Abstract
The vulnerability stress model, as related to mental disorders, has gained much attention since it captures the multifactorial nature of the disorders. In the last few years, research activities have aimed at identifying biological and genetic components that interact with psychosocial factors, such as stressful life events and city living, which according to this model increase or lower vulnerability to mental disorders. Interplay between environmental factors and biological systems (e.g., stress-axis/hypothalamus-pituitary-adrenal-axis) and clinical outcomes will be analyzed from an anticipatory perspective. Previous studies on brain structure and childhood traumatization have implicated limbic regions like the hippocampus, amygdala, anterior cingulate cortex, and frontal areas. Functional changes in those areas are proposed to mediate the long-term risk of mental disorders. The societal and clinical impact of those findings and models will be discussed.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Anticipating Depressive Disorders
Anticipation of the onset of disease is a very timely but complex topic of high relevance to the course and outcome of psychiatric treatment. Anticipatory medicine is concomitant with preventive medicine: both have the capacity to change the usual approach to mental disorders. In the last decades, numerous risk factors that contribute to the onset of mental disorders have been identified. For severe mental disorders, like schizophrenia and bipolar disorder, several environmental factors—such as antenatal maternal virus infections, obstetric complications comprising hypoxia-related conditions or stress during neurodevelopment—were already discovered. Those factors converge in the disturbance of neuro-circuits within the hippocampal [1]. Thus, knowledge of risk factors and their interaction could enable clinicians to anticipate the risk of disorders such as bipolarism and schizophrenia. Needless to state, real life is highly complex, and simple point-by-point associations are rarely observable. The complexity in mood disorders may even be higher since environmental factors seem to interact with the individual’s genetic vulnerability. In major depressive disorders, psychosocial stress and traumatization during the perinatal period, infancy, or adulthood are important risk factors. Genetic risk in terms of less adaptive biological systems may put individuals at risk of disease in stressful life situations. On the other hand, protective factors such as resiliency might increase the likelihood of the ability to cope with stressful life situations and thus facilitate better outcomes regarding mental and physical health.
2 Risk Factors
Major depressive disorders (MDD) belong to the prevalent causes of morbidity, disability, and impaired quality of life. Previous studies have also provided robust evidence that depression is a risk factor for type 2 diabetes (T2DM) [2, 3], cardiovascular disease (CVD), and mortality [4–6]. Generally, abuse and mistreatment during childhood (CA) are strong environmental risk factors for the development of depressive disorders [7, 8]. Although a great deal of research has investigated the immediate and delayed pathogenic effects of childhood abuse, only a small body of literature has focused on adaptive outcomes in the aftermath of CA [9, 10]. Yet it seems that in addition to trauma-related factors, such as frequency and intrusiveness, individual biological and psychological factors modify the risk of long-term consequences of childhood abuse [11]. Research has consistently shown that a considerable number of childhood abuse victims show little or no long-term psychological damage [12].
2.1 Resilience
Over the years the vague term “resilience” was established to describe the phenomenon mentioned above. There is no generally agreed upon definition of “resilience”. One can assign the attempted definitions to (at least) two mainstream thoughts: (1) the end result of a complex adaptation process; (2) the dispositional ability to access and use resources in the face of traumatic events. Nadin [13] describes resilience:
“the capacity to cope with unanticipated dangers after they have become manifest, learning to bounce back.” Not surprising is the inference that “anticipation seeks to preserve stability: the less fluctuation, the better. Resilience accommodates variability…”
Wagnild et al. [14, 15] related resilience to a “dynamic personality trait” arising in “the aftermath of adversity.” They acknowledged the existence of certain inherent resources, which are, however, fluid and alterable rather than determined and inflexible. In a recent empirical study, we investigated 2046 subjects between the ages of 29–89 years (SD = 13.9) from a community-based sample who were free of MDD during the 12 months prior to data collection. They had been examined and diagnosed for Lifetime diagnosis of MDD by the Munich-Composite International Diagnostic Interview (M-CIDI) according to Diagnostic and Statistical Manual of Mental Disorders–(4th edition) (DSM-IV) criteria [7]. Childhood trauma and resilience were assessed with the Childhood Trauma Questionnaire (CTQ) and the Resilience-Scale (RS-25). Both childhood maltreatment and resilience were associated with MDD later in life. The detrimental effects of low resilience on MDD were especially prominent in subjects with a history of childhood abuse (odds ratio [OR] = 3.18, 95 % confidence interval [CI] [1.84, 5.50]), but also effective in subjects without CM (OR = 2.62, 95 %CI [1.41, 4.88]). The findings supported the clinical assumption that resilient subjects may be partly protected against the detrimental long-term effects of child abuse and neglect.
2.2 Gene-Environment Interaction
To date, it has been difficult to identify genetic factors that directly put individuals at higher risk of depressive disorders. Although large-scale genetic studies with sufficient statistical power are about to be published, clear-cut genetic risk factors are missing. Nevertheless, research in the field of gene-environment interaction has provided an important model of functional and dysfunctional adaptation to stressful or averse experiences and life conditions.
Nadin [16] distinguishes the various discussions of the concept (including the areas of behavior, cognition, neurology, and in medicine in general). Generally, anticipation is described as behavior defined by possible future states as well as by previous states [17].
In the field of gene-environment interaction research, I would propose a new facet of the concept of “anticipation”: In light of the genetic variation at a given genetic risk locus, one genetic variant increases the risk and the other one decreases the risk of disease in stressful conditions. Those stressful conditions can constitute previous states like childhood abuse that still shape behavior and emotional states in adulthood. In addition, biological variation contributes to the anticipation: The environmental stressor determines in interaction with the genetic risk variant the risk of mental disorder or the degree of burden caused by psychopathological symptoms and thereby emotional states and behavior. Traditionally, “anticipation” in the field of genetics denotes to the phenomenon that “the signs and symptoms of some genetic conditions tend to become more severe and appear at an earlier age as the disorder is passed from one generation to the next” [18]. Anticipation is typically seen in disorders that are caused by a type of mutation called a trinucleotide repeat expansion. Those repeats are associated with neurological conditions like Huntington disease, myotonic dystrophy, and fragile X syndrome. This repeat expansion can increase from generation to generