Abstract
Application of equiatomic NiTi alloys in cardiovascular system has been expanding over last decades. By modification of chemical and phase composition the limit of biocompatibility has been reached. Further development is connected with surface modification. Among many methods of surface treatment of NiTi alloys, passivation has been often chosen as the first choice method. Resistance to pitting and crevice corrosion of the surface modified NiTi alloy in artificial plasma was investigated by means of electrochemical methods (potentidynamic polarization and chronoamperometric method respectively). The obtained results indicate that the proposed surface treatment ensures good corrosion resistance in artificial plasma and can be applied in shaping final functional properties of NiTi alloys used in cardiovascular system.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Nearly equiatomic nickel-titanium alloys have been attracting both scientific and engineering interest for biomedical applications due to their unique mechanical properties and performance (shape memory and superelasticity), and biocompatibility. The shape memory effect is based on a phase transformation induced by the temperature or applied stresses. When a shape memory alloy is in its cold state (below As), the material can be easily deformed into a variety of new shapes and will remain in this shape until it is heated above the transition temperature (Af). After heating, the material recovers its original shape. Superelasticity refers to the ability of the alloy to undergo large elastic deformations, during mechanical loading-unloading cycles performed at constant temperatures.
Nowadays, due to the mentioned unique properties, NiTi shape memory alloys are widely used in numerous biomedical applications, focused mostly on minimally invasive procedures (e.g. orthodontics - orthodontic archives, endodontic files; orthopaedics - staples for foot surgery, bone plates, intramedullary nails; urology and gastroenterology). The use of NiTi alloys in biomedical application results from the unique functional properties and performance considering: elastic deployment, thermal deployment, kink resistance, biocompatibility, constant unloading stresses, biomechanical compatibility, dynamic interference, hysteresis, MR compatibility, fatigue resistance and uniform plastic deformation [1]. However, the most common is the application of these alloys in a cardiovascular field (e.g. vena cava filters, atrial septal occlusion device, ablation devices) with the special attention focused on stents. Stenting has become the standard procedure in treatment of cardiovascular diseases. Intravascular stents are extensively used in conjunction with conventional angioplasty to improve the final outcome of percutaneous revascularization procedures.
In spite of the mentioned interesting properties and application in biomedical field, special attention should be put concerning the implantation of alloys containing Ni. Although nickel is considered as the nutrition, trace element which plays important role in metabolic processes, it is well known that Ni is also considered as allergic, toxic, and carcinogenic element [2–6]. Therefore, describing the biocompatibility of NiTi alloys, nickel release should be taken into account. Since biocompatibility is strongly correlated with corrosion resistance, it is extremely important for alloys to exhibit excellent electrochemical, protective properties. Issues of corrosion resistance of metal biomaterials and its influence on functional properties have been widely described in the literature [7–16].
Good corrosion resistance and associated good biocompatibility can be ascribed to a passive oxide layer formed spontaneously on the alloy surface. The passive layer consist mostly of \({\text {TiO}_2}\). Depending on its structure, phase composition, stability and thickness the layer may act as a barrier against Ni release. Native oxide layers consisting mostly of \({\text {TiO}_2}\) seem to be the most appropriate in cardiovascular applications, especially taking into account deformability of surface layers corresponding to phenomenon of superelasticity. Thus issues of surface treatment of NiTi alloys play extremely important role in their biocompatibility. Different methods and protocols have been used for surface treatments - mechanical and electrochemical treatments, chemical etching, heat treatments, physical and chemical plasma methods, ion implantation, laser and electron-beam irradiation. Many studies of corrosion resistance of NiTi alloys in simulated body fluids have been reported [17–24]. However, due to diverse test regimes, and what is the most important different surface treatments applied, the obtained results are incomparable and questionable.
2 Materials and Methods
The aim of the study was evaluation of pitting and crevice corrosion resistance of the surface treated NiTi alloy. Due to possible cardiovascular applications, corrosion studies were carried out in artificial plasma - Table 1 - according to the requirements enclosed in the ISO 10993-15 and ASTM F746 standards. The chemical composition of the alloy (Ni - \(55,5\,\%\), Ti - balance) met the requirements of the ASTM 2063 standard. The tests were carried out on flat samples (\(10\,\times \,10\,\times \,1\,\text {mm}\)).
