was no statistically significant difference in sensitivities between the different reviewer groups (neurophysiologists, technologists, and neuroscience ICU nurses). Among four qEEG trends used in this study, rhythmicity spectrogram seemed to help the most in seizure identification [43]. These data further support the role for qEEG as a reliable tool for screening EEG and targeting raw EEG review by both experienced and non-experienced readers. qEEG-guided EEG review may save as much as 78% of EEG reading time without carrying a significant impact on sensitivity [44].
In our experience, no one qEEG tool is appropriate for all patients or even for the same patient at all times. Situations may occur where one tool is more susceptible to certain artifacts or is less sensitive to the seizures the individual patient may have. Instead, we employ multiple tools simultaneously to screen the initial cEEG record and focus particularly on reviewing the raw EEG data at times where there appear to be clear changes in the qEEG measures from baseline. Once the patient’s seizure pattern is identified, the parameters of the qEEG tools can be further refined to highlight this pattern and improve the recognition of subsequent seizures.
With Internet-based networking, it is now practical to monitor dozens of patients in multiple ICUs. If there is sufficient network capability in the hospital, cEEG can be streamed live over the network and can be interpreted in real-time if needed (and personnel are available). In addition, cEEG can be reviewed remotely from home or from a distant hospital site using virtual private networks and virtual network computing [25]. However, in current practice, cEEG is not yet truly real-time “monitoring” at most centers. In both our centers, records are routinely reviewed by neurophysiologists or technologists two-three times daily; ACNS guidelines suggest a minimum of twice daily [34]. All new records should be interpreted as soon as possible. Ongoing records should be reviewed more frequently than just a few times per day if there are suspicious clinical events or medications are being titrated. However, as most NCSzs have little or no detectable clinical correlate, they may go unrecognized for several hours with only intermittent review. It is clear that we need to move toward continuous real-time monitoring via use of quantitative EEG alarms and around-the-clock “neurotelemetrists” to respond to the alarms and review the long-term trends. Several academic centers are already doing this.
Who to Monitor
Recent studies using routine and continuous EEG monitoring have helped to identify which patients are at risk for NCSz and, therefore, may benefit from cEEG. The causes of NCSz and NCSE in ICU patients are similar to the causes of convulsive seizures in these patients. These include acute structural lesions, infections (including sepsis), metabolic derangements, toxins, withdrawal, and epilepsy, all common diagnoses in the critically ill patient [45]. It is important to stress that the majority of seizures in these critically ill patients are nonconvulsive and can only be diagnosed with EEG [1, 6, 46–48]. NCSzs are even more common in the pediatric population, especially in infants [1, 25, 49, 50]. There are many studies using cEEG that have identified the incidence of NCSz and NCSE in various patient populations. These studies are summarized in Fig. 3.1.
While it may not be surprising that patients with acute brain injuries [1, 51] and recent convulsive status epilepticus [52] have a high risk of NCSz, NCSzs are not uncommon in medical or surgical ICU patients, including in those without known structural brain injury. Critically ill medical and surgical patients are susceptible to many toxic, electrolyte, and metabolic abnormalities that may cause both mental status changes and seizures [7, 47]. 17–21% of patients with toxic-metabolic encephalopathy and impaired mental status had electrographic seizures on cEEG monitoring in two retrospective studies [1, 2]. Moreover, in one study of 201 medical ICU patients without known brain injury that underwent cEEG monitoring, 22% of patients had periodic discharges (PDs) or seizures; sepsis and acute renal failure were significantly associated with both PDs and seizures [53]. A more recent prospective study found that among 100 episodes of sepsis in 98 patients without diagnosed acute primary neurological illness, periodic discharges were identified in 25 episodes; 11 of whom had nonconvulsive seizures [54]. Meanwhile, 16% of patients admitted to the surgical ICU undergoing cEEG monitoring had electrographic seizures in one recent study, while 29% had periodic discharges (PDs) [55].
The American Clinical Neurophysiology Society (ACNS) recently published an official guideline entitled “Consensus Statement on Continuous EEG in Critically Ill Adults and Children.” The following are the proposed indications [56]:
-
1.
Persistent alteration of mental state following generalized convulsive status epilepticus (GCSE)
-
2.
Altered mental state in association with acute supratentorial brain injury
-
3.
Unexplained alteration of mental status without evidence of acute brain injury
-
4.
Periodic discharges on routine or emergent EEG
-
5.
Pharmacological paralysis in patients at high risk for seizures
-
6.
Paroxysmal events suspected to be seizures to determine ictal vs. non-ictal nature of these events
Other indications recommended by ACNS include monitoring response to treatment of seizures and SE. In addition, the ACNS taskforce suggested the use of continuous EEG monitoring for detection of cerebral ischemia in high risk individuals, as an adjunct to other methods [56]. Similar statements discussing indications of continuous EEG monitoring have been released by the European Society of Intensive Care Medicine (ESICM) [57], as well as the Neurocritical Care Society as part of recommendations on management of SE [10].
EEG Patterns Encountered During EEG Monitoring
The background, interictal, and ictal EEG patterns of the critically ill patient are significantly different from those encountered in ambulatory patients [58, 59]. Ictal patterns may include rhythmic epileptiform discharges or rhythmic waves at greater than 3 Hz (as with most seizures). However, in critically ill patients, rhythmic or periodic patterns occurring at a rate of less than three per second can be ictal as well. One set of criteria for defining NCSz are shown in Table 3.1. It should be noted that these criteria reflect expert consensus and there are periodic patterns common in critically ill patients where the relationship to seizures is unknown [60]. In practice, it is often difficult to determine whether periodic or rhythmic activity at 1–3 Hz in a comatose patient reflects seizure activity or a brain at risk for seizures or is merely a marker of severe brain injury. These patterns have been considered to lie along the ictal-interictal continuum [61]. Aiming to create common terminology for use by critical care electroencephalographers worldwide, the ACNS published standardized terminology for describing these patterns, initially proposed in 2005 and then revised and published as an official guideline in late 2012 [62, 63]. The current terminology is summarized in Table 3.2. In one recent study, the interrater reliability for ACNS terminology was near perfect for main terms (1) and (2), which describe the location and the nature of the pattern, respectively. However, the interrater reliability for evolution and some of the other modifiers was not as good [64].
There is accumulating evidence that certain periodic discharges may reflect injured tissue at high risk for seizures such as lateralized periodic discharges (LPDs; previously called periodic lateralized epileptiform discharges (PLEDs)) and generalized periodic discharges (GPDs) (Fig. 3.6) [60]. There is convincing evidence to suggest that LPDs are sometimes ictal. For instance, LPDs can be time-locked to focal clonic movements in some patients with focal motor status epilepticus [21]. This seems to be more common in cases in which LPDs primarily involve Rolandic cortex (not surprisingly) [65]. Positron emission tomography in one patient with frequent LPDs demonstrated increased regional glucose metabolism similar to what is seen with focal seizures [66]. Single-photon emission CT (SPECT) imaging in patients with LPDs demonstrated increased regional cerebral perfusion in some patients that normalized when the LPDs resolved [67, 68]. In addition, frequent LPDs in elderly patients have been associated with a confusional state that resolves spontaneously or with diazepam treatment [69]. However, other studies have described cases where LPDs are clearly non-ictal such as in some epilepsy patients with chronic interictal LPDs [70]. In addition, when some patients with LPDs and acute brain injury demonstrate seizures, the EEG pattern is often faster and with different morphology [71]. Given the close association with seizures and the fact they are at times clearly associated with behavioral changes, some authors view LPDs as an unstable state in an “irritable” brain, lying along an ictal-interictal continuum [60, 72].
