Abstract
BMPs are originally identified based on their ability to induce new bone in vivo and represent large members of the TGF-β superfamily of proteins. BMPs serve as inductive signals for cell migration, growth, and subsequently differentiation in many organ developments during embryogenesis and are shown to modulate inflammation, angiogenesis, and immune responses and thus provide biological cues for adult tissue repair, protection, and regeneration. BMP-2- and BMP-7-containing osteogenic devices have been approved for use as bone graft substitutes for spine fusion and long bone fractures. BMP-7 biology has been considered positively against parenchymal tissue fibrosis to improve function. In this chapter, I summarize the biology of BMPs to emphasize its (1) morphogenic role in skeletal tissue repair and regeneration; (2) modulatory role in curtailing inflammation, governing angiogenesis, suppressing apoptosis, and reducing fibrosis following immunological and mechanical insults; (3) metabolic role in glucose, calcium, and phosphate and iron homeostasis; and (4) cytoprotective role to maintain skeletal and vascular integrity. The importance of BMP biology is further corroborated in rare genetic disorders (e.g., pulmonary arterial hypertension, hemochromatosis, fibrodysplasia ossificans progressiva, and osteogenesis imperfecta) and in cancer.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- BMP receptors and signaling
- BMP antagonists
- BMPs in cartilage, bone and dentin repair
- BMP-7 in inflammation, angiogenesis and fibrosis
- BMP-7 in caclium and posphate homeostasis
- BMPs in diabetes
- BMP type II receptor in pulmonary arterial hypertension
- BMP-6 in hemochromatosis and anemia
- BMP signaling in skeletal rare disorders
1 BMPs During Development
BMPs are potent chemoattractants (motogens) [1, 2], mitogens [3], and morphogens [4, 5] which act across a concentration gradient during embryogenesis [6, 7]. BMPs recruit stem cells and determine the fate of the responding cells to undergo condensation (proliferation) and subsequently trigger their differentiation by serving as an inductive signal at specific tissue compartment in order to promote morphogenesis. During embryogenesis, in general, ectoderm expresses BMPs as secretary proteins, which bind to extracellular matrix (e.g., heparin sulfate proteoglycans and type IV collagen) and specific BMP antagonists and subsequently released as needed for mesoderm to respond. The cells that express BMPs also express BMP antagonists in order to establish a concentration gradient for ligand-receptor binding to induce downstream signaling [7, 8]. For example, during embryogenesis, the ureteric bud synthesizes BMP-7 and nephrogenic mesenchyme response to it, which then undergo condensation and differentiation into S- and comma-shaped tubules that become a functional nephron [9, 10]. Likewise, BMP-2 is required for cardiac mesoderm condensation and morphogenesis [11], while BMP-4 is responsible for lung epithelial morphogenesis [12]. Hence, the loss of function of BMP-2 and BMP-4 is embryonically lethal, and they die early at days 11–14 of embryo due to impaired cardiac function, whereas the loss of BMP-7 function results in death during birth due to the lack of functional kidney. The BMP signal-based tissue morphogenesis is so tightly controlled in space and time during embryogenesis, and thus the loss of a given BMP function at given tissue compartment can result in tissue malformation. Furthermore, BMP signaling cross talks with TGF-beta and activin signaling, the other members of TGF-beta superfamily proteins, as well as with Wnt and hedgehog signaling to govern tissue morphogenesis [8, 13].
BMPs are responsible for endochondral bone formation during development, and the cellular events that are responsible for embryonic endochondral ossification can be recapitulated in postnatal life by implanting an osteogenic BMP with appropriate collagenous scaffold at subcutaneous sites to induce mesenchymal cell migration, proliferation, and differentiation to form the cartilage and bone [14–16]. The biological function of BMP is concentration-dependent, the lower amount is motogenic (chemotaxis), medium concentrations are mitogenic (proliferation), and higher concentrations are morphogenic (differentiation). The biological activities of BMPs with respect to chemotaxis, proliferation, and differentiation have been demonstrated in vitro using Boyden chamber, cell proliferation, and differentiation assays in cultures using a BMP and responding mesenchymal stem cells. The role of BMPs and its canonical downstream signaling with cross talk with Wnt signaling during embryonic skeletal and craniofacial development and osteo- and dentinogenesis are described in the chapter on “Embryonic Skeletogenesis and Craniofacial Development”.
2 Structure and Function
BMPs are homodimers, and all have the hallmark of “7-cysteine domain” held by an inter-disulfide bridge at the fourth cysteine between two monomers and are highly conserved from fly to humans. BMPs are produced as a large precursor with signal peptide, pro-domain, and mature “7-cystein TGF-beta domain.” They synthesized as monomer with three intra-disulfide bridges and then undergo dimerization in endoplasmic reticulum by forming inter-disulfide bridge at the fourth cysteine and processing at RXXR site before they are secreted into extracellular space [17, 18]. The secreted BMP protein is a dimer at the mature TGF-beta domain, which is biologically active, whereas pro-domain is not active but can interact with mature, processed dimer by non-covalent interactions. The mature protein loses its biological activity if inter-disulfide bridge is broken. The crystal structure reveals that the BMP dimer is aligned antiparallel with Finger 1 and Finger 2 and Heal region [19]. A cysteine knot with intra- and inter-disulfide bridges holds the dimer protein, and because of this, it is very stable, even against proteases like trypsin.
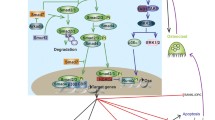
BMPs signal through ser-thr kinase receptors type I and type II. Although both type I and type II bind to the ligand and form a complex, type I receptor renders specificity and recruits intracellular kinases signaling SMAD-1/5/8 and subsequently triggers phosphorylation, which forms a complex with a co-smad-4 and translocates into the nucleus to switch on and off a set of genes responsible for tissue morphogenesis, repair, and regeneration [20]. ALK-2, ALK-3, and ALK-6 are known BMP-specific type I receptors, and BMPRII, ActRII-A, and ActRII-B serve as type II receptors; BMPs employ a specific type I receptor and type II receptor depending on the cell type and type of cellular responses it triggers [21]. There are several BMP co-receptors that have been described to activate or inhibit BMP signaling to trigger specific cellular function and outcome [22]. These include the Dragon family of protein, hemojuvelin, receptor tyrosine kinases (RTKs) TrkC, TGF-β type III receptors, BAMBI, betaglycan, and endoglin. Two downstream inhibitors, smads 6 and 7, are identified to play a functional role as checkpoints by de-plugging the BMP downstream signaling to modulate the biological activity. BMP ligands can also trigger non-canonical downstream signaling directly or indirectly that are SMAD independent, such as MAPK, ERK, NK, p38, PI3K, Akt, RANK and RANKL, as well as substantial cross talk with the Wnt, hedgehog, and VEGF signaling cascades. In addition, known BMP antagonists like noggin, chordin, follistatin, gremlin, sclerostin, and USAG-1 are shown to govern the availability of BMP ligand to its receptor by binding avidly at the extracellular space to render specificity and establish a concentration gradient [23]. For more details, refer to the chapter on “BMP and BMP Regulation: Structure and Function”.
3 BMP: In Vitro and In Vivo Model Systems for Endochondral Bone Differentiation
The systems biology of BMP with respect to skeletal tissue morphogenesis has been well documented in vivo [24]. The embryonic cellular events that culminate the formation of the new cartilage and bone can be recapitulated in post-fetal life by implanting an osteogenic BMP (e.g., BMP-2, BMP-4, BMP-6, and BMP-7) with a carrier in the rat subcutaneous site and in diaphysis fracture, segmental defect, and lumbar spine fusion models. The presence of BMP is a must in the implant in order to attract sufficient amount of mesenchymal stem cells and induce proliferation and differentiation into the bone.
In Vitro Model Systems
Several in vitro cell cultures have been used to examine BMP-like activity. Primary cultures generated from the chick [25] and mouse limb bud [26], synovial tissue [27], periosteum [28], primary bovine articular chondrocytes [29], calvarial-derived primary osteoblasts [16], established rat osteosarcoma cell lines [30], C2C12 mouse myoblast cell line [31], and bone marrow-derived W-29 stromal cells [32] have been routinely employed. To examine for chondrogenic and osteogenic responses, the early responsive genes like id-1, id-2, and id-3 [33], differentiation determinants like sox-5 and sox-9 [34] for chondrocyte and osterix and Runs-2 for osteoblast [35, 36], markers of chondrocyte phenotype like type II collagen and cartilage-specific proteoglycan [37], and markers of osteoblast phenotype, alkaline phosphatase, and osteocalcin are routinely monitored [16]. Identification of BMP-responding elements in the promotor region of the BMP-SMAD-dependent responding genes has allowed to engineer several established stable cell lines linking with luciferase enzyme to specifically qualify the biological activity of BMP from cell and tissue extracts and body fluids and for release assays for the recombinant BMP production [38]. Furthermore, pluripotent stem cells generated from patients from musculoskeletal disorder are being employed to drive chondrogenesis and osteogenesis in order to understand the loss or gain of function and to establish screens to select small molecules [39]. For more information, refer to the chapter “Novel In Vitro Assay Models to Study Osteogenesis and Chondrogenesis for Human Skeletal Disorders”.
In Vivo Model Systems
BMP alone when implanted with an appropriate collagenous matrix can induce new bone formation at ectopic or orthotopic sites. This serves as a prototype for tissue engineering [40]. BMP serves as signal and collagen serves as scaffold. The local implant site provides a microenvironment to recruit the responding cells, and they attach onto the collagenous scaffold in order to promote the differentiation into endochondral bone. This BMP-induced new bone formation is dose-dependent [16] up to certain doses based on a given substratum used; however, at a higher dose, BMP can trigger a more number of progenitors’ recruitment and proliferation, which results in hematoma and cyst-like condensation and delays the differentiation into the bone. This high-dose cyst phenomenon is observed both in ectopic and orthotopic sites.
The most important component in BMP-based osteogenic device is scaffold. The current BMP-based osteogenic device utilizes bovine-derived collagen alone or in combination with ceramics (hydroxyapatite and tricalcium phosphate), and because of ceramics and an animal-derived collagen, the device triggers initially inflammation and immune responses and promotes the expression of makers associated with fibroblast phenotype. In order to overcome this unwanted fibrogenic biology, high doses of BMP-2 (12–40 mg) are employed in the current osteogenic device. In addition, because of low affinity to collagen/ceramics, BMPs are diffused out readily from the implant site and induce unwanted ossification at the distant sites. These unwanted safety issues were observed in the clinical studies for posterolateral fusion which has been ascribed to a high dose of BMP and animal-derived collagen.
As the cells are prerequisite for BMP to signal, a situation wherein the site is compromised due to nonunion as seen in tibial diaphysis where the responding cells are not readily available in sufficient quantity, Efforts are being attempted to implant autologous bone marrow with BMP-containing scaffold. Autologous bone marrow-derived mesenchymal stem cells and periosteal-derived mesenchymal stem cells are also being considered for such BMP implants. It is likely that selecting autologous mesenchymal stem cells with specific cell surface markers that have high levels of BMP receptor expression at the cell surface may be beneficial to implant with a BMP and scaffold in certain rare indications like tibial nonunion, pseudo anthrosis and atypical fractures associated with long-term bisphosphonate or steroid use. The preferred components of bone tissue engineering are (1) BMP that lacks affinity for BMP antagonists as a signal, (2) autologous substratum (instead of animal-derived collagen), and (3) autologous responding cells, where they are short supplied. More details on this subject are discussed in the chapter “Towards Advanced Therapy Medicinal Products (ATMPs) Combining Bone Morphogenetic Proteins (BMP) and Cells for Bone Regeneration”.
4 Role of BMPs in Cartilage Repair and Regeneration
The therapeutic engineering of tissue formation requires three biological components: signaling molecules, responding cells, scaffold and permissive microenvironment. Carticel® (autologous chondrocyte implantation, ACI), the first FDA-approved cell-based therapy for the articular cartilage repair, employs the autologous cells and the live periosteum as scaffold, two of the biological components required for tissue engineering [41]. Bone morphogenetic proteins (BMPs) are potent chondrogenic morphogens and are capable of inducing differentiation of MSCs into cell lineage of hyaline cartilage and maintenance of the expression of markers associated with chondrocyte phenotype in vitro and in vivo [42, 43]. Several studies have demonstrated that BMPs when applied alone or in combination with appropriate scaffold onto chondral or osteochondral defects are capable of inducing new articular cartilage formation in vivo [44]. However, the newly formed chondrocytes fail to maintain the cellular morphology and expression of articular cartilage phenotype over time, thus leading to the degeneration of the repaired tissue in the preclinical studies. It is likely that providing BMPs continuously or at periodic intervals instead of a one-time application in the beginning as used to repair bone fractures may induce sustainable cartilage differentiation readily and maintain the regenerated cartilage to attain articularization (surface, mid- and deeper zone) and function over time under mechanical loading [45, 46]. The combination of responding cells with an appropriate scaffold and providing BMP signaling in situ will have added advantage in the enhancement of chondrocyte differentiation and maintenance of phenotypic expression in order to sustain function over long time. As BMP-2, BMP-4, BMP-6, and BMP-7 are more osteogenic and CDMP-1/GDF-5/BMP-14 and CDMP-2/GD-6/BMP-13 are more chondrogenic in vitro and in vivo model systems [42], it remains to be seen which BMP is likely to render an expected outcome in articular cartilage and intervertebral disk repair and regeneration in the human clinical trials.
The first human clinical trial for cartilage repair was conducted to evaluate BMP-7 to treat symptomatic knee OA with emphasis to reduce pain [47]. This was a double-blind, randomized, multicenter, placebo-controlled, single-dose escalation safety study that examined four doses 0 (placebo), 0.03, 0.1, 0.3, and 1.0 mg in 5 % lactose, injected intra-articularly, evaluated at 4, 8, 12, and 24 weeks. Patients receiving the BMP-7 injections at the midrange doses (0.1 and 0.3 mg) reported some symptomatic improvement, while high- and low-dose cohorts do not have the same. For more details, refer to the chapter “BMP Signaling in Articular Cartilage Repair and Regeneration: Potential Therapeutic Opportunity for Osteoarthritis”.
5 Role of BMPs in Bone Repair and Regeneration
Several clinical trials have been conducted to assess the safety and efficacy of recombinant human BMP-containing devices for the treatment of acute diaphysis bone fractures and delayed union, tibial nonunion, and anterior lumbar interbody fusion (ALIF) and posterolateral lumbar fusion (PLF). Two BMP products, rhBMP2 (InFUSE®) [48] and rhBMP-7 (OP-1® [49] and OP-1 Putty®) [50], are licensed under PMA and HDE for marketing and clinical application in the USA.
OP-1® Implant: The first human clinical study was performed to assess the efficacy of recombinant human rhBMP-7 (OP-1®) for the treatment of tibial nonunion in a prospective, randomized, and controlled clinical trial [51]. The conclusion of this clinical study demonstrated that OP-1® Implant was a safe and effective treatment modality for tibial nonunion and the outcome was comparable to the use of bone autograft but failed to achieve a statistical significance as the number of patients included in the study is not sufficient, and because of this, it has gotten only HDE approval in the USA.
OP-1 Putty®: It is an OP-1® Implant containing 230 mg of sterile carboxymethyl cellulose to provide putty-like property. The OP-1 Putty® device was evaluated in the PLF clinical study to treat symptomatic single-level degenerative lumbar spondylolisthesis and spinal stenosis without instrumentation [52, 53]. Outcomes measured at 12 months of follow-up showed a promise but did not again meet a statistical difference. Therefore, OP-1 Putty® received again HDE approval for use as an alternative to autograft in compromised patients requiring revision posterolateral (inter-transverse) lumbar spinal fusion.
InFUSE® (rhBMP-2) was approved by FDA via premarketing approval (PMA) process, in conjunction with the LT-Cage Lumbar Tapered Fusion device for spinal fusion procedures via an anterior approach; the specific indication is for spinal fusion procedures in skeletally mature patients with degenerative disk disease (DDD) at one level from L2-S1 [54–56]. However, large clinical studies conducted using a high dose (40 mg/single-level fusion) of InFUSE® with compressive resistant matrices bulking agents (Amplify™) did not result in a positive outcome; autologous ICBG was used as comparator [57, 58].
The FDA issued a public health notification regarding life-threatening complications associated with InFUSE® in cervical spine fusion used as off-label [59]. These complications were associated with swelling of the neck and throat tissue, which resulted in compression of the airway and/or neurological structures in the neck. Some reports described difficulty in swallowing, breathing, or speaking. Though fewer documented adverse events can be attributed to BMP, certain complications and safety issues are of concern. Adverse events that have been reported include but are not limited to inflammation, unwanted ectopic bone formation, infection, immune responses, vertebral osteolysis, and vertebral edema.
Regulatory agencies, clinical and patient communities, and payers are concerned with the off-label use of current BMP products. The concern is centered on whopping dose of BMPs (e.g., hrBMP-2 applied 12–40 mg for single-level fusion) and the use of animal-sourced collagen (bovine type I collagen) and synthetic ceramics (hydroxyapatite and tricalcium phosphate) as substratum to deliver rhBMP-2 at the implant site [60]. Animal-sourced collagens and ceramics as carriers induce inflammatory cytokine release and immune reactions at the local implant sites. Lower doses of BMPs with appropriate biocompatible and bio-friendly autologous scaffold may provide the optimal bone formation without provoking unwanted ectopic bone formation detailed in the chapter “Osteogrow: A Novel Bone Graft Substitute for Orthopedic Reconstruction.” Future BMP studies are directed to utilize BMPs that have little or no affinity to endogenous BMP antagonists [61] and delivered with an autologous substratum, which does not provoke inflammatory signals and immune responses. For more details, refer to the chapters “BMPs in Orthopedic Medicine: Promises and Challenges” and “Biology of Spine Fusion and Application of Osteobiologics in Spine Surgery”.
