Abstract
To find out the influences on the emotionality and attentional deployment caused by depression, we recruited 19 MDD patients and 19 healthy controls, and implemented a task-state fMRI experiment using a distraction task paradigm. Our results showed relatively decreased brain activation in the right precuneus and left DLPFC, in the MDD group compared with the healthy group across the positive, neutral, and negative task conditions. During only the positive condition, decreased subcortical responses and concurrently reduced brain activation in the salience network were found only in MDD patients. Further brain-symptom analysis demonstrated significant correlation between alterations in the key region of the salience network and the depressive severity of the patients. Our findings suggest a crucial role of aberrant salience processes (especially in the anterior insulae) in the abnormal perception of positive stimuli in MDD patients, which is likely to be the underlying pathology of the anhedonia.
Y. Yang and L. Feng—These authors contributed equally to this work.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
- Major Depressive Disorder
- Default Mode Network
- Anterior Insula
- Positive Stimulus
- Major Depressive Disorder Patient
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
As a hallmark symptom of MDD, the anhedonia was defined in DSM-IV-TR (American Psychiatric Association, 1994) as diminished interest or pleasure in response to stimuli that were previously perceived as rewarding during a pre-morbid state. Because the capacity to feel pleasure is a critical step during the normal processing of rewards, anhedonia has been greatly implicated in the reward deficits of MDD patients [1]. Neuroimaging studies, especially functional magnetic resonance imaging (fMRI) studies, have played an important role in revealing brain abnormalities in major depressive disorder (MDD) [2]. However, previous findings are still ambiguous to tell the underlying mechanism of anhedonia.
Several brain areas showing alterations in MDD patients have been indicated, including cortical, subcortical, (para) limbic, and midbrain regions that mediate cognition, emotion, as well as metabolism. Most of these areas are associated with emotional and reward processing. A number of neurobiological models of MDD have been proposed to interpret the observed alterations in the patients, such as the limbic-cortical model that suggests the association of over-activity in limbic areas traditionally linked to emotional processing and inadequate inhibition by prefrontal areas in MDD patients [3]; the corticostriatal model that highlights the subcortical structures in information processing and their dysfunction associated with symptoms such as psychomotor retardation [4]; furthermore, an increasing emphasis has been put on the relationship between the default mode network (DMN) and depressive symptoms where patients were reported to present increased self-reflective rumination [5]. Although each of the hypothesized models can be supported by abundant neuroimaging evidence, a consensus about the neural pathology of the depressive anhedonia has not been achieved.
As a newly proposed hypothesis, Uddin [6] indicates that abnormally functioning in the salience system associated with detection of behaviourally relevant stimuli of the outward environment is a key factor to many neuropsychiatric disorders, such as schizophrenia, autism, and depression. Alterations in the salience network comprised of the anterior parts of insular and cingulate cortices might result in misappropriated salience detection and altered attentional processes. Abnormalities in recognition of reward or positive stimuli have been identified in MDD patients. For instance, depressed patients showed decreased perceptual sensitivity to positive words and pictures, but exhibited increased vigilance towards negative information [7]. Therefore, emerging evidence suggests the relationship between the biased attentional processing and the morbid processes on reward or positive stimuli in MDD patients.
In the present study, we employed a distraction task paradigm to examine the responses to affective images and attentional control of MDD and healthy cohorts. We hypothesized that abnormal emotionality in MDD patients may be implicated in altered attentional activities.
2 Materials and Methods
2.1 Participants
Nineteen right-handed MDD outpatients (8 males and 11 females) from Beijing Anding Hospital, China, and 19 healthy controls (HC) matched for gender, age, and years of education with MDD patients recruited from community participated in our experiment. Clinically trained and experienced raters (T. Tian and B. Fu) performed diagnostic assessments for all the participants, by means of the DSM-IV-based Mini International Neuropsychiatric Interview 6.0 (MINI 6.0) [8]. Clinical symptom severity of depression was evaluated for only patients using Hamilton Depression Rating Scale 17 items (HDRS-17). The demographics and clinical characteristics of participants are presented in Table 1. The following criteria were applied to exclude participants who are unsuitable for our fMRI experiment: (1) depressive patients with any mania episode or history of any comorbid major psychiatric illness on Axis I or Axis II; (2) concurrent serious medical illness or primary neurological illness; (3) history of head injury resulting in loss of consciousness; (4) abuse of or dependence on alcohol or other substances; (5) and contraindication for MRI. All subjects signed the informed consent and this study was approved by the Ethics committee of Beijing Anding Hospital, Capital Medical University.
2.2 Experimental Design
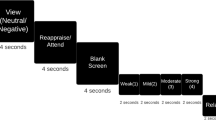
Participants were displayed with pictures and then required to solve mental arithmetic problems presented as overlays on the pictures. Three types of pictures were applied, with positive (e.g., joyful, exciting), neutral, and negative (e.g., aversive) valences, respectively, corresponding to three task conditions. As distractors, 2-digit simple mental addition and subtraction problems without carrying and borrowing were employed to avoid ceiling and floor effects. The difficulty level of such arithmetic problems was verified to be appropriate for attracting attention of both MDD and healthy groups by performing a pre-experiment. All the visual stimuli were presented in a block-designed pattern.
Each trial consisted of an emotion induction phase and a distraction phase. During the induction phase (2000 ms), a valenced picture was displayed. Participants passively viewed the picture to elicit an initial emotional response. During the distraction phase (4000 ms), participants needed to shift attention from the picture to an arithmetic problem, and then decide whether the displayed solution was correct or incorrect by pressing two response keys using the left and right thumbs. The accuracy and reaction time of each response were recorded. Incorrect displayed solutions deviated by ±1 or ±10 from the correct solutions in 50 % of all the trials. The frequency of occurrence of each number was balanced and the proportion of each arithmetic operation was 50 % for all conditions. Twelve successive trials with same task condition constituted a task block. Blocks of three conditions were mixed and counterbalanced, and every two task blocks were separated by a rest block. Data were acquired in three functional runs with a total of 36 trials for each type of task.
2.3 MRI Data Acquisition
All the participants were scanned by using a 3.0 T MRI system (Siemens Trio Tim; Siemens Medical System, Erlanger, Germany) and a 12-channel phased array head coil. To limit head motion and reduce scanning noise, foam padding and headphone were employed. 192 slices of structural images with a thickness of 1 mm were acquired by using a T1 weighted 3D MPRAGE sequence (TR = 1600 ms, TE = 3.28 ms, TI = 800 ms, FOV = 256 × 256 mm2, flip angle = 9°, voxel size = 1 × 1 × 1 mm3). Functional images were collected through a T2 gradient-echo EPI sequence (TR = 2000 ms, TE = 31 ms, flip angle = 90°, FOV = 240 × 240 mm2, matrix size = 64 × 64). Thirty axial slices with a thickness of 4 mm and an interslice gap of 0.8 mm were acquired.
2.4 Data Preprocessing
The preprocessing of fMRI data was performed with SPM12 software (Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk) based on MATLAB platform (MathWorks, Natick, MA). The first two images were discarded to allow the magnetization to approach dynamic equilibrium. Temporal and spatial corrections were performed on the functional images to eliminate influences from slice-timing differences and rigid body motion. Patients with head movement exceeding 3 mm or 3° were rejected. The high resolution anatomical image was co-registered with the mean image of the EPI series and then spatially normalized to the MNI template. After applying the normalization parameters to the EPI images, all volumes were resampled into 3 × 3 × 3 mm3. Then the normalized task-state images were smoothed with an 8-mm FWHM isotropic Gaussian kernel.
2.5 Functional MRI Analysis
Statistical analysis was performed on the preprocessed data with SPM12. After specifying the design matrix, each participant’s hemodynamic responses induced by the trials were modeled with a box-car function convolved with a hemodynamic function. The parameters for the effects of the positive task (PT), neutral task (NEUT), and negative task (NT) which displayed pictures with respective valences were estimated. Contrast images were constructed individually based on the general linear model (GLM). Due to the involvement of two factors in the present study, the group-level analysis was implemented based on a 2 by 3 factorial design with factors of “Group” (2 levels) and “Condition” (3 levels). Main effects of “Group” and “Condition” were analyzed to confirm whether differences in brain activation pattern exist between MDD patients and healthy controls (HC), and among PT, NEUT, and NT. Interaction was also examined for the two factors. Further inspections for the simple effects could be computed following a significant interaction, by which comparisons between groups under either emotion state would be allowed. Thresholds were set at a voxel-level p < 0.005, cluster size > 1242 mm3, corresponding to a corrected p < 0.05 as determined by AlphaSim correction.
Finally, the mean percentage BOLD signal change acquired from each region of interest (ROI) was extracted by a 6 mm-radius sphere for each subject, and correlated with the symptom scores of depression to investigate the interaction between altered brain functions and severity of clinical symptoms. SPSS 19.0 software (SPSS, Chicago, IL, USA) was used for the statistical analyses.
3 Results
3.1 Behavioral Results
We carried out two-way analyses of variance on the accuracy (ACC) and reaction time (RT) by specifying the 3 task conditions as within-group factor and the 2 groups as between-group factor. In the MDD group, the average ACC was 86.84 ± 11.51 % (mean ± SD) for the positive task (PT), 85.96 ± 15.94 % for the neutral task (NEUT), and 85.75 ± 12.46 % for the negative task (NT). In the HC group, the average ACC was 92.25 ± 7.49 % for the PT, 92.98 ± 6.10 % for the NEUT, and 90.94 ± 5.23 % for the NT. Only the main effect of group was significant, with the F (1, 108) = 8.897, p = 0.004. MDD patients showed significantly lower ACC than the healthy subjects.
In the MDD group, the average RT was 2461.96 ± 463.03 ms for the PT, 2467.21 ± 498.99 ms for the NEUT, and 2500.29 ± 485.92 ms for the NT. In the HC group, the average RT was 2391.31 ± 375.99 ms for the PT, 2405.70 ± 346.38 ms for the NEUT, and 2452.68 ± 384.96 ms for the NT. Neither main effect nor interaction reached significance.
3.2 fMRI Results
The group-level analysis based on factorial design exhibited significant main effects of group and condition, as well as significant interaction between group and condition. Post hoc 2-sample t-tests were implemented to examine brain activation differences of emotional responses and attentional control between MDD and HC groups under different emotional state. In the positive condition, MDD patients showed only decreased brain activation in the left anterior insula (AI), right orbital part of inferior frontal gyrus around the AI, dorsal part of anterior cingulate cortex (dACC), left precuneus, bilateral angular gyri (AG), bilateral dorsolateral prefrontal cortices (DLPFC), and bilateral thalamus extending to putamen, caudate nuclei, pallidum, and other subcortical areas (see Fig. 1). In the neutral condition, decreased brain activations were observed in the right precuneus and left DLPFC in MDD patients compared with healthy subjects (see Fig. 2A). In the negative condition, MDD group showed a similar pattern as in neutral condition with only decreased activation in the right precuneus and left DLPFC (see Fig. 2B). All the regions with significant activation are listed in Table 2. No increased activation was found for MDD patients in either condition.
3.3 Brain-Symptom Associations
According to the aforementioned results of fMRI analyses, more prominent discrepancies in brain activation between the MDD and HC groups can be found during the positive condition. Particularly, the salience network that consists of AI and dACC showed significant group differences during only positive condition. Therefore, we further focused on the BOLD signal in salience-related regions. In the MDD group, the mean percentage BOLD signal change of the right AI during the positive task exhibited significant negative correlations with the total score of Hamilton Depression Rating Scale 17 Items (r = −0.48, p = 0.038) and the subscore for feeling down, depressed or hopeless of 9-item Patient Health Questionnaire (r = −0.58, p = 0.009), suggesting that patients with more severe MDD symptoms show lower BOLD signal change when engaging in positive task. No other significant correlation was found between other task-induced brain activation and clinical data in MDD patients. In the HC group, no significant correlation was found between fMRI results and clinical data. Results of the brain-symptom associations are shown in Fig. 3.
4 Discussion
As post hoc results of the group-level analyses, only significantly decreased activation was revealed by comparing MDD patients with HC subjects in all the three task conditions. Hypo-activity was found in the left DLPFC and precuneus across the three conditions in MDD group. The left DLPFC is well-known for its role in central executive of working memory and top-down voluntary modulation of positive and negative emotions [9]. Given that the decreased activation in the left DLPFC was consistent even during neutral task in this study, abnormalities in this region is more likely to associate with difficulties in active cognitive control, i.e., cognitive manipulation in mental calculation. This inference was borne out by the behavioral results which showed significantly lower accuracy in patients relative to healthy subjects. The precuneus, serving as a component of the default mode network (DMN) which is always deactivated during goal-oriented activities, is particularly critical for the facilitation of self-referential cognitive activity and autobiographical memory [10]. It has been evidenced that the self-projection related to personal past experience relies closely on the precuneus [10]. The relatively greater deactivation in precuneus might imply the endeavor to suppress depressive rumination while MDD patients attempted to control their attention. The general pattern of the hypo-activity across task conditions indicated the poor executive control and maladaptive rumination, especially difficulties in shifting from the DMN activity to the task-positive network activity in MDD patients during participating in distraction tasks.
As a hallmark clinical symptom, anhedonia rates highly in making a diagnosis of depression. Lack of reactivity to pleasurable stimuli within brain is conceived as a cardinal feature of anhedonia, reflected in the dysfunction of midbrain, striatum, and limbic areas [11]. Given the prior evidence indicating reduced response to positive stimuli and selective attention to negative stimuli in MDD patients [12], the abnormalities in the salience detection of positive stimuli is likely to contribute to the diminished pleasure. In the present study, concurrent hypo-activation was found in the reward-related subcortical areas and regions in charge of salience processing, suggesting an impaired incentive salience processing, that is, a possible neglect of positive stimuli in MDD patients. When allocating the attention from viewing pictures to arithmetic problems, multiple salient targets are supposed to elicit more increased activation in the salience-related regions. This pattern can be observed across the three conditions in HC subjects (see Fig. 4). However, positive pictures failed to induce activation in the bilateral insulae of MDD patients, even when the corresponding activation could be elicited by neutral pictures. Furthermore, the subsequent ROI analysis demonstrated that patients with higher depressive symptom rates showed lower BOLD signal changes in the right anterior insula (AI) when evoked by positive stimuli. While the right AI is conceived as a critical note in the salience network for salience detection [6].
Taken together, it can be speculated that the abnormalities in reward processing related to the anhedonia of MDD patients are possibly caused by a morbid detection for the salience of positive stimuli. This task-based finding is consistent with previous resting-state study [13].
5 Conclusion
In conclusion, the present study applied a distraction task paradigm to examine the emotionality and attentional control of MDD and healthy cohorts. Across all the tasks, MDD patients showed poorer executive control and maladaptive rumination relative to healthy participants. Moreover, close relations were identified between salience processing and brain responses to positively valenced stimuli/rewards. Our findings suggest a crucial role of aberrant salience processes (especially in the anterior insulae) in the abnormal perception of positive stimuli in MDD patients, which is likely to be the pathology underlying the anhedonia.