Periodic discharges in critically ill patients. (a) Right frontal LPDs occurring at 1 Hz (arrow) in an 82-year-old man after resection of a bifrontal meningioma. The patient subsequently developed right frontal electrographic seizures. (b) Generalized periodic discharges at 1–2 Hz in a 79-year-old patient with dementia, renal disease, and altered mental status. Although these waveforms have a triphasic morphology at times, the pattern subsequently evolved to 2.5–3 Hz GPDs consistent with NCSz and was associated with modest elevations in neuron-specific enolase to 14 (reference range 3.7–8.9). Low-frequency filter (LFF) = 1 Hz, HFF = 70 Hz, notch off (Published with kind permission from © Lawrence J. Hirsch, MD 2013. All Rights Reserved)
A common practice used to distinguish ictal from non-ictal periodic EEG patterns in the critically ill is to see if they are abolished by a trial of short-acting benzodiazepines (Table 3.3). However, almost all periodic discharges are attenuated by benzodiazepines [75]. Thus, unless there is clinical improvement accompanying the EEG change, the test is not helpful. Unfortunately, clinical improvement can take substantial time even if the activity represents NCSE and is aborted with benzodiazepines. However, a substantial portion of ICU patients with nonconvulsive seizures or NCSE will improve neurologically and usually within a day of treatment. For example, Hopp et al. [76] showed that 35% of patients with suspected NCSE receiving IV benzodiazepine (BZP) trial achieved positive clinical response. Moreover, positive clinical response correlated well with survival, recovery of consciousness, and achieving good functional outcome [76]. In order to avoid the confounding effect of sedation, we often use loading doses of nonsedating IV antiepileptic drugs (AEDs) such as valproate, lacosamide, levetiracetam, and phenytoin, for these diagnostic trials (see Table 3.3). One recent retrospective study evaluated clinical response to antiepileptic drug trial in patients with unexplained encephalopathy and triphasic wave pattern on EEG and found that 42.2% of patients receiving nonsedating IV AED trial achieved positive clinical response, whereas only 18.9% of patients receiving IV BZP trial did [77]. Our protocol for attempting to prove the presence of NCSE is shown in Table 3.3. It is important to recognize that lack of clinical improvement does not exclude NCSE—it simply does not help determine its presence or absence. This situation (EEG improvement without clinical improvement) has been referred to as “possible NCSE” [74].
There is fairly consistent evidence that the presence of PDs and frequent nonconvulsive seizures are an independent risk factor for worse prognosis in ICH [78], SAH [79], and sepsis [53] and after GCSE [52, 80], even in patients without evidence of acute brain injury [81]. In addition, there is accumulating evidence that increased electrographic seizure burden is correlated with worse outcome in both pediatric and adult populations [9, 82, 83]. Both NCSzs and LPDs have been shown to be independently associated with later epilepsy as well [84]. Nonetheless, it is unclear whether these and other periodic discharges require treatment and how aggressive this treatment should be. Laboratory studies and computer modeling are beginning to probe the network mechanisms that mediate periodic discharges in the injured brain [85].
Another recently described pattern in critically ill patients is lateralized rhythmic delta activity (LRDA) . This pattern was identified as an independent predictor of increased risk of acute seizures in critically ill individuals. It has been suggested that it carries similar implication as LPDs as regards risk of acute seizures [86]. One recent study suggested that LRDA is more likely to be associated with seizures if it occurs at a frequency of ≥1.5 Hz or is associated with a plus modifier [87].On the other hand, the presence of generalized rhythmic delta activity (GRDA), even when sharply contoured or superimposed by sharp waves or fast frequency activity (GRDA + S or GRDA + F, respectively), doesn’t seem to carry a significant increase in risk of electrographic seizures [87], at least based on the single retrospective study that has looked at this [87].
Epileptiform or rhythmic activity triggered by stimulation or arousal is also a common pattern in encephalopathic ICU patients. The evoked activity may be anywhere on the interictal-ictal spectrum and is collectively known as stimulus-induced rhythmic, periodic, or ictal discharges [22] (SIRPIDs) . There is usually no clinical correlate, as with most ICU seizures, but a small portion of patients will have focal motor seizures consistently elicited by alerting stimuli [23]. On the other hand, there have been two case reports of SPECT-negative SIRPIDs, suggesting that some of these poststimulation discharges do not represent clear ictal phenomena or at least do not have the usual seizure-associated increased blood flow [88, 89]. SIRPIDs most likely occur as a result of hyperexcitable cortex that is activated by normal arousal pathways, which involve the upper brainstem, thalamus, and widespread thalamocortical projections. This epileptiform activity may become clinically apparent if it causes synchronous activation, propagates caudally in an organized fashion, and involves motor pathways. At both our centers, technologists stimulate patients twice daily to assess for state-dependent changes in the EEG including the appearance of SIRPIDs, but the relationship between ictal discharges and arousals raises the possibility that limiting unnecessary stimulation in patients with SIRPIDs may be beneficial. One recent study demonstrated that the presence of SIRPIDs was not independently associated with in-hospital mortality in critically ill patients [90]. Another study reported that the presence of SIRPIDs is a poor prognostic marker in postanoxic patients, particularly when recorded during therapeutic hypothermia [91]. The effect of SIRPIDs on long-term outcome in other settings as well as its therapeutic implication remains unclear. We treat stimulus-induced patterns the same as spontaneous patterns (other than potentially limiting stimulation) as there is no theoretical reason or evidence that they differ in their ability to cause neuronal injury.
Lastly, brief potentially ictal rhythmic discharges (B(I)RDs), a pattern previously described in neonates [92], has been recently reported in critically ill patients and shown to be associated with a high risk (75%) of acute seizures during continuous electroencephalography [93]. This pattern consists of focal rhythmic discharges that are 5 Hz or faster and last less than 10 s, whether evolving or not (often too short to determine); the most common form is sharply contoured rhythmic theta lasting a few seconds [93]. When seizures are successfully treated, B(I)RDs usually resolve. More research is needed to determine the exact prognostic implication and effect of treatment of these patterns [93].
Assessing response to treatment in status epilepticus is one of the indications for cEEG monitoring mentioned earlier in this chapter. Delorenzo et al. [52] found that 48% of patients with treated GCSE develop nonconvulsive seizures. Most authorities recommend monitoring for 24 h after the last electrographic seizure [10]. The optimum EEG end point of treatment of refractory status epilepticus (RSE) is not well established, but commonly used targets include seizure suppression, burst suppression, or in some cases complete EEG suppression to a nearly flat record. Two retrospective analyses demonstrated that achieving burst suppression is not correlated with outcome in patients with RSE [94, 95]. Another retrospective series [96] included 35 patients treated with pentobarbital infusion for RSE. Of the 35 assessed patients, 12 reached burst suppression pattern, 20 attained complete EEG suppression to a nearly flat record, and 3 became seizure free without needing to reach burst suppression or complete EEG suppression. 17/20 (85%) patients who had nearly flat records, 6/12 (50%) patients who reached burst suppression pattern, and 3/3 (100%) patients who became seizure free without attaining burst suppression or nearly flat EEG remained seizure free. All of the latter patients survived, while 12/20 (60%) patients reaching nearly flat EEG and 3/12 (25%) patients reaching burst suppression survived [96]. The key message from these limited data is that in some cases, good outcome may be achieved without reaching burst suppression, while in other cases, near-complete EEG suppression may be needed. The underlying etiology appears to be the most important prognostic determinant [94–96]. On the other hand, the duration of interburst suppression doesn’t seem to correlate with outcome [97]. However, the presence of highly epileptiform bursts may indicate increased risk for seizure recurrence, suggesting the need for a more aggressive therapeutic end point [97, 98]. In general, we recommend treating to seizure suppression (not to a specific background pattern) as long as continuous EEG is being monitored. We often tolerate brief nonconvulsive seizures as well, especially in cases requiring prolonged iatrogenic coma.
Why Monitor
While NCSzs are common in the critically ill, the evidence that they worsen outcomes and require prompt identification and treatment is mixed [99, 100]. In several studies, the presence of NCSE and delay to diagnosis and treatment were each associated with significantly higher mortality [7, 101] though mortality in patients with NCSE may be most related to the underlying cause [102]. Recent evidence indicate that NCSE is more likely to be associated with worse outcome in critically ill children than NCSz [9] and that increased seizure burden is correlated with more significant degrees of neurological decline in that population [82]. One recent prospective study showed that increased seizure burden was associated with poor functional and cognitive outcome at 3 months following subarachnoid hemorrhage [83]. Nonetheless, while NCSE may be associated with poor prognosis in the critically ill elderly [103], one retrospective study from almost 20 years ago showed that aggressive treatment of NCSz and NCSE was associated with worse outcomes in this population [104]. In addition, three recent studies have shown that IV anesthetic use is correlated with poor outcome in status epilepticus [105–107]. This correlation was more significant in patients with complex partial status epilepticus in the latter study [107]. However, all of these studies failed to completely account for the severity and refractoriness of SE, and none of them included assessment of long-term cognitive or seizure outcomes [108]. Nevertheless, because of the conflicting outcome data, much of the justification for identifying and treating NCSz in the critically ill comes from human and animal data demonstrating that seizures can lead to neuronal injury. To date, there have been no prospective controlled trials to determine if treating NCSz or NCSE improves neurologic outcomes; this type of study may not be feasible as most neurologists and intensivists are not willing to leave seizures untreated based on the above evidence.
There is a large body of evidence that prolonged seizures in animals, even if nonconvulsive, can lead to neuronal damage. In a seminal study, Meldrum et al. [109] found that paralyzed and artificially ventilated baboons had hippocampal cell loss after treatment with a convulsant. Cell death occurred after 60 min of continuous electrographic seizures despite careful control of oxygenation, temperature, and metabolic status. In rodent models, electrical and chemoconvulsant-induced SE is associated with cell loss, free radical production, inflammation, gliosis, and synaptic reorganization [110]. Pathological changes can be seen in the absence of overt convulsions and can have profound long-term effects such as impaired performance on cognitive tasks after one episode of NCSE [111] and the development of epilepsy [112]. There is also some evidence from animal models that even single or multiple brief seizures may lead to cell death and cognitive impairment [113, 114]. Even in the absence of cell death, brief seizures in certain animal models can lead to alterations in gene expression [115]; impaired long-term potentiation , which is related to memory [116]; and reduced threshold for subsequent seizures [116]. SE in humans has also been associated with hippocampal cell loss in postmortem studies [117], and acute posttraumatic nonconvulsive seizures have been shown to be associated with significant long-term hippocampal atrophy [3]. In hospitalized patients, SE is associated with neuronal injury as demonstrated by elevated levels of serum neuron-specific enolase (NSE) , including in patients without detectable acute brain injury (e.g., from seizure activity alone) [118, 119]. While the sequelae of NCSz and NCSE are not as well understood, evidence suggests that they can lead to neuronal damage in humans. DeGiorgio et al. [120] showed that NSE levels, though elevated after all seizures, were especially high following NCSz and seizures of partial onset even in absence of acute brain injury.In addition to direct pathological effects of seizures themselves, seizures may also worsen the extent of injury from the inciting neurological injury. Seizures can place increased metabolic, excitotoxic, and oxidative stress on at-risk brain leading to irreversible injury. For instance, microdialysis studies in patients with TBI demonstrated increases of extracellular glutamate to excitotoxic levels following NCSz [121] as well as associated elevated lactate/pyruvate ratios and ICP [122]. Glycerol, a marker of cellular breakdown, has also been found to be elevated in the microdialysate after NCSz in TBI patients [123]. Compared to patients without NCSz that had similar injuries, impaired brain metabolism and increased intracranial pressure (ICP) could be seen up to 100 h after injury [122]. NCSzs in ICH were associated with increased mass effect on serial imaging, as well as worse NIHSS scores in one study [4] and expansion of hematoma size in another [78]; there was a trend toward worse outcomes in those with NCSzs in both studies. Seizures are also associated with increased metabolic demand that may worsen injury to ischemic brain, particularly the penumbra. NCSzs were associated with increased infarct volumes and higher mortality rates following middle cerebral artery occlusion in rats [124], and treatment resulted in reduced volumes [125]. In addition, even brief seizures can lead to hemodynamic changes, such as increased cerebral blood flow [126], which may lead to transient and potentially injurious elevations in ICP even in the absence of tonic-clonic activity [127, 128]. Finally, seizures are associated with peri-injury depolarizations, a process related to cortical spreading depression that seems to be very common and to contribute to secondary neuronal injury itself [129, 130].
How Long to Monitor
Several studies have addressed the duration of cEEG monitoring required to diagnose NCSz in critically ill patients. In their study of NICU patients, Pandian et al. [46] found that routine EEGs (30 min) detected seizures in only 11% of patients, while subsequent cEEG (mean duration of 2.9 days) detected seizures in 28%. In 110 critically ill patients with seizures detected by cEEG (92% of patients had purely nonconvulsive seizures), Claassen et al. [1] found that only half of patients had their first seizure within the first hour of monitoring. Although 95% of non-comatose patients had their first seizure within 24 h, only 80% of comatose patients had a seizure by this time. After 48 h of monitoring, the first seizure had occurred in 98% of non-comatose versus 87% of comatose patients. Coma and the presence of PDs predicted a delay in the time to first seizure (>24 h). Similarly, Jette et al. [50] found that 50% of 51 children with nonconvulsive seizures had their first seizure within 1 h and 80% within 24 h. Therefore, we feel monitoring for 24 h is probably sufficient to rule out NCSz in non-comatose patients without PDs, but longer periods may be required for comatose patients. However, recently it has been proposed that shorter monitoring may be sufficient in patients with no epileptiform abnormalities on EEG after 2 h of recording. One recent retrospective study evaluated time-dependent seizure probability in critically ill patients undergoing ≥18 h of recording and demonstrated that 72 h risk of seizures declined to less than 5% in patients with no epileptiform discharges in their initial 2 h of recording [2]. In addition, one study showed that the presence of generalized slowing as the sole finding on initial 30 min of EEG monitoring was predictive of low risk of electrographic seizures in patients undergoing at least 24 h of continuous EEG monitoring [5]. None of the 112 patients in this study with generalized slowing as the initial EEG finding developed electrographic seizures [43]. More studies are needed to confirm and refine these recommendations.
