Abstract
Multiple sclerosis (MS) is normally considered a chronic inflammatory disease of the central nervous system (CNS), where T-cells breaching the blood brain barrier react against proteins of the axonal myelin sheaths, leading to focal plaques and demyelination in the brain and spinal cord. Many current therapies are immunosuppressive in nature and are designed to target the immune system at an early stage of the disease. But there is no cure and MS may evolve into a neurodegenerative disease, where immunomodulatory treatments appear less effective. Neurodegeneration is influenced by oxidative and endoplasmic reticulum (ER) mediated stress which can be induced independently of immune processes. Since 1970, MS patients have been self-managing their long term symptoms using hyperbaric oxygen and reporting improvement in their symptoms, especially bladder control. In contrast, the majority of clinical trial evidence does not support the views of patients. Therefore does oxygen under pressure affect brain tissue by modulating oxidative or ER stress at the cellular level resulting in CNS tissue repair or deterioration? This chapter reviews our understanding and the role of oxidative and ER stress in the context of employing hyperoxia treatments to treat MS and evaluate its effects on neural cells.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
Multiple sclerosis (MS) is a demyelinating autoimmune disease whereby damage to cells of the central nervous system (CNS) results in the generation of lesions that results in loss of neurological function. The disease is categorized to various degrees of severity beginning with preclinical, followed by in most cases relapsing remitting, primary progressive and finally secondary progressive, suggesting a chronic onslaught of inflammation which leads to an increase in neurodegeneration to the CNS. The greatest genetic risk factor comes from carrying the class II HLA-DRB1*1501 allele which can increase susceptibility by 2–4-fold (Odds ratio 3.06; 95 % CI, 2.30–4.08), while Epstein-Barr virus infection has a similar risk association (Odds ratio 2.60; 95 % CI, 1.48–4.59) (Xiao et al. 2015) Geographical latitude (Kinoshita et al. 2015) and ethnic considerations (Langer-Gould et al. 2013) also contribute to the overall chance of developing MS. It is well established that the adaptive immune system plays a role in MS pathology, especially proinflammatory T-cells (Cao et al. 2015; Hong et al. 2009). Autoreactive T-cells can be found in the peripheral blood of autoimmune patients and healthy control subjects, but such cells appear to be more resistant to apoptosis and reactive against myelin proteins in MS patients (Mandel et al. 2009; Vergelli et al. 2001). The cause of development of peripheral blood autoreactive T-cells against CNS tissue derived myelin, prior to T-cell exposure to such tissue is largely unknown. In MS, the transmigration of autoreactive T-cells across the blood brain barrier (BBB) can ultimately lead to an escalation of pro-inflammatory damage to myelin-producing oligodendrocytes in close proximity to neuronal axons, leading to major damage and cell death. The oxidative damage and endoplasmic reticulum (ER) stress that ensues (Mhaille et al. 2008), requires the cells of the CNS to either undergo apoptosis or repair, which is controlled to a large extent by the unfolded protein response (UPR) (Stone and Lin 2015). Moreover, the UPR can also influence the ability of various cells to resist apoptosis and influence their cytokine phenotypes (Chan et al. 2011; Kim et al. 2006). Therefore pathways such as the UPR that regulate many aspects of cell survival and repair might be a fruitful area of research in developing therapeutics to alleviate or prevent MS pathology, and are already being investigated for other neurodegenerative diseases (Rozpedek et al. 2015; Torres et al. 2015).
We and others have shown that cells in vitro exposed to 100 % oxygen under hyperbaric pressure (HBO) alter the expression of a wide variety of genes involved in immunity and inflammation (Kendall et al. 2011, 2012, 2013a; Thom 2011). Consequently, HBO might work as a therapy by promoting or suppressing selective genes and their products in a non-invasive manner. But, how HBO works downstream, at the cellular and biochemical level remains largely unknown and more work is required, but it does not appear to damage DNA in the longer term (Yuan et al. 2011). Hyperbaric oxygen therapy (HBOT), which involves breathing pure oxygen under pressure is used to treat a number of clinical conditions including non-healing wounds (Eggleton et al. 2015) and to ameliorate the side-effects of radiation therapy (Clarke et al. 2008; Glover et al. 2015). However HBOT as a treatment for MS is highly contentious and does not have approval from the USA Food and Drug Administration (US Food and Drug Administration 2013) or The National Institute for Health and Care Excellence (The Guideline Development Group NICE 2014). Despite the non-recommendation by health governance authorities, many patients continue to use HBOT to treat their symptoms and frequently report symptomatic improvement. In the late 1970s and 1980s when HBOT began to be trialled, some clinicians supported the use of HBOT for MS sufferers (Boschetty et al. 1970; Fischer 1983; Fischer et al. 1983; James 1984; James 1983; Neubauer 1978, 1980; Neubauer et al. 2005), while others did not (Barnes et al. 1985b; Neiman et al. 1985; Wiles et al. 1986). This has led to confusion for both patients and clinicians alike. Here we evaluate the pros and cons of HBO treatment in the context of oxidative and ER stress, the unfolded protein response and the changes that occur in cells and their genes under hyperbaric conditions.
2.2 Oxidative and ER Stress in MS Pathology
The cellular damage induced in the CNS of MS patients directly accounts for many of the dysfunctional changes observed in the well-being, mobility and motor processes of individual MS sufferers. Within all nucleated cells, a number of cell repair molecules, the UPR sensor molecules, are sensitive to changes in their environment, and this is particularly so of CNS cells (Giovannoni and Ebers 2007; Hedstrom et al. 2015). Inflammatory cells and the molecules they release can attack oligodendrocytes and the neuronal axons and signal to these cells to shut down and die by apoptotic death. Under the appropriate conditions the UPR can attempt to repair the cell. Whenever the UPR response signal is one of repair, regeneration and remyelination of axons can occur (Gow and Wrabetz 2009). One potential way of driving the decision to repair rather than destroy a cell is to manipulate the ER-mediated UPR stress response. There are several diverse environment factors that can trigger cellular and ultimately ER-stress, namely virus, microbial toxins, oxidative stress and nutrient deficiency (Mkhikian et al. 2011). These stimuli can all trigger additional rapid protein production within the ER to help maintain the status quo of the cell. The rapidity of this process can lead to errors in amino acid biosynthesis, protein folding and glycosylation, inducing degradation factors to deal with the disruption in cellular homeostasis and triggering reactive oxidative (ROS) and nitrosative species (RNS) production. Similarly, activation of ROS and RNS can also activate the UPR, and the UPR has been shown to be elevated in myelin-generating oligodendrocytes of the CNS, as well as other cells of the peripheral nervous system (Lin and Popko 2009).