In order to evaluate the influence of diverse methods of surface modification on the corrosion resistance of the alloy, the following subsequent surface treatments were applied:
-
grinding - abrasive paper (#600 grit).
-
electropolishing,
-
\({\text {H}_2\text {O}}\) chemical passivation,
-
\(\text {H}_2\text {SO}_4\) electrochemical passivation.
Since passivation is often considered as the first choice surface treatment assuring formation of the dense, stable \({\text {TiO}_2}\) oxide layer, different methods of passivation were adopted in the study. Both chemical and electrochemical methods were adopted. The applied methods of surface treatment and their parameters were presented in Table 2.
The electrochemical tests of the investigated alloy were performed with the use of a potentiodynamic method by recording of anodic polarization curves. In the tests the scan rate was equal to 1 mV/sec. The PGP 201 (Radiometer) potentiostat with the software for electrochemical tests was applied. The saturated calomel electrode (SCE) was applied as the reference electrode and the auxiliary electrode was a platinum wire. All samples were immersed in the artificial plasma for 60 min before the scanning started at a potential of about 100 mV below the recorded open circuit potential (EOCP). The scanning direction was reversed when the anodic current density reached 1000 \(\upmu \text {A/cm}^2\). The tests were carried out at the temperature of \(37 \pm 1^{\circ }\text {C}\). On the basis of the recorded curves characteristic values describing the resistance to pitting corrosion i.e.: corrosion potential Ecorr (V), breakdown potential Eb (V) or transpassivation potential Etr (V), polarization resistance Rp (\(\Omega *\text {cm}^2\)) and corrosion current density \((\text {A/cm}^2)\) were determined. To determine the value of polarization resistance Rp the Stern method was applied. Corrosion current density was determined from the simplified formula: icorr = 0.026/Rp.
The ASTM F746 standard test method was applied to assess crevice corrosion resistance. According to the standard, stimulation of localized corrosion is marked by one of the following conditions: the polarization current density exceeds 500 \(\upmu \text {A/cm}^2\) instantly; the current density does not exceed 500 \( \upmu \text {A/cm}^2\) within 20 s, but is increasing in general; these two conditions are not met in the first 20 s, but are met in a period of 15 min. The crevice corrosion tests were carried out at the temperature of \(37^{\circ }\text {C}\). The corrosion potential of the sample was continuously monitored for 1 h, starting immediately after immersion in the electrolyte. According to the ASTM standard, damage of the passive film is performed electrochemically by applying a potential of +800 mV versus SCE for durations up to 15 min on a creviced sample. If during 15 min localized corrosion is not stimulated the test is terminated and the material is considered resistant to localized corrosion, otherwise a voltage step back to a preselected potential is conducted. The test consists of alternating steps between stimulation at +800 mV and repassivation to a preselected potential up to a critical potential, for which repassivation does not take place, is attained (the increase of the preselected potential value between the steps is 50 mV).
3 Results
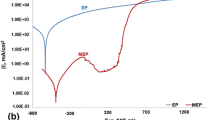
The electrochemical tests carried out in the artificial plasma showed diverse resistance of NiTi alloy to pitting corrosion, depending on the applied surface treatment. Results of the pitting corrosion tests for the ground, electropolished and passivated with the use of both chemical and electrochemical methods samples are presented in Table 3 and in Fig. 1. The results presented in the tables are mean values.
Similarly to the results of pitting corrosion, the results of the crevice corrosion tests showed also diverse resistance of NiTi alloy to crevice corrosion depending on the applied surface treatment. The results of the crevice corrosion resistance for the ground, electropolished, and passivated samples are presented in Table 4 and in Fig. 2.
4 Discusion
Assessment of corrosion resistance is essential in determining biocompatibility of metal implant materials. By changes in chemical and phase composition the given level of biocompatibility has been reached. Further development of biocompatibility is related with surface modification. Different surface treatment methods have been applied in order to enhance corrosion resistance and biocompatibility in consequence. Due to application of shape memory alloys as cardiovascular implants, appropriate methods of surface treatment must be applied. Since the implants are miniaturized the only method ensuring required surface roughness and chemistry is electropolishing. And due to ease of oxidation of NiTi alloys the next first choice surface treatment is passivation. Passivation can be realized by means of both chemical and electrochemical methods.
In the presented work the following subsequent surface treatment methods were applied: grinding, electropolishing, \(\text {H}_2\text {O}\) chemical passivation and \(\text {H}_2\text {SO}_4\) electrochemical passivation.
The potentiodynamic method is widely used in determining the susceptibility of alloys to both pitting and crevice corrosion. Thus both, the polarization method and the chromoamperometry method were applied respectively.
In general the obtained results of pitting corrosion showed that all the NiTi alloy samples were characterized by high resistance to this type of corrosion with the exception of the ground samples. For the electropolished and the passivated samples transpassivation values above +1300 mV were recorded whereas for the ground samples the breakdown potential was observed (+ 346 mV). The applied passivation both chemical and electrochemical significantly increased polarization resistance of the tested NiTi alloy. The mechanism of improving corrosion resistance in reference to ground and even electropolished samples is related to the formation of thicker and denser oxide layers.
Similar behavior of the tested NiTi samples was observed in the crevice corrosion studies. Resistance to this type of corrosion is important because of the geometry of cardiovascular implants (for example stents). The obtained results have shown that grinding does not ensure resistance to this type of corrosion. The applied surface treatment, consisting of the electropolishing and the two types of passivation, significantly increased resistance to crevice corrosion. For all these samples no signs of corrosion were observed on their surfaces.
References
Duerig, T., Pelton, A., Stoeckel, D.: An overview of nitinol medical applications. Mater. Sci. Eng. A273–275, 149–160 (1999)
Symeonides, P.P., Paschologlou, C., Papageorgiou, S.: An allergic reaction after internal fixation of a fracture using a vitallium plate. J. Allergy Clin. Immunol. 51, 251 (1973)
Elves, M.W., Wilson, J.N., Scales, H.S., Kemp, S.B.: Incidence of metal sensitivity in patients with total joint replacements. British Med. J. 4, 376 (1975)
Veien, N.: In: Maibach, H., Menne, T. (eds.) Nickel and the Skin: Immunology and Toxicology, pp. 165–178. CRC, Boca Raton (1989)
Pulletikurthi, C., Munroe, N., Gill, P., Pandya, S., Persaud, D., Haider, W., Iyer, K., McGoron, A.: Cytotoxicity of Ni from surface-treated porous nitinol (PNT) on osteoblast cells. J. Mater. Eng. Perform. 20, 824–829 (2011)
Rocher, P., et al.: Biocorrosion and cytocompatibility assessment of NiTi shape memory alloys. Scripta Materialia 50, 255–260 (2004)
Basiaga, M., Jendrus, R., Walke, W., Paszenda, Z., Kaczmarek, M., Popczyk, M.: Influence of surface modification on properties of stainless steel used for implants. Arch. Metall. Mater. 60(4), 2965–2969 (2015)
Basiaga, M., Staszuk, M., Walke, W., Opilski, Z.: Mechanical properties of ALD TiO2 layers on stainless steel substrate. Materialwissenschaft & Werkstofftechnik 47(5), 1–9 (2016)
Kajzer, A., Kajzer, W., Dzielicki, J., Matejczyk, D.: The study of physicochemical properties of stabilizing plates removed from the body after treatment of pectus excavatum. Acta Bioeng. Biomech. 2, 35–44 (2015)
Kajzer, A., Kajzer, W., Golombek, K., Knol, M., Dzielicki, J., Walke, W.: Corrosion resistance, EIS and wettability of the implants made of 316 LVM steel used in chest deformation treatment. Arch. Metall. Mater. 61(2a), 767–770 (2016)
Szewczenko, J., Marciniak, J., Kajzer, W., Kajzer, A.: Evaluation of corrosive resistance of titanium alloys used for medical implants. Arch. Metall. Mater. 61(2a), 695–770 (2016)
Basiaga, M., Walke, W., Paszenda, Z., Karasinski, P.: Research on electrochemical properties \({\text{ SiO }_2}\) layer intended for contact with blood deposited by sol-gel method. Eur. Cells Mater. 26, 157 (2013)
Marciniak, J., Szewczenko, J., Kajzer, W.: Surface modification of implants for bone surgery. Arch. Metall. Mater. 60(3), 2123–2129 (2015)
Szewczenko, J., Pochrzast, M., Walke, W.: Evaluation of electrochemical properties of modified Ti-6Al-4V ELI alloy. Przeglad Elektrotechniczny 87(12b), 177–180 (2011)
Marciniak, J., Szewczenko, J., Walke, W., Basiaga, M., Kiel, M., Manka, I.: Biomechanical analysis of lumbar spine stabilization by means of transpedicular stabilizer. In: Pietka, E., Kawa, J. (eds.) Information Technologies in Biomedicine, pp. 529–536. Springer, Berlin (2008). Advances in Soft Computing, vol. 47, pp. 1615–3871
Kiel-Jamrozik, M., Szewczenko, J., Basiaga, M., Nowińska, K.: Technological capabilities of surface layers formation on implant made of Ti-6Al-4V ELI alloy. Acta Bioeng. Biomech. 17(1), 31–37 (2015)
Vojtech, D., Fojt, J., Joska, L., Novak, P.: Surface treatment of NiTi shape memory alloy and its influence on corrosion behavior. Surf. Coat. Technol. 204, 3895–3901 (2010)
Khalil-Allafi, J., Amin-Ahmadi, B., Zare, M.: Bio-compatibility and corrosion behavior of the shape memory NiTi alloy in the physiological environments simulated with body fluids for medical applications. Mater. Sci. Eng. C 30, 1112–1117 (2010)
Chan, C.W., Man, H.C., Yue, T.M.: Susceptibility to stress corrosion cracking of NiTi laser weldment in Hanks’ solution. Corros. Sci. 57, 260–269 (2012)
Zhenga, C.Y., Niea, F.L., Zhenga, Y.F., Chengc, Y., Weid, S.C., Ruand, L., Valiev, R.Z.: Enhanced corrosion resistance and cellular behavior of ultrafine-grained biomedical NiTi alloy with a novel \(\text{ SrO }-{\text{ SiO }_2}-{\text{ TiO }_2}\) sol-gel coating. Appl. Surf. Sci. 257, 5913–5918 (2011)
Freiberg, K.E., Bremer-Streck, S., Kiehntopf, M., Rettenmayr, M., Undisz, A.: Effect of thermo-mechanical pre-treatment on short- and long-term Ni release from biomedical NiTi. Acta Biomaterialia 10, 2290–2295 (2014)
Pound, B.G.: The electro-chemical behavior of nitinol in simulated physiological solutions. J. Biomed. Mater. Res. 85A, 1103–1113 (2008)
Michiardi, A., Aparicio, C., Planell, J.A., Gil, F.J.: Electro-chemical behaviour of oxidized NiTi shape memory alloys for biomedical applications. Surf. Coat. Technol. 201, 6484–6488 (2007)
Warner, C.P.: The effect of exposure to simulated body fluids on breakdown potentials. JMEPEG 18, 754–759 (2009)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Kaczmarek, M., Kurtyka, P. (2017). Corrosion Resistance of Surface Treated NiTi Alloy Tested in Artificial Plasma. In: Gzik, M., Tkacz, E., Paszenda, Z., Piętka, E. (eds) Innovations in Biomedical Engineering. Advances in Intelligent Systems and Computing, vol 526. Springer, Cham. https://doi.org/10.1007/978-3-319-47154-9_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-47154-9_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47153-2
Online ISBN: 978-3-319-47154-9
eBook Packages: EngineeringEngineering (R0)