Abstract
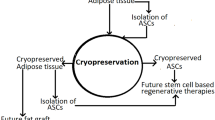
Adipose-Derived Stromal/Stem Cells (ASC) have considerable potential for regenerative medicine due to their abilities to proliferate, differentiate into multiple cell lineages, high cell yield, relative ease of acquisition, and almost no ethical concerns since they are derived from adult tissue. Storage of ASC by cryopreservation has been well described that maintains high cell yield and viability, stable immunophenotype, and robust differentiation potential post-thaw. This ability is crucial for banking research and for clinical therapeutic purposes that avoid the morbidity related to repetitive liposuction tissue harvests. ASC secrete various biomolecules such as cytokines which are reported to have immunomodulatory properties and therapeutic potential to reverse symptoms of multiple degenerative diseases/disorders. Nevertheless, safety regarding the use of these cells clinically is still under investigation. This chapter focuses on the different aspects of cryopreserved ASC and the methods to evaluate their functionality for future clinical use.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Adipose-derived stromal/stem cells
- Regenerative medicine
- Adipose tissue
- Human cells
- Cell differentiation
- Immunophenotype
- Cryopreservation
- Clinical trials
11.1 Introduction
Regenerative medicine aims to restore tissue or organ form and function with the use of cell therapy , organ transplant, tissue engineering among other techniques and the combination of biological tissues with natural or synthetic materials [1]. Mesenchymal stem cells (MSC) including Adipose-Derived Stromal/Stem Cells (ASC) are multipotent with the ability to differentiate into tissues of mesodermal origin (bone, cartilage and fat). This property, associated to other mechanisms such as the release of paracrine factors, scavenging of reactive oxygen species, immunomodulatory function are ASC mechanisms contribute for tissue regeneration and possible translational applications [2–5]. Adipose tissue is a useful source for stromal/stem cells with no ethical restriction given its adult tissue origin and accessibility, targeting, ease of collection, ability to expand, maintenance in culture, and ability to contribute to tissue regeneration after implantation [6]. The Stromal Vascular Fraction (SVF), the uncultured heterogeneous cell population obtained after adipose tissue digestion, have also increasing research interest due to less manipulation related to cell culture and immunomodulatory effects [7, 8].
The potential challenge to the clinical translation of MSC-based products is the diversity in how MSC are defined. This can be based on multiple criteria, including the tissue source, methods for processing/manufacturing, cell surface marker expression and other in vitro and in vivo characteristics as described in regulatory submissions to the FDA [9]. Current Good Manufacturing Practice (cGMP) protocols for generating clinical-grade cells are necessary before beginning a clinical trial based on guidance documents [10]. Donor tissue and cell products must be screened for viral agents (such as HIV, Hepatitis C in case of allogenic use), aerobic and anaerobic bacteria, endotoxin, and mycoplasma. Cell biological characterization and product quality control must be included. The manufacturing facility must meet and be routinely monitored based on rigorous standards which include documentation of all processes based on established and validated standard operating procedures [11]. In this chapter we discuss the critical aspects for the clinical use of ASC for regenerative/reconstruction purposes.
11.2 Adipose-Derived Stromal/Stem Cells Cryopreservation
Long-term cryopreservation is fundamental for a reliable product supply for research or clinical purposes. Current cryopreservation techniques already allow easier access and use of the products for clinical use such as blood transfusion, bone marrow transplantation , in vitro fertilization, vascular grafts, bone grafts and skin grafts [12]. This knowledge can be transferred for ASC cryopreservation and some principles can be kept such as addition of cryoprotective agents (CPA) before controlled freezing at which the cells are stored , rapidly thaw cells and remove the CPA for use [13]. Simply freezing a cell or tissue is not enough to maintain its long-term viability since ice crystal formation will damage the cells by direct disruption or due to the increase in solute concentrations as water within the cytoplasm is sequestered [14]. Permeable CPA prevent this outcome by entering the cell and equilibrating across cell membranes where it replaces the lost water and maintains an acceptable volume allowing cell survival [15]. Dimethyl sulfoxide (DMSO) is the most commonly employed permeable CPA. Bone marrow and blood cell product cryopreservation routinely combines the use of albumin and DMSO; however, DMSO can be clinically associated with toxicity including genotypic changes, impaired differentiation potentiality, and symptomatic side effects in the recipient, thus making dilution or washing steps to reduce or remove DMSO fundamental to protect against such complications [16, 17]. Alternative permeable CPA include glycerol, ethylene glycol and propylene glycol. Although these do not cause the same degree of toxicity as DMSO, none has displayed equivalent or better cell viability post-thaw after cryopreservation. Impermeable CPA act extracellularly to prevent ice crystal formation and protect against plasma membrane damage. Impermeable CPAs include both low molecular weight compounds (glucose, sucrose, trehalose, hydroxyethylstarch/HES) and high molecular weight macromolecular polymers (dextran sulfate/DS, methylcellulose/MC, Polyvinylpyrollidone/PVP) [18, 19].