It is established that oxidative stress plays a role in cellular damage and particularly so in MS neuropathology, where the cerebro spinal fluid (CSF) and plasma are observed to have increased amounts of lipid peroxidation (Calabrese et al. 1998). During lesion formation activated microglia cells release superoxide, which in part can be defended by the antioxidant systems of the brain such as superoxide dismutases (SOD) and reduced glutathione. Free iron can promote CNS damage by catalyzing the production of hydroxyl and peroxyl-based free radicals from hydrogen peroxide and lipid peroxides (Halliwell 2001). The balance between free radical and antioxidant production undoubtedly plays a role in whether inflammation subsides or progresses, leading to lesion development (Gilgun-Sherki et al. 2004; Syburra and Passi 1999), although it has been questioned whether the formation of ROS in MS is in fact deleterious (Koch et al. 2006). At the cellular level in vitro, the myelin producing oligodendrocytes are thought to be more susceptible to damage by ROS/RNS compared to astrocytes and microglia possibly due to higher iron content and diminished antioxidant defenses (Smith et al. 1999). The molecular events that lead to oligodendrocyte loss and lesion formation are not fully understood, but are known to involve signaling pathways associated with both the ER (Kraus and Michalak 2011) and mitochondria organelles (Aboul-Enein and Lassmann 2005; Dutta et al. 2006; Gilgun-Sherki et al. 2004; Lu et al. 2000). Furthermore, dysfunctional mitochondria are an additional source of ROS production (Mahad et al. 2009; Nickel et al. 2014). Ultimately, inflammation, oxidative stress, demyelination of axons and the lack of remyelination and restoration of axonal function will be partially dependent on the cellular activation of the UPR to these various insults. The response can manifest itself in various ways including accumulation of unfolded or misfolded proteins in the ER. The main UPR sensor pathways are regulated by three proteins inositol requiring kinase 1 (IRE1), activating transcription factor 6 (ATF6), and PKR-like ER kinase (PERK) (Fig. 2.1). The signalling pathways that these sensors regulate have been well documented and described in detail with regards to MS (Getts et al. 2008; Stone and Lin 2015), but are triggered initially when B-cell immunoglobulin heavy chain binding protein (BiP) detaches from PERK. Key components downstream of the UPR initiating signal are phosphorylated eukaryotic initiation factor alpha (p-eIF2α) and C/EBP homologous protein (CHOP) which drive cells toward survival (Walter and Ron 2011) or apoptosis (Szegezdi et al. 2006) respectively.
Oxidative stress induces ER stress than can activate the unfolded protein response (UPR) pathways. (a) Oxidative stress can arise from localized activated inflammatory cells, secretion of proinflammatory cytokines and induction of ROS and NOS by activated macrophages and microglia in the brain. (b) The resulting oxidative stress can lead to damage of lipid, DNA and protein. This in turn can disrupt lipid and protein biosynthesis, resulting in the accumulation of misfolded proteins in the ER and ER stress. In turn, ER stress activates one or more of the three ER-transmembrane transducers of the UPR. Individual stressed cells are then programmed to survive or undergo cell death. Localized regions of the brain where oxidative and ER stress are present can result in the formation of lesions in the CNS white matter
Over a period of time the chronic inflammation, oxidative and ER-stress leads to visible MS pathology in the CNS. Affecting predominantly white matter, demyelinating lesions become clearly distinguishable from the surrounding normal appearing white matter (NAWM) tissue (Fig. 2.2a–c). Evidence of myelin-specific T-cell accumulation leads to the development of lesions which can be acute or sub-acute (sometimes referred to as - chronic active), in which myelin is progressively stripped from the axon sheaths of neurons and is engulfed by macrophages and microglial cells. An additional type of lesion is the chronic lesion (sometimes referred to as chronic silent) in which inflammation has abated and scarred lesions devoid of myelin present within the CNS. Lesions can be seen on MRI scans (Fig. 2.2 d–f). MS lesions defined in terms of inflammatory destruction and neurodegeneration are useful for studies designed to identify differences in gene expression at the DNA and mRNA level of diverse cellular and molecular biomarkers of pathology at distinctive stages of disease progression. As shown in Fig. 2.2a, during the acute phase of lesion formation there is a gradation of infiltrating inflammatory (microglia and macrophages) cells, with more cells close to the lesion border engorged with oil red O stained myelin, providing evidence of demyelination of axons. In the sub-acute stage (Fig. 2.2b) the lesion border appears more distinct, with the central region of the lesion becoming devoid of myelin and oligodendrocytes. The chronic lesions have little evidence of inflammatory cells, typically appear hypocellular and are devoid of a visible inflammatory border with the NAWM and represent a scarred region of irreversible demyelination (Fig. 2.2 c). However we have recently identified a novel proinflammatory subset of T-cells (CD20+/IL17+) associated with the chronic and acute lesions of MS patients (Holley et al. 2014). The NAWM tissue in MS differs from that of white matter in non-MS brain, in that greater numbers of T-cell infiltrates are detected (Allen et al. 2001; Kutzelnigg et al. 2005), indicative of pre-lesion inflammation and breach of the blood brain barrier (BBB).
Classification and imaging of MS lesions. Oil red ‘O’/hematoxylin staining of 10 μm sections of MS brain tissue, showing lesion areas at the top of each image and NAWM at the bottom, demarcated by a dashed lined. (a) Depicts an acute lesion with increasing numbers of oil red ‘O’ positive macrophages containing myelin and more densely packed towards the lesion border. (b) Illustrates oil red ‘O’ positive macrophages located mainly at the lesion border (black arrow heads) and a demyelinated area of the lesion. (c) Shows a chronic lesion, devoid of myelin and oil red ‘O’ positive macrophages. All images are at 100x magnification. (d) Normal brain axial T2 weighted MRI scan. (e) Axial T2-weighted MRI in a patient with MS demonstrating several white matter hyper-intense lesions. (f) Coronal fluid-attenuated inversion recovery (FLAIR) MRI in a patient with MS demonstrating high-signal intensity lesions in the deep white matter and the periventricular regions. Key: R right, L left, A anterior, P posterior, S superior, I inferior. White arrows depict lesions
Through analysis of significant changes in UPR genes in various MS lesions, a better understanding of the cell response to oxidative and ER stress with respect to MS pathology can be established. A number of microarray studies have identified elevated levels of expression of certain genes including UPR pathway genes in biopsy material obtained from the demyelinating lesions in the CNS of MS patients (Cwiklinska et al. 2003; Lock and Heller 2003; Mycko et al. 2003, 2004; Tajouri et al. 2003). Mycko and colleagues examined differences in gene expression from cell extracts from the border and centres of active lesions, with varying degrees of inflammatory infiltrates. Not surprisingly more genes were upregulated at the DNA level in active lesions compared to inactive lesions both at the lesion borders and centres (87 vs. 69 genes and 65 vs. 22 genes) respectively, which included a number of intracellular signalling and transcription factors (Mycko et al. 2003). The same group went on to look at mRNA gene expression in the same tissue regions and observed a number of ER-stress and heat shock protein genes upregulated in both active and inactive regions of MS lesions including activated transcription factor (ATF4) and heat shock protein 70 (HSP70) (Cwiklinska et al. 2003; Mycko et al. 2004). Tajouri and co-workers also examined NAWM and chronic and acute lesion material from five MS patients with secondary progressive disease and non-MS subjects (Tajouri et al. 2003). The authors observed 139 genes that were differentially regulated >1.5 fold in the five MS lesions compared with NAWM. Several of the genes upregulated were associated with tissue damage and oxidative stress including transferrin (TF), superoxide dismutase 1 (SOD1), glutathione peroxidase (GPX1) and glutathione S-transferase (GSTP1) peroxiredoxin I (PRDX1), which are all expressed during free radical formation and in some cases as antioxidants to counteract oxidative stress. More recently, Cunnea and associates have detected elevated expression levels at the mRNA level of a number of ER and hypoxic stress genes in actively demyelinating lesions of MS patients with primary or secondary progressive disease compared to control white matter (Cunnea et al. 2011). Specifically they observed a 2–8 fold elevated expression of BiP, CHOP and ATF4. Interestingly the elevation of these classical ER-stress proteins were not restricted to lesions but also in the NAWM of MS patients, indicative of ER stress occurring prior to lesion formation. Increases in UPR gene products are not restricted to the white matter of MS patients and various grey matter lesions have been shown to have significantly increased levels of CHOP compared to normal grey matter. The increased CHOP appeared to be predominantly associated with microglial cells. Whether increased CHOP in microglial cells predestines such inflammatory cells to undergo apoptosis remains to be elucidated (McMahon et al. 2012). The function and over expression of CHOP and other UPR genes should be considered on an individual cellular basis, especially in the knowledge that elevated CHOP protects oligodendrocytes from cell death (Gow and Wrabetz 2009).
Oxidative and ER stress appears to have a dynamic affect and differential sensitivity on various UPR response genes and the proteins they encode in human CNS biopsy tissue of specific lesions. Specifically many UPR genes appear to be elevated in MS. However, the underlying mechanisms through which UPR genes act in individual cell types (e.g. oligodendrocytes, neurons, microglial cells) or individual MS patients requires more work. The knowledge gained from such studies might aid the development of therapeutic strategies that protect both oligodendrocytes and neurons in patients with MS. One overall impression is that the UPR appears to be ‘over activated’ in MS lesions and mechanisms that can suppress the UPR or at least alter it may be of benefit.
2.3 HBOT and MS: Clinical and Patient Perspectives
The data above describes a number of human studies post-mortem, in which evidence of oxidative and ER-stress is clearly implicated in altering CNS tissue cell survival and degeneration. So the logical question is how does environmental oxygen affect MS patients? In 1970, 26 MS patients were treated with 100 % O2 under hyperbaric pressure (HBOT) at 2 ATA (Boschetty and Cernoch 1970) and fifteen patients symptoms were observed to improve. Over the past 45 years, both clinicians and patients have reported or observed improvements in MS symptoms after HBOT treatment, often as anecdotal reports or in randomized control trials. But the use of HBOT as a treatment for MS remains highly contentious. Indeed HBOT has been regarded by some as no better than other ‘alternative’ treatments such as oral arsenic, intrathecal injections of tuberculin, oral seaweed and snake venom (Bates 1986). The early history and controversy in using HBOT to treat MS patients has been eloquently described by an advocate pioneer in the field, RA Neubauer (Neubauer et al. 2005). The main conclusion of his and his colleagues report was that HBOT is not a cure, but does stabilize the symptoms in the majority of patients and slows progression in 17–33 % of patients. They also recommend that additional treatments might be required as treatment is transient and the effect of HBOT diminishes over time. The first formal small placebo-controlled, double-blinded study conducted in 1983 produced positive results for HBOT treatment of MS with ‘objective’ improvement in 12/17 patients compared with 1/20 patients treated with a placebo (Fischer et al. 1983). This was despite using 100 % O2 at 2 ATA (pure oxygen at 1.5–1.75 ATA has generally been recommended since this initial study) for 90 min. An age-sex placebo group of match MS patients were exposed to 10 % O2/90 % N2 for the same time period. To assess MS disability as a whole disease severity must be monitored. A number of clinical scales have been developed to this end, the most well established is the Kurtzke’s Expanded Disability Status Score (EDSS) which was originally described in 1955 (Kurtzke 1955) and has been modified through the following decades (Kurtzke 1965, 1970, 1983, 1989, 2000, 2008). Clinical parameters can also be monitored using the Multiple Sclerosis Functional Composite (MSFC), Symbol Digit Modality Test (SDMT) and low contrast visual acuity. In the original Fischer trial in 1983, the successfully treated patients showed improvements in a number of features on the EDSS scale by 1–2 points in mobility, coordination, bladder control and fatigability. Historically, this study was significant as it also provided evidence that MS may be an autoimmune disease whereby oxygen might have immunosuppressive properties. At the time of the study MS was thought by some to consist of venous infarction in the CNS (James 1983) which was disputed by Mertin (Mertin and Mcdonald 1984).
After the Fischer trial of 1983, at least 14 additional double blinded control studies were conducted (Barnes et al. 1985a, 1987; Confavreux et al. 1986; Harpur et al. 1986; Lhermitte et al. 1986; Oriani et al. 1990; Wiles et al. 1986; Wood et al. 1985), and many of these refuted the original findings. It is fair to say that in hindsight these studies were poorly controlled. In some of these studies all MS patients irrespective of their disease severity as judged by their EDSS were given the same number of treatments, and therefore this was not treating ‘like with like’. This has led to inconsistency in results and confusion in the clinical community as to whether HBOT is useful (Adamson 1985; Bates 1986; Jacoby 2001; Kleijnen and Knipschild 1995; Monks 1988; Wynne and Monks 1989). One consistency is that HBOT treatment at pressures below 2.0 ATA for short periods of time are not detrimental to patients, indeed O2 used at higher ATA have been indicated to improve recovery from brain trauma in patients (Rockswold et al. 2010). To address the controversy two meta-analysis reports have addressed the use of HBOT for MS. In 2004, a Cochrane report evaluated nine randomized control trials comprising of 504 participants. Only two of the nine trials showed a reduction in EDSS score at 12 months (−0.85 compared to sham). The conclusions suggested better well-designed trials would be required to confirm this improvement but overall did not recommend such trials to be performed (Bennett and Heard 2004). The same authors revaluated the use of HBOT in 2010, in trials they had previously analyzed between 1983 and 1987 and came to the same conclusions that HBOT for MS was ineffective. They suggested that ‘only staunch advocates would be willing to pursue such investigations’, (Bennett and Heard 2010).
Such ‘staunch advocates’ come in the form of MS individuals. The internet is full of testimonials from MS subjects (http://www.oxygenunderpressure.com/category/multiple-sclerosis/) and advocate clinicians (Maxfield 2005) who have personally used or employed HBOT, patients have reported feeling better in terms of pain relief, gait, bladder control and overall mobility. The justification for using HBOT for MS is most likely governed by the ability of the treatment to suppress disease symptoms for long periods of time. Despite the resistance and skepticism from many clinicians to prescribe HBOT for MS, several thousand MS individuals use such treatment in the UK and elsewhere every year and report positive outcomes, including decreased fatigue and depression. There are more than 50 hyperbaric centers throughout the UK (Fig. 2.3), where individuals can book a HBOT session, either in a monoplace or multiplace chamber that can accommodate up to 12 people (Fig. 2.3 b). MS subjects who are more mobile and in the early stages of the disease have anecdotally reported the use of HBOT to be beneficial. Ideally it would be useful to gauge the effectiveness of HBOT on MS individuals with different stages of the disease (e.g. relapsing remitting vs. secondary chronic progressive with and without conventional medication), but no such data exists. The word used frequently by MS individuals is ‘stabilize’. Again qualitative testimonials from MS subjects who self-administer HBOT, commonly report a stabilization of their symptoms or mild improvement. One difference between the clinical trials and the practical use of HBO by MS subjects is the frequency of HBOT use by the individuals themselves. Whereas trial protocols in the past, used HBOT on MS patients suffering from differing degrees of severity on ~20 occasions over a period of a month (Fig. 2.4), many of these protocols have not been used over longer periods. In reality MS subjects voluntarily use HBOT more frequently and over a longer period of time. Perrins and James reported on 1384 MS subjects employing long-term treatment of HBOT (Perrins and James 2005). About 9 % were regularly treated with HBOT for 5–15 years and 11 % were treated for 17 years or more. Better stabilization and retardation of MS progression was reported if treatment was used soon after MS was diagnosed and before irreversible lesions developed. As HBOT treatment is not offered by the UK National Health Service (NHS) there are no official numbers of clients or treatments in the public domain. However there are a large number of MS therapy centers located around the UK (Fig. 2.3). One such center in Exeter, Devon, UK opened in 1982. The Exeter Center recommends that ‘MS clients’ begin with 15 session (5 daily sessions/week for 3 weeks at between 1.5 and 2.0 ATA) and then ‘top up’ with HBO on a weekly basis, depending on how the client is responding. The client is placed in a three or seven seater chamber and gently placed under pressure at a rate of 1 m/min until the appropriate pressure is reached. Clients breathe 100 % oxygen for 60 min and then the pressure is reversed at 1 m/min until normobaric pressure is reached. Some clients at this center have used the facility for decades and feel it prevents their condition deteriorating (personal communication - Esme Gibbins, Therapies Manager). In 2011, Professor Philip B. James (Emeritus Professor of Medicine, University of Dundee, UK) wrote an open letter (http://www.hjernebarnet.dk/fileadmin/_temp_/Philip_James_-110405.pdf) suggesting over 2.5 million HBOT sessions have been safely provided to over 20,000 individuals in MS National Therapy centers since they began to operate in 1982 (figures up to 2011).
Frequency of multiple sclerosis and quantity and location of HBO chambers for MS patients in the UK. (a) The frequency of MS increases with latitude; England & Wales < Northern Island < Scotland. (b) Patients frequently use single and multiplace HBO chambers to help alleviate their symptoms. (c) There are HBOT chambers located in many major cities and regions around the UK, including the islands of the Scottish coast, where incidences of MS are some of the highest in the world: 420/100,000 in the Orkney Islands and 295/100,000 in the Shetland Islands
Examples of MS HBOT protocols in comparison to wound treatment HBOT. (a) & (b) Examples of protocols to treat MS patients with oxygen under pressure. The protocols over the past 25–35 years have evolved but have retained consistently the same treatment time (60 min) and pressure protocols (1.5–2 ATA). (c) The HBOT protocols used to successfully treat chronic wounds, commonly employs the use of oxygen at >2 ATA for longer periods ~90 min, with air breaks
2.4 ER Targeted Therapeutics and MS
2.4.1 Effect of HBOT on Cell Function and Gene Expression
Neurologists may agree or disagree with the merits of using HBOT for MS, but HBOT is used successfully to treat many other conditions, and more information as to the effect of HBOT at the cellular and molecular level is required to aid in the further understanding of the mechanism of action of HBOT. We have investigated the role of hyperoxia on various cells under hyperbaric pressures (HBOT) at 2.4 ATA, including platelets (Shaw et al. 2009), endothelial cells and neutrophils (Almzaiel et al. 2013, 2015; Kendall et al. 2013a; Kendall et al. 2012; Kendall et al. 2013b) and bone tissue (Al Hadi et al. 2015; Al Hadi et al. 2013) We developed a chronic wound model to study neutrophil-endothelial interactions to study the effect of HBOT on individual cell types in chronic wounds (Kendall et al. 2011). The culmination of these and other studies suggested HBO reduces the surface expression of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) on endothelial cells and reduces neutrophil adhesion. Although we did not observe changes in neutrophil adhesion molecule expression CD18, CD11b, CD62L, CD31, we proposed HBOT inhibited neutrophil adhesion to endothelial cells by S-nitrosation (Kendall et al. 2013b). In the context of MS, similar effects of HBOT could possibly inhibit T-cell interaction with brain vascular endothelial cells.
Chronic wounds that are normally exposed to 2 % O2 are frequently treated with HBO at a pressure of 2.4 ATA. In contrast, MS patients whose brain tissue is normally exposed to 4 % O2 are normally treated with HBO at 1.5 −2.0 ATA (Fig. 2.4). Recently we examined how oxidative and inflammatory gene expression alters under different pressures. We have cultured human endothelial cells under hypoxic conditions (2 % O2) as a model because they are important in both wound healing and immune cell interaction in the BBB. We studied the effect of a single 90 min exposure of HBO on a number of categories of genes, including adhesion molecules, apoptosis, angiogenesis and tissue remodeling, inflammation, intracellular signaling and oxygen responses and redox signaling (Kendall et al. 2013a). In these studies a number of genes were sensitive to HBO at both 1.5 and 2.4 ATA compared to cells treated under pressure at 1 ATA and showed reduced levels of expression at the mRNA level that was sustained for at least 22.5 h (the time RNA was extracted from the cells). Notably, 1–4-fold decreases in adhesion molecules; Platelet endothelial cell adhesion molecule1, fibronectin1, angiogenesis factors; angiopoietin 2, connective tissue growth factor, vascular endothelial growth factor receptor 2, endothelial tyrosine kinase, tissue inhibitor of metalloproteinases 3, the chemokine; Interleukin 8, and oxygen response genes; endothelial PAS domain protein 1- HIF-2α and glutathione peroxidase 1. In most cases treatment of endothelial cells with 96.7 % O2 at 1.5 ATA produced greater reductions in the above genes than when treated with 97.9 % O2 at 2.4 ATA. When mRNA was quantified from the same endothelial cells, 5 h post HBOT treatment a whole serious of oxygen response genes were downregulated 2–3 fold at both 1.5 and 2.4 ATA, but more so when exposed to O2 at 1.5 ATA compared with cells treated with O2 at 1ATA or 2.4 ATA pressure. These included HIF-1α and −2α, peroxiredoxin 2 and 6, glutathione peroxidase 1, superoxide dismutase 1 and 2, catalase, thioredoxin and glyoxalase 1. We have observed similar increases in antioxidants of the peroxiredoxin family at the protein levels in chronic lesions of MS patients (Holley et al. 2007). Interestingly, HBOT at 1.5 ATA reduced the expression of the ER stress chaperone calreticulin by two fold and in MS, calreticulin levels being known to increase as part of the UPR (Mhaille et al. 2008). This illustrated that several antioxidants and chaperones are sensitive to rapid changes to oxygen levels and adjust their expression accordingly. Perhaps of more interest is that O2 administered at 1.5 ATA altered the expression of many more genes more effectively than 2.4 ATA. The reason for this is unknown, but raises the possibility that at least for MS, HBOT treatment, oxygen used at 1.5 ATA, that has been adopted over the past 30–40 years by patients, is more effective at altering gene expression in a number of oxidative and ER stress conditions. One question not answered by the above experiments is how long are changes in gene expression retained post HBOT? Our work suggests at least in vitro that the effect is transient and might require multiple and regular exposures to have any long term beneficial effect on oxidative response, ER stress, UPR and inflammatory gene products. This would support the recommendations of Perrins and James, who suggest MS patients should have regular HBOT treatments for it to have a significant effect in improving EDSS scores or preventing further deterioration (Perrins and James 2005).
2.4.2 Neural UPR Sensor Genes
As HBOT can alter gene expression, it would be of great interest to be able to alter gene expression in neural cells in a non-invasive manner. More specifically to arrest or activate UPR and ER-stress regulatory genes that are involved in clearing misfolded proteins, cell repair and death (Fig. 2.5 a). These processes and their regulation are known to be important in neurodegenerative diseases (Oyadomari and Mori 2004; Soto and Estrada 2008). Oxidative stress in the form of lipid peroxidation (Wang et al. 2014), oxygen consumption to form ROS during myelin sheath attack and mitochondrial injury (Haider 2015) and nitrosative stress (Kallaur et al. 2015) have all been observed to precede the inflammatory response in MS patients.
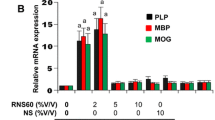
Effect of Oxidative Stress on ER stress regulators in human CNS cells. (a) Changes in cellular oxidative status is one condition that can lead to ER stress. The three recognized UPR sensor pathways PERK, IRE1α and ATF6α induce a number of downstream genes that stimulate changes of a number of important enzyme, oxidoreductase and chaperone pathways that aid in the regulated cell death or survival of individual cell types. (b) The mechanisms by which changes in oxidative stress induced by hyperoxia effect demyelinating diseases such as MS as induced by HBOT treatment remain unknown. We assessed the effect of hyperoxia under pressure (HBO) and pressure alone (PC) on UPR gene pathways in human oligodendrocytes (HOGs) and neuronal cells (SH-SY5Y). (c) The effect of either HBO or PC affected the expression of UPR pathway sensors differently in neuronal or oligodendrocyte cells
We therefore examined the effect of HBOT exposure specifically on both differentiated neuron-like SH-SY5Y cells and myelin-producing human oligodendrocytes (HOGs). These neural cells were cultured under the appropriate optimal growth conditions for differentiation and maturation in the presence of 4 % oxygen (Normoxia; 4.0 % O2/CO2 at 1.0 ATA) for 4–6 days (neural cells normally exist in a low-oxygen environment) (Ndubuizu and LaManna 2007). Next the cells were treated with HBO (96.7%O2/CO2 at 1.5 ATA), or pressure control (2.67 % O2/CO2 at 1.5 ATA) treatments for 90 min (Fig. 2.5 b). All of the gas mixtures contained CO2 at a level to give a final pCO2 of 5 kPa, representing the respiration-derived CO2 at the cellular level. The cells were then placed in their former culture conditions for 5 h or 22.5 h. RNA was isolated from the cells for quantitative real time PCR and analyzed for differences in unfolded protein response (UPR) gene expression pre- and post- exposure to HBO or pressure control (PC) treatment. mRNA expression was analyzed by our previously described methods (Eggleton et al. 2010; Kendall et al. 2011; Kendall et al. 2013a; Kendall et al. 2012). The results revealed that mRNA expression in the major UPR regulatory genes PERK, IRE1α and ATF6α were largely unaffected by HBO or PC treatment 5 h after treatments in SH-SY5Y cells. But 22.5 h post-treatment the PERK and ATF6α mRNA expression levels had reduced by 50 % in PERK in cells treated with HBO or hyperbaric pressure (Fig. 2.5 c, left panel). Similar reductions in PERK and ATF6α genes were seen in HOG cells after 22.5 h post-treatment with HBO. However, in contrast, to SH-SY5Y cells, IRE1α mRNA levels were reduced by over 50 % following HBO treatment at 1.5 ATA 5 h post-treatment (Fig. 2.5 c, right panel). In general, hyperbaric pressure and not oxygen accounted for some but not all of the reduced gene expression in the UPR sensor genes, but did appear to act synergistically in down regulating the UPR genes tested. This might in part explain why in clinical trials of HBOT for MS patients in which a pressure control is used as a placebo, some placebo treated patients report a benefit for the treatment. In the Cochrane analysis of HBO trials conducted in 2004 (Bennett and Heard 2004), all of the trials evaluated administered oxygen to patients at between 1.75 ATA and 2.5 ATA for 90 min. This is despite the recommended protocols suggesting 1.5 ATA should be initially used (Fig. 2.4). In our gene expression study on neural cells we chose 1.5 ATA because this pressure is used to treat brain injury (Stoller 2011, 2015) and we have seen greater reductions in inflammatory gene expression in cells exposed to oxygen at 1.5 ATA compared to 2.4 ATA (Kendall et al. 2013a). These results are encouraging and demonstrate the use of oxygen at a relatively low hyperbaric pressure can markedly reduce the regulatory genes of the UPR pathways responsible for controlling cell death and repair, which are known to be over-expressed in lesions of MS patients as described above.
2.5 Conclusion
There is growing evidence that both ER (Cunnea et al. 2011; Mhaille et al. 2008) and oxidative stress (Guan et al. 2015; Karlik et al. 2015; Lassmann and van Horssen 2015; Ohl et al. 2015; Pasquali et al. 2015) play a role in the pathology of MS. These stress pathways are also the focus of attention to down-regulate inflammation and aid remyelination within the CNS of MS patients. (Getts et al. 2008). A number of pharmacological agents and small molecule therapeutics have or are being trialed in an attempt to reduce ER and/or oxidative stress in MS. (Bahamonde et al. 2014; Khalili et al. 2014; Miller et al. 2013; Naziroglu et al. 2014; Ramirez-Ramirez et al. 2013; Sanoobar et al. 2013; Seven et al. 2013). The problem with all drugs is their ability to target specific cells, and this is made more difficult when attempting to target pharmacological agents across the BBB. Despite this problem, a number of agents are being developed to suppress ROS/RNS and ER stress in the CNS (Chiurchiu 2014). While drug development continues and MS patients await new treatments, many other MS patients continue to seek solace in HBOT treatment. The fact that so many HBOT treatment centers exist worldwide and are used regularly by MS patients is a testament to their usefulness. Despite HBOT treatment not being officially approved for the treatment of MS by the clinical community, the lack of approval is probably of no consequence to individuals who use HBOT and feel they benefit from its effects.
The debate on the pros and cons of using HBOT as a MS therapy will continue ad infinitum until proper regulated trials are conducted, but this may never happen due to lack of patent protection, low financial gains and importantly a lack of understanding as to the precise mechanisms of how oxygen under hyperbaric pressure can reduce the symptoms of MS. For example the paradox that oxidative stress is detrimental to CNS tissue, but brain tissue may benefit from being exposed to 100 % oxygen under pressure warrants a cautious approach. Further studies investigating the effect of hyperoxia under normobaric and hyperbaric conditions at the cellular level may help us understand what some patients with MS already believe and feel - that HBOT is an efficacious therapy.
Abbreviations
- ATA:
-
atmospheres absolute
- BBB:
-
blood brain barrier
- CL:
-
chronic lesion
- CNS:
-
central nervous system
- EDSS:
-
expanded disability status scale
- ER:
-
endoplasmic reticulum
- HBOT:
-
hyperbaric oxygen therapy
- MS:
-
multiple sclerosis
- NAWM:
-
normal appearing white matter
- pO2 :
-
partial pressure of oxygen
- ROS:
-
Reactive oxygen species
- UPR:
-
unfolded protein response
References
Aboul-Enein F, Lassmann H (2005) Mitochondrial damage and histotoxic hypoxia: a pathway of tissue injury in inflammatory brain disease? Acta Neuropathol 109:49–55
Adamson L (1985) Hyperbaric oxygen treatment for multiple sclerosis. Nurs Times 81:47–48
Al Hadi H, Smerdon GR, Fox SW (2013) Hyperbaric oxygen therapy suppresses osteoclast formation and bone resorption. J Orthop Res 31:1839–1844
Al Hadi H, Smerdon G, Fox SW (2015) Osteoclastic resorptive capacity is suppressed in patients receiving hyperbaric oxygen therapy. Acta Orthop 86:264–269
Allen IV, McQuaid S, Mirakhur M, Nevin G (2001) Pathological abnormalities in the normal-appearing white matter in multiple sclerosis. Neurol Sci 22:141–144
Almzaiel AJ, Billington R, Smerdon G, Moody AJ (2013) Effects of hyperbaric oxygen treatment on antimicrobial function and apoptosis of differentiated HL-60 (neutrophil-like) cells. Life Sci 93:125–131
Almzaiel AJ, Billington R, Smerdon G, Moody AJ (2015) Hyperbaric oxygen enhances neutrophil apoptosis and their clearance by monocyte-derived macrophages. Biochem Cell Biol = Biochimie et biologie cellulaire 93:405–416
Bahamonde C, Conde C, Aguera E et al (2014) Elevated melatonin levels in natalizumab-treated female patients with relapsing-remitting multiple sclerosis: relationship to oxidative stress. Eur J Pharmacol 730:26–30
Barnes MP, Bates D, Cartlidge NE, French JM, Shaw DA (1985a) Hyperbaric oxygen and multiple sclerosis: short-term results of a placebo-controlled, double-blind trial. Lancet 1:297–300
Barnes MP, Bates D, Cartlidge NEF, French JM, Shaw DA (1985b) Hyperbaric-oxygen for multiple-sclerosis – reply. Lancet 1:810–811
Barnes MP, Bates D, Cartlidge NE, French JM, Shaw DA (1987) Hyperbaric oxygen and multiple sclerosis: final results of a placebo-controlled, double-blind trial. J Neurol Neurosurg Psychiatry 50:1402–1406
Bates D (1986) Hyperbaric oxygen in multiple sclerosis: discussion paper. J R Soc Med 79:535–537
Bennett, M and Heard, R (2004) Hyperbaric oxygen therapy for multiple sclerosis. Cochrane Database Syst Rev CD003057
Bennett M, Heard R (2010) Hyperbaric oxygen therapy for multiple sclerosis. CNS Neurosci Ther 16:115–124
Boschetty V, Cernoch J (1970) Use of hyperbaric oxygen in various neurologic diseases. (Preliminary report). Bratisl Lek Listy 53:298–302
Boschetty V, Dostal J, Holek J (1970) Use of hyperbaric oxygenation in acute cerebrovascular accidents. (Preliminary report). Bratisl Lek Listy 53:160–164
Calabrese V, Bella R, Testa D et al (1998) Increased cerebrospinal fluid and plasma levels of ultraweak chemiluminescence are associated with changes in the thiol pool and lipid-soluble fluorescence in multiple sclerosis: the pathogenic role of oxidative stress. Drugs Exp Clin Res 24:125–131
Cao Y, Goods BA, Raddassi K et al (2015) Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci Transl Med 7:287ra274
Chan JY, Cooney GJ, Biden TJ, Laybutt DR (2011) Differential regulation of adaptive and apoptotic unfolded protein response signalling by cytokine-induced nitric oxide production in mouse pancreatic beta cells. Diabetologia 54:1766–1776
Chiurchiu V (2014) Novel targets in multiple sclerosis: to oxidative stress and beyond. Curr Top Med Chem 14:2590–2599
Clarke RE, Tenorio LM, Hussey JR et al (2008) Hyperbaric oxygen treatment of chronic refractory radiation proctitis: a randomized and controlled double-blind crossover trial with long-term follow-up. Int J Radiat Oncol Biol Phys 72:134–143
Confavreux, C, Mathieu, C, Chacornac, R, Aimard, G and Devic, M (1986) [Ineffectiveness of hyperbaric oxygen therapy in multiple sclerosis. A randomized placebo-controlled double-blind study]. Presse Med 15:1319–1322
Cunnea P, Mhaille AN, McQuaid S, Farrell M, McMahon J, FitzGerald U (2011) Expression profiles of endoplasmic reticulum stress-related molecules in demyelinating lesions and multiple sclerosis. Mult Scler 17:808–818
Cwiklinska H, Mycko MP, Luvsannorov O et al (2003) Heat shock protein 70 associations with myelin basic protein and proteolipid protein in multiple sclerosis brains. Int Immunol 15:241–249
Dutta R, McDonough J, Yin X et al (2006) Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol 59:478–489
Eggleton P, Harries LW, Alberigo G et al (2010) Changes in apoptotic gene expression in lymphocytes from rheumatoid arthritis and systemic lupus erythematosus patients compared with healthy lymphocytes. J Clin Immunol 30:649–658
Eggleton P, Bishop AJ, Smerdon GR (2015) Safety and efficacy of hyperbaric oxygen therapy in chronic wound management: current evidence. Chron Wound Care Manag Res 2:81–93
Fischer BH (1983) Evoked-potentials after hyperbaric-oxygen treatment of multiple-sclerosis – reply. New Engl J Med 309:242–242
Fischer BH, Marks M, Reich T (1983) Hyperbaric-oxygen treatment of multiple-sclerosis - a randomized, placebo-controlled, double-blind-study. New Engl J Med 308:181–186
Getts MT, Getts DR, Kohm AP, Miller SD (2008) Endoplasmic reticulum stress response as a potential therapeutic target in multiple sclerosis. Therapy 5:631–640
Gilgun-Sherki Y, Melamed E, Offen D (2004) The role of oxidative stress in the pathogenesis of multiple sclerosis: the need for effective antioxidant therapy. J Neurol 251:261–268
Giovannoni G, Ebers G (2007) Multiple sclerosis: the environment and causation. Curr Opin Neurol 20:261–268
Glover M, Smerdon GR, Andreyev HJ et al (2015) Hyperbaric oxygen for patients with chronic bowel dysfunction after pelvic radiotherapy (HOT2): a randomised, double-blind, sham-controlled phase 3 trial. Lancet Oncol 17:224–233
Gow A, Wrabetz L (2009) CHOP and the endoplasmic reticulum stress response in myelinating glia. Curr Opin Neurobiol 19:505–510
Guan JZ, Guan WP, Maeda T, Guoqing X, GuangZhi W, Makino N (2015) Patients with multiple sclerosis show increased oxidative stress markers and somatic telomere length shortening. Mol Cell Biochem 400:183–187
Haider L (2015) Inflammation, iron, energy failure, and oxidative stress in the pathogenesis of multiple sclerosis. Oxid Med Cell Longev 2015:725370
Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drug Aging 18:685–716
Harpur GD, Suke R, Bass BH et al (1986) Hyperbaric oxygen therapy in chronic stable multiple sclerosis: double-blind study. Neurology 36:988–991
Hedstrom AK, Olsson T, Alfredsson L (2015) The role of environment and lifestyle in determining the risk of multiple sclerosis. Curr Top Behav Neurosci 26:87–104
Holley JE, Newcombe J, Winyard PG, Gutowski NJ (2007) Peroxiredoxin V in multiple sclerosis lesions: predominant expression by astrocytes. Mult Scler 13:955–961