Cost-Effectiveness
Continuous EEG monitoring is labor intensive and requires substantial amount of resources. The main challenge is to prove that it actually leads to changes in management, that these changes in management improve outcome, and perhaps that the magnitude of these effects outweighs the cost of cEEG monitoring. One prospective study showed that continuous EEG monitoring led to significant changes in AED prescribing in 52% of critically ill adults [131]. A similar study in pediatric population showed that cEEG monitoring led to significant changes in management in 59% of critically ill children [132]. These changes include prescribing AEDs to patients not previously receiving them, as well as stopping unneeded medications for patients not having NCSz or rhythmic/periodic patterns on EEG monitoring, thus protecting them from side effects and toxicity of unnecessary medications. The question that remains elusive is: to what extent do these changes impact outcome? Observational data from UCLA suggested that cEEG monitoring implementation led to significant decrease in hospital cost, via shortening of length of hospital stay, and improved recovery on discharge. In the meantime, cEEG monitoring contributed to only 1% of hospital cost [133]. However, these observations didn’t control for other simultaneous changes in hospital protocols. More recently, Abend et al. [56] performed decision analysis to examine variables affecting the decision of whether or not to perform cEEG monitoring. They postulated that for 24 h cEEG monitoring to be considered a cost-effective measure, detection of NCSE/NCSz should lead to at least 3% improvement in outcome [56]. Nonetheless, data on the effect of cEEG monitoring on outcome remain scarce, and research in this field is of utmost importance to both practitioners in the field and healthcare administration.
Future Directions
In addition to detecting seizures, cEEG can be used to identify other changes in brain physiology. In recent years, there has been renewed interest in using cEEG for the detection of brain ischemia. It has been known for some time that EEG changes occur within seconds of reduction in cerebral blood flow (CBF) [134, 135], which is the basis for intraoperative EEG monitoring for ischemia during carotid endarterectomy [136–138]. In these patients, as CBF falls below 25–30 mL/100 g/min, there is a progressive loss of higher frequencies and prominent slowing of background EEG activity, yet cell death does not occur at this level. When CBF falls below 8–10 mL/100 g/min, low enough to cause irreversible cell death, all EEG frequencies are suppressed [139, 140]. Therefore, cEEG can detect a window where intervention can potentially prevent permanent brain injury.
Recent advances in computing have allowed for the real-time application of qEEG tools for extracting time-frequency data to measure changes in the background EEG rhythms. The ability to reduce EEG patterns usually identified by visual review to scalar values allows for prolonged use of cEEG monitoring in the ICU to detect cerebral hypoperfusion or other acute processes and is especially useful in comatose or sedated patients where clinical examination is limited. In a study of 32 primarily good-grade SAH patients, Vespa et al. [24] found that a reduction in the variability of relative alpha-frequency (a visual scoring of a tracing displaying 6–14 Hz expressed as a percentage of total power between 1 and 20 Hz) was 100% sensitive and 50% specific for vasospasm as detected by TCD or angiography. In the majority of patients, qEEG changes preceded the diagnosis of vasospasm by over 2 days. In a study of 34 poor-grade SAH patients (Hunt-Hess grades 4 and 5), Claassen et al. [141] found that the poststimulation alpha/delta ratio (ADR) was the most useful quantitative EEG parameter for detection of delayed cerebral ischemia (DCI): a reduction in the poststimulation ratio of alpha- to delta-frequency power of >10% relative to baseline in six consecutive epochs of cEEG was 100% sensitive and 76% specific for delayed cerebral ischemia. A reduction of >50% in a single epoch was 89% sensitive and 84% specific. Furthermore, in a recent prospective cohort study involving 20 patients with aneurysmal SAH, Rots et al. [142] found that ADR was the most reliable parameter in detecting DCI, showing the most significant change in patients with CT-detected DCI. Quantitative EEG changes preceded clinical diagnosis of DCI by a median of 7 h and preceded CT changes by a median of 44 h [142]. Further research is needed to determine the effect of early identification of DCI by qEEG on management and overall outcome.
Real-time application of cEEG monitoring—neurotelemetry—including using automated alarm systems at the bedside, as exists with cardiac telemetry in almost all hospitals today, is becoming an approachable goal. Reducing the raw cEEG to a few displayed variables using qEEG tools will make it a practical tool that can be interpreted by nurses and intensivists or by neurotelemetry technologists. In addition, trend and critical value alarms can be used to alert staff to potential changes in neurological status [143]. Computer algorithms have been successfully used to detect ongoing seizures in epilepsy monitoring unit patients [144]. Because seizure pattern in the critically ill are different from ambulatory patients, new algorithms must be designed to detect seizures in this patient population [143]. Refining techniques to help identify patterns of interest is an area of active research [145, 146]. Improvement is needed as many qEEG and data reduction tools are not sufficiently specific [147] and susceptible to contamination by artifact. While ICU staff can be trained to review raw cEEG traces for obvious artifacts and even pathological patterns [148], a neurophysiologist must still be available to verify the interpretation.
In parallel with these technical advancements, continued research is needed to confirm that real-time monitoring is a necessary goal. Further studies need to be performed in both laboratory models and in prospective clinical trials to examine if identifying and treating NCSz early improves outcomes. It is also necessary to determine the relationship of the different periodic and rhythmic EEG patterns in the critically ill to ongoing brain injury to identify targets for intervention [60]. Studies are also needed to determine whether using cEEG to detect ischemia improves patient outcomes and to identify the time window for intervention after a change is detected by cEEG.
Continuous EEG monitoring is just one of the modalities available to evaluate brain physiology in the ICU. Intracranial pressure monitoring using intraventricular catheters or intraparenchymal probes, brain tissue oxygenation monitors, CBF monitoring, and brain metabolism monitoring using microdialysis probes [149] all provide critical data about brain physiology. The use of these methods in combination with cEEG may help further understand the complex relationships between cerebral blood flow, tissue oxygenation, cerebral metabolism, and neuronal activity in the injured brain. In addition, multimodal physiological monitoring (Fig. 3.7) offers critical information on physiological changes in response to seizures, e.g., changes in heart rate (HR), respiratory rate (RR), and mean arterial pressure (MAP) [150]. Recent evidence indicates that these physiological changes may precede demonstrable EEG seizure onset on scalp EEG, often coinciding with intracortical seizure onset detected by mini-depth recording (Fig. 3.7) [150]. The combined use of these methods together may be able to compensate for some of the shortcomings of the individual methods. For instance, microdialysis and tissue oxygenation probes sample only the immediate area of the brain into which they are inserted and can miss new injury to a remote area of the brain that may be detected by cEEG because of the wide spatial coverage.
Physiologic changes associated with intracortical seizures. Increase in HR, RR, MAP, and CPP is seen very early, correlating with increased spectral power on depth EEG recording. Rise in ICP is seen later. A transient decline in jugular bulb oxygen saturation is seen around 2 min after seizure onset, followed by a drop in partial brain tissue oxygenation. Regional cerebral blood flow increase is seen around 10 min after seizure onset (Reproduced from Claassen et al. [150] with permission from Wiley-Blackwell Publishing)
Finally, new research is examining the utility of electrophysiological monitoring beyond conventional scalp EEG. Recent studies in patients with severe TBI using subdural electrodes found episodes of cortical spreading depression, slow and prolonged peri-injury depolarizations lasting several minutes or longer, and near-injured brain [129]. In another recent study involving 48 patients with poor-grade aneurysmal SAH undergoing invasive EEG monitoring by mini-depth electrodes, 38% of patients had seizures on mini-depth recording. 43% of these seizures were only detected by mini-depth recording. Interestingly, 19% of intracortical seizures were associated with ictal-interictal continuum patterns on scalp EEG [150]. It is possible that many focal seizures occurring across the cerebral cortex, but not synchronized sufficiently to generate scalp EEG changes, may contribute to impaired consciousness in some comatose patients without evidence of seizures on cEEG. Whether targeting these events for therapy improves patient outcomes needs to be determined. When possible, physiology-driven data (such as the lactate/pyruvate ratio and glutamate on microdialysis) can be used to decide which EEG patterns require additional treatment and which do not.
Summary
Nonconvulsive seizures are common in brain-injured patients with altered mental status and even in critically ill patients without structural brain injury. Seizures can contribute to depressed level of consciousness and cause secondary neuronal injury. Therefore, in both of our centers, we recommend cEEG for all critically ill patients with acute brain injury and altered mental status and for patients with fluctuating or unexplained impaired mental status. Patients who are encephalopathic, but not comatose, are typically monitored for 24 h to exclude NCSz. However, patients who are comatose, who have PDs, or who are having sedation/AEDs withdrawn undergo at least 48 h of cEEG. Once NCSz or equivocal periodic patterns are identified, monitoring can continue for several days. If NCSzs are identified, cEEG is necessary to monitor the response to treatment and, more importantly, correlate improvement in the cEEG findings with improvement in the patient’s clinical status. If the cEEG demonstrates periodic activity that is suspicious for, but not definitively, seizure activity, further monitoring can help the neurophysiologist gather additional evidence for or against the ictal nature of the pattern (e.g., to see if there are unequivocal seizures). This monitoring requires 24 h technologist coverage to connect patients and perform maintenance, appropriate information technology infrastructure, and available neurophysiologists to review the data and tools (quantitative EEG) to speed data review. While this requires a substantial amount of resources, it is feasible, and cEEG is routinely employed in many neuroscience ICUs around the world. In addition, cEEG has applications outside of NCSz detection that can expand the number of patients who may benefit from monitoring. Advances in the use of cEEG for ischemia detection and general brain function monitoring can make it a widely applicable tool for dynamic assessment of neurological function, in combination with other monitoring modalities, with the potential to detect brain injury moments after it occurs and even to prevent it.
References
Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–8.
Westover MB, Shafi MM, Bianchi MT, et al. The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol. 2015;126:463–71.
Vespa PM, McArthur DL, Xu Y, et al. Nonconvulsive seizures after traumatic brain injury are associated with hippocampal atrophy. Neurology. 2010;75:792–8.
Vespa PM, O'Phelan K, Shah M, et al. Acute seizures after intracerebral hdeemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441–6.
Swisher CB, Shah D, Sinha SR, Husain AM. Baseline EEG pattern on continuous ICU EEG monitoring and incidence of seizures. J Clin Neurophysiol. 2015;32:147–51.
Jordan K. Nonconvulsive status epilepticus in acute brain injury. J Clin Neurophysiol. 1999;16:332–40.
Young GB, Jordan KG, Doig GS. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83–9.
Walker M, Cross H, Smith S, et al. Nonconvulsive status epilepticus: epilepsy research foundation workshop reports. Epileptic Dis. 2005;7:253–96.
Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children*. Crit Care Med. 2013;41:215–23.
Brophy G, Bell R, Claassen J, et al. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17:3–23.
Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus—report of the ILAE task force on classification of status epilepticus. Epilepsia. 2015;56:1515–23.
Kaplan PW. Behavioral manifestations of nonconvulsive status epilepticus. Epilepsy Behav. 2002;3:122–39.
Husain AM, Horn GJ, Jacobson MP. Non-convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry. 2003;74:189–91.
Jirsch J, Hirsch LJ. Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophysiol. 2007;118:1660–70.
Young GB, Ives JR, Chapman MG, Mirsattari SM. A comparison of subdermal wire electrodes with collodion-applied disk electrodes in long-term EEG recordings in ICU. Clin Neurophysiol. 2006;117:1376–9.
Mirsattari SM, Lee DH, Jones D, Bihari F, Ives JR. MRI compatible EEG electrode system for routine use in the epilepsy monitoring unit and intensive care unit. Clin Neurophysiol. 2004;115:2175–80.
Alvarez V, Rossetti AO. Clinical use of EEG in the ICU: technical setting. J Clin Neurophysiol. 2015;32:481–5.
Young GB, Campbell VC. EEG monitoring in the intensive care unit: pitfalls and caveats. J Clin Neurophysiol. 1999;16:40.
Hirsch LJ. Continuous EEG monitoring in the intensive care unit: an overview. J Clin Neurophysiol. 2004;21:332–40.
Gaspard N, Hirsch LJ. Pitfalls in ictal EEG interpretation: critical care and intracranial recordings. Neurology. 2013;80:S26–42.
Snodgrass SM, Tsuburaya K, Ajmone-Marsan C. Clinical significance of periodic lateralized epileptiform discharges: relationship with status epilepticus. J Clin Neurophysiol. 1989;6:159–72.
Hirsch LJ, Claassen J, Mayer SA, Emerson RG. Stimulus-induced rhythmic, periodic, or ictal discharges (SIRPIDs): a common EEG phenomenon in the critically ill. Epilepsia. 2004;45:109–23.
Hirsch LJ, Pang T, Claassen J, et al. Focal motor seizures induced by alerting stimuli in critically ill patients. Epilepsia. 2008;49:968–73.
Vespa PM, Nuwer MR, Juhász C, et al. Early detection of vasospasm after acute subarachnoid hemorrhage using continuous EEG ICU monitoring. Electroencephalogr Clin Neurophysiol. 1997;103:607–15.
Kull LL, Emerson RG. Continuous EEG monitoring in the intensive care unit: technical and staffing considerations. J Clin Neurophysiol. 2005;22:107.
Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–7.
Kolls BJ, Husain AM. Assessment of hairline EEG as a screening tool for nonconvulsive status epilepticus. Epilepsia. 2007;48:959–65.
StatNet™. (Accessed 17 Nov 2015, at http://www.hydrodot.net/Products/statnet.html.)
Hirsch LJ, Sinha SR. Continuous EEG monitoring in the intensive care unit. In: Ebersole JS, editor. Current practice of clinical electroencephalography. Philadelphia: LWW; 2014. p. 567.
Claassen J, Hirsch LJ, Kreiter KT, et al. Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clin Neurophysiol. 2004;115:2699–710.
Toet MC, van der Meij W, de Vries LS, Uiterwaal CS, van Huffelen KC. Comparison between simultaneously recorded amplitude integrated electroencephalogram (cerebral function monitor) and standard electroencephalogram in neonates. Pediatrics. 2002;109:772–9.
Akman CI, Micic V, Thompson A, Riviello Jr JJ. Seizure detection using digital trend analysis: factors affecting utility. Epilepsy Res. 93:66–72.
Sackellares JC, Shiau DS, Halford JJ, LaRoche SM, Kelly KM. Quantitative EEG analysis for automated detection of nonconvulsive seizures in intensive care units. Epilepsy Behav. 2011;22(Suppl 1):S69–73.
Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, Part II: personnel, technical specifications, and clinical practice. J Clin Neurophysiol. 2015;32:96–108.
Eisenberg HM, Frankowski RF, Contant CF, Marshall LF, Walker MD. High-dose barbiturate control of elevated intracranial pressure in patients with severe head injury. J Neurosurg. 1988;69:15–23.