ASC isolation, expansion and cryopreservation methods typically use animal or human products such as serum [20]. Historically, serum is employed as a source of proteins and chemicals promoting cell growth, attachment, and neutralization of toxins and oxidants. Bovine or human serum derived products added to DMSO or other low molecular weight chemical CPA serves as a source of additional high molecular weight impermeable colloid CPA; however, even with rigorous screening for common infectious agents (such as HIV, hepatitis B or C), human serum proteins remain a potential source of bacterial, prion, or viral contaminants [21]. Consequently, it is important to establish xeno-free defined protocols capable of preserving cell function post-thaw to levels comparable to that seen in freshly isolated tissue at the various steps during ASC processing in order to avoid contamination risks associated with human or animal cell culture and/or cryopreservation components [4, 9–11, 22, 23]. While autologous donor serum or platelet-derived supplements have potential as acceptable alternatives, an optimal human serum reagent would need to have any antibodies or complement proteins depleted to remove any risk of cell damage [24].
Systematic analyses combining different CPA to optimize a CPA solution’s function could minimize cytotoxicity and provide either an allogenic or xenogeneic serum-free cryopreservation solution. Published studies have begun to pursue such an approach [24]. After storage in liquid nitrogen, 10 % PVP in the absence of serum or DMSO at concentrations as low as 2 % maintained post-thaw cell viability at levels close to those displayed by freshly isolated cells [17]. The favorable outcomes using DMSO and/or PVP were not observed with the use of glycerol or MC as CPA, which resulted in sub-optimal cryopreservation based on in vitro assays [25, 26]. Trehalose has been used to preserve not only cells but also intact tissues (including pancreatic islets, skin, oocytes, testis, and lipoaspirate fragments) that displayed functional viability when transplanted in vivo [27]. With the aim to avoid the use of xenogenic product, a CPA solution with antioxidants (reduced glutathione and ascorbic acid), polymers (PVA and ficoll), permeating CPA (ethylene glycol/EG and DMSO), a disaccharide (trehalose), and a calcium chelator (EGTA) were added to HEPES-buffered DMEM/F12 and ASC retained their multipotency and chromosomal normality [20].
The optimization of CPA solution has so far focused on the clinical translation of adipose tissue and cells for regenerative medical applications and the complete characterization of the cells is of fundamental importance for product development, regulatory approval and patient safety [17, 25, 26, 28–31]. Extensive published studies have addressed optimized approaches to SVF and ASC cryopreservation based on in vitro assays. In vitro assays include adipogenic, osteogenic and chondrogenic differentiation, cell apoptosis , glycerophosphate dehydrogenase (GPDH) enzyme activity, cell proliferation rate, surface immunophenotype (FACS) and Magnetic Cell Sorting (MACS), adipose-derived cell recovery rates, viability (MTT), karyotype and gene expression [20, 28, 29, 31, 32]. The vast majority of the protocols utilize storage in liquid nitrogen after controlled rate cooling [20, 28, 31–34] (Table 11.1).
11.3 Why ASC Cryopreservation Remains a Relevant Research Question
Recent studies have found that the age, body mass index, and overall health condition of donors impacts the functionality of isolated ASC. The ASC from elderly donors expressed elevated levels of senescence markers, oxidative stress, lower antioxidant enzyme (superoxide dimutase) activity, increased cell doubling times and reduced clonogenic potential [35, 36]. The differentiation potential of ASC especially towards osteogenic and chondrogenic pathways was compromised from the aged donors whereas the adipogenic ability tended to increase [35, 36]. The Angiogenic differentiation potential and the ability to secrete pro-angiogenic growth factors such as ANGPT1, HGF, and VEGF, was also hampered in ASC isolated from older donors [37, 38]. Furthermore, ASC isolated from the obese individuals display impaired functionality. The therapeutic efficacy of ASC from obese donors to treat multiple sclerosis was decreased in comparison to ASC from lean donors [39]. The osteogenic potential of ASC from obese donors was also reduced relative to ASC from lean donors [39]. Collectively, these published findings suggest that ASC from young, healthy, lean donors will have the greatest potential for efficacy in clinical applications . However, most of the patients who benefit from ASC therapies are elderly with co-morbidities associated with obesity. Thus, their autologous ASC may not display the desired therapeutic benefits, making such patients dependent on the use of allogeneic ASC. Consequently, cryopreservation of ASC from young and healthy individuals may emerge as an opportunity to create a new cell product, similar to type O erythrocytes that can be transplanted to multiple recipients with minimal risk and maximal benefit. For these reasons, it remains important to develop cryopreservation protocols that retain the post thaw functionality of ASC while minimizing risks of infectious agent contamination or transmission .
11.4 Pre-clinical Models and Clinical Use
Despite variation in processing methods, isolated ASC have displayed regenerative, anti-inflammatory and immunomodulatory potential. Studies in animal models have determined the beneficial effects of ASC in the healing of Acute Myocardial Infarction, Chronic Ulcers, Peripheral Vascular Disease, and Bone defects among other conditions [11, 40–44]. There remain challenges to the development of clinical grade quality cell products without biological contaminants, with high cell viability , low product variability and constant and reproducible potency [45]. In addition to the impact of cryopreservation and thawing on the cell characteristics, studies must consider the final success of the ASC when administered to the recipient in vivo [32]. For example, the intravenous infusion of MSC has been found to modulate the cytokines released by resident lung immune cells [46]. Furthermore, the gene expression profile can change after MSC have been exposed to the host/recipient [32]. Thus, pre-clinical animal models are critically necessary to define the mechanisms of action and to identify the ideal method for cell transplantation [45].
The existing body of basic science investigations has contributed to an increased number of clinical trials using ASC for Soft Tissue Reconstruction, Osteoarticular Defects, and Cardiovascular, Pulmonary and Neurological diseases, among others (www.clinicaltrials.org); however, at present, there are no U.S. FDA-approved ASC-based products (Table 11.2).
11.5 Potential Reconstructive and Chronic Disease Targets for ASC Therapies
11.5.1 Soft Tissue Reconstruction
Fat grafting is a useful tool for soft tissue reconstruction to repair volume loss or improve shape. Recently, the use of Cell Assisted Lipotransfer (CAL), which combines autologous SVF cells with fat grafts, has gained considerable interest among plastic surgeons internationally. The SVF cell enriched fat graft contains increased numbers of ASC and associated cells which have been reported to improve breast reconstruction outcomes by increasing volume retention and reducing irregularities or retractions [47, 48]. Facial atrophy [49] and other craniofacial malformations [50] classically treated with fat grafts have also displayed improved outcomes when treated with CAL.
11.5.2 Hard Tissue Reconstruction
Although autologous or allogeneic bone graft remains the bone regeneration “gold standard” for treatment, this approach is flawed due to volume limitation and morbidity associated with the tissue harvesting. Thus, ongoing efforts are underway to seek an improved solution [42]. Published studies have achieved the successful reconstruction of large mandibular defects using a tissue-engineered construct containing beta-tricalcium phosphate, recombinant bone morphogenetic protein and Good Manufacturing Practice-level autologous ASC allowing for full functional recover and patient rehabilitation [51]. Likewise, similar approaches have used autologous ASC to repair calvarian defects through local ossification, thereby improving the patient’s quality of life [52].
11.5.3 Multiple Sclerosis
Administration of SVF cells has ameliorated the severity of experimental autoimmune encephalitis (EAE) in mice (an animal model of Multiple Sclerosis) by decreasing the magnitude of the pro-inflammatory cytokines interleukin (IL-12) and interferon gamma (IFN-γ) which contribute to the progression of the disease [53]. Furthermore, administration of culture expanded ASC after the establishment of the disease could still alleviate the signs and symptoms associated with chronic EAE [54]. In contrast, bone marrow-derived mesenchymal stem cells (BM-MSCs) were only able cure the disease when they were injected at the initiation stage of the EAE model [55]. Currently, at least one company is sponsoring a registered clinical trial to treat multiple sclerosis using autologous SVF with an estimated enrolment of 100 participants [ClinicalTrials.gov Identifier: NCT02157064]. Their goal is to complete the study by May 2017.
11.5.4 Osteoarthritis
A phase I/II clinical study designed to test the effect of injected ASC in to intra-articular cartilage of knee in patients suffering with osteoarthritis has so far shown reported promising results [56]. This trial has enrolled 18 patients with osteoarthiritis divided into groups who received autologous ASC at one of three different cell concentrations: low (107 cells), intermediate (5 × 107 cells), and high (108 cells) respectively [56]. In the group that received high dose of ASC hyaline-like cartilage was regenerated at cartilage defects resulting in improved function and relief of knee pain without any severe side effects [56]. Another clinical trial has reported that the 14 out of 16 elderly osteoarthritis patients injected intra-articularly with ASC suspended in platelet rich plasma showed significant cartilage regeneration, reduced pain relief, and improved ambulatory function [57].
11.5.5 Crohn’s Disease
Crohn’s disease is an inflammatory disease that affects the entire digestive tract and commonly is associated with the development of fistulas. The use of ASC to treat Crohn’s disease has shown some success in a phase I clinical study [58]. Patients suffering from a perianal fistula received 2 × 107 ASC which resulted in complete healing of fistula 8 weeks post injection with no adverse events [58, 59]. There were no signs of recurrence of fistula 8 months after the ASC injection [58]. In a phase II clinical study, the same group demonstrated that administration of ASC improved recovery of fistulas in 27 out of 33 [59]. A follow up phase II trial investigation conducted in 43 patients showed the long-term sustainability of ASC treatment since 75 % of the patients maintained complete closure of the fistula without recurrence [60]. Other groups have reported similar beneficial results in phase I and II clinical trials [61, 62]. Anterogen Co., Ltd. a South Korean based company, is currently marketing an ASC based product, (Cupistem®) as an injection to treat Crohn’s disease.
11.5.6 Parkinson’s Disease
Parkinson’s disease (PD) is a progressive central nervous system disorder that results in the chronic degeneration of dopamine producing neurons in the substantia nigra region of brain [63] and the accumulation of alpha-synuclein protein aggregates called Lewy Bodies [64]. ASC transplanted into the subventicular region of the brain in a rat PD model significantly improved neurogenesis in comparison to controls [65]. Furthermore, there was an increase in the levels of brain-derived neurotrophic factor and the number of tyrosine hydroxylase and glial fibrillary acidic secreting cells resulting in betterment of motor function in the PD model rats transplanted with ASC [66]. At present a clinical study sponsored by StemGenex medical group is recruiting participants with PD to study the safety and efficacy of autologous SVF transplantation for up to 12 months [ClinicalTrials.gov Identifier: NCT02184546]. The study is expected to be complete by June 2017.
11.5.7 Diabetes
Diabetes is a chronic disease that affects the insulin secretion and glucose metabolism leading to hyperglycemia. An intravenous infusion of autologous ASC in diabetic rats significantly reduced hyperglycemia [67, 68]. The mechanism involved the regeneration of pancreatic β cells and increased insulin secretion [67]. Additionally, ASC infusion significantly lowered the level of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 in comparison to untreated diabetic controls [67, 68]. Moreover, cells in the recipient animal’s liver and adipose tissues showed improved insulin sensitivity based on enhanced expression levels of both the glucose transporter (GLUT4) and the insulin receptor (INSR) [67]. In addition to ameliorating the major diabetic symptoms such as hyperglycemia, impaired insulin secretion, and insulin resistance ASC infusion reduced other adverse effects such as diabetic nephropathy and diabetic wounds [68, 69]. Adistem Ltd is conducting phase I and II clinical studies to treat diabetes type 1 and 2 using intravenous administration of ASC [ClinicalTrials.gov Identifiers: NCT00703599; NCT00703612].
11.6 Summary
Although ASC are relatively easy to harvest, ASC cryopreservation will allow for their use as an “of the shelf” product immediately available at the bedside. Although clinical applications for ASC and SVF cells remains experimental, their future success will rely in part on the development of fully tested protocols relating to tissue acquisition, processing, cryopreservation, thawing , CPA dilution and infusion techniques. These may need to be modified depending on the target organ or tissue to be treated. These advances will require the use of internationally harmonized assays predictive of both functionality and potency.
Abbreviations
- ASC:
-
Adipose-derived stromal/stem cells
- ANGPT1:
-
Angiopoietin 1
- CAL:
-
Cell Assisted Lipotranfer
- CPA:
-
Cryoprotectant agents
- cGMP:
-
Current Good Manufacturing Practice
- DS:
-
Dextran sulfate
- DMSO:
-
Dimethyl sulfoxide
- EG:
-
Ethylene glycol
- EAE:
-
Experimental autoimmune encephalitis
- FDA:
-
U.S. Food and Drug Administration
- GLUT4:
-
Glucose transporter
- HGF:
-
Hepatocyte growth factor
- HES:
-
Hydroxyethylstarch
- INSR:
-
Insulin receptor
- IL-1β:
-
Interleukin 1 beta
- IL-6:
-
Interleukin 6
- IL-12:
-
Interleukin 12
- IFN-γ:
-
Interferon gamma
- MSC:
-
Mesenchymal stem cell
- SVF:
-
Stromal vascular fraction
- MC:
-
Methylcellulose
- PD:
-
Parkinson’s disease
- PVP:
-
Polyvinylpyrollidone
- TNF-α:
-
Tumor necrosis factor alpha
- VEGF:
-
Vascular endothelial growth factor
References
Jenkins DD, Yang GP, Lorenz HP et al (2003) Tissue engineering and regenerative medicine. Clin Plast Surg 30:581
Horwitz EM, Le Blanc K, Dominici M et al (2005) Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy 7:393–395
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
McIntosh KR, Frazier T, Rowan BG et al (2013) Evolution and future prospects of adipose-derived immunomodulatory cell therapeutics. Expert Rev Clin Immunol 9:175–184
Davis TA, Anam K, Lazdun Y et al (2014) Adipose-derived stromal cells promote allograft tolerance induction. Stem Cells Transl Med 3:1444–1450
Gimble JM, Bunnell BA, Frazier T et al (2013) Adipose-derived stromal/stem cells: a primer. Organogenesis 9:3–10, United States
Cawthorn WP, Scheller EL, MacDougald OA (2012) Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res 53:227–246
McIntosh K, Zvonic S, Garrett S et al (2006) The immunogenicity of human adipose-derived cells: temporal changes in vitro. Stem Cells 24:1246–1253
Mendicino M, Bailey AM, Wonnacott K et al (2014) MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell 14:141–145
Halme DG, Kessler DA (2006) FDA regulation of stem-cell-based therapies. N Engl J Med 355:1730–1735
Gimble JM, Guilak F, Bunnell BA (2010) Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther 1:19
Hunt CJ (2011) Cryopreservation of human stem cells for clinical application: a review. Transfus Med Hemother 38:107–123
Pegg DE (2015) Principles of cryopreservation. Methods Mol Biol 1257:3–19
Mazur P (1984) Freezing of living cells: mechanisms and implications. Am J Physiol 247:C125–C142
Saragusty J (2015) Directional freezing for large volume cryopreservation. Methods Mol Biol 1257:381–397
Gorin NC (1986) Collection, manipulation and freezing of haemopoietic stem cells. Clin Haematol 15:19–48
Thirumala S, Gimble JM, Devireddy RV (2010) Cryopreservation of stromal vascular fraction of adipose tissue in a serum-free freezing medium. J Tissue Eng Regen Med 4:224–232
Balci D, Can A (2013) The assessment of cryopreservation conditions for human umbilical cord stroma-derived mesenchymal stem cells towards a potential use for stem cell banking. Curr Stem Cell Res Ther 8:60–72
Pegg DE (2002) The history and principles of cryopreservation. Semin Reprod Med 20:5–13
Lopez M, Bollag RJ, Yu JC et al (2016) Chemically defined and xeno-free cryopreservation of human adipose-derived stem cells. PLoS One 11:e0152161
Brockbank KG, Heacox AE, Schenke-Layland K (2011) Guidance for removal of fetal bovine serum from cryopreserved heart valve processing. Cells Tissues Organs 193:264–273
Carvalho PP, Wu X, Yu G et al (2011) Use of animal protein-free products for passaging adherent human adipose-derived stromal/stem cells. Cytotherapy 13:594–597
Dos Santos F, Campbell A, Fernandes-Platzgummer A et al (2014) A xenogeneic-free bioreactor system for the clinical-scale expansion of human mesenchymal stem/stromal cells. Biotechnol Bioeng 111:1116–1127
Gimble JM, Bunnell BA, Chiu ES et al (2011) Concise review: adipose-derived stromal vascular fraction cells and stem cells: let’s not get lost in translation. Stem Cells 29:749–754
Thirumala S, Wu X, Gimble JM et al (2010) Evaluation of polyvinylpyrollidone as a cryoprotectant for adipose tissue-derived adult stem cells. Tissue Eng Part C Methods 16:783–792
Thirumala S, Gimble JM, Devireddy RV (2010) Evaluation of methylcellulose and dimethyl sulfoxide as the cryoprotectants in a serum-free freezing media for cryopreservation of adipose-derived adult stem cells. Stem Cells Dev 19:513–522
Pu LL, Cui X, Fink BF et al (2005) Cryopreservation of adipose tissues: the role of trehalose. Aesthet Surg J 25:126–131
Devireddy RV, Thirumala S, Gimble JM (2005) Cellular response of adipose derived passage-4 adult stem cells to freezing stress. J Biomech Eng 127:1081–1086
Goh BC, Thirumala S, Kilroy G et al (2007) Cryopreservation characteristics of adipose-derived stem cells: maintenance of differentiation potential and viability. J Tissue Eng Regen Med 1:322–324
Thirumala S, Gimble JM, Devireddy RV (2005) Transport phenomena during freezing of adipose tissue derived adult stem cells. Biotechnol Bioeng 92:372–383
Thirumala S, Zvonic S, Floyd E et al (2005) Effect of various freezing parameters on the immediate post-thaw membrane integrity of adipose tissue derived adult stem cells. Biotechnol Prog 21:1511–1524
Hoogduijn MJ, de Witte SF, Luk F et al (2016) Effects of freeze-thawing and intravenous infusion on mesenchymal stromal cell gene expression. Stem Cells Dev 25:586–597
De Rosa A, De Francesco F, Tirino V et al (2009) A new method for cryopreserving adipose-derived stem cells: an attractive and suitable large-scale and long-term cell banking technology. Tissue Eng Part C Methods 15:659–667
Gonda K, Shigeura T, Sato T et al (2008) Preserved proliferative capacity and multipotency of human adipose-derived stem cells after long-term cryopreservation. Plast Reconstr Surg 121:401–410
Choudhery MS, Badowski M, Muise A et al (2014) Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J Transl Med 12:8
Kornicka K, Marycz K, Tomaszewski KA et al (2015) The effect of age on osteogenic and adipogenic differentiation potential of human adipose derived stromal stem cells (hASCs) and the impact of stress factors in the course of the differentiation process. Oxid Med Cell Longev 2015:309169
Efimenko A, Dzhoyashvili N, Kalinina N et al (2014) Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl Med 3:32–41
Duscher D, Rennert RC, Januszyk M et al (2014) Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci Rep 4:7144
Strong AL, Bowles AC, Wise RM et al (2016) Human adipose stromal/stem cells from obese donors show reduced efficacy in halting disease progression in the experimental autoimmune encephalomyelitis model of multiple sclerosis. Stem Cells 34:614–626
Gauglitz GG, Jeschke MG (2011) Combined gene and stem cell therapy for cutaneous wound healing. Mol Pharm 8:1471–1479
Gimble JM, Grayson W, Guilak F et al (2011) Adipose tissue as a stem cell source for musculoskeletal regeneration. Front Biosci (Schol Ed) 3:69–81
Grayson WL, Bunnell BA, Martin E et al (2015) Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol 11:140–150
Sultan SM, Stern CS, Allen RJ Jr et al (2011) Human fat grafting alleviates radiation skin damage in a murine model. Plast Reconstr Surg 128:363–372
Cowan CM, Shi YY, Aalami OO et al (2004) Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol 22:560–567
Galipeau J, Krampera M, Barrett J et al (2016) International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy 18:151–159
Fischer UM, Harting MT, Jimenez F et al (2009) Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev 18:683–692
Yoshimura K, Sato K, Aoi N et al (2008) Cell-assisted lipotransfer for cosmetic breast augmentation: supportive use of adipose-derived stem/stromal cells. Aesthetic Plast Surg 32:48–55, discussion 56–47
Tissiani LA, Alonso N (2016) A prospective and controlled clinical trial on stromal vascular fraction enriched fat grafts in secondary breast reconstruction. Stem Cells Int 2016:2636454
Yoshimura K, Sato K, Aoi N et al (2008) Cell-assisted lipotransfer for facial lipoatrophy: efficacy of clinical use of adipose-derived stem cells. Dermatol Surg 34:1178–1185
Tanikawa DY, Aguena M, Bueno DF et al (2013) Fat grafts supplemented with adipose-derived stromal cells in the rehabilitation of patients with craniofacial microsomia. Plast Reconstr Surg 132:141–152
Sandor GK, Tuovinen VJ, Wolff J et al (2013) Adipose stem cell tissue-engineered construct used to treat large anterior mandibular defect: a case report and review of the clinical application of good manufacturing practice-level adipose stem cells for bone regeneration. J Oral Maxillofac Surg 71:938–950
Lendeckel S, Jodicke A, Christophis P et al (2004) Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg 32:370–373
Semon JA, Maness C, Zhang X et al (2014) Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res Ther 5:2
Constantin G, Marconi S, Rossi B et al (2009) Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells 27:2624–2635
Zappia E, Casazza S, Pedemonte E et al (2005) Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 106:1755–1761
Jo CH, Lee YG, Shin WH et al (2014) Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells 32:1254–1266
Koh YG, Choi YJ, Kwon SK et al (2015) Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc 23:1308–1316
Cho YB, Lee WY, Park KJ et al (2013) Autologous adipose tissue-derived stem cells for the treatment of Crohn’s fistula: a phase I clinical study. Cell Transplant 22:279–285
Lee WY, Park KJ, Cho YB et al (2013) Autologous adipose tissue-derived stem cells treatment demonstrated favorable and sustainable therapeutic effect for Crohn’s fistula. Stem Cells 31:2575–2581
Cho YB, Park KJ, Yoon SN et al (2015) Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med 4:532–537
Garcia-Olmo D, Garcia-Arranz M, Herreros D et al (2005) A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum 48:1416–1423
Garcia-Olmo D, Herreros D, Pascual I et al (2009) Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum 52:79–86
Braak H, Del Tredici K, Rub U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Ubeda-Banon I, Saiz-Sanchez D, de la Rosa-Prieto C et al (2010) alpha-synucleinopathy in the human olfactory system in Parkinson’s disease: involvement of calcium-binding protein- and substance P-positive cells. Acta Neuropathol 119:723–735
Schwerk A, Altschuler J, Roch M et al (2015) Human adipose-derived mesenchymal stromal cells increase endogenous neurogenesis in the rat subventricular zone acutely after 6-hydroxydopamine lesioning. Cytotherapy 17:199–214
Berg J, Roch M, Altschuler J et al (2015) Human adipose-derived mesenchymal stem cells improve motor functions and are neuroprotective in the 6-hydroxydopamine-rat model for Parkinson’s disease when cultured in monolayer cultures but suppress hippocampal neurogenesis and hippocampal memory function when cultured in spheroids. Stem Cell Rev 11:133–149
Hu J, Fu Z, Chen Y et al (2015) Effects of autologous adipose-derived stem cell infusion on type 2 diabetic rats. Endocr J 62:339–352
Fang Y, Tian X, Bai S et al (2012) Autologous transplantation of adipose-derived mesenchymal stem cells ameliorates streptozotocin-induced diabetic nephropathy in rats by inhibiting oxidative stress, pro-inflammatory cytokines and the p38 MAPK signaling pathway. Int J Mol Med 30:85–92
Zografou A, Papadopoulos O, Tsigris C et al (2013) Autologous transplantation of adipose-derived stem cells enhances skin graft survival and wound healing in diabetic rats. Ann Plast Surg 71:225–232
Disclosures
Fabiana Zanata received financial support from CAPES, Brazil (Process BEX 1524/15-1). Jeffrey Gimble and Xiying Wu are co-owners and employees of LaCell LLC, a biotechnology company focusing on research and clinical translation involving ASC and SVF cells.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Zanata, F., Shaik, S., Devireddy, R.V., Wu, X., Ferreira, L.M., Gimble, J.M. (2016). Cryopreserved Adipose Tissue-Derived Stromal/Stem Cells: Potential for Applications in Clinic and Therapy. In: Karimi-Busheri, F., Weinfeld, M. (eds) Biobanking and Cryopreservation of Stem Cells. Advances in Experimental Medicine and Biology, vol 951. Springer, Cham. https://doi.org/10.1007/978-3-319-45457-3_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-45457-3_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45455-9
Online ISBN: 978-3-319-45457-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)