Abstract
Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis, and amyotrophic lateral sclerosis are neurodegenerative disorders that result in progressive dysfunction and loss of neurons in the central nervous system (CNS). A strong link between neurodegeneration and chronic inflammation has recently been demonstrated. Neuropathological studies suggest that the neuroinflammatory responses might begin before significant neuronal loss, which supports the hypothesis that neuroinflammation might play an important role in the pathogenesis of most neurodegenerative disorders. Chronic neuroinflammation contributes to increased glial activation and proliferation, leading to the release of detrimental pro-inflammatory factors. The inflammatory processes promote changes in brain capillaries, such as loss of tight junction proteins, atrophy of pericytes, thickening of the basement membrane as a result of the accumulation of basement membrane proteins, and increased permeability to small molecules and plasma proteins. These changes accelerate transmigration of peripheral cells into the brain parenchyma. In this work, we discuss the role of neuroinflammation in neurodegenerative diseases. We review the impact of immune responses on the CNS, resulting in blood–brain barrier changes during neurodegeneration.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
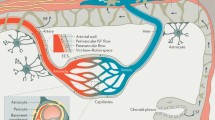
Homeostasis of the central nervous system (CNS) is essential for its normal functioning and is maintained by the highly specialized brain endothelial structure, the blood–brain barrier (BBB). Astrocytes, neurons, pericytes, and microglia communicate with endothelial cells and are collectively referred to as the neurovascular unit. The BBB strictly controls the exchange of cells and molecules between blood and the CNS [30]. BBB disruption is associated with numerous pathological conditions that affect the CNS, such as ischemia, infections, epilepsy, tumors, and neuroinflammatory diseases including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS).
Neuroinflammatory events may begin before significant loss of neural tissue during the process of neurodegeneration, which supports the hypothesis that neuroinflammation might be associated with the progress of neurodegenerative diseases and the modulation of pathogenesis. Whether inflammatory processes modulating BBB permeability precede the process of neurodegeneration or are the consequence of disease pathology remains to be demonstrated.
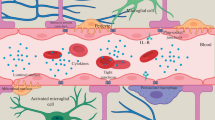
In neurodegenerative disorders associated with chronic neuroinflammation, immune response driven by glial cells triggers the disruption of the BBB. Inflammatory processes affect the BBB by increasing vascular permeability, enhancing migration of immune cells, altering transport systems, or influencing the role of the BBB as a signaling interface. These changes can range from mild and transient “BBB opening” to chronic breakdown, impairing neuronal activity and leading to neuronal damage and cognitive dysfunction [39]. Proinflammatory signaling molecules, such as cytokines, chemokines, and adhesion molecules produced by glial cells, neurons, and endothelial cells, respectively, cooperate to determine BBB properties and to control leukocyte–endothelial adhesion. These mediators play a prominent role in regulating blood-to-brain cell migration, perpetuating inflammation, and thus exacerbating the disease pathology [23, 104] (Fig. 15.1
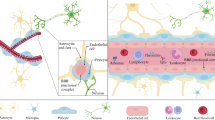
Role of inflammatory processes in CNS diseases. Increased concentration of inflammatory agents (reactive oxygen species, cytokines, chemokines, etc.) is related to numerous neurodegenerative diseases such as Alzheimer´s disease, multiple sclerosis, Parkinson disease´s and Huntington´s disease. In Multiple Sclerosis T-cells proliferate and infiltrate the CNS through the upregulation of adhesions molecules on the brain endothelial cells. T-cells in the presence of cytokines differentiate into Th17 cells, which secrete IL-17, that can stimulate further production of inflammatory agents in astrocytes. T-cell contact induces expression of IL-6, reactive oxygen species and nitric oxide in astrocytes, which contribute to damaging myelin sheath on neurons and to fully development of MS. In Alzheimer’s disease, amyloid-β peptides (Aβ) produced by cleavage of amyloid precursor protein (APP) and misfolded tau species, induce microgliosis, astrogliosis and trigger increased expression of inflammatory agents. Production of inflammatory molecules upregulate APP, further post-translational modifications of tau protein in neuronal cells and neurovascular unit changes. On the other hand, inflammatory agents such as cytokines could have a protective role, they could differentiate microglia into phagocytic cells capable of degrading Aβ and tau. Huntington’s disease is associated with mutant form of Huntingtin (mHtt) protein. Toxic intracellular polyglutamine inclusions increase the intracellular Ca2+ due to NMDA receptor binding, lead to mitochondrial dysfunction with ROS production, and to axonal transport disruption due to mHtt/HAP1 complexes. Subsequently, increased amount of intracellular Ca2+ activated enzymes such as caspases and calpains, which finally cleaved mHtt into toxic N-terminal fragments and triggering apoptosis. Microglia cells expressing mHtt contribute to neuronal degeneration. Pathogenesis of Parkinson’s disease is characterized by abnormal intracellular accumulation of insoluble alpha-synuclein aggregations in the form of Lewy bodies in dopaminergic (DA) neurons due to a mutation. Compare to HD, neuronal death is a result of mitochondrial dysfunction with ROS production, an intracellular increase of Ca2+, oxidative stress and alterations in the ubiquitinproteasomal system. Created alpha-synuclein aggregates trigger microglia cells to produce ROS
).
Although the role of neuroinflammation during neurodegeneration remains unclear, findings from experimental models and clinical studies have demonstrated a significant contribution of inflammation to pathological features and symptoms.
2 Multiple Sclerosis
Multiple sclerosis is a human chronic inflammatory disease of the CNS, leading to demyelination and neurodegeneration. MS, as an autoimmune disease, affects both the brain and the spinal cord. The most common form is relapsing-remitting MS, which affects more than 85 % of patients with MS. MS is more common in women than in men [20]. MS occurs in genetically predisposed young adults exposed to unknown environmental triggers [19]. Genome-wide association studies and meta-analysis identified 23 associated loci outside of the human leukocyte antigen genomic region [3, 53, 64].
Neuropathologically, MS is characterized by extensive focal and disseminated infiltration of mononuclear cells in the white and gray matter. Infiltration of autoreactive immune cells causes inflammatory response and neurodegenerative processes characterized by the development of multiple demyelinated plaques found in proximity to blood vessels, significant axonal damage and loss, and finally irreversible damage to the CNS [103]. Acute lesions display disruption of the BBB, as demonstrated by intravenous administration of gadolinium chelate diethylenetriamine pentaacetic acid, a contrast dye that can be visualized by magnetic resonance imaging (MRI), and postmortem evidence of focal microvascular leakage [75, 82]. Whether BBB dysfunction precedes immune cell infiltration or is the consequence of perivascular leukocyte accumulation remains to be established.
Recruitment of CD4+ T cells into the cerebral interstitium is the most significant consequence of BBB inflammation in MS. In physiological conditions, only a few peripheral immune cells are present in CNS. Nevertheless, the luminal side of BBB is in constant contact with patrolling cells and this immune surveillance is critical for the organism to respond to any pathological process in the CNS [74]. Studies using a rat model for experimental autoimmune encephalomyelitis showed T-cell binding and diapedesis through leptomeningeal vessels and through the BBB [5]. Acute inflammatory lesions are infiltrated mainly by CD4+ and CD8+ T-and B-cells. Active (demyelinating) lesions at a later stage show an abundance of macrophages and reactive proliferating astrocytes.
The migration of immune cells from blood into the brain parenchyma occurs through a process involving tethering, rolling, adhesion, and finally extravasation across the BBB. The capture and rolling are mediated by the selectin family of adhesion molecules and their sulfated, sialylated, and fucosylated glycoprotein ligands [89]. The most efficient tethering molecules are P-selectin and L-selectin. Their most important ligand is P-selectin glycoprotein ligand-1 (PSGL-1), which is glycosylated sialomucin expressed on leukocytes. In vivo studies using mice deficient in PSGL-1 showed that PSGL-1 is the predominant P-selectin ligand expressed during inflammation. The anchoring of rolling leukocytes is achieved by interactions between antigen-4 and vascular cell adhesion molecule (VCAM-1) [99]. Leukocytes extravasate the BBB through tiny spaces into the brain parenchyma [32]. The process is regulated by proinflammatory cytokines [117] and leads to pathological lesions of MS (sclerotic plaques). The plaques growing by radial expansion result in abnormalities in normal-appearing white matter [38, 72].
The interaction of T-cell receptors on migrated CD4+ T lymphocytes with myelin antigens, presented by major histocompatibility complex (MHC) class II expressed on brain-resident microglia and astrocytes leads to the activation of glia, subsequent to an immune attack on the myelin–oligodendrocyte complex and a destructive inflammatory response. The increase in the local concentration of proinflammatory mediators, such as cytokines and chemokines, reactive oxygen species (ROS), and enzymes, induces alterations of the endothelium of the BBB, leads to leukocyte–endothelium interactions, enhanced leukocyte transmigration across the BBB, and perpetuating inflammation, thus exacerbating the MS pathology [82].
The further leukocyte migration may be stimulated by reduced junctional integrity and may contribute to structural modifications of endothelial junctions and thus increased BBB permeability during inflammatory processes.
In active lesions, immune active T-cells, microglia, and astrocytes release Th1 cytokines, including interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), interleukin-1-beta (IL-1β), and interleukin-6 (IL-6), that initiate and sustain inflammatory responses. The cytokines induce increased expression of endothelial selectins and immunoglobulin superfamily molecules: intercellular adhesion molecule-1 (ICAM-1) and VCAM-1. IFN-γ can alter the organization of the tight (occludin) and adherens junctions (vascular endothelial cadherin [VE-cadherin]) of endothelial cells, and TNF-α and IL-1β induce expression of nitric oxide synthase, together promoting injury of the BBB [75, 97].
The cytokines act as the main stimuli for chemokine production. Elevated levels of CCL (2, 3, 4, 5, 7, 8) and CXCL10 have been described in MS patients [113]. Chemokines then change low affinity, selectin-mediated interaction of leukocytes with endothelial cells into the higher affinity, integrin-mediated interaction that leads to transendothelial migration of blood-borne cells. Taken together, in MS, cytokines, chemokines, and adhesion molecules cooperate to control leukocyte–endothelial adhesion and transmigration of blood-borne cells through the BBB, thus escalating the disease process.
Alterations of BBB integrity not only involve the alterations of the tight junctions, but also include changes in expression of the ATP-binding cassette transporters. The P-glycoprotein (P-gp) is upregulated on astrocytes and downregulated on endothelial cells within the active and inactive MS lesions, whereas ABCG2 is unaltered on endothelial cells in active lesions and increased in chronic lesions [69].
In summary, observations derived from in vitro experiments, animal models, and patient studies support the hypothesis that BBB disruption represents an early event in MS pathogenesis, preceding the infiltration of blood-borne cells that leads to myelin degradation and destruction of the CNS.
2.1 Alzheimer’s Disease
Alzheimer’s disease, the most common form of dementia, is characterized by cerebrovascular and neuronal dysfunctions leading to a progressive decrease in cognitive functions [7]. On the histopathological level, AD is defined by the presence of extracellular amyloid plaques composed of amyloid-beta (Aβ) peptide aggregates and neurofibrillary tangles formed of hyperphosphorylated, truncated, and aggregated tau protein [51, 54, 87]. In addition to the classic neuropathological features, accumulation of activated immune cells has been documented in the AD brain, indicating a contribution of neuroinflammation to the pathogenesis of this disease [122].
Microglia are the key players in the brain immune system. The loss of cellular branching, transition from ramified to round shape morphology, and modified expression of numerous cell surface receptors are characteristic of activated microglia that are present in areas affected by AD pathology. Clusters of reactive microglia with upregulated expression of a variety of inflammatory cytokines (IL-1β, IL-6, and TNF-α) are often associated with amyloid plaques [96], and in and around neurofibrillary tangles [24, 98]. Activated microglial cells showed increased expression of class II histocompatibility antigen near amyloid deposits in the senile plaque [81]. Microglia in AD also express high levels of MHC class I receptors [112], C3 and C1q [66], IL-1 or ferritin [92]. In AD changes in astrocytes occur. Glial fibrillary acidic protein (GFAP)-positive, hypertrophically activated astrocytes have been located in the proximity of senile plaques. The number of S100 calcium-binding protein B-positive astrocytes correlates with the number of neurofibrillary tangles [42]. However, no significant correlation between GFAP upregulation or excitatory amino-acid transporter 2 (EAAT2) downregulation and amyloid or tau pathology was observed [102].
Transgenic (Tg) animal models recapitulate many neuroinflammatory changes seen in humans. Dense clusters of activated microglia with hundreds of upregulated genes are associated with extracellular deposits of amyloid beta protein in APP23 amyloid Tg mouse. Mutations in one of them, TREM-2, have been linked to the development of dementia [40]. In P301S tau transgenic mice, microglia activation preceded tangle formation, immunosuppression with FK506-attenuated tau pathology, and increased lifespan of the animals [123]. We have shown that expression of truncated tau-induced inflammatory response manifested as upregulation of immune molecules, such as CD11a, CD11b, CD18, CD4, CD45, and CD68. The number of immune reactive microglia and astrocytes progressively increased with neurofibrillary tangle load, suggesting that activated glial cells might be involved in the immune response targeting tau pathology [126]. Reactive astrocytes have been found in the brain parenchyma of transgenic mice overexpressing the London mutant of the amyloid precursor protein, APP [V717I]. These reactive astrocytes produced an increased amount of proinflammatory molecules and upregulated expression of nitric oxide synthase [56].
Besides activation of immune cells, numerous cerebrovascular abnormalities, including endothelial and pericyte damage, reduced glucose transport, increased expression of proinflammatory molecules by activated cells and microvascular degeneration, were observed in AD [12, 127].
The idea that cerebrovascular changes might be the initial events of AD pathogenesis was proposed more than 30 years ago [48]. According to the two-hit vascular hypothesis, vascular changes lead to BBB dysfunction and cerebral hypoperfusion, initiating a cascade of events resulting in dementia [128].
In AD, the brain endothelium is often degenerated and this leads to the accumulation of Aβ on the outer side of the basement membrane of capillaries, promoting a local neuroinflammatory vascular response. A high number of AD patients exhibit vascular pathology and develop cerebral amyloid angiopathy (CAA) and cerebral infarcts. In patients with predominantly capillary CAA, loss of tight junction proteins of the BBB is accompanied by a massive inflammatory response [15].
Inflammatory changes in cerebrovascular endothelium are an integral part of AD pathology. There is an increased immunoreactivity for ICAM-1 and microvessel-associated monocyte chemoattractant protein (MCP-1) on the cerebrovascular endothelium of AD patients [41, 49]. In comparison with non-AD microvessels, the AD microvessels release significantly higher levels of a number of inflammatory factors including TNF-α, transforming growth factor-β (TGF-β), nitric oxide (NO), thrombin, cytokines such as IL-1β, IL-6, IL-8, and matrix metalloproteinases (MMPs) [49]. TGF-β1 is a multifunctional cytokine that has an intense effect on vasculogenesis, angiogenesis, and maintenance of vessel wall integrity. In AD, TGF-β1 has been detected to form part of senile plaques and neurofibrillary tangles [114]. Significantly higher levels of TGF-β1 were also found in serum and CSF of AD patients compared with nondemented elderly controls [17]. The chronic overexpression of TGF-β1 triggered an accumulation of basement membrane proteins and resulted in AD-like cerebrovascular amyloidosis and microvascular degeneration in a Tg mouse model, confirming its critical role in BBB changes seen in AD [121].
Many independent studies showed that various Aβ species are toxic to endothelial cells from the brain [90, 116] or other organs [9, 107, 110]. Treatment of endothelial cells with Aβ has been shown to induce activation of mitogen-activated protein kinases and increased production of proinflammatory cytokines and ROS [80].
Plasma-derived Aβ is transported through the BBB by the receptor for advanced glycation end products (RAGE) [22]. RAGE is upregulated in brain vasculature from AD, suggesting that it might play a role in the accumulation of Aβ within the brain. Interestingly, after interaction of Aβ with RAGE, endothelial cells upregulate expression of C-C chemokine receptor type 5 and MMP-2, which promotes T cells crossing the BBB [27, 78]. Elimination of Aβ from the brain is effected via the bulk flow of CSF and through the transcytosis process mediated by low-density lipoprotein receptor-related protein (LRP-1) and P-gp [33]. Systemic inflammation induced by injection of lipopolysaccharide (LPS) into mice downregulated the expression of transporters LRP-1 and P-gp, which correlated with impaired Aβ efflux [34, 63].
In contrast to Aβ, very little is known about the interaction between BBB and tau. In one of our studies, we showed that exposure of brain endothelial cells to tau does not evoke any significant responses. However, when glial cells were present, inflammatory mediators produced by these cells, such as NO, cytokines, and chemokines, significantly modified endothelial properties, such as transendothelial electrical resistance and permeability for small molecules [71].
Whether the blood-borne immune cells infiltrate the brain in AD has been highly controversial. Several authors reported that the chronic neuroinflammation seen in neurodegeneration is provided exclusively by resident CNS cells without influx of leukocytes from the blood [28, 105]. Others described hematopoietic cells entering the brain in AD and possibly contributing to inflammatory processes [13, 37, 91, 111]. Recently, accumulating evidence has supported the notion that infiltrating peripheral cells play a significant and critical role in regulating amyloid depositions in the brain [45].
In summary, the inflammatory changes in the cerebrovascular endothelium are common in AD and despite intensive research, the exact mechanisms by which they contribute to the pathogenesis of AD are not completely understood. Moreover, it is clear that BBB and inflammation both play an important role in AD and it is therefore worth putting more effort into understanding their interplay in the course of this devastating neurological disease.
3 Amyotrophic Lateral Sclerosis
Amyotrophic lateral sclerosis is a neurodegenerative disease affecting upper and lower motor neurons in the brain and spinal cord resulting in progressive muscle atrophy, fatal paralysis, and death [94]. Most cases of ALS are sporadic. About 5–10 % are cases of genetically linked familiar ALS that can be caused by mutation in the Cu/Zn superoxide dismutase (SOD1) gene [68, 93].
The neurodegenerative process in ALS is accompanied by sustained inflammation in the brain and spinal cord [11]. In humans and animal models of ALS, gliosis with accumulation of a large number of microglia and astrocytes is observed in brain and spinal cord tissue [76, 124]. Astrocytes in ALS are defective in clearing glutamate because of a loss of EAAT2/GLT1 transporter. Approximately 60–70 % of ALS patients have up to 95 % loss of the EAAT2 protein in the motor cortex and spinal cord. Loss of EAAT2/GLT1 transporter was also described in SOD1 mice and correlates with neuronal loss [79].
The generation and wide use of transgenic rodent models expressing mutant SOD1 has significantly contributed to the understanding of ALS pathogenesis. A functional impairment of the BBB and the blood–spinal cord barrier (BSCB) that might contribute to disease pathogenesis and precede motor neuron death was described in the G93A SOD1 transgenic mouse strain, which carries a human mutant Cu/Zn superoxide dismutase transgene. SOD1 mutant mice display protein aggregates in the mitochondrial intermembrane space [120]. The mitochondria from SOD1 transgenic mice have altered calcium-buffering properties, which have an effect on calcium-mediated excitotoxicity, leading to neuronal death [21]. Other authors have also shown that overexpression of SOD1 in a transgenic mouse model attenuated BBB disruption by superoxide anion during ischemia [67]. Garbuzova-Davis et al. [43] demonstrated capillary alterations and increased albumin permeability in the brainstem and spinal cord at initial (presymptomatic) and late stages (symptomatic) of the disease in SOD1 mice. Electron microscopy showed highly vacuolated and degenerated endothelial cells, perivascular edema, downregulation of tight junction proteins, microhemorrhages, and swelling of astrocyte end-feet adjacent to capillaries. Compared with SOD1 transgenic mice, the SOD1 rat model of ALS demonstrated alterations of the capillaries, such as perivascular swollen astrocyte end-feet, reduced ZO-1 mRNA synthesis and IgG leakage only at a late (symptomatic) stage [86].
These observations were confirmed by Zhong et al. [125], who also showed microvascular barrier damage in the spinal cord preceded by neuroinflammation. Their analyses showed decreased expression of tight junction proteins such as ZO-1, occludin, claudin-5 before disease onset. On the other hand, markers of endothelial activation, such as ICAM-1, and inflammation, such as MCP-1 and cycloxygenase-2 (COX-2), remained unchanged.
Damage to BSCB and BBB was demonstrated in studies on post-mortem tissues from sporadic and familiar ALS patients. In the brains of ALS patients, inflammation and activation of immune cells are associated with neuronal death. Studies in the 1980s reported deposits of IgG and C3/C4 complement in the spinal cord and cortex in the brain from ALS patients, suggesting BSCB and BBB damage [25]. Engelhardt and Appel [31] observed perivascular inflammation and breakdown of the BBB, leading to leakage in the brain. They detected the presence of IgG in motor neurons and the presence of activated macrophages, mainly in the territory of degenerating pyramidal tracts and ventral horns. Henkel et al. [57] demonstrated decreased synthesis of the mRNA of occludin and ZO-1 in lumbar spinal cord tissue from ALS patients. Similarly, Garbuzova-Davis et al. [44] showed a significant decrease in the expression of ZO-1, occludin, and claudin-5 proteins in the white and gray matter of the medulla and the cervical spinal cord in patients with the sporadic form of ALS. Angiogenesis, compensating for vascular insufficiency, was also detected. Additionally, the increased expression of P-gp and breast cancer resistance protein (BCRP) was determined in the spinal cords of ALS patients and SOD1 animal models. This suggests that rather than dose adjustments, the combination of P-gp/BCRP inhibitors and anti-ALS therapies might be necessary [62].
The human ALS tissues showed abnormal perivascular accumulation of basement membrane protein collagen IV, possibly resulting from an imbalance between MMPs and the tissue inhibitors of metalloproteinases. This may, over a long period of time, alter the BBB/BSCB transport mechanisms [44]. However, studies showing opposing results are also available [83].
In summary, the inflammatory changes, together with BBB and BCSB damage, are widely observed in humans and animal models of ALS and should be considered a primary target for successful drug development.
3.1 Parkinson’s Disease
Parkinson’s disease is a complex progressive neurodegenerative disorder characterized by motor symptoms, including bradykinesia with resting tremor, rigidity, and gait disturbance [46]. The major neuropathological hallmarks of PD are progressive degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc), the presence of α-synuclein (α-syn) inclusions called Lewy bodies, and chronic inflammation. The cause of PD is unknown, but chronic inflammation can act as an environmental factor and may increase the susceptibility to PD and finally promote the degeneration of dopaminergic neurons. PD can be triggered by diseases that induce systemic infections, such as pneumonia and respiratory and gastrointestinal infections [1].
Inflammatory responses manifested by glial reactions, T-cell infiltration, and increased expression of detrimental proinflammatory cytokines are recognized as prominent features of PD. Activated microglia can be seen in early stages of the disease and parallels the degeneration of dopaminergic neurons [47]. They are distributed not only in the SNpc and putamen, but also in other brain regions of PD patients and are associated with α-syn-positive Lewy neurites [61]. Accumulation of intrinsically disordered protein α-syn actively secreted or released by dying neurons to the extracellular space of the brain leads to microglial activation, CD4+ and CD8+ T-cell infiltration, and increased production of proinflammatory cytokines, such as IL-1α, IFN-γ, IL-1β, TNF-α, and IL-10 [55]. The higher levels of cytokines, mainly IL-1β, TNF-α, and IL-2, were also found in the CSF and serum of PD patients, indicating peripheral inflammation [6, 84, 85]. A massive astrogliosis is present in SNpc in some PD patients [58]; the majority of cases showed only a mild increase in the number of astrocytes and in immunoreactivity for GFAP [109].
The microgliosis and astrogliosis alter BBB permeability. Increased levels of cytotoxic peripheral CD4+ and CD8+ T-lymphocytes infiltrate the SNpc of PD patients and animal models [14]. Clinical studies also demonstrated progressive impairment of barrier integrity and IgG depositions surrounding degenerating neurons during PD progression [50, 70, 77, 88]. Additionally, deficiencies in cerebral blood flow have been demonstrated with PET imaging [2]. These findings support early work by Faucheux et al. [36], who found that PD patients have alterations in the histological appearance of endothelial cells within the SNpc.
Evidence from animal studies indicate a direct link among inflammatory processes, α-syn, and BBB permeability during PD pathogenesis. Peripheral inflammation induced by LPS injection does not have any effect on BBB permeability in α-syn knock-out mice; however, it significantly alters the barrier in wild-type animals [65]. A recent study showed that α-syn can be transported bi-directionally through the BBB, and LRP-1, but not P-gp, may be involved in its efflux from the brain. Interestingly, LPS-induced inflammation increased the uptake of α-syn in the blood-to-brain direction, indicating the possible role of blood-borne α-syn in brain pathology [106]. Increased expression of LRP-1 was observed in PD patients, suggesting that alteration in α-syn transport might contribute to PD pathogenesis [119].
The risk of PD may be influenced by environmental exposure and nongenetic factors. The role of environmental factors in PD development was first described in the 1980s. Various toxin-induced animal PD models, including the 6-hydroxydopamine rats and 1-methyl-1,2,3,6-tetrahydropyridine (MPTP) mice, also show BBB disruption, as demonstrated by increased permeability for FITC-albumin and horse radish peroxidase and decreased expression of the tight junction proteins ZO-1 and occludin [16, 18]. In PD models, neuroinflammation as a consequence of the action of environmental factors is integrally associated within the areas affected by pathology and may be a major contributor to the BBB changes, finally promoting neurodegeneration.
Recent publications showed that there is a decreased expression of P-gp in BBB disruption areas [4, 118]. As P-gp is one of the major efflux transporters at the BBB, the accumulation of xenobiotics, such as MPTP or 1,1-Bis(4-chlorophenyl)-2,2,2-trichloroethane (DDT), in the brain could be partially associated with P-gp reduction or dysfunction [115].
In summary, neuroinflammation and BBB changes are integral parts of PD and should be considered an important therapeutic target in future drug development programs.
3.2 Huntington’s Disease
Huntington’s disease is an autosomal dominant neurodegenerative disease linked to mutations in the huntingtin (htt) gene leading to degeneration of neurons, predominantly in the caudate putamen and cortex [52]. The mutant htt causes movement disturbances, psychiatric symptoms and cognitive decline. Although the mechanism by which mutant htt causes neurodegeneration remains unclear, evidence supports inflammation as being a key player in HD pathogenesis. It is possible that increased inflammation in HD brains is a consequence of neuronal death that is a direct result of mutant htt neurotoxicity. On the other hand, accumulation of mutant htt in glia may increase the vulnerability of neurons to excitotoxic stimuli and directly cause inflammation in the CNS [100].
The role of inflammation in HD pathogenesis was supported by microarray profiling, which revealed expression of inflammation-related genes in brain regions from HD patients [59]. Postmortem studies of HD brains revealed accumulation of activated microglial cells in regions affected by HD, especially in the basal ganglia and the frontal cortex [95]. The presence of immunoreactive microglia was seen in the presymptomatic stage of HD and increased as it progressed [108]. Increased microglial activation was also shown in a R6/2 mouse model of HD [101]. Astrocyte reactivity is an early feature of HD. GFAP immunoreactivity is detected in the striatum of presymptomatic carriers and it increases with disease progression [35]. Furthermore, the astrocytes from HD produce more VEGF through an IkB kinase-nuclear factor kB-dependent pathway [60]. Interestingly, no clear evidence for the activation of astrocytes in most models of HD exists.
Cytokines are increased in HD. Bjorkqvist et al. [8] determined increased amounts of proinflammatory cytokines, such as IL-6 and IL-8, in plasma samples and striatum. Both cytokines, IL-6 and IL-1b, are also increased in the R6/2 mouse. Studies with neutralizing antibody confirmed the hypothesis that IL-6 produced by peripheral immune cells might contribute to pathology in the R6/2 model [10]. IL-1b, another member of the proinflammatory cytokine family, is increased in HD sera and in brain lysates of the R6/2 model [29]. Increased production of proinflammatory cytokines together with impaired migration properties of microglia and peripheral monocytes [73] may lead to chronic exacerbated inflammation, and thus contribute to HD pathology.
Two recent studies investigated the impairment of BBB in HD. Drouin-Ouellet et al. [26] found that mutant huntingtin protein aggregates were present in components of the neurovascular unit of R6/2 mice and HD patients. This was accompanied by an increase in blood vessel density, in addition to BBB leakage in the striatum of R6/2 mice, which correlated with the decreased expression of occludin and claudin-5. The study revealed a significant increase in cerebral blood flow in the cortical gray matter of HD patients. The results published by Hsiao et al. [60] further broaden the field, by measuring the blood vessel density and vascular reactivity using MRI. The results in several different knock-in models indicate that vascular density and reactivity are noticeably changed when mutant htt is expressed in both neurons and astrocytes.
In summary, all the above-mentioned studies clearly demonstrated that BBB is compromised in both HD patients and animal models of the disease. However, further studies are needed to investigate at what stage of the disease this process begins.
4 Conclusion
It is becoming increasingly evident that neuroinflammation plays a crucial role in the development and progression of many neurodegenerative disorders. Chronic neuroinflammation associated with neuronal damage includes extended activation of microglia and astrocytes followed by increased secretion of detrimental proinflammatory cytokines and chemokines. The prolonged inflammation affects the BBB, which in turn supports the infiltration of blood-borne cells into the brain parenchyma that further intensifies the inflammatory process. In future research, suppression of the inflammatory events at the site of the BBB should be explored as a therapeutic strategy against neuroinflammatory diseases.