References
Der-Avakian, A., Markou, A.: The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 35, 68–77 (2012)
Diener, C., Kuehner, C., Brusniak, W., Ubl, B., Wessa, M., Flor, H.: A meta-analysis of neurofunctional imaging studies of emotion and cognition in major depression. NeuroImage 61, 677–685 (2012)
Mayberg, H.S., Liotti, M., Brannan, S.K., McGinnis, S., Mahurin, R.K., Jerabek, P.A., Silva, J.A., Tekell, J.L., Martin, C.C., Lancaster, J.L., Fox, P.T.: Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry 156, 675–682 (1999)
Bora, E., Harrison, B.J., Davey, C.G., Yucel, M., Pantelis, C.: Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol. Med. 42, 671–681 (2012)
Sheline, Y.I., Barch, D.M., Price, J.L., Rundle, M.M., Vaishnavi, S.N., Snyder, A.Z., Mintun, M.A., Wang, S., Coalson, R.S., Raichle, M.E.: The default mode network and self-referential processes in depression. Proc. Natl. Acad. Sci. U.S.A. 106, 1942–1947 (2009)
Uddin, L.Q.: Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 16, 55–61 (2015)
Henderson, S.E., Vallejo, A.I., Ely, B.A., Kang, G., Krain Roy, A., Pine, D.S., Stern, E.R., Gabbay, V.: The neural correlates of emotional face-processing in adolescent depression: a dimensional approach focusing on anhedonia and illness severity. Psychiatry Res. 224, 234–241 (2014)
Sheehan, D.V., Sheehan, K.H., Shytle, R.D., Janavs, J., Bannon, Y., Rogers, J.E., Milo, K.M., Stock, S.L., Wilkinson, B.: Reliability and validity of the Mini International Neuropsychiatric Interview for Children and Adolescents (MINI-KID). J. Clin. Psychiatry 71, 313–326 (2010)
Phillips, M.L., Ladouceur, C.D., Drevets, W.C.: A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry 13(829), 833–857 (2008)
Buckner, R.L., Carroll, D.C.: Self-projection and the brain. Trends Cogn. Sci. 11, 49–57 (2007)
Pizzagalli, D.A., Holmes, A.J., Dillon, D.G., Goetz, E.L., Birk, J.L., Bogdan, R., Dougherty, D.D., Iosifescu, D.V., Rauch, S.L., Fava, M.: Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry 166, 702–710 (2009)
Asthana, H.S., Mandal, M.K., Khurana, H., Haque-Nizamie, S.: Visuospatial and affect recognition deficit in depression. J. Affect. Disord. 48, 57–62 (1998)
Tahmasian, M., Knight, D.C., Manoliu, A., Schwerthoffer, D., Scherr, M., Meng, C., Shao, J., Peters, H., Doll, A., Khazaie, H., Drzezga, A., Bauml, J., Zimmer, C., Forstl, H., Wohlschlager, A.M., Riedl, V., Sorg, C.: Aberrant intrinsic connectivity of hippocampus and amygdala overlap in the fronto-insular and dorsomedial-prefrontal cortex in major depressive disorder. Front. Hum. Neurosci. 7, 639 (2013)
Acknowledgements
This work was funded by National Basic Research Program of China (2014CB744600), International Science & Technology Cooperation Program of China (2013DFA32180), National Natural Science Foundation of China (61420106005, 61272345), and JSPS Grants-in-Aid for Scientific Research of Japan (26350994), and supported by Beijing Municipal Commission of Education, and Beijing Xuanwu Hospital.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing AG
About this paper
Cite this paper
Yang, Y. et al. (2016). Alterations in Emotional and Salience Responses to Positive Stimuli in Major Depressive Disorder. In: Ascoli, G., Hawrylycz, M., Ali, H., Khazanchi, D., Shi, Y. (eds) Brain Informatics and Health. BIH 2016. Lecture Notes in Computer Science(), vol 9919. Springer, Cham. https://doi.org/10.1007/978-3-319-47103-7_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-47103-7_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-47102-0
Online ISBN: 978-3-319-47103-7
eBook Packages: Computer ScienceComputer Science (R0)