6 BMPs in Dentin Repair and Regeneration
Although autograft is a gold standard in dental medicine, because of donor site-associated mobility, BMP-containing bone graft substitutes (BGS) are preferred as it provides robust therapeutic benefit than osteoinductive (e.g., DBM) and osteoconductive (e.g., HA/TCP) biomaterials [62, 63]. The application of BMP-based BGS has its clinical utility in several dentin indications that include alveolar ridge and maxillary sinus augmentation, alveolar cleft and mandibular reconstruction, osteointegration following dentin implants, and periodontium repair. BMP-2- and BMP-7-containing collagen implants and GDF-5-containing hyaluronic implants have been evaluated in dentin preclinical models and in the clinic for various dental indications [64–66]. Obtaining a robust bone formation to speed up the osteointegration for dental implants and avoiding ankyloses to regenerate periodontium with new cementum, ligaments containing sharpie fibers and regeneration of alveolar bone are unmet needs in dental medicine [67]. Application of a given BMP with an appropriate dose and acceptable autologous scaffold in a permissive microenvironment is lacking. The promises and challenges still remain in order to deliver BMP locally with a bio-scaffold that allows lesser inflammation and immune responses and thus allow dental tissue repair and regeneration in space and time. It is unlikely the same dose and same bio-scaffold will serve as therapeutic benefit for all the dental tissue repair and regeneration. For details refer to the chapter “BMPs in Dental Medicine: Promises and Challenges”.
7 BMP-7 in Acute and Chronic Kidney Failure
Although BMP-7 is originally isolated from bone matrix, the predominant site for its synthesis is the kidney [68]. The loss-of-function studies revealed that it is absolutely required for kidney development during embryogenesis [69] and it plays a functional role in the adult kidney and is responsible for vascular and skeletal integrity and modulates calcium and phosphate homeostasis. In preclinical studies, BMP-7 has been shown to provide protection against acute kidney injury (AKI) [70], glomerulosclerosis, diabetic nephropathy, chronic kidney disease (CKD), renal osteodystrophy, lupus nephropathy, and Alport’s syndrome [71, 72]. BMP-7 is available in circulation, and its level correlates with renal function. The mechanism of action studies indicates that BMP-7 suppresses inflammation, improves renal blood flow, preserves tubular structure, reduces interstitial fibrosis, and governs calcium and phosphate homeostasis and subsequently vascular calcification by improving disordered bone remodeling. As BMP-7 is a potent bone-inducing morphogenic protein and forms ectopic ossification at the injection sites, it is believed that enhancing its biology through mimetics and secretagogoues may provide a safe and viable therapy than administering BMP-7 protein systemically. More details can be found in the chapter “Bone Morphogenetic Protein-7 and Its Role in Acute Kidney Injury and Chronic Kidney Failure”.
8 BMPs in Glucose Homeostasis
By employing a functional genomic approach, BMP-9, expressed in the liver, was first identified as a factor that regulates glucose homeostasis as it was shown to suppress hepatic glucose production to reduce insulin resistance and glycemia in diabetic mice [73]. In concurrence with the observation that the kidney is a major site for BMP-7 expression, it serves as autocrine survival factor for podocytes [74] and maintains expression of structural proteins of the foot processes such as synaptopodin and podocin. BMP-7 also inhibits the TGF-β1-activated signaling pathway in mesangial cells and podocytes in vitro. In preclinical models of diabetic nephropathy, BMP-7 was shown to attenuate tubular pro-inflammatory responses by suppressing oxidative stress and multiple inflammatory signaling pathways in the mesangium and proximal tubular epithelium [75]. It is likely that BMP-7 may be useful in delaying diabetic glomerulosclerosis and reversing early podocyte injury. To support BMP-7 biology role in diabetics, a recent study indicates that removal of USAGA-1/Sostdc1, a BMP-7 antagonist, is able to enhance insulin secretion and glucose homeostasis by improving β-cell function under metabolic stress [76]. A metabolic approach of managing glucose homeostasis is through systemic energy homeostasis. Brown adipose tissue (BAT) is responsible for energy utilization by promoting thermogenesis [77]. Again BMP-7 has been shown to promote BAT differentiation and promote thermogenesis in vitro and in vivo suggesting a therapeutic role against obesity [78] and thus to improve glucose uptake and reduce insulin sensitivity. For details, refer to the chapter “Role of BMPs in Inflammation.”
9 BMP-7 and Calcium and Phosphate Homeostasis
The kidney is the site for the production of active 1,25-dihydroxy vitamin D3 from its precursor 25-dihroxy vitamin D3, and the loss of renal function results in vitamin D deficiency (Rickets) which then leads to secondary parathyroidism. The secondary hyperparathyroidism occurs in CKD, which produces a high turnover osteodystrophy that is associated with peritrabecular fibrosis. In animal models of CKD, BMP-7 treatment was shown to eliminate peritrabecular fibrosis, increased “active” osteoblast number, osteoblast surface, mineralizing surface, and significant decrease in the eroded surface [79, 80]. Loss of renal function is also associated with hyperphosphatemia and elevated calcium x phosphate (Ca x P) product, leading to vascular stiffness, dysfunction, and calcification. Hyperphosphatemia has been a known predictor of cardiovascular death, particularly in hemodialysis patients. Vascular smooth muscle cells (VSMC) are very responsive to changes in elevated serum phosphate and undergo a loss of phenotypic expression and differentiate into cell types of the osteoblast lineage. Although phosphate is managed through binders, it is becoming increasingly important to improve vascular tone and elastic modulus of vessel in ESRD patients. Hyperphosphatemia induces the loss of phenotype in VSMCs and induces dedifferentiation into myofibroblast and subsequently their proliferation in culture. In CKD models of hyperphosphatemia, BMP-7 treatment reduces the loss of VSMC phenotype and vascular calcification [81]. The effect of BMP-7 on osteoblast differentiation also reduces the systemic phosphate level thus indirectly has a positive influence on reducing phosphate levels in circulation. In summary, application of BMP-7 biology agonists may likely reduce hyperphosphatemia, secondary parathyroidism-associated osteodystrophy (osteitis fibrosa), and the loss of VSMC phenotype, thus reducing vascular stiffness, dysfunction, and calcification, bone pain, and high fracture incidence in patients with loss of kidney function.
10 BMPs in Iron Homeostasis
Currently, erythropoiesis-stimulating agents (ESA) like erythropoietin, EPO, or iron supplements have been used to manage anemia in CKD/ESRD patients. About 1/3 of patients, however, do not respond to EPO. Oral dietary iron serves as an alternative but is not effective, and IV iron supplement provides some relief but does not overcome anemia successfully. High doses of EPO to manage anemia led to cardiovascular events, stroke, progression of cancer, and death, and because of this, the FDA issued black box warning on the EPO label. Patients nonresponsive to IV iron and EPO end up in iron overloading that associates with high levels of hepcidin in the blood.
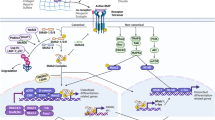
Hepcidin is the iron regulatory hormone (25 amino acid peptides), and its expression is regulated tightly by circulating iron levels [82, 83]. Hepcidin is a ligand for ferroportin, an iron exporter [84]. Upon binding to ferroportin, hepcidin induces an internalization (endocytosis) and subsequently its degradation (proteolysis in lysosomes) [85]. Hepcidin inhibits the export of iron from enterocytes in the duodenum (obtained through dietary intake), reticular endothelial macrophages (recycled through senescent erythrocytes), and hepatocytes (stored intracellularly through ferritin) into the plasma. High level of hepcidin results in “anemia,” and low level of hepcidin results in “hemochromatosis,” a rare hematological disorder.
BMP-6 has been shown to regulate the expression of hepcidin through its downstream smad-1/5/8-dependent pathway [86]. Hemojuvelin (HJV), a glycophospholipid inositol (GPI)-anchored membrane protein, functions as a co-receptor for BMP-6 to enhance the effectiveness of BMP signaling-dependent SMAD pathway to stimulate hepcidin expression by acting on its promoter [87].
Inflammatory cytokine IL-6/JAK2/STAT3 pathway can also stimulate hepcidin expression; however, BMP-HJV-SMAD pathway-based functional SMAD binding is necessary for IL-6/JAK2/STAT3 pathway to effectively enhance hepcidin expression. BMP-6 (−/−) knockout mice showed reduced hepcidin levels in circulation and resemble “hemochromatosis” phenotype [88, 89]. A similar phenotype was also observed in HJV (−/−) mice [90]. Recently, three heterozygous missense mutations in BMP-6 were identified in patients with unexplained iron overload; these mutations lead to loss of signaling to SMAD proteins and reduced hepcidin production [91].
Inhibition of BMP-HJV-SMAD pathway is therefore a novel target to reduce the production of hepcidin in the liver. There are several ways one could approach, for example, the use of a drug that can antagonize BMP signaling (dorsomorphin) and the use of BMP antagonist proteins like gremlin, anti-BMP-6-neutralizing monoclonal antibody, Fc-soluble ActRII-A receptor, activin/BMP/GDF ligand trap, anti-hemojuvelin-neutralizing antibody, and Fc-soluble hemojuvelin, all of which may have some safety concerns, as they are not addressing the specific role of iron-sensing BMP-6 in regulating hepcidin expression with respect to iron homeostasis. For more details on the role of TGF-beta superfamily of proteins in iron homeostasis, refer to the chapter on “The Central Role of BMP Signaling in Regulating Iron Homeostasis”.
11 BMPs’ Role in Rare Genetic Disorders
Pulmonary Arterial Hypertension (PAH)
PAH is a rare disease that occurs neonatal and young children due to poor vascular dilation and abnormal muscularization characterized by a progressive increase in pulmonary vascular resistance [92]. In older children and adults, abnormal vessel and enhanced muscularization occurs in the distal artery [93], all results in progressive intimal and medial thickening leading to occlusive changes and hence elevation in pulmonary arterial pressure [94]. An imbalance between vasodilators and vasoconstrictors has been linked to the onset of PAH [95]. Genetic studies showed a link to mutations in BMPRII among familial PAH (60 %) and idiopathic PAH (10 %–20 %) patients [96–98]. The mutations are spread along the ligand binding domain, kinase domain, and long cytoplasmic tail, all of which can affect negatively BMP-smad downstream signaling. id, a BMP-responding gene, is paramount in governing endothelial and smooth muscle cell growth, perturbing id expression will have consequences [99]. That said, there are people who have BMPRII mutations who do not develop PAH [100]. This makes sense that BMPs do also engage the other type II receptors, ActRII-A and ActRII-B, for signaling, and likely in the absence of functional BMPRII, these receptors may compensate function in certain PAH patients. BMP-9 and TGF-beta utilize ALK-1, type I receptor, and endoglin, a co-receptor to mediate signaling in endothelial cells (ECs). Mutations in endoglin have also been linked to hereditary hemorrhagic telangiectasia which has been linked in some patients with PAH [101, 102]. Overall, no doubt BMP signaling is paramount in governing normal growth of EC and SMC of the pulmonary artery, and perturbation of BMP-smad signaling may have detrimental effects for the onset of PAH. For more information, refer to the chapter on “BMP Signaling in Pulmonary Arterial Hypertension”.
Hereditary Hemochromatosis (HH)
HH is a genetic disorder of iron overload characterized by an excess iron entry into the bloodstream surpassing the requirements for erythropoiesis, resulting in tissue iron deposition and organ dysfunction [103]. As there is no regulated mechanism for the removal of excess iron from the body and the excess iron in patients with HH deposits in other tissues, most notably parenchymal cells of the liver, pancreas, heart, and pituitary gland generate reactive oxygen species leading to tissue damage and ultimately resulting in cirrhosis, diabetes, cardiomyopathy, hypogonadism, arthropathy, and increased skin pigmentation that is characteristic of this disease. Mutations in hfe gene are identified as a causal for HH [104–106]. hfe is atypical major histocompatibility class-I-like protein [107] that competes with transferrin for binding to transferrin receptor-1 as well as transferrin receptor-2 (TRF1/TRF2). Hence, mutations in TRF1 or TRF2 can also result in HH. It is believed that TFR1 in the liver sequesters HFE and when serum levels increase, iron-saturated transferrin displaces hfe from TFF1; thereby, HFE can regulate hepcidin expression possibly by interacting with TRF2 [108, 109]. The precise mechanism by which hfe regulates hepcidin expression is still unknown. The loss of function of hfe studies in mice showed impaired BMP downstream smad signaling and low level of hepcidin expression. This is further corroborated that BMP-6 (−/−) mice and HJV (−/−) mice both exhibit hemochromatosis phenotype and have low level of hepcidin in circulation. For more details, refer to the chapter on “The Central Role of BMP Signaling in Regulating Iron Homeostasis”.
Fibrodysplasia Ossificans Progressiva
Fibrodysplasia ossificans progressiva (FOP) is a rare genetic disorder characterized by progressive extra-skeletal (heterotopic) ossification [110]. Patients with FOP develop progressive heterotopic ossification within soft connective tissues by recapitulating a developmental cascade of endochondral ossification in which cartilage forms initially at the lesion site and is subsequently replaced by the bone [111]. The effects of FOP are accelerated by inflammation and trauma, precluding surgical intervention, and there is an urgent need for an effective treatment. Linkage analysis has led to the identification of a recurrent heterozygous mutation (617G A; R206H) in the type I BMP receptor ALK-2 (ACVR1) [112, 113]. Additional FOP mutations have since been identified in both the GS and kinase domains of ALK-2 that differentially affect the age of onset of ossification, as well as the extent of skeletal malformation. Analyses of a subset of ALK-2 FOP mutants including L196P, R206H, and G356D suggest that FOP mutations are more weakly activating than constitutively active ALK-2, but show similar potential to induce osteogenic differentiation through reduced FKBP12 binding to ALK-2 and increased Smad1/5/8 phosphorylation [114]. A recent study suggests that nonenzymatic scaffolding function provided by type II receptors is required for mutant ALK-2 to exert its function independent of a BMP ligand [115].
The FOP condition can be recapitulated in cultures using muscle cell lines transfected with mutant ALK-2 and in animal models by transgenic overexpression of caALK-2 [116], a classic constitutively active ALK-2 receptor containing the artificial mutation Q207D and knock-in R206H mutation in mice [117]. Furthermore, pluripotent stem cells generated from FOP patients are also being pursued to screen for small molecules that could inhibit chondrocyte/osteoblast differentiation [118, 119]. By using a dorsalization function assay in zebrafish, researchers in Harvard (MGH/Brigham) have identified a BMP inhibitor called dorsomorphin that led to the development of LDN compounds which tend to render a specificity to ALK-2 kinase inhibition and functionally inhibit ALK-2 kinase activity in vitro and ectopic endochondral ossification in mutant ALK-2 FOP transgenic mouse model [120]. Based on ALK-2 crystal structure and kinase inhibition assay, researchers at Oxford have identified yet another BMP inhibitor specific to ALK-2 [121]. In addition to SM BMP inhibitors, researchers are looking at the possibility of intervening FOP mutant ALK-2 activity using siRNA and/or antisense oligonucleotide. Attempts are also being made to inhibit the ectopic differentiation of endochondral ossification using retinoic acid receptor γ agonist [122], a potent stimulator of chondrocyte differentiation.
However, it remains to be established what are the cell types that are cued to manifest heterotopic ossification as a result of FOP-ALK-2 insult. Fascia/skeletal muscle-derived satellite cells/myoblasts, vascular endothelium-derived pericytes/smooth muscle cells, blood-borne inflammatory cells, and endothelial-mesenchymal transition, all of these are contemplated as potential responding cell types. Still it remains elusive how the mechanical/inflammatory signals promote the FOP-ALK-2 insult in vivo. A recent study suggests that anti-activin antibody and ActRII-A/ActRII-B trap are shown to provide therapeutic benefit against FOP mice [123]. For more details, refer to the chapter “BMP Signaling in Fibrodysplasia Ossificans Progressiva, a Rare Genetic Disorder of Heterotopic Ossification”.
Osteogenesis Imperfecta
Osteogenesis imperfecta (OI), also known as “brittle bone disease,” is a collagen-related disorder characterized by low bone mass, increased bone fragility, and decreased bone strength. Dominant osteogenesis imperfecta is caused by defects in the quantity or quality (structure) of type I procollagen, which affects the bone at multiple levels, for example, matrix structure and mineralization. Recessive osteogenesis imperfecta is caused by deficiency of proteins that interact with collagen process collagen and/or affect its posttranslational modification or folding, such as CRTAP, P3H1, and PPIB and Serpin H1 and FKBP10 [124]. The common features of dominant and/or recessive osteogenesis imperfecta are delayed collagen folding and increased endoplasmic reticulum stress effects in the bone and are likely to be the key to understanding its pathogenesis. Bisphosphonates are widely administered to individuals with osteogenesis imperfecta, with positive effects on bone mass and vertebral geometry, but cause a decline in bone material quality in time [125]. In its various types, OI occurs in ~1 in 15,000 in the USA (~20,000–50,000) with mostly autosomal dominant inheritance (about 85 %) and lesser with autosomal recessive (15 %).
The clinical overlap in both dominant and recessive phenotypes of OI is comparable. A recent study for the first time demonstrated an excessive TGF-β signaling as evidenced by an increased ratio of pSMAD2/SMAD2 proteins and higher in vivo SMAD2 reporter activity that corresponds with higher expression of TGF-beta target genes. It is suggested that an alteration in collagen posttranslational modifications results in a dysregulation of matrix-cell signaling contributing to phenotype manifestation [126, 127]. Furthermore, anti-TGF-beta antibody (1D11) treatment demonstrated that treatments restored bone volume, trabecular number, trabecular thickness, and reduced trabecular separation in the lumbar and femur of OI mice comparable to WT mice. Biomechanical testing of femurs showed mice treated with the 1D11 showed significant improvements in bone strength as well. Hence, altered TGF-β matrix-cell signaling is a primary mechanism in the pathogenesis of OI.
As BMP downstream signaling counteracts TGF-β activity, it is likely that BMP biology may serve as therapeutic avenue for OI. To support this notion, recent study showed anti-sclerostin, a BMP antagonist, antibody also effectively restored OI phenotype in mice [128]. Genetic linkage studies found mutations in BMP-1 and collagen C-peptidase as a causal for OI in man [129]. BMP-1 is also responsible for processing certain BMP family proteins from pro-form into active and BMP antagonists like chordin [130]. Since BMPs have direct influence on the differentiation of both bone-forming (osteoblast) and bone-resorbing (osteoclast) cells and the bone undergoes a high turnover in OI skeleton, BMP biology-based therapy could be administered intermittently in combination with antiresorptive agents like bisphosphonate.
12 BMP in Oncology
The BMP signaling pathway involves many ligands, receptors, and antagonists extracellularly and downstream signaling smads-1/5/8 and co-smad-4 and inhibitory smads-6/7 intracellularly, all of which are capable of impacting tumor growth and progression, both positively and negatively [131]. The effects of BMP on tumor growth are based on specific BMP, are dose- and context-dependent, and are associated with either increased or decreased survival. For example, in ovarian carcinoma, the MSCs that recruited at the tumor microenvironment exhibit a phenotype that expresses high levels of BMP-2, BMP-4, and BMP-6 [132]. On the contrary, in primary mammary tumor, BMP-7 expression is reduced which is accompanied by enhanced TGF-beta activity and EMT transition that leads to bone metastasis [133]. Aberrant expression of BMP ligands and their respective receptors and subsequently dysregulation of downstream signaling can influence growth inhibitory genes (e.g., id1-3) [134] and tumor suppressor genes (e.g., p53) [135, 136] and promote epithelial-mesenchymal transition [137], stromal cell proliferation [132], angiogenesis [138], inflammation, and immunosuppression to promote tumor growth and metastasis. Depending on the tumor cell type (carcinoma versus sarcoma) and stage (primary versus metastasis), BMPs can affect cancer growth and its progression and modulate responsiveness to endocrine and metabolic factors [139].
As an example, low expression of BMP-7 can shift a cell phenotype from androgen-dependent to androgen-independent activity in primary prostate tumor cells, and the loss of endogenous BMP-7 may encourage the prostate cancer cells to be more aggressive [133]. However, BMP-7 can be reexpressed once cancer cells metastasized in the bone suggesting when to consider BMP-based therapy for targeting to curtail cancer growth [140, 141]. Likewise, not all BMPs are the same when it comes to angiogenesis; BMP-2, BMP-4, BMP-6, BMP-7, and GDF-5 are pro-angiogenic, while BMP-9 and BMP-10 are anti-angiogenic; thus, to inhibit angiogenesis, natural BMP antagonists like noggin can be used to target pro-angiogenic BMPs, and recombinant BMP-9 and BMP-10 can be used to suppress angiogenesis [142, 143]. However, in certain cancers, the attenuation of BMP-9-induced ALK-1, a BMP type I receptor, signaling with neutralizing antibody and small molecule was able to inhibit endothelial cell sprouting [144–146]. PF- 03446962, an antibody against ALK-1 (Pfizer), and dalantercept, a soluble chimeric protein (ALK1-Fc) which displays high-affinity binding with BMP-9 and BMP-10, have been shown as potent inhibitors for blocking the development of blood vessels [147, 148]. An endoglin antibody, also known as CD105, a co-receptor of BMP-9 and TGF-β that mediates a transition of endothelial cells from quiescent to active status during angiogenesis through preferential phosphorylation of SMAD 1/5/8, has also exhibited anti-angiogenic potential [149, 150]. Overall, BMPs and their signaling pathways play critical roles in the development, progression, and metastasis of various cancers in part by governing with their involvement in angiogenesis, inflammation, and immunosuppression and thus may serve as promising targets for therapeutic potential. Taken together, it remains to be seen that targeting one specific receptor with small molecule or an antibody or Fc conjugates could render the required outcome, as tumorigenesis is a result of a disturbed cascade of several biological events. For more details of the role of BMP signaling in mammary tumor growth and regulation, refer to the chapter on “Bone Morphogenetic Proteins in the Initiation and Progression of Breast Cancer”.
13 Conclusion
BMPs are highly conserved from fly to man. The systems biology of BMP is a prerequisite for most of tissue induction during development and recapitulates it in adult tissue repair, regeneration, and homeostasis. The outcome of tissue induction/responsiveness is dictated by the responding cell than by BMP signal. BMP governs its function through a concentration gradient and is context-dependent in a permissive microenvironment. There are several BMPs, BMP antagonists, and receptors to govern its function as and when needed and to govern the inductive events in control fashion. Extracellular matrices and various BMP-specific antagonists that interact with BMP ligands add to that regulation. An aberrant expression in either ligand or receptor or antagonist can dictate unwanted cell growth and differentiation than required for normalcy. Thus far, BMP-based biologics have been approved for use only for local bone formation. There are several BMP-based therapeutics that are being evaluated in the clinic as drugs and/or biologics to improve tissue function against parenchymal fibrosis and to curtail angiogenesis in certain rare genetic disorders like FOP and anemia. Overall, the systems biology of BMP is promising, but the challenges are abundant as it comes to applying safely to achieve the required outcome in the clinic.
References
Cunningham NS, Paralkar V, Reddi AH (1992) Osteogenin and recombinant bone morphogenetic protein 2b are chemotactic for human monocytes and stimulate transforming growth factor β1 mRNA expression. Proc Natl Acad Sci U S A 89:11740–11744
Zhang W, Zhu C, Wu Y, Ye D, Wang S, Zou D, Zhang X, Kaplan DL, Jiang X (2014) VEGF and BMP-2 promote bone regeneration by facilitating bone marrow stem cell homing and differentiation. Eur Cell Mater 27:1–11
Kann S, Chiu R, T M, SB G (2010) OP-1 (BMP-7) stimulates osteoprogenitor cell differentiation in the presence of polymethylmethacrylate particles. J Biomed Mater Res A 94(2):485–488
Reddi AH, Reddi A (2009) Bone morphogenetic proteins (BMPs): from morphogens to metabologens. Cytokine Growth Factor Rev 20(5–6):341–342
Zhang J, Li L (2005) BMP signaling and stem cell regulation. Dev Biol 284(1):1–11
Matsuda S, Harmansa S, Affolter M (2016) BMP morphogen gradients in flies. Cytokine Growth F Rev 27:119–127
Bier E, De Robertis EM (2015) EMBRYO DEVELOPMENT. BMP gradients: a paradigm for morphogen-mediated developmental patterning. Science 348(6242):aaa5838. doi:10.1126/science.aaa5838
Hogan BL (1996) Bone morphogenetic proteins in development. Curr Opin Genet Dev 6:432–443
Dudley AT, Lyons KM, Robertson EJ (1995) A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9:2795–2807
Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G (1995) BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9:2808–2820
Zhang H, Bradley A (1996) Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122:2977–2986
Bellusci S, Henderson R, Winnier G, Oikawa T, BL H (1996) Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development 122(6):1693–1702
Kishigami S, Mishina Y (2005) BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev 16:265–278
Reddi AH, Huggins CB (1972) Biochemical sequence in the transformation of fibroblasts into cartilage and bone. Proc Natl Acad Sci U S A 69:1601–1605
Sampath TK, Reddi AH (1981) Dissociative extraction and reconstitution of extracellular matrix components involved in local bone differentiation. Proc Natl Acad Sci U S A 78:7599–7602
Sampath TK, Maliakal JC, Hauschka PV, Jones WK, Sasak H, Tucker RF, White KH, Coughlin JE, Tucker MM, Pang RH et al (1992) Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem 267:20352–20362
Sampath TK, Rueger DC (1994) Structure, function and orthopedic application of osteogenic protein-1 (OP-1). Complicat Orthopedics 9:101–107
Massague J (1990) The transforming growth factor-β family. Annu Rev Cell Biol 6:597–641
Griffith DL, Keck PC, Sampath TK, Rueger DC, Carlson WD (1996) Three-dimensional structure of recombinant human Osteogenic Protein-1: structure paradigm for the transforming growth factor β superfamily. Proc Natl Acad Sci U S A 93:878–883
Massagué J (1998) TGF-β signal transduction. Annu Rev Biochem 67:753–791
ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin C-H, Miyazono K (1994) Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem 269:16985–16988
Heldin C-H, Miyazono K, ten Dijke P (1997) TGF-β signaling from cell membrane to nucleus via Smad proteins. Nature 390:465–471
Yanagita M (2008) Bone morphogenetic protein antagonists and kidney. In: Vukicevic S, Sampath TK (eds) Bone morphogenetic proteins: from local to systemic therapeutics. Birkhauser, Basel, pp. 213–232
Vukicevic S, Sampath TK (eds)Bone morphogenetic proteins: from local to systemic therapeutics. Birkhauser Verlag, Basel
Macias D, Gañan Y, Sampath TK, Piedra ME, Ros MA, Hurle JM (1997) Role of BMP-2 and OP-1 (BMP-7) in programmed cell death and skeletogenesis during chick limb development. Development 124(6):1109–1117
Rosen V, Nove J, Song JJ, Thies RS, Cox K, Wozney JM (1994) Responsiveness of clonal limb bud cell lines to bone morphogenetic protein 2 reveals a sequential relationship between cartilage and bone cell phenotypes. J Bone Miner Res 9(11):1759–1768