References
Arai H, Furuya T, Mizuno Y, Mochizuki H (2006) Inflammation and infection in Parkinson’s disease. Histol Histopathol 21(6):673–678
Ballanger B, Lozano AM, Moro E, van Eimeren T, Hamani C, Chen R, Cilia R, Houle S, Poon YY, Lang AE, Strafella AP (2009) Cerebral blood flow changes induced by pedunculopontine nucleus stimulation in patients with advanced Parkinson’s disease: a [(15)O] H2O PET study. Hum Brain Mapp 30(12):3901–3909. doi:10.1002/hbm.20815
Baranzini SE, Galwey NW, Wang J, Khankhanian P, Lindberg R, Pelletier D et al (2009) Pathway and network-based analysis of genome-wide association studies in multiple sclerosis. Hum Mol Genet 18(11):2078–2090. Epub 2009/03/17
Bartels AL, Willemsen AT, Kortekaas R, de Jong BM, de Vries R, de Klerk O, van Oostrom JC, Portman A, Leenders KL (2008) Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J Neural Transm 115(7):1001–1009. doi:10.1007/s00702-008-0030-y
Bartholomaus I, Kawakami N, Odoardi F, Schlager C, Miljkovic D, Ellwart JW, Klinkert WE, Flugel-Koch C, Issekutz TB, Wekerle H, Flugel A (2009) Effector T cell interactions with meningeal vascular structures in nascent autoimmune CNS lesions. Nature 462(7269):94–98. doi:10.1038/nature08478
Bas J, Calopa M, Mestre M, Mollevi DG, Cutillas B, Ambrosio S, Buendia E (2001) Lymphocyte populations in Parkinson’s disease and in rat models of parkinsonism. J Neuroimmunol 113(1):146–152
Bell RD, Zlokovic BV (2009) Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer’s disease. Acta Neuropathol 118(1):103–113. doi:10.1007/s00401-009-0522-3
Bjorkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N et al (2008) A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. J Exp Med 205(8):1869–1877. Epub 2008/07/16
Blanc EM, Toborek M, Mark RJ, Hennig B, Mattson MP (1997) Amyloid beta-peptide induces cell monolayer albumin permeability, impairs glucose transport, and induces apoptosis in vascular endothelial cells. J Neurochem 68(5):1870–1881
Bouchard J, Truong J, Bouchard K, Dunkelberger D, Desrayaud S, Moussaoui S et al (2012) Cannabinoid receptor 2 signaling in peripheral immune cells modulates disease onset and severity in mouse models of Huntington’s disease. J Neurosci Off J Soc Neurosci 32(50):18259–18268. Epub 2012/12/15
Bowerman M, Vincent T, Scamps F, Perrin FE, Camu W, Raoul C (2013) Neuroimmunity dynamics and the development of therapeutic strategies for amyotrophic lateral sclerosis. Front Cell Neurosci 7:214. doi:10.3389/fncel.2013.00214
Bowman GL, Quinn JF (2008) Alzheimer’s disease and the blood-brain barrier: past, present and future. Aging Health 4(1):47–55. doi:10.2217/1745509X.4.1.47
Britschgi M, Wyss-Coray T (2007) Systemic and acquired immune responses in Alzheimer’s disease. Int Rev Neurobiol 82:205–233. doi:10.1016/S0074-7742(07)82011-3
Brochard V, Combadiere B, Prigent A, Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer D, Callebert J, Launay JM, Duyckaerts C, Flavell RA, Hirsch EC, Hunot S (2009) Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J Clin Invest 119(1):182–192. doi:10.1172/JCI36470
Carrano A, Hoozemans JJ, van der Vies SM, van Horssen J, de Vries HE, Rozemuller AJ (2012) Neuroinflammation and blood-brain barrier changes in capillary amyloid angiopathy. Neuro Degener Dis 10(1-4):329–331. doi:10.1159/000334916
Carvey PM, Zhao CH, Hendey B, Lum H, Trachtenberg J, Desai BS, Snyder J, Zhu YG, Ling ZD (2005) 6-Hydroxydopamine-induced alterations in blood-brain barrier permeability. Eur J Neurosci 22(5):1158–1168. doi:10.1111/j.1460-9568.2005.04281.x
Chao CC, Hu S, Frey WH 2nd, Ala TA, Tourtellotte WW, Peterson PK (1994) Transforming growth factor beta in Alzheimer’s disease. Clin Diagn Lab Immunol 1(1):109–110
Chen X, Lan X, Roche I, Liu R, Geiger JD (2008) Caffeine protects against MPTP-induced blood-brain barrier dysfunction in mouse striatum. J Neurochem 107(4):1147–1157. doi:10.1111/j.1471-4159.2008.05697.x
Compston A, Coles A (2008) Multiple sclerosis. Lancet 372(9648):1502–1517. doi:10.1016/S0140-6736(08)61620-7
Cruz-Orengo L, Daniels BP, Dorsey D, Basak SA, Grajales-Reyes JG, McCandless EE, Piccio L, Schmidt RE, Cross AH, Crosby SD, Klein RS (2014) Enhanced sphingosine-1-phosphate receptor 2 expression underlies female CNS autoimmunity susceptibility. J Clin Invest 124(6):2571–2584. doi:10.1172/JCI73408
Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M et al (2006) Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice. J Neurochem 96(5):1349–1361. Epub 2006/02/16
Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, Welch D, Manness L, Lin C, Yu J, Zhu H, Ghiso J, Frangione B, Stern A, Schmidt AM, Armstrong DL, Arnold B, Liliensiek B, Nawroth P, Hofman F, Kindy M, Stern D, Zlokovic B (2003) RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med 9(7):907–913. doi:10.1038/nm890
Dietrich JB (2002) The adhesion molecule ICAM-1 and its regulation in relation with the blood-brain barrier. J Neuroimmunol 128(1-2):58–68
DiPatre PL, Gelman BB (1997) Microglial cell activation in aging and Alzheimer disease: partial linkage with neurofibrillary tangle burden in the hippocampus. J Neuropathol Exp Neurol 56(2):143–149
Donnenfeld H, Kascsak RJ, Bartfeld H (1984) Deposits of IgG and C3 in the spinal cord and motor cortex of ALS patients. J Neuroimmunol 6(1):51–57
Drouin-Ouellet J, Sawiak SJ, Cisbani G, Lagace M, Kuan WL, Saint-Pierre M et al (2015) Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: potential implications for its pathophysiology. Ann Neurol 78(2):160–177. Epub 2015/04/14
Du H, Li P, Wang J, Qing X, Li W (2012) The interaction of amyloid beta and the receptor for advanced glycation endproducts induces matrix metalloproteinase-2 expression in brain endothelial cells. Cell Mol Neurobiol 32(1):141–147. doi:10.1007/s10571-011-9744-8
Eikelenboom P, Veerhuis R, Scheper W, Rozemuller AJ, van Gool WA, Hoozemans JJ (2006) The significance of neuroinflammation in understanding Alzheimer’s disease. J Neural Transm 113(11):1685–1695. doi:10.1007/s00702-006-0575-6
Ellrichmann G, Reick C, Saft C, Linker RA (2013) The role of the immune system in Huntington’s disease. Clin Dev Immunol 2013:541259. Epub 2013/08/21
Engelhardt B (2008) The blood-central nervous system barriers actively control immune cell entry into the central nervous system. Curr Pharm Des 14(16):1555–1565
Engelhardt JI, Appel SH (1990) IgG reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch Neurol 47(11):1210–1216
Engelhardt B, Ransohoff RM (2005) The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol 26(9):485–495. Epub 2005/07/26
Erickson MA, Banks WA (2013) Blood-brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab 33(10):1500–1513. doi:10.1038/jcbfm.2013.135
Erickson MA, Hartvigson PE, Morofuji Y, Owen JB, Butterfield DA, Banks WA (2012) Lipopolysaccharide impairs amyloid beta efflux from brain: altered vascular sequestration, cerebrospinal fluid reabsorption, peripheral clearance and transporter function at the blood-brain barrier. J Neuroinflammation 9:150. doi:10.1186/1742-2094-9-150
Faideau M, Kim J, Cormier K, Gilmore R, Welch M, Auregan G, Dufour N, Guillermier M, Brouillet E, Hantraye P, Deglon N, Ferrante RJ, Bonvento G (2010) In vivo expression of polyglutamine-expanded huntingtin by mouse striatal astrocytes impairs glutamate transport: a correlation with Huntington’s disease subjects. Hum Mol Genet 19(15):3053–3067. doi:10.1093/hmg/ddq212
Faucheux BA, Bonnet AM, Agid Y, Hirsch EC (1999) Blood vessels change in the mesencephalon of patients with Parkinson’s disease. Lancet 353(9157):981–982. doi:10.1016/S0140-6736(99)00641-8
Fiala M, Liu QN, Sayre J, Pop V, Brahmandam V, Graves MC, Vinters HV (2002) Cyclooxygenase-2-positive macrophages infiltrate the Alzheimer’s disease brain and damage the blood-brain barrier. Eur J Clin Invest 32(5):360–371
Filli L, Hofstetter L, Kuster P, Traud S, Mueller-Lenke N, Naegelin Y et al (2012) Spatiotemporal distribution of white matter lesions in relapsing-remitting and secondary progressive multiple sclerosis. Mult Scler 18(11):1577–1584. Epub 2012/04/13
Forster C (2008) Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol 130(1):55–70. doi:10.1007/s00418-008-0424-9
Frank S, Burbach GJ, Bonin M, Walter M, Streit W, Bechmann I, Deller T (2008) TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia 56(13):1438–1447. doi:10.1002/glia.20710
Frohman EM, Frohman TC, Gupta S, de Fougerolles A, van den Noort S (1991) Expression of intercellular adhesion molecule 1 (ICAM-1) in Alzheimer’s disease. J Neurol Sci 106(1):105–111
Fuller S, Munch G, Steele M (2009) Activated astrocytes: a therapeutic target in Alzheimer’s disease? Expert Rev Neurother 9(11):1585–1594. doi:10.1586/ern.09.111
Garbuzova-Davis S, Haller E, Saporta S, Kolomey I, Nicosia SV, Sanberg PR (2007) Ultrastructure of blood-brain barrier and blood-spinal cord barrier in SOD1 mice modeling ALS. Brain Res 1157:126–137. doi:10.1016/j.brainres.2007.04.044
Garbuzova-Davis S, Hernandez-Ontiveros DG, Rodrigues MC, Haller E, Frisina-Deyo A, Mirtyl S, Sallot S, Saporta S, Borlongan CV, Sanberg PR (2012) Impaired blood-brain/spinal cord barrier in ALS patients. Brain Res 1469:114–128. doi:10.1016/j.brainres.2012.05.056
Gate D, Rezai-Zadeh K, Jodry D, Rentsendorj A, Town T (2010) Macrophages in Alzheimer’s disease: the blood-borne identity. J Neural Transm 117(8):961–970. doi:10.1007/s00702-010-0422-7
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56(1):33–39
Gerhard A, Pavese N, Hotton G, Turkheimer F, Es M, Hammers A, Eggert K, Oertel W, Banati RB, Brooks DJ (2006) In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol Dis 21(2):404–412. doi:10.1016/j.nbd.2005.08.002
Grammas P (2011) Neurovascular dysfunction, inflammation and endothelial activation: implications for the pathogenesis of Alzheimer’s disease. J Neuroinflammation 8:26. doi:10.1186/1742-2094-8-26
Grammas P, Ovase R (2001) Inflammatory factors are elevated in brain microvessels in Alzheimer’s disease. Neurobiol Aging 22(6):837–842
Gray MT, Woulfe JM (2015) Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab 35(5):747–750. doi:10.1038/jcbfm.2015.32
Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM (1986) Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem 261(13):6084–6089
Gusella JF, MacDonald ME (1993) Hunting for Huntington’s disease. Mol Genet Med 3:139–158. Epub 1993/01/01
Hafler DA, Compston A, Sawcer S, Lander ES, Daly MJ, De Jager PL et al (2007) Risk alleles for multiple sclerosis identified by a genomewide study. N Engl J Med 357(9):851–862. Epub 2007/07/31
Hardy JA, Higgins GA (1992) Alzheimer’s disease: the amyloid cascade hypothesis. Science 256(5054):184–185
Harms AS, Cao S, Rowse AL, Thome AD, Li X, Mangieri LR, Cron RQ, Shacka JJ, Raman C, Standaert DG (2013) MHCII is required for alpha-synuclein-induced activation of microglia, CD4 T cell proliferation, and dopaminergic neurodegeneration. J Neurosci 33(23):9592–9600. doi:10.1523/JNEUROSCI.5610-12.2013
Heneka MT, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, Van Leuven F (2005) Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation 2:22. doi:10.1186/1742-2094-2-22
Henkel JS, Beers DR, Wen S, Bowser R, Appel SH (2009) Decreased mRNA expression of tight junction proteins in lumbar spinal cords of patients with ALS. Neurology 72(18):1614–1616. doi:10.1212/WNL.0b013e3181a41228
Hirsch EC, Breidert T, Rousselet E, Hunot S, Hartmann A, Michel PP. (2003) The Role of Glial Reaction and Inflammation in Parkinson’s Disease. Annals of the New York Academy of Sciences, 991: 214–228
Hodges A, Strand AD, Aragaki AK, Kuhn A, Sengstag T, Hughes G et al (2006) Regional and cellular gene expression changes in human Huntington’s disease brain. Hum Mol Genet 15(6):965–977. Epub 2006/02/10
Hsiao HY, Chen YC, Huang CH, Chen CC, Hsu YH, Chen HM, Chiu FL, Kuo HC, Chang C, Chern Y (2015) Aberrant astrocytes impair vascular reactivity in Huntington disease. Ann Neurol 78(2):178–192. doi:10.1002/ana.24428
Imamura K, Hishikawa N, Sawada M, Nagatsu T, Yoshida M, Hashizume Y (2003) Distribution of major histocompatibility complex class II-positive microglia and cytokine profile of Parkinson’s disease brains. Acta Neuropathol 106(6):518–526. doi:10.1007/s00401-003-0766-2
Jablonski MR, Jacob DA, Campos C, Miller DS, Maragakis NJ, Pasinelli P, Trotti D (2012) Selective increase of two ABC drug efflux transporters at the blood-spinal cord barrier suggests induced pharmacoresistance in ALS. Neurobiol Dis 47(2):194–200. doi:10.1016/j.nbd.2012.03.040
Jaeger LB, Dohgu S, Sultana R, Lynch JL, Owen JB, Erickson MA, Shah GN, Price TO, Fleegal-Demotta MA, Butterfield DA, Banks WA (2009) Lipopolysaccharide alters the blood-brain barrier transport of amyloid beta protein: a mechanism for inflammation in the progression of Alzheimer’s disease. Brain Behav Immun 23(4):507–517. doi:10.1016/j.bbi.2009.01.017
Jakkula E, Leppa V, Sulonen AM, Varilo T, Kallio S, Kemppinen A et al (2010) Genome-wide association study in a high-risk isolate for multiple sclerosis reveals associated variants in STAT3 gene. Am J Hum Genet 86(2):285–291. Epub 2010/02/18
Jangula A, Murphy EJ (2013) Lipopolysaccharide-induced blood brain barrier permeability is enhanced by alpha-synuclein expression. Neurosci Lett 551:23–27. doi:10.1016/j.neulet.2013.06.058
Johnson SA, Lampert-Etchells M, Pasinetti GM, Rozovsky I, Finch CE (1992) Complement mRNA in the mammalian brain: responses to Alzheimer’s disease and experimental brain lesioning. Neurobiol Aging 13(6):641–648
Kim GW, Lewen A, Copin J, Watson BD, Chan PH (2001) The cytosolic antioxidant, copper/zinc superoxide dismutase, attenuates blood-brain barrier disruption and oxidative cellular injury after photothrombotic cortical ischemia in mice. Neuroscience 105(4):1007–1018. Epub 2001/09/01
Komine O, Yamanaka K (2015) Neuroinflammation in motor neuron disease. Nagoya J Med Sci 77(4):537–549
Kooij G, Mizee MR, van Horssen J, Reijerkerk A, Witte ME, Drexhage JA, van der Pol SM, van Het Hof B, Scheffer G, Scheper R, Dijkstra CD, van der Valk P, de Vries HE (2011) Adenosine triphosphate-binding cassette transporters mediate chemokine (C-C motif) ligand 2 secretion from reactive astrocytes: relevance to multiple sclerosis pathogenesis. Brain 134(Pt 2):555–570. doi:10.1093/brain/awq330
Kortekaas R, Leenders KL, van Oostrom JC, Vaalburg W, Bart J, Willemsen AT, Hendrikse NH (2005) Blood-brain barrier dysfunction in parkinsonian midbrain in vivo. Ann Neurol 57(2):176–179. doi:10.1002/ana.20369
Kovac A, Zilkova M, Deli MA, Zilka N, Novak M (2009) Human truncated tau is using a different mechanism from amyloid-beta to damage the blood-brain barrier. J Alzheimer’s Dis JAD 18(4):897–906. doi:10.3233/JAD-2009-1197
Kutzelnigg A, Lassmann H (2005) Cortical lesions and brain atrophy in MS. J Neurol Sci 233(1–2):55–59. Epub 2005/05/17
Kwan W, Trager U, Davalos D, Chou A, Bouchard J, Andre R et al (2012) Mutant huntingtin impairs immune cell migration in Huntington disease. J Clin Invest 122(12):4737–4747. Epub 2012/11/20
Lampron A, Elali A, Rivest S (2013) Innate immunity in the CNS: redefining the relationship between the CNS and Its environment. Neuron 78(2):214–232. Epub 2013/04/30
Larochelle C, Alvarez JI, Prat A (2011) How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett 585(23):3770–3780. doi:10.1016/j.febslet.2011.04.066. Epub 2011/05/10
Lasiene J, Yamanaka K (2011) Glial cells in amyotrophic lateral sclerosis. Neurol Res Int 2011:718987. doi:10.1155/2011/718987
Lee H, Pienaar IS (2014) Disruption of the blood-brain barrier in Parkinson’s disease: curse or route to a cure? Front Biosci 19:272–280
Li H, Ke H, Ren G, Qiu X, Weng YX, Wang CC (2009) Thermal-induced dissociation and unfolding of homodimeric DsbC revealed by temperature-jump time-resolved infrared spectra. Biophys J 97(10):2811–2819. doi:10.1016/j.bpj.2009.08.049
Lin CL, Kong Q, Cuny GD, Glicksman MA (2012) Glutamate transporter EAAT2: a new target for the treatment of neurodegenerative diseases. Future Med Chem 4(13):1689–1700. doi:10.4155/fmc.12.122
Liu R, Li JZ, Song JK, Sun JL, Li YJ, Zhou SB, Zhang TT, Du GH (2014) Pinocembrin protects human brain microvascular endothelial cells against fibrillar amyloid-beta(1-40) injury by suppressing the MAPK/NF-kappaB inflammatory pathways. Biomed Res Int 2014:470393. doi:10.1155/2014/470393
McGeer PL, McGeer EG (2011) History of innate immunity in neurodegenerative disorders. Front Pharmacol 2:77. doi:10.3389/fphar.2011.00077
Minagar A, Alexander JS (2003) Blood-brain barrier disruption in multiple sclerosis. Mult Scler 9(6):540–549
Miyazaki K, Ohta Y, Nagai M, Morimoto N, Kurata T, Takehisa Y, Ikeda Y, Matsuura T, Abe K (2011) Disruption of neurovascular unit prior to motor neuron degeneration in amyotrophic lateral sclerosis. J Neurosci Res 89(5):718–728. doi:10.1002/jnr.22594
Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T (1994) Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci Lett 180(2):147–150
Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T (1996) Interleukin (IL)-1 beta, IL-2, IL-4, IL-6 and transforming growth factor-alpha levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson’s disease. Neurosci Lett 211(1):13–16
Nicaise C, Mitrecic D, Demetter P, De Decker R, Authelet M, Boom A, Pochet R (2009) Impaired blood-brain and blood-spinal cord barriers in mutant SOD1-linked ALS rat. Brain Res 1301:152–162. doi:10.1016/j.brainres.2009.09.018
Novak M, Jakes R, Edwards PC, Milstein C, Wischik CM (1991) Difference between the tau protein of Alzheimer paired helical filament core and normal tau revealed by epitope analysis of monoclonal antibodies 423 and 7.51. Proc Natl Acad Sci U S A 88(13):5837–5841
Orr CF, Rowe DB, Mizuno Y, Mori H, Halliday GM (2005) A possible role for humoral immunity in the pathogenesis of Parkinson’s disease. Brain J Neurol 128(Pt 11):2665–2674. Epub 2005/10/13
Palmer AM (2013) Multiple sclerosis and the blood-central nervous system barrier. Cardiovasc Psychiatry Neurol 2013:530356. Epub 2013/02/13
Qosa H, LeVine H 3rd, Keller JN, Kaddoumi A (2014) Mixed oligomers and monomeric amyloid-beta disrupts endothelial cells integrity and reduces monomeric amyloid-beta transport across hCMEC/D3 cell line as an in vitro blood-brain barrier model. Biochim Biophys Acta 1842(9):1806–1815. doi:10.1016/j.bbadis.2014.06.029
Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T (2007) Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med 13(11):1359–1362. doi:10.1038/nm1653
Rogers JT, Bush AI, Cho HH, Smith DH, Thomson AM, Friedlich AL, Lahiri DK, Leedman PJ, Huang X, Cahill CM (2008) Iron and the translation of the amyloid precursor protein (APP) and ferritin mRNAs: riboregulation against neural oxidative damage in Alzheimer’s disease. Biochem Soc Trans 36(Pt 6):1282–1287. doi:10.1042/BST0361282
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX et al (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362(6415):59–62. doi:10.1038/362059a0
Rowland LP, Shneider NA (2001) Amyotrophic lateral sclerosis. N Engl J Med 344(22):1688–1700. doi:10.1056/NEJM200105313442207
Sapp E, Kegel KB, Aronin N, Hashikawa T, Uchiyama Y, Tohyama K et al (2001) Early and progressive accumulation of reactive microglia in the Huntington disease brain. J Neuropathol Exp Neurol 60(2):161–172. Epub 2001/03/29
Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT (2011) Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspect Med 1(1):a006189. doi:10.1101/cshperspect.a006189
Sharief MK (1998) Cytokines in multiple sclerosis: pro-inflammation or pro-remyelination? Mult Scler 4(3):169–173
Sheffield LG, Marquis JG, Berman NE (2000) Regional distribution of cortical microglia parallels that of neurofibrillary tangles in Alzheimer’s disease. Neurosci Lett 285(3):165–168
Sheremata WA, Minagar A, Alexander JS, Vollmer T (2005) The role of alpha-4 integrin in the aetiology of multiple sclerosis: current knowledge and therapeutic implications. CNS Drugs 19(11):909–922. Epub 2005/11/05
Shin JY, Fang ZH, Yu ZX, Wang CE, Li SH, Li XJ (2005) Expression of mutant huntingtin in glial cells contributes to neuronal excitotoxicity. J Cell Biol 171(6):1001–1012. Epub 2005/12/21
Simmons DA, Casale M, Alcon B, Pham N, Narayan N, Lynch G (2007) Ferritin accumulation in dystrophic microglia is an early event in the development of Huntington’s disease. Glia 55(10):1074–1084. Epub 2007/06/07
Simpson JE, Ince PG, Lace G, Forster G, Shaw PJ, Matthews F, Savva G, Brayne C, Wharton SB, Function MRCC, Ageing Neuropathology Study G (2010) Astrocyte phenotype in relation to Alzheimer-type pathology in the ageing brain. Neurobiol Aging 31(4):578–590. doi:10.1016/j.neurobiolaging.2008.05.015
Sospedra M, Martin R (2005) Immunology of multiple sclerosis. Annu Rev Immunol 23:683–747. doi:10.1146/annurev.immunol.23.021704.115707
Stanimirovic D, Satoh K (2000) Inflammatory mediators of cerebral endothelium: a role in ischemic brain inflammation. Brain Pathol 10(1):113–126
Streit WJ, Mrak RE, Griffin WS (2004) Microglia and neuroinflammation: a pathological perspective. J Neuroinflammation 1(1):14. doi:10.1186/1742-2094-1-14
Sui YT, Bullock KM, Erickson MA, Zhang J, Banks WA (2014) Alpha synuclein is transported into and out of the brain by the blood-brain barrier. Peptides 62:197–202. doi:10.1016/j.peptides.2014.09.018
Suo Z, Fang C, Crawford F, Mullan M (1997) Superoxide free radical and intracellular calcium mediate A beta(1-42) induced endothelial toxicity. Brain Res 762(1-2):144–152
Tai YF, Pavese N, Gerhard A, Tabrizi SJ, Barker RA, Brooks DJ et al (2007) Microglial activation in presymptomatic Huntington’s disease gene carriers. Brain J Neurol 130(Pt 7):1759–1766. Epub 2007/04/03
Teismann P, Schulz JB (2004) Cellular pathology of Parkinson’s disease: astrocytes, microglia and inflammation. Cell Tissue Res 318(1):149–161. Epub 2004/09/01
Thomas T, Thomas G, McLendon C, Sutton T, Mullan M (1996) beta-Amyloid-mediated vasoactivity and vascular endothelial damage. Nature 380(6570):168–171. doi:10.1038/380168a0
Togo T, Akiyama H, Iseki E, Kondo H, Ikeda K, Kato M, Oda T, Tsuchiya K, Kosaka K (2002) Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J Neuroimmunol 124(1-2):83–92
Tooyama I, Kimura H, Akiyama H, McGeer PL (1990) Reactive microglia express class I and class II major histocompatibility complex antigens in Alzheimer’s disease. Brain Res 523(2):273–280
Trebst C, Ransohoff RM (2001) Investigating chemokines and chemokine receptors in patients with multiple sclerosis: opportunities and challenges. Arch Neurol 58(12):1975–1980
van der Wal EA, Gomez-Pinilla F, Cotman CW (1993) Transforming growth factor-beta 1 is in plaques in Alzheimer and Down pathologies. Neuroreport 4(1):69–72
Vautier S, Fernandez C (2009) ABCB1: the role in Parkinson’s disease and pharmacokinetics of antiparkinsonian drugs. Expert Opin Drug Metab Toxicol 5(11):1349–1358. doi:10.1517/17425250903193079
Veszelka S, Toth AE, Walter FR, Datki Z, Mozes E, Fulop L, Bozso Z, Hellinger E, Vastag M, Orsolits B, Kornyei Z, Penke B, Deli MA (2013) Docosahexaenoic acid reduces amyloid-beta induced toxicity in cells of the neurovascular unit. J Alzheimer’s Dis JAD 36(3):487–501. doi:10.3233/JAD-120163
Wekerle H (2005) Immune pathogenesis of multiple sclerosis. Neurol Sci Off J Ital Neurol Soc Ital Soc Clin Neurophysiol 26(Suppl 1):S1–S2. Epub 2005/05/11
Westerlund M, Belin AC, Olson L, Galter D (2008) Expression of multi-drug resistance 1 mRNA in human and rodent tissues: reduced levels in Parkinson patients. Cell Tissue Res 334(2):179–185. doi:10.1007/s00441-008-0686-5
Wilhelmus MM, Bol JG, Van Haastert ES, Rozemuller AJ, Bu G, Drukarch B, Hoozemans JJ (2011) Apolipoprotein E and LRP1 increase early in Parkinson’s disease pathogenesis. Am J Pathol 179(5):2152–2156. doi:10.1016/j.ajpath.2011.07.021
Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA et al (1995) An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14(6):1105–1116. Epub 1995/06/01
Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E (2000) Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am J Pathol 156(1):139–150
Wyss-Coray T, Rogers J (2012) Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harbor Perspect Med 2(1):a006346. doi:10.1101/cshperspect.a006346
Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM (2007) Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53(3):337–351. doi:10.1016/j.neuron.2007.01.010
Zhao W, Beers DR, Appel SH (2013) Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J Neuroimmune Pharmacol 8(4):888–899. doi:10.1007/s11481-013-9489-x
Zhong Z, Deane R, Ali Z, Parisi M, Shapovalov Y, O’Banion MK, Stojanovic K, Sagare A, Boillee S, Cleveland DW, Zlokovic BV (2008) ALS-causing SOD1 mutants generate vascular changes prior to motor neuron degeneration. Nat Neurosci 11(4):420–422. doi:10.1038/nn2073
Zilka N, Stozicka Z, Kovac A, Pilipcinec E, Bugos O, Novak M (2009) Human misfolded truncated tau protein promotes activation of microglia and leukocyte infiltration in the transgenic rat model of tauopathy. J Neuroimmunol 209(1-2):16–25. doi:10.1016/j.jneuroim.2009.01.013
Zlokovic BV (2008) The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 57(2):178–201. doi:10.1016/j.neuron.2008.01.003
Zlokovic BV (2011) Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 12(12):723–738. doi:10.1038/nrn3114
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Majerova, P., Kovac, A. (2017). Pathophysiology of the Blood–Brain Barrier in Neuroinflammatory Diseases. In: Lyck, R., Enzmann, G. (eds) The Blood Brain Barrier and Inflammation. Progress in Inflammation Research. Springer, Cham. https://doi.org/10.1007/978-3-319-45514-3_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-45514-3_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45512-9
Online ISBN: 978-3-319-45514-3
eBook Packages: MedicineMedicine (R0)