Simmons LE, Riker RR, Prato BS, Fraser GL. Assessing sedation during intensive care unit mechanical ventilation with the bispectral index and the sedation-agitation scale. Crit Care Med. 1999;27:1499–504.
Prichep LS, Gugino LD, John ER, et al. The patient state index as an indicator of the level of hypnosis under general anaesthesia. Br J Anaesth. 2004;92:393–9.
Al-Kadi MI, Reaz MBI, Ali MAM. Evolution of electroencephalogram signal analysis techniques during anesthesia. Sensors (Basel, Switzerland). 2013;13:6605–35.
Roustan JP, Valette S, Aubas P, Rondouin G, Capdevila X. Can electroencephalographic analysis be used to determine sedation levels in critically ill patients? Anesth Analg. 2005;101:1141–51.
Bousselmi R, Lebbi A, Ferjani M. Bispectral index changes during generalised tonic-clonic seizures. Anaesthesia. 2013;68:1084–5.
Stewart CP, Otsubo H, Ochi A, Sharma R, Hutchison JS, Hahn CD. Seizure identification in the ICU using quantitative EEG displays. Neurology. 2010;75:1501–8.
Williamson C, Wahlster S, Shafi M, Westover MB. Sensitivity of compressed spectral arrays for detecting seizures in acutely ill adults. Neurocrit Care. 2014;20:32–9.
Swisher CB, White CR, Mace BE, et al. Diagnostic accuracy of electrographic seizure detection by neurophysiologists and non-neurophysiologists in the adult ICU using a panel of quantitative EEG trends. J Clin Neurophysiol. 2015;32:324–30.
Moura LMVR, Shafi MM, Ng M, et al. Spectrogram screening of adult EEGs is sensitive and efficient. Neurology. 2014;83:56–64.
Abou Khaled KJ, Hirsch LJ. Advances in the management of seizures and status epilepticus in critically ill patients. Crit Care Clin. 2006;22:637–59. abstract viii
Pandian JD, Cascino GD, So EL, Manno E, Fulgham JR. Digital video-electroencephalographic monitoring in the neurological-neurosurgical intensive care unit: clinical features and outcome. Arch Neurol. 2004;61:1090–4.
Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–5.
Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res. 1994;18:155–66.
Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–61.
Jette N, Claassen J, Emerson RG, Hirsch LJ. Frequency and predictors of nonconvulsive seizures during continuous electroencephalographic monitoring in critically ill children. Arch Neurol. 2006;63:1750–5.
Vespa P. Continuous EEG monitoring for the detection of seizures in traumatic brain injury, infarction, and intracerebral hemorrhage: “to detect and protect”. J Clin Neurophysiol. 2005;22:99–106.
DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833–40.
Oddo M, Carrera E, Claassen J, Mayer SA, Hirsch LJ. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051–6.
Gilmore EJ, Gaspard N, Choi HA, et al. Acute brain failure in severe sepsis: a prospective study in the medical intensive care unit utilizing continuous EEG monitoring. Intensive Care Med. 2015;41:686–94.
Kurtz P, Gaspard N, Wahl AS, et al. Continuous electroencephalography in a surgical intensive care unit. Intensive Care Med. 2014;40:228–34.
Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32:87–95.
Claassen J, Taccone FS, Horn P, Holtkamp M, Stocchetti N, Oddo M. Recommendations on the use of EEG monitoring in critically ill patients: consensus statement from the neurointensive care section of the ESICM. Intensive Care Med. 2013;39:1337–51.
Young GB. The EEG in Coma. J Clin Neurophysiol. 2000;17:473.
Kaplan PW. The EEG in metabolic encephalopathy and coma. J Clin Neurophysiol. 2004;21:307–18.
Chong DJ, Hirsch LJ. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79–91.
Claassen J, How I. Treat patients with EEG patterns on the ictal–interictal continuum in the neuro ICU. Neurocrit Care. 2009;11:437–44.
Hirsch LJ, Brenner RP, Drislane FW, et al. The ACNS subcommittee on research terminology for continuous EEG monitoring: proposed standardized terminology for rhythmic and periodic EEG patterns encountered in critically ill patients. J Clin Neurophysiol. 2005;22:128–35.
Hirsch LJ, LaRoche SM, Gaspard N, et al. American clinical neurophysiology society's standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27.
Gaspard N, Hirsch LJ, LaRoche SM, Hahn CD, Westover MB, the Critical Care EEGMRC. Interrater agreement for Critical Care EEG Terminology. Epilepsia. 2014;55:1366–73.
Sen-Gupta I, Schuele SU, Macken MP, Kwasny MJ, Gerard EE. “Ictal” lateralized periodic discharges. Epilepsy Behav. 2014;36:165–70.
Handforth A, Cheng JT, Mandelkern MA, Treiman DM. Markedly increased mesiotemporal lobe metabolism in a case with PLEDs: further evidence that PLEDs are a manifestation of partial status epilepticus. Epilepsia. 1994;35:876–81.
Assal F, Papazyan JP, Slosman DO, Jallon P, Goerres GW. SPECT in periodic lateralized epileptiform discharges (PLEDs): a form of partial status epilepticus? Seizure. 2001;10:260–5.
Bozkurt MF, Saygi S, Erbas B. SPECT in a patient with postictal PLEDs: is hyperperfusion evidence of electrical seizure? Clin Electroencephalogr. 2002;33:171–3.
Terzano MG, Parrino L, Mazzucchi A, Moretti G. Confusional states with periodic lateralized epileptiform discharges (PLEDs): a peculiar epileptic syndrome in the elderly. Epilepsia. 1986;27:446–57.
Westmoreland BF, Klass DW, Sharbrough FW. Chronic periodic lateralized epileptiform discharges. Arch Neurol. 1986;43:494–6.
Brenner RP. Is it status? Epilepsia. 2002;43(Suppl 3):103–13.
Pohlmann-Eden B, Hoch DB, Cochius JI, Chiappa KH. Periodic lateralized epileptiform discharges—a critical review. J Clin Neurophysiol. 1996;13:519–30.
Hirsch LJ, Gaspard N. Status epilepticus. Continuum (Minneapolis, Minn). 2013;19:767–94.
Beniczky S, Hirsch LJ, Kaplan PW, et al. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia. 2013;54:28–9.
Fountain NB, Waldman WA. Effects of benzodiazepines on triphasic waves: implications for nonconvulsive status epilepticus. J Clin Neurophysiol. 2001;18:345–52.
Hopp JL, Sanchez A, Krumholz A, Hart G, Barry E. Nonconvulsive status epilepticus: value of a benzodiazepine trial for predicting outcomes. Neurologist. 2011;17:325–9.
O'Rourke D, Chen PM, Gaspard N, et al. Response rates to anticonvulsant trials in patients with triphasic-wave EEG patterns of uncertain significance. Neurocrit Care 2015.
Claassen J, Jette N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–65.
Claassen J, Hirsch LJ, Frontera JA, et al. Prognostic significance of continuous EEG monitoring in patients with poor-grade subarachnoid hemorrhage. Neurocrit Care. 2006;4:103–12.
Jaitly R, Sgro JA, Towne AR, Ko D, DeLorenzo RJ. Prognostic value of EEG monitoring after status epilepticus: a prospective adult study. J Clin Neurophysiol. 1997;14:326–34.