Sato K, Miura T, Iwata H (1988) Cartilaginous transdifferentiation of rat synovial cells under the influence of bone morphogenetic protein in tissue culture. Clin Orthop Relat Res 236:233–239
Olnot C (2009) Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res 24:274–282
Lietman SA, Yamagishita M, Sampath TK, Reddi AH (1997) Stimulation of proteoglycan synthesis in porcine articular cartilage explants by recombinant Osteogenic Protein-1 (BMP-7). J Bone Joint Surg 79:1132–1136
Maliakal JC, Asahina I, Hauschka PV, Sampath TK (1994) Osteogenic protein-1 (BMP-7) inhibits cell proliferation and stimulates the expression of markers characteristic of osteoblast phenotype in rat osteosarcoma (17/2.8) cells. Growth Factors 11(3):227–234
Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T (1994) Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127(6 Pt 1):1755–1766
Thies RS, Bauduy M, Ashton BA, Kurtzberg L, Wozney JM, Rosen V (1992) Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology 130(3):1318–1324
Hollnagel A, Oehlmann V, Heymer J, Rüther U, Nordheim A (1999) Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem 274(28):19838–19845
Zehentner BK, Dony C, Burtscher H (1999) The transcription factor Sox9 is involved in BMP-2 signaling. J Bone Miner Res 14(10):1734–1741
Ito Y, Miyazono K (2003) RUNX transcription factors as key targets of TGF-beta superfamily signaling. Curr Opin Genet Dev 13(1):43–47
Huang L, Teng XY, Cheng YY, Lee KM, Kumta SM (2004) Expression of preosteoblast markers and Cbfa-1 and Osterix gene transcripts in stromal tumour cells of giant cell tumour of bone. Bone 34(3):393–401
Flechtenmacher J, Huch K, Thonar EJ-MA, Mollenhauer JA, Davies SR, Schmid TM, Puhl W, Sampath TK, Adelotte MB, Kuettner KE (1996) Recombinant human osteogenic protein-1is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum 39:1896–1904
Logeart-Avramoglou D, Bourguignon M, Oudina K, Ten Dijke P, Petite H (2006) An assay for the determination of biologically active bone morphogenetic proteins using cells transfected with an inhibitor of differentiation promoter-luciferase construct. Anal Biochem 349(1):78–86
Matsumoto Y, Ikeya M, Hino K, Horigome K, Fukuta M, Watanabe M, Nagata S, Yamamoto T, Otsuka T, Toguchida J (2015) New protocol to optimize iPS cells for genome analysis of fibrodysplasia ossificans progressiva. Stem Cells 33:1730–1742
Reddi AH (1998) Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol 16:247–252
Minas T, Chiu R (2000) Autologous chondrocyte implantation. Am J Knee Surg 13(1):41–50
Rueger DC, Chubinskaya S (2004) Bone morphogenetic proteins in articular cartilage repair. In: Vukicevic S, Sampath TK (eds) Bone morphogenetic proteins: regeneration of bone and beyond. Birkhauser Verlag, Basel
Flechtenmacher J, Huch K, Thonar EJ-MA, Mollenhauer JA, Davies SR, Schmid TM, Puhl W, Sampath TK, Adelotte MB, Kuettner KE (1996) Recombinant human osteogenic protein-1is a potent stimulator of the synthesis of cartilage proteoglycans and collagens by human articular chondrocytes. Arthritis Rheum 39:1896–1904
Merrihew K, Kumar B, Heretis K, Rueger DC, Kuettner KE, Chubinskaya S (2003) Alterations in endogenous osteogenic protein-1 with degeneration of human articular cartilage. J Orthop Res 21:899–907
Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Marticic D, McCartney J, Yin s, Rueger D, Vukicevic S (2001) Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors 19:101–113
Hayashi M, Muneta T, Takahashi T, Ju YJ, Tsuji K, Sekiya I (2010) Intra-articular injections of bone morphogenetic protein-7 retard progression of existing cartilage degeneration. J Orthop Res 28:1502–1506
Hunter DJ, Pike MC, Jonas BL, Kissin E, Krop J, McAlindon T (2010) Phase 1 safety and tolerability study of BMP-7 in symptomatic knee osteoarthritis. BMC Musculoskelet Disord 11:232
Medtronic Sofamor Danek USA, Inc. (2006) INFUSE bone graft product information: oral/facial. Memphis. Available online at www.accessdata.fda.gov/cdrh_docs/pdf5/P050053c.pdf. Last accessed Feb 2010
Stryker Biotech (2009) OP-1 Implant® product information. Hopkinton. Available online at www.stryker.com/stellent/groups/public/documents/webprod/126737.pdf. Last accessed Feb 2010
Stryker Biotech (2009) OP-1 Putty® product information. Hopkinton. Available online at www.stryker.com/stellent/groups/public/documents/webprod/127024.pdf. Last accessed Feb 2010
Friedlaender GE, Perry CR, Cole JD, Cook SD, Clerny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S (2001) Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial non-unions: a prospective randomized clinical trial comparing rhOP-1 with fresh bone autograft. J Bone Joint Surg Am 83(Suppl 1):S151–S158
Vaccaro AR, Patel T, Fischgrund J et al (2004) A pilot study evaluating the safety and efficacy of OP-1 Putty (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis for degenerative spondylolisthesis. Spine 29:1885–1892
Vaccaro AR, Lawrence JP, Patel T et al (2008) The safety and efficacy of OP-1 (rhBMP-7) as a replacement for iliac crest autograft in posterolateral lumbar arthrodesis: a long-term (>4 years) pivotal study. Spine 33:2850–2862
Boden SD, Zdeblick TA, Sandhu HS et al (2000) The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine 25:376–381
Burkus JK, Gornet MF, Dickman CA et al (2002) Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech 15:337–349
Burkus JK, Dorchak JD, Sanders DL (2003) Radiographic assessment of interbody fusion using recombinant human bone morphogenetic protein type 2. Spine 28:372–377
Dawson E, Bae HW, Burkus JK et al (2009) Recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge with an osteoconductive-bulking agent in posterolateral arthrodesis with instrumentation. A prospective randomized trial. J Bone Joint Surg Am 91:1604–1613
Dimar JR 2nd, Glassman SD, Burkus JK et al (2009) Clinical and radiographic analysis of an optimized rhBMP-2 formulation as an autograft replacement in posterolateral lumbar spine arthrodesis. J Bone Joint Surg Am 91:1377–1386
Crawford CH 3rd, Carreon LY et al (2009) Perioperative complications of recombinant human bone morphogenetic protein-2 on an absorbable collagen sponge versus iliac crest bone graft for posterior cervical arthrodesis. Spine 34:1390–1394
Wong DA, Kumar A, Jatana S et al (2008) Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J 8:1011–1018
Song K, Krause C, Shi S et al (2010) Identification of a key residue mediating bone morphogenetic Protein (BMP)-6 resistance to noggin inhibition allows for engineered BMPs with superior agonist activity. J Biol Chem 285:12169–12180
Wikesjö UME, Qahash M, Huang Y-H, Xiropaidis AV, Polimeni G, Susin C (2009) Bone morphogenetic proteins for periodontal and alveolar indications; Biological observations – clinical implications. Orthod Craniofac Res 12:263–270
Ripamonti U, Herbst NN, Ramoshebi LN (2005) Bone morphogenetic proteins in craniofacial and periodontal tissue engineering: experimental studies in the non-human primate Papio ursinus. Cytokine Growth Factor Rev 16(3):357–368
Weng D, Pöhling S, Pippig S, Bell M, Richter EJ, Zuhr O, Hürzeler MB (2009) The effects of recombinant human growth/differentiation factor-5 (rhGDF-5) on bone regeneration around titanium dental implants in barrier membrane-protected defects: a pilot study in the mandible of beagle dogs. Int J Oral Maxillofac Implants 24:31–37
Roldán JC, Jepsen S, Schmidt C, Knüppel H, Rueger DC, Açil Y, Terheyden H (2004) Sinus floor augmentation with simultaneous placement of dental implants in the presence of platelet-rich plasma or recombinant human bone morphogenetic protein-7. Clin Oral Implants Res 15:716–723
Koch FP, Becker J, Terheyden H, Capsius B, Wagner W (2010) A prospective, randomized pilot study on the safety and efficacy of recombinant human growth and differentiation factor-5 coated onto β -tricalcium phosphate for sinus lift augmentation. Clin Oral Implants Res 21:1301–1308
Nakashima M, Reddi AH (2003) The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol 21(9):1025–1032
Ozkaynak E, Schnegelsberg PN, Oppermann H (1991) Murine osteogenic protein-1 (OP-1): high levels of mRNA in kidney. Biochem Biophys Res Commun 179:116–123
Vukicevic S, Kopp JB, Luyten FB, Sampath TK (1996) Induction of nephrogenic mesenchyme by osteogenic protein-1 (bone morphogenetic protein 7). Proc Natl Acad Sci U S A 93:9021–9026
Vukicevic S, Basic V, Rogic D, Basic N, Shih M, Shepard A, Jin D, Dattatreyamurty B, Jones W, Dorai H et al (1998) Osteogenic protein-1 (bone morphogenetic protein-7) reduces severity of injury after ischemic acute renal failure in rat. J Clin Invest 102:202–214
Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R (2003) BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nat Med 9:964–968