Holley JE, Bremer E, Kendall AC et al (2014) CD20+inflammatory T-cells are present in blood and brain of multiple sclerosis patients and can be selectively targeted for apoptotic elimination. Multiple Scler Relat Disord 3:650–658
Hong J, Li H, Chen M et al (2009) Regulatory and pro-inflammatory phenotypes of myelin basic protein-autoreactive T cells in multiple sclerosis. Int Immunol 21:1329–1340
Jacoby I (2001) Hyperbaric oxygen therapy, multiple sclerosis, and unapproved indications: taking a stand. Undersea Hyperb Med 28:113–115
James PB (1983) Hyperbaric oxygen for multiple sclerosis. World Med 18:33–34
James P (1984) Hyperbaric-oxygen for patients with multiple-sclerosis. Br Med J 288:1831–1831
Kallaur AP, Lopes J, Oliveira SR et al (2015) Immune-inflammatory and oxidative and nitrosative stress biomarkers of depression symptoms in subjects with multiple Sclerosis: increased peripheral inflammation but less acute neuroinflammation. Mol Neurobiol 53:5191–5202
Karlik M, Valkovic P, Hancinova V, Krizova L, Tothova L, Celec P (2015) Markers of oxidative stress in plasma and saliva in patients with multiple sclerosis. Clin Biochem 48:24–28
Kendall AC, Smerdon GR, Harries LW, Winyard PG, Eggleton P, Whatmore JL (2011) Hyperbaric oxygen therapy and chronic wound healing. In: Middleton JE (ed) Wound healing: process, phases and promoting. Nova Science Publishing Inc., New York, pp 145–177
Kendall AC, Whatmore JL, Harries LW, Winyard PG, Smerdon GR, Eggleton P (2012) Changes in inflammatory gene expression induced by hyperbaric oxygen treatment in human endothelial cells under chronic wound conditions. Exp Cell Res 318:207–216
Kendall AC, Whatmore JL, Harries LW, Winyard PG, Eggleton P, Smerdon GR (2013a) Different oxygen treatment pressures alter inflammatory gene expression in human endothelial cells. Undersea Hyperb Med 40:115–123
Kendall AC, Whatmore JL, Winyard PG, Smerdon GR, Eggleton P (2013b) Hyperbaric oxygen treatment reduces neutrophil-endothelial adhesion in chronic wound conditions through S-nitrosation. Wound Repair Regen 21:860–868
Khalili M, Eghtesadi S, Mirshafiey A et al (2014) Effect of lipoic acid consumption on oxidative stress among multiple sclerosis patients: a randomized controlled clinical trial. Nutr Neurosci 17:16–20
Kim R, Emi M, Tanabe K, Murakami S (2006) Role of the unfolded protein response in cell death. Apoptosis 11:5–13
Kinoshita M, Obata K, Tanaka M (2015) Latitude has more significant impact on prevalence of multiple sclerosis than ultraviolet level or sunshine duration in Japanese population. Neurol Sci 36:1147–1151
Kleijnen J, Knipschild P (1995) Hyperbaric oxygen for multiple sclerosis. Rev Controlled Trials Act Neurol Scand 91:330–334
Koch M, Ramsaransing GS, Arutjunyan AV et al (2006) Oxidative stress in serum and peripheral blood leukocytes in patients with different disease courses of multiple sclerosis. J Neurol 253:483–487
Kraus A, Michalak M (2011) Endoplasmic reticulum quality control and dysmyelination. Biodivers Conserv 2:261–274
Kurtzke JF (1955) A new scale for evaluating disability in multiple sclerosis. Neurology 5:580–583
Kurtzke JF (1965) Further notes on disability evaluation in multiple sclerosis, with scale modifications. Neurology 15:654–661
Kurtzke JF (1970) Neurologic impairment in multiple sclerosis and the disability status scale. Act Neurol Scand 46:493–512
Kurtzke JF (1983) Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 33:1444–1452
Kurtzke JF (1989) The disability status scale for multiple sclerosis: apologia pro DSS sua. Neurology 39:291–302
Kurtzke JF (2000) Natural history and clinical outcome measures for multiple sclerosis studies. Why at the present time does EDSS scale remain a preferred outcome measure to evaluate disease evolution? Neurol Sci 21:339–341
Kurtzke JF (2008) Historical and clinical perspectives of the expanded disability status scale. Neuroepidemiology 31:1–9
Kutzelnigg A, Lucchinetti CF, Stadelmann C et al (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain J Neurol 128:2705–2712
Langer-Gould A, Brara SM, Beaber BE, Zhang JL (2013) Incidence of multiple sclerosis in multiple racial and ethnic groups. Neurology 80:1734–1739
Lassmann H, van Horssen J (2015) Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochimica et biophysica acta 1862(3):506–510
Lhermitte F, Roullet E, Lyon-Caen O et al (1986) Double-blind treatment of 49 cases of chronic multiple sclerosis using hyperbaric oxygen. Rev Neurol 142:201–206
Lin W, Popko B (2009) Endoplasmic reticulum stress in disorders of myelinating cells. Nat Neurosci 12:379–385
Lock CB, Heller RA (2003) Gene microarray analysis of multiple sclerosis lesions. Trends Mol Med 9:535–541
Lu F, Selak M, O’Connor J et al (2000) Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci 177:95–103
Mahad DJ, Ziabreva I, Campbell G et al (2009) Mitochondrial changes within axons in multiple sclerosis. Brain J Neurol 132:1161–1174
Mandel M, Achiron A, Tuller T et al (2009) Clone clusters in autoreactive CD4 T-cell lines from probable multiple sclerosis patients form disease-characteristic signatures. Immunology 128:287–300
Maxfield WS (2005) Hyperbaric oxygen therapy for multiple sclerosis: my experience. J Am Physic Surg 10:116
McMahon JM, McQuaid S, Reynolds R, FitzGerald UF (2012) Increased expression of ER stress- and hypoxia-associated molecules in grey matter lesions in multiple sclerosis. Mult Scler 18:1437–1447
Mertin J, Mcdonald WI (1984) Hyperbaric-oxygen for patients with multiple-sclerosis. Br Med J 288:957–960
Mhaille AN, McQuaid S, Windebank A et al (2008) Increased expression of endoplasmic reticulum stress-related signaling pathway molecules in multiple sclerosis lesions. J Neuropathol Exp Neurol 67:200–211
Miller E, Walczak A, Majsterek I, Kedziora J (2013) Melatonin reduces oxidative stress in the erythrocytes of multiple sclerosis patients with secondary progressive clinical course. J Neuroimmunol 257:97–101
Mkhikian H, Grigorian A, Li CF et al (2011) Genetics and the environment converge to dysregulate N-glycosylation in multiple sclerosis. Nat Commun 2:334
Monks J (1988) Interpretation of subjective measures in a clinical trial of hyperbaric oxygen therapy for multiple sclerosis. J Psychosom Res 32:365–372
Mycko MP, Papoian R, Boschert U, Raine CS, Selmaj KW (2003) cDNA microarray analysis in multiple sclerosis lesions: detection of genes associated with disease activity. Brain J Neurol 126:1048–1057
Mycko MP, Papoian R, Boschert U, Raine CS, Selmaj KW (2004) Microarray gene expression profiling of chronic active and inactive lesions in multiple sclerosis. Clin Neurol Neurosurg 106:223–229
Naziroglu M, Kutluhan S, Ovey IS, Aykur M, Yurekli VA (2014) Modulation of oxidative stress, apoptosis, and calcium entry in leukocytes of patients with multiple sclerosis by Hypericum perforatum. Nutr Neurosci 17:214–221
Ndubuizu O, LaManna JC (2007) Brain tissue oxygen concentration measurements. Antioxid Redox Signal 9:1207–1219
Neiman J, Nilsson BY, Barr PO, Perrins DJD (1985) Hyperbaric-oxygen in chronic progressive multiple-sclerosis – visual evoked-potentials and clinical effects. J Neurol Neurosurg Psychiatry 48:497–500
Neubauer RA (1978) Treatment of multiple-sclerosis with monoplace hyperbaric oxygenation. J Fla Med Assoc 65:101–101
Neubauer RA (1980) Exposure of multiple-sclerosis patients to hyperbaric-oxygen at 1.5-2 Ata – a preliminary-report. J Fla Med Assoc 67:498–504
Neubauer RA, Neubauer V, Gottlieb SF (2005) The controversy over hyperbaric oxygenation therapy for multiple sclerosis. J Am Phys Surg 10:112–115
Nickel A, Kohlhaas M, Maack C (2014) Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol 73:26–33
Ohl K, Tenbrock K, Kipp M (2015) Oxidative stress in multiple sclerosis: central and peripheral mode of action. Exper Neurol 277:58–67
Oriani G, Barbieri S, Cislaghi G et al (1990) Long-term hyperbaric oxygen in multiple sclerosis: A placebo controlled double-blind trial with evoked potentials studies. J Hyperb Med 5:237–245
Oyadomari S, Mori M (2004) Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ 11:381–389
Pasquali L, Pecori C, Lucchesi C et al (2015) Plasmatic oxidative stress biomarkers in multiple sclerosis: relation with clinical and demographic characteristics. Clin Biochem 48:19–23
Perrins DJ, James PB (2005) Long-term hyperbaric oxygenation retards progression in multiple sclerosis patients. IJNN 2:45–48
Ramirez-Ramirez V, Macias-Islas MA, Ortiz GG et al (2013) Efficacy of fish oil on serum of TNF alpha, IL-1 beta, and IL-6 oxidative stress markers in multiple sclerosis treated with interferon beta-1b. Oxidative Med Cell Longev 2013:709493
Rockswold SB, Rockswold GL, Zaun DA et al (2010) A prospective, randomized clinical trial to compare the effect of hyperbaric to normobaric hyperoxia on cerebral metabolism, intracranial pressure, and oxygen toxicity in severe traumatic brain injury. J Neurosurg 112:1080–1094
Rozpedek W, Markiewicz L, Diehl JA, Pytel D, Majsterek I (2015) Unfolded protein response and PERK kinase as a new therapeutic target in the pathogenesis of Alzheimer’s disease. Curr Med Chem 22:3169–3184
Sanoobar M, Eghtesadi S, Azimi A, Khalili M, Jazayeri S, Reza Gohari M (2013) Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with relapsing-remitting multiple sclerosis. Int J Neurosci 123:776–782
Seven A, Aslan M, Incir S, Altintas A (2013) Evaluation of oxidative and nitrosative stress in relapsing remitting multiple sclerosis: effect of corticosteroid therapy. Folia neuropathologica/Association of Polish Neuropathologists and Medical Research Centre. Polish Acad Sci 51:58–64
Shaw FL, Winyard PG, Smerdon GR, Bryson PJ, Moody AJ, Eggleton P (2009) Hyperbaric oxygen treatment induces platelet aggregation and protein release, without altering expression of activation molecules. Clin Biochem 42:467–476
Smith KJ, Kapoor R, Felts PA (1999) Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol 9:69–92
Soto C, Estrada LD (2008) Protein misfolding and neurodegeneration. Arch Neurol 65:184–189
Stoller KP (2011) Hyperbaric oxygen therapy (1.5 ATA) in treating sports related TBI/CTE: two case reports. Med Gas Res 1:17
Stoller KP (2015) All the right moves: the need for the timely use of hyperbaric oxygen therapy for treating TBI/CTE/PTSD. Med Gas Res 5:7
Stone S, Lin W (2015) The unfolded protein response in multiple sclerosis. Front Neurosci 9:264
Syburra C, Passi S (1999) Oxidative stress in patients with multiple sclerosis. Ukr Biokhim Zh 71:112–115
Szegezdi E, Logue SE, Gorman AM, Samali A (2006) Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7:880–885
Tajouri L, Mellick AS, Ashton KJ et al (2003) Quantitative and qualitative changes in gene expression patterns characterize the activity of plaques in multiple sclerosis. Brain Res Mol Brain Res 119:170–183
The Guideline Development Group NICE (2014) Multiple sclerosis in adults: management. In: Excellence, NIfHaCs (eds) Clinical guideline, vol 186. NICE, UK, pp 1–35
Thom SR (2011) Hyperbaric oxygen: its mechanisms and efficacy. Plast Reconstr Surg 127(Suppl 1):131S–141S
Torres M, Marcilla-Etxenike A, Fiol-deRoque MA, Escriba PV, Busquets X (2015) The unfolded protein response in the therapeutic effect of hydroxy-DHA against Alzheimer’s disease. Apoptosis: An Int J Program Cell Death 20:712–724
US Food and Drug Administration (2013) Hyperbaric oxygen therapy: don’t be misled. In: Administration, UFaDs (ed) Consumer updates, pp 1–2, USA
Vergelli M, Mazzanti B, Traggiai E et al (2001) Short-term evolution of autoreactive T cell repertoire in multiple sclerosis. J Neurosci Res 66:517–524
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086
Wang P, Xie K, Wang C, Bi J (2014) Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur Neurol 72:249–254
Wiles CM, Clarke CR, Irwin HP, Edgar EF, Swan AV (1986) Hyperbaric oxygen in multiple sclerosis: a double blind trial. Br Med J 292:367–371
Wood J, Stell R, Unsworth I, Lance JW, Skuse N (1985) A double-blind trial of hyperbaric oxygen in the treatment of multiple sclerosis. Med J Aust 143:238–240
Wynne A, Monks J (1989) Patients’ decisions about continuing with therapy in chronic illness: a study of hyperbaric oxygen therapy in multiple sclerosis. Fam Pract 6:268–273
Xiao D, Ye X, Zhang N et al (2015) A meta-analysis of interaction between Epstein-Barr virus and HLA-DRB1*1501 on risk of multiple sclerosis. Sci Report 5:18083
Yuan J, Handy RD, Moody AJ, Smerdon G, Bryson P (2011) Limited DNA damage in human endothelial cells after hyperbaric oxygen treatment and protection from subsequent hydrogen peroxide exposure. Biochim Biophys Acta 1810:526–531
Acknowledgements
The authors thank Drs. Debra Green and Lorna W Harries for assistance in the generation and analysis of the UPR gene expression work. We also thank Dr. Anthony Campagnoni (Brain Research Institute, UCLA, USA) for providing HOGs. This work was supported by grants from DDRC Healthcare, Royal Devon and Exeter Foundation Trust and Medical Research Council UK. Additional thanks are extended to the NeuroResource Tissue Bank, UCL Institute of Neurology, London and UK MS Tissue Bank London, U.K. We also thank Buzzy Eggleton for the design and construction of some of the figures.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Eggleton, P., Smerdon, G.R., Holley, J.E., Gutowski, N.J. (2017). Manipulation of Oxygen and Endoplasmic Reticulum Stress Factors as Possible Interventions for Treatment of Multiple Sclerosis: Evidence for and Against. In: Asea, A., Geraci, F., Kaur, P. (eds) Multiple Sclerosis: Bench to Bedside. Advances in Experimental Medicine and Biology, vol 958. Springer, Cham. https://doi.org/10.1007/978-3-319-47861-6_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-47861-6_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47860-9
Online ISBN: 978-3-319-47861-6
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)