Sainju RK, Manganas LN, Gilmore EJ, et al. Clinical correlates and prognostic significance of lateralized periodic discharges in patients without acute or progressive brain injury: a case–control study. J Clin Neurophysiol. 2015;32:495–500.
Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137:1429–38.
De Marchis GM, Pugin D, Meyers E, et al. Seizure burden in subarachnoid hemorrhage associated with functional and cognitive outcome. Neurology. 2015; Epub ahead of print23
Punia V, Garcia CG, Hantus S. Incidence of recurrent seizures following hospital discharge in patients with LPDs (PLEDs) and nonconvulsive seizures recorded on continuous EEG in the critical care setting. Epilepsy Behav. 2015;49:250–4.
Frohlich F, Bazhenov M, Sejnowski TJ. Pathological effect of homeostatic synaptic scaling on network dynamics in diseases of the cortex. J Neurosci. 2008;28:1709.
Gaspard N, Manganas L, Rampal N, Petroff OA, Hirsch LJ. Similarity of lateralized rhythmic delta activity to periodic lateralized epileptiform discharges in critically ill patients. JAMA Neurol. 2013;70:1288–95.
Rodriguez A, Vlachy J, Lee JW, et al. Periodic and rhythmic patterns in the critically ill: Characteristics associated with seizures. 69th Annual meeting of the American Epilepsy Society. Philadephia, PA, USA. 2015.
Zeiler SR, Turtzo LC, Kaplan PW. SPECT–negative SIRPIDs argues against treatment as seizures. J Clin Neurophysiol. 2011;28:493–6.
Smith CC, Tatum WO, Gupta V, Pooley RA, Freeman WD. SPECT-negative SIRPIDs: less aggressive neurointensive care? J Clin Neurophysiol. 2014;31:e6–e10.
Braksick A, Burkholder DB, Tsetsou S, et al. Stimulus-induced rhythmic, periodic or ictal discharges (SRIPIDs): associated factors and prognostic implications. 69th Annual meeting of the American Epilepsy Society. Philadelphia, PA, USA 2015.
Alvarez V, Oddo M, Rossetti AO. Stimulus-induced rhythmic, periodic or ictal discharges (SIRPIDs) in comatose survivors of cardiac arrest: characteristics and prognostic value. Clin Neurophysiol. 2013;124:204–8.
Tsuchida TN, Wusthoff CJ, Shellhaas RA, et al. American clinical neurophysiology society standardized EEG terminology and categorization for the description of continuous EEG monitoring in neonates: report of the American clinical neurophysiology society critical care monitoring committee. J Clin Neurophysiol. 2013;30:161–73.
Yoo J, Rampal N, Petroff OA, Hirsch LJ, Gaspard N. BRief potentially ictal rhythmic discharges in critically ill adults. JAMA Neurol. 2014;71:454–62.
Rossetti AO, Logroscino G, Bromfield EB. Refractory status epilepticus: effect of treatment aggressiveness on prognosis. Arch Neurol. 2005;62:1698–702.
Kang BS, Jung KH, Shin JW, et al. Induction of burst suppression or coma using intravenous anesthetics in refractory status epilepticus. J Clin Neurosci. 2015;22:854–8.
Krishnamurthy KB, Drislane FW. Depth of EEG suppression and outcome in barbiturate anesthetic treatment for refractory status epilepticus. Epilepsia. 1999;40:759–62.
Johnson E, Ritzl EK. EEG characteristics of successful burst suppression for status epilepticus. 69th Annual meeting of the American Epilepsy Society. Philadelphia, PA, USA. 2015.
Thompson SA, Hantus S. Highly epileptiform bursts are associated with seizure recurrence. J Clin Neurophysiol. 2015; Publish Ahead of Print
Aminoff MJ. Do nonconvulsive seizures damage the brain?–No. Arch Neurol. 1998;55:119–20.
Young GB, Jordan KG. Do nonconvulsive seizures damage the brain?–Yes. Arch Neurol. 1998;55:117–9.
Vespa PM, Nuwer MR, Nenov V, et al. Increased incidence and impact of nonconvulsive and convulsive seizures after traumatic brain injury as detected by continuous electroencephalographic monitoring. J Neurosurg. 1999;91:750–60.
Shneker BF, Fountain NB. Assessment of acute morbidity and mortality in nonconvulsive status epilepticus. Neurology. 2003;61:1066–73.
Bottaro FJ, Martinez OA, Pardal MM, Bruetman JE, Reisin RC. Nonconvulsive status epilepticus in the elderly: a case-control study. Epilepsia. 2007;48:966–72.
Litt B, Wityk RJ, Hertz SH, et al. Nonconvulsive status epilepticus in the critically ill elderly. Epilepsia. 1998;39:1194–202.
Kowalski RG, Ziai WC, Rees RN, et al. Third-line antiepileptic therapy and outcome in status epilepticus: The impact of vasopressor use and prolonged mechanical ventilation*. Crit Care Med. 2012;40:2677–84.
Sutter R, Marsch S, Fuhr P, Kaplan PW, Ruegg S. Anesthetic drugs in status epilepticus: risk or rescue? A 6-year cohort study. Neurology. 2014;82:656–64.
Marchi NA, Novy J, Faouzi M, Stahli C, Burnand B, Rossetti AO. Status epilepticus: impact of therapeutic coma on outcome. Crit Care Med. 2015;43:1003–9.
Hirsch LJ. Finding the lesser of two evils: treating refractory status epilepticus. Epilepsy Curr. 2015;15:313–6.
Meldrum BS, Vigouroux RA, Brierley JB. Systemic factors and epileptic brain damage. Prolonged seizures in paralyzed, artificially ventilated baboons. Arch Neurol. 1973;29:82–7.
Holmes GL. Seizure-induced neuronal injury: animal data. Neurology. 2002;59:S3–6.
Krsek P, Mikulecka A, Druga R, et al. Long-term behavioral and morphological consequences of nonconvulsive status epilepticus in rats. Epilepsy Behav. 2004;5:180–91.
Lothman EW, Bertram EH, Bekenstein JW, Perlin JB. Self-sustaining limbic status epilepticus induced by ‘continuous’ hippocampal stimulation: electrographic and behavioral characteristics. Epilepsy Res. 1989;3:107–19.
Cavazos JE, Das I, Sutula TP. Neuronal loss induced in limbic pathways by kindling: evidence for induction of hippocampal sclerosis by repeated brief seizures. J Neurosci. 1994;14:3106–21.
Kotloski R, Lynch M, Lauersdorf S, Sutula T. Repeated brief seizures induce progressive hippocampal neuron loss and memory deficits. Prog Brain Res. 2002;135:95–110.
Saffen DW, Cole AJ, Worley PF, Christy BA, Ryder K, Baraban JM. Convulsant-induced increase in transcription factor messenger RNAs in rat brain. Proc Natl Acad Sci U S A. 1988;85:7795–9.
Dube C, Chen K, Eghbal-Ahmadi M, Brunson K, Soltesz I, Baram TZ. Prolonged febrile seizures in the immature rat model enhance hippocampal excitability long term. Ann Neurol. 2000;47:336–44.
DeGiorgio CM, Tomiyasu U, Gott PS, Treiman DM. Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia. 1992;33:23–7.
Rabinowicz AL, Correale J, Boutros RB, Couldwell WT, Henderson CW, DeGiorgio CM. Neuron-specific enolase is increased after single seizures during inpatient video/EEG monitoring. Epilepsia. 1996;37:122–5.