Zeisberg M, Bottiglio C, Kumar N, Maeshima Y, Strutz F, Müller GA, Kalluri R (2003) Bone morphogenic protein-7 inhibits progression of chronic renal fibrosis associated with two genetic mouse models. Am J Physiol Renal Physiol 285:F1060
Chen C, Grzegorzewski KJ, Barash S, Zhao Q, Schneider H, Wang Q, Singh M, Pukac L, Bell AC, Duan R, Coleman T, Duttaroy A, Cheng S, Hirsch J, Zhang L, Lazard Y, Fischer C, Barber MC, Ma ZD, Zhang YQ, Reavey P, Zhong L, Teng B, Sanyal I, Ruben SM, Blondel O, Birse CE (2003) An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat Biotechnol 21(3):294–301
Mitu GM, Wang S, Hirschberg R (2007) BMP7 is a podocyte survival factor and rescues podocytes from diabetic injury. Am J Physiol Renal Physiol 293(5):F1641–F1648
Wang SN, Lapage J, Hirschberg R (2001) Loss of tubular bone morphogenetic protein 7 in diabetic nephropathy. J Am Soc Nephrol 12:2392–2399
Henley KD, Gooding KA, Economides AN, Gannon M (2012) Inactivation of the dual Bmp/Wnt inhibitor Sostdc1 enhances pancreatic islet function. Am J Physiol Endocrinol Metab 303(6):E752–E761
Tseng YH et al (2008) New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454:1000–1004
Boon MR, van den Berg SA, Wang Y, van den Bossche J, Karkampouna S, Bauwens M, De Saint-Hubert M, van der Horst G, Vukicevic S, de Winther MP, Havekes LM, Jukema JW, Tamsma JT, van der Pluijm G, van Dijk KW, Rensen PC (2013) BMP7 activates brown adipose tissue and reduces diet-induced obesity only at sub-thermoneutrality. PLoS One 8(9):e74083
González EA, Lund RJ, Martin KJ, McCartney JE, Tondravi MM, Sampath TK, Hruska KA (2002) Treatment of a murine model of high-turnover renal osteodystrophy by exogenous BMP-7. Kidney Int 61(4):1322–1331
Lund RJ, Davies MR, Hruska KA (2002) Bone morphogenetic protein-7: an anti-fibrotic morphogenetic protein with therapeutic importance in renal disease. Curr Opin Nephrol Hypertens 11(1):31–36
Davies MR, Lund RJ, Hruska KA (2003) BMP-7 is an efficacious treatment of vascular calcification in a murine model of atherosclerosis and chronic renal failure. Am Soc Nephrol 14(6):1559–1567
Nicolas G et al (2002) The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest 110:1037–1044. doi:10.1172/JCI200215686
Ganz T (2003) Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. Blood 102:783–788
Donovan A, Brownlie A, Zhou Y et al (2000) Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403:776–781
Nemeth E, Tuttle MS, Powelson J (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306:2090–2093
Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY (2007) Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest 117(7):1933–1939
Babitt JL, Huang FW, Wrighting DM, Xia Y, Sidis Y, Samad TA, Campagna JA, Chung RT, Schneyer AL, Woolf CJ et al (2006) Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet 38:531–553
Andriopoulos B Jr, Corradini E, Xia Y, Faasse SA, Chen S, Grgurevic L, Knutson MD, Pietrangelo A, Vukicevic S, Lin HY, Babitt JL (2009) BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet 41:482–487
Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP (2009) Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet 41:478–481
Bartnikas TB, Fleming MD (2012) Hemojuvelin is essential for transferrin-dependent and transferrin-independent hepcidin expression in mice. Haematologica 97(2):189–192
Daher R, Kannengiesser C, Houamel D, Lefebvre T, Bardou-Jacquet E, Ducrot N, de Kerguenec C, Jouanolle AM, Robreau AM, Oudin C, Le Gac G, Moulouel B, Loustaud-Ratti V, Bedossa P, Valla D, Gouya L, Beaumont C, Brissot P, Puy H, Karim Z, Tchernitchko D (2016) Heterozygous mutations in BMP6 pro-peptide lead to inappropriate hepcidin synthesis and moderate iron overload in humans. Gastroenterol 150(3):672–683
Morrell NW et al (2009) Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 54:S20–S31
Masri FA et al (2007) Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 293:L548–L554
McLaughlin VV, Archer SL, Badesch DB, Barst HW, Linder JR et al (2009) ACCF/AHA 2009 expert consensus document on Pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society Inc.’ and the Pulmonary Hypertension Association. J Am Coll Cardiol 53:1573–619
Christman BW et al (1992) An imbalance between the excretion of thromboxane and prostacyclin metabolites in pulmonary hypertension. N Engl J Med 327:70–75
Yu PB, Deng DY, Beppu H, Hong CC, Lai C, Hoyng SA et al (2008) Bone morphogenetic protein (BMP) type II receptor is required for BMP-mediated growth arrest and differentiation in pulmonary artery smooth muscle cells. J Biol Chem 283(7):3877–3888
Lane KB et al (2000) Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. Inter PPH Consortium Nat Genet 26:81–84
Hong KH et al (2008) Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension. Circulation 118:722–730
Yang J et al (2008) Mutations in bone morphogenetic protein type II receptor cause dysregulation of Id gene expression in pulmonary artery smooth muscle cells: implications for familial pulmonary arterial hypertension. Circ Res 102:1212–1221
Trembath RC et al (2001) Clinical and molecular genetic features of pulmonary hypertension in patients with hereditary hemorrhagic telangiectasia. N Engl J Med 345:325–334
Yang J et al (2008) Mutations in bone morphogenetic protein type II receptor cause dysregulation of Id gene expression in pulmonary artery smooth muscle cells: implications for familial pulmonary arterial hypertension. Circ Res 102:1212–1221
Harrison RE et al (2003) Molecular and functional analysis identifies ALK-1 as the predominant cause of pulmonary hypertension related to hereditary haemorrhagic telangiectasia. J Med Genet 40:865–871
Powell LW, Seckington RC, Deugnier Y (2016). Haemochromatosis. Lancet 388(10045):706–16. doi:10.1016/S0140–6736(15)01315-X. [Epub ahead of print]
Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN (2009) Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology 50:1992–2000
Ahmad KA, Ahmann JR, Migas MC et al (2002) Decreased liver hepcidin expression in the Hfe knockout mouse. Blood Cells Mol Dis 29(3):361–366
Corradini E, Garuti C, Montosi G, Ventura P, Andriopoulos B Jr, Lin HY, Pietrangelo A, Babitt JL (2009) Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology 137:1489–1497
Feder JN, Gnirke A, Thomas W et al (1996) A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet 13(4):399–408
Goswami T, Andrews NC (2006) Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem 281:28494–28498
Gao J, Chen J, Kramer M, Tsukamoto H, Zhang AS, Enns CA (2009) Interaction of the hereditary hemochromatosis protein HFE with transferrin receptor 2 is required for transferrin-induced hepcidin expression. Cell Metab 9:217–227
Cohen RB, Hahn GV, Tabas JA, Peeper J, Levitz CL, Sando A, Sando N, Zasloff M, Kaplan FS (1993) The natural history of heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. A study of forty-four patients. J Bone Joint Surg Am 75(2):215–219
Kaplan FS, Tabas JA, Gannon FH, Finkel G, Hahn GV, Zasloff MA (1993) The histopathology of fibrodysplasia ossificans progressiva. An endochondral process. J BoneJoint Surg Am 75(2):220–230
Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS (2006) A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressive. Nat Genet 38:525–527
Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Koster B, Pauli RM, Reardon W, Zaidi SA, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM (2009) Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat 30:379–390
Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, Mundlos S, Seemann P, Kaplan FS, Mullins MC, Shore EM (2009) The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest 119:3462–3472
Bagarova J, Vonner AJ, Armstrong KA, Börgermann J, Lai CS, Deng DY, Beppu H, Alfano I, Filippakopoulos P, Morrell NW, Bullock AN, Knaus P, Mishina Y, Yu PB (2013) Constitutively active ALK2 receptor mutants require type II receptor cooperation. Mol Cell Biol 33(12):2413–2424
Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, Endo K, Awakura T, Fukushi J, Nakashima Y, Chiyonobu T, Kawara A, Nishida Y, Wada I, Akita M, Komori T, Nakayama K, Nanba A, Maruki Y, Yoda T, Tomoda H, Yu PB, Shore EM, Kaplan FS, Miyazono K, Matsuoka M, Ikebuchi K, Ohtake A, Oda H, Jimi E, Owan I, Okazaki Y, Katagiri T (2009) Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem 284(11):7149–7156
Chaikuad A, Alfano I, Kerr G, Sanvitale CE, Boergermann JH, Triffitt JT, von Delft F, Knapp S, Knaus P, Bullock AN (2012) Structure of the bone morphogenetic protein receptor ALK2 and implications for fibrodysplasia ossificans progressiva. J Biol Chem 287(44):36990–36998
Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD (2008) BMP type I receptor inhibition reduces heterotopic ossification. Nat Med 14:1363–1369
Cai J, Orlova VV, Cai X, Eekhoff EM, Zhang K, Pei D, Pan G, Mummery CL, Ten Dijke P (2015) Induced pluripotent stem cells to model human fibrodysplasia ossificans progressiva. Stem Cell Reports 5(6):963–970. doi: 10.1016/j.stemcr.2015.10.020. Epub 2015 Nov 26
Hao J, Daleo MA, Murphy CK, Yu PB, Ho JN, Hu J, Peterson RT, Hatzopoulos AK, Hong CC (2008) Dorsomorphin, a selective small molecule inhibitor of BMP signaling, promotes cardiomyogenesis in embryonic stem cells. PLoS One 3(8):e2904. doi:10.1371/journal.pone.0002904
Sanvitale CE, Kerr G, Chaikuad A, Ramel M-C, Mohedas AH, Reichert S, Wang Y, Triffitt JT, Cuny GD, Paul YB, Hill CS, Bullock AN (2013) A new class of small molecule inhibitor of BMP signaling. PLoS One 8:e62721
Chakkalakal SA, Uchibe K, Convente MR, Zhang D, Economides AN, Kaplan FS, Pacifici M, Iwamoto M, Shore EM 2016. Palovarotene inhibits heterotopic ossification and maintains limb mobility and growth in mice with the human ACVR1R206H Fibrodysplasia Ossificans Progressiva (FOP) mutation. J Bone Miner Res. doi: 10.1002/jbmr.2820. [Epub ahead of print]
Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L, Wen X, Nannuru KC, Jimenez J, Xie L, Das N, Makhoul G, Chernomorsky R, D’Ambrosio D, Corpina RA, Schoenherr CJ, Feeley K, Yu PB, Yancopoulos GD, Murphy AJ, Economides AN (2015) ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med 7(303):303ra137. doi:10.1126/scitranslmed.aac4358
Forlino A, Marini JC (2016) Osteogenesis imperfecta. Lancet 387(10028):1657–1671
Palomo T, Fassier F, Ouellet J, Sato A, Montpetit K, Glorieux FH, Rauch F (2015) Intravenous bisphosphonate therapy of young children with osteogenesis imperfecta: skeletal findings during follow up throughout the growing years. J Bone Miner Res 30(12):2150–2157. doi:10.1002/jbmr.2567. Epub 2015 Jun 30
Gebken J, Brenner R, Feydt A, Notbohm H, Brinckmann J, Müller PK, Bätge B (2000) Increased cell surface expression of receptors for transforming growth factor-beta on osteoblasts from patients with Osteogenesis imperfecta. Pathobiol 68(3):106–112
Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, Bertin T, Munivez E, Chen Y, Dawson B, Ishikawa Y, Weis MA, Sampath TK, Ambrose C, Eyre D, HP B, Lee B (2014) Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med 20:670–675
Grafe I, Alexander S, Yang T, Lietman C, EP H, Munivez E, Chen Y, MM J, Bertin T, Dawson B, Asuncion F, HZ K, MS O, Lee B (2016) Sclerostin antibody treatment improves the bone phenotype of Crtap (−/−) mice, a model of recessive osteogenesis imperfecta. J Bone Miner Res 31:1030–1040
Cho SY, Asharani PV, Kim OH, Iida A, Miyake N, Matsumoto N, Nishimura G, Ki CS, Hong G, Kim SJ, Sohn YB, Park SW, Lee J, Kwun Y, Carney TJ, Huh R, Ikegawa S, Jin DK (2015) Identification and in vivo functional characterization of novel compound heterozygous BMP1 variants in osteogenesis imperfecta. Hum Mutat 36(2):191–195
Vadon-Le Goff S, DJ H, Moali C (2015) BMP-1/tolloid-like proteinases synchronize matrix assembly with growth factor activation to promote morphogenesis and tissue remodeling. Matrix Biol 44–46:14–23
Sánchez-Duffhues G, Hiepen C, Knaus P, Ten Dijke P (2015) Bone morphogenetic protein signaling in bone homeostasis. Bone 80:43–59
McLean K, Gong Y, Choi Y, Deng N, Yang K, Bai S, Cabrera L, Keller E, McCauley L, Cho KR, RJ B (2011) Human ovarian carcinoma–associated mesenchymal stem cells regulate cancer stem cells and tumorigenesis via altered BMP production. J Clin Invest 121:3206–3219
Buijs JT, Henriquez NV, van Overveld PG, van der Horst G, ten Dijke P, van der Pluijm G (2007) TGF-β and BMP7 interactions in tumour progression and bone metastasis. Clin Exp Metastasis 24:609–617
Langenfeld E, Deen M, Zachariah E, Langenfeld J (2013) Small molecule antagonist of the bone morphogenetic protein type I receptors suppresses growth and expression of Id1 and Id3 in lung cancer cells expressing Oct4 or nestin. Mol Cancer 12(1):129
Liu H, Jia D, Li A, Chau J, He D, Ruan X, Liu F, Li J, He L, Li B (2013) p53 regulates neural stem cell proliferation and differentiation via BMP-Smad1 signaling and Id1. Stem Cells Dev 22(6):913–927. doi:10.1089/scd.2012.0370. Epub 2013 Jan 30
Yan W, Chen X (2007) Targeted repression of bone morphogenetic protein 7, a novel target of the p53 family, triggers proliferative defect in p53-deficient breast cancer cells. Cancer Res 67:9117–9124
Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, Wu CC, LeBleu VS, Kalluri R (2015) Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527(7579):525–530
Ye L, Jiang WG (2016) Bone morphogenetic proteins in tumour associated angiogenesis and implication in cancer therapies. Cancer Lett 380(2):586–597
Ye L, Mason MD, Jiang WG (2011) Bone morphogenetic protein and bone metastasis, implication and therapeutic potential. Front Biosci (Landmark Ed) 16:865–897
Morrissey C, Brown LG, Pitts TE, Vessella RL, Corey E (2010) Bone morphogenetic protein 7 is expressed in prostate cancer metastases and its effects on prostate tumor cells depend on cell phenotype and the tumor microenvironment. Neoplasia 12(2):192–205
Katsuno Y, Hanyu A, Kanda H, Ishikawa Y, Akiyama F, Iwase T, Ogata E, Ehata S, Miyazono K, Imamura T (2008) Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 27:6322–6333
Yoshimatsu Y, Lee YG, Akatsu Y, Taguchi L, Suzuki HI, Cunha SI, Maruyama K, Suzuki Y, Yamazaki T, Katsura A, Oh SP, Zimmers TA, Lee SJ, Pietras K, Koh GY, Miyazono K, Watabe T (2013) Bone morphogenetic protein-9 inhibits lymphatic vessel formation via activin receptor-like kinase 1 during development and cancer progression. Proc Natl Acad Sci U S A 110(47):18940–18945
Hawinkels LJ, Garcia de Vinuesa A, Ten Dijke P (2013) Activin receptor-like kinase 1 as a target for anti-angiogenesis therapy. Expert Opin Investig Drugs 22(11):1371–1383
Kerr G, Sheldon H, Chaikuad A, Alfano I, von Delft F, Bullock AN, Harris AL (2015) A small molecule targeting ALK1 prevents Notch cooperativity and inhibits functional angiogenesis. Angiogenesis 18(2):209–217
Simonelli M, Zucali P, Santoro A, Thomas MB, de Braud FG, Borghaei H, Berlin J, Denlinger CS, Noberasco C, Rimassa L, Kim TY, English PA, Abbattista A, Gallo Stampino C, Carpentieri M, Williams JA (2016) Phase I study of PF-03446962, a fully human monoclonal antibody against activin receptor-like kinase 1 in patients with hepatocellular carcinoma Ann Oncol. pii: mdw240. [Epub ahead of print]
Doi T, Lee KH, Kim TM, Ohtsu A, Kim TY, Ikeda M, Yoh K, Gallo Stampino C, Hirohashi T, Suzuki A, Fujii Y, Andrew Williams J, Bang YJ (2016) A phase I study of the human anti-activin receptor-like kinase 1 antibody PF-03446962 in Asian patients with advanced solid tumors Cancer Med. doi:10.1002/cam4.724. [Epub ahead of print]
Hawinkels LJ, de Vinuesa AG, Paauwe M, Kruithof-de Julio M, Wiercinska E, Pardali E, Mezzanotte L, Keereweer S, Braumuller TM, Heijkants RC, Jonkers J, Löwik CW, Goumans MJ, ten Hagen TL, ten Dijke P (2016) Activin receptor-like Kinase 1 ligand trap reduces microvascular density and improves chemotherapy efficiency to various solid tumors. Clin Cancer Res 22(1):96–106
Makker V, Filiaci VL, Chen LM, Darus CJ, Kendrick JE, Sutton G, Moxley K, Aghajanian C (2015) Phase II evaluation of dalantercept, a soluble recombinant activin receptor-like kinase 1 (ALK1) receptor fusion protein, for the treatment of recurrent or persistent endometrial cancer: an NRG Oncology/Gynecologic Oncology Group Study 0229N. Gynecol Oncol 138(1):24–29
Apolo AB, Karzai FH, Trepel JB, Alarcon S, Lee S, Lee MJ, Tomita Y, Cao L, Yu Y, Merino MJ, Madan RA, Parnes HL, Steinberg SM, Rodriguez BW, Seon BK, Gulley JL, Arlen PM, Dawson NA, Figg WD, Dahut WL (2016) A phase II clinical trial of TRC105 (anti-endoglin antibody) in adults with advanced/metastatic urothelial carcinoma. Clin Genitourin Cancer. pii: S1558–7673(16)30136–7
Duffy AG, Ulahannan SV, Cao L, Rahma OE, Makarova-Rusher OV, Kleiner DE, Fioravanti S, Walker M, Carey S, Yu Y, Venkatesan AM, Turkbey B, Choyke P, Trepel J, Bollen KC, Steinberg SM, Figg WD, Greten TF (2015) A phase II study of TRC105 in patients with hepatocellular carcinoma who have progressed on sorafenib United. Eur Gastroenterol J 3(5):453–461
Acknowledgments
I thank all of my past colleagues and collaborators with whom I have had the privilege to work with over 25 plus years; they made this chapter possible. Because of page constraints, I have included selective references; a lot more of them are available in respective chapters by other authors of the book.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Sampath, K.T. (2017). The Systems Biology of Bone Morphogenetic Proteins. In: Vukicevic, S., Sampath, K. (eds) Bone Morphogenetic Proteins: Systems Biology Regulators. Progress in Inflammation Research. Springer, Cham. https://doi.org/10.1007/978-3-319-47507-3_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-47507-3_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47505-9
Online ISBN: 978-3-319-47507-3
eBook Packages: MedicineMedicine (R0)