DeGiorgio CM, Gott PS, Rabinowicz AL, Heck CN, Smith TD, Correale JD. Neuron-specific enolase, a marker of acute neuronal injury, is increased in complex partial status epilepticus. Epilepsia. 1996;37:606–9.
DeGiorgio CM, Heck CN, Rabinowicz AL, Gott PS, Smith T, Correale J. Serum neuron-specific enolase in the major subtypes of status epilepticus. Neurology. 1999;52:746–9.
Vespa P, Prins M, Ronne-Engstrom E, et al. Increase in extracellular glutamate caused by reduced cerebral perfusion pressure and seizures after human traumatic brain injury: a microdialysis study. J Neurosurg. 1998;89:971–82.
Vespa PM, Miller C, McArthur D, et al. Nonconvulsive electrographic seizures after traumatic brain injury result in a delayed, prolonged increase in intracranial pressure and metabolic crisis. Crit Care Med. 2007;35(12):2830–6.
Vespa P, Martin NA, Nenov V, et al. Delayed increase in extracellular glycerol with post-traumatic electrographic epileptic activity: support for the theory that seizures induce secondary injury. Acta Neurochir Suppl. 2002;81:355–7.
Hartings JA, Williams AJ, Tortella FC. Occurrence of nonconvulsive seizures, periodic epileptiform discharges, and intermittent rhythmic delta activity in rat focal ischemia. Exp Neurol. 2003;179:139–49.
Williams AJ, Tortella FC, Lu XM, Moreton JE, Hartings JA. Antiepileptic drug treatment of nonconvulsive seizures induced by experimental focal brain ischemia. J Pharmacol Exp Ther. 2004;311:220–7.
Johnson DW, Hogg JP, Dasheiff R, Yonas H, Pentheny S, Jumao-as A. Xenon/CT cerebral blood flow studies during continuous depth electrode monitoring in epilepsy patients. AJNR Am J Neuroradiol. 1993;14:245–52.
Gabor AJ, Brooks AG, Scobey RP, Parsons GH. Intracranial pressure during epileptic seizures. Electroencephalogr Clin Neurophysiol. 1984;57:497–506.
Marienne JP, Robert G, Bagnat E. Post-traumatic acute rise of ICP related to subclinical epileptic seizures. Acta Neurochir Suppl (Wien). 1979;28:89–92.
Fabricius M, Fuhr S, Bhatia R, et al. Cortical spreading depression and peri-infarct depolarization in acutely injured human cerebral cortex. Brain. 2006;129:778–90.
Fabricius M, Fuhr S, Willumsen L, et al. Association of seizures with cortical spreading depression and peri-infarct depolarisations in the acutely injured human brain. Clin Neurophysiol. 2008;119:1973–84.
Kilbride RD, Costello DJ, Chiappa KH. How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol. 2009;66:723–8.
Abend N, Topjian A, Gutierrez-Colina A, Donnelly M, Clancy R, Dlugos D. Impact of continuous EEG monitoring on clinical management in critically ill children. Neurocrit Care. 2011;15:70–5.
Vespa PM, Nenov V, Nuwer MR. Continuous EEG Monitoring in the intensive care unit: early findings and clinical efficacy. J Clin Neurophysiol. 1999;16:1–13.
Sundt Jr TM, Sharbrough FW, Anderson RE, Michenfelder JD. Cerebral blood flow measurements and electroencephalograms during carotid endarterectomy. J Neurosurg. 1974;41:310–20.
Sundt Jr TM, Sharbrough FW, Piepgras DG, Kearns TP, Messick Jr JM, O'Fallon WM. Correlation of cerebral blood flow and electroencephalographic changes during carotid endarterectomy: with results of surgery and hemodynamics of cerebral ischemia. Mayo Clin Proc. 1981;56:533–43.
Sharbrough FW, Messick Jr JM, Sundt Jr TM. Correlation of continuous electroencephalograms with cerebral blood flow measurements during carotid endarterectomy. Stroke. 1973;4:674–83.
Zampella E, Morawetz RB, McDowell HA, et al. The importance of cerebral ischemia during carotid endarterectomy. Neurosurgery. 1991;29:727–30. discussion 30-1
Arnold M, Sturzenegger M, Schaffler L, Seiler RW. Continuous intraoperative monitoring of middle cerebral artery blood flow velocities and electroencephalography during carotid endarterectomy. A comparison of the two methods to detect cerebral ischemia. Stroke. 1997;28:1345–50.
Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia—the ischemic penumbra. Stroke. 1981;12:723–5.
Jordan KG. Emergency EEG and continuous EEG monitoring in acute ischemic stroke. J Clin Neurophysiol. 2004;21:341–52.
Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia. 2002;43:146–53.
Rots ML, van Putten MJAM, Hoedemaekers CWE, Horn J. Continuous EEG monitoring for early detection of delayed cerebral ischemia in subarachnoid hemorrhage: a pilot study. Neurocrit Care. 2015;24:207–16.
Scheuer ML, Wilson SB. Data analysis for continuous EEG monitoring in the ICU: seeing the forest and the trees. J Clin Neurophysiol. 2004;21:353–78.
Gotman J. Automatic detection of seizures and spikes. J Clin Neurophysiol. 1999;16:130.
Shah AK, Agarwal R, Carhuapoma JR, Loeb JA. Compressed EEG pattern analysis for critically III neurological-neurosurgical patients. Neurocrit Care. 2006;5:124–33.
Fürbass F, Hartmann MM, Halford JJ, et al. Automatic detection of rhythmic and periodic patterns in critical care EEG based on American Clinical Neurophysiology Society (ACNS) standardized terminology. Neurophysiol Clin. 2015;45:203–13.
Adams DC, Heyer EJ, Emerson RG, et al. The reliability of quantitative electroencephalography as an indicator of cerebral ischemia. Anesth Analg. 1995;81:80–3.
Jordan KG, Continuous EEG. Monitoring in the neuroscience intensive care unit and emergency department. J Clin Neurophysiol. 1999;16:14.
Wartenberg KE, Schmidt JM, Mayer SA. Multimodality monitoring in neurocritical care. Crit Care Clin. 2007;23:507–38.
Claassen J, Perotte A, Albers D, et al. Nonconvulsive seizures after subarachnoid hemorrhage: multimodal detection and outcomes. Ann Neurol. 2013;74:53–64.
Kaplan PW. EEG criteria for nonconvulsive status epilepticus. Epilepsia. 2007;48:39–41.
Bauerle K, Greim CA, Schroth M, Geisselbrecht M, Kobler A, Roewer N. Prediction of depth of sedation and anaesthesia by the NarcotrendTM EEG monitor. Br J Anaesth. 2004;92:841–5.
Zhou JL, Shatskikh TN, Liu X, Holmes GL. Impaired single cell firing and long-term potentiation parallels memory impairment following recurrent seizures. Eur J Neurosci. 2007;25:3667–77.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Osman, G., Friedman, D., Hirsch, L.J. (2017). Diagnosing and Monitoring Seizures in the ICU: The Role of Continuous EEG for Detection and Management of Seizures in Critically Ill Patients, Including the Ictal-Interictal Continuum. In: Varelas, P., Claassen, J. (eds) Seizures in Critical Care. Current Clinical Neurology. Humana Press, Cham. https://doi.org/10.1007/978-3-319-49557-6_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-49557-6_3
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-49555-2
Online ISBN: 978-3-319-49557-6
eBook Packages: MedicineMedicine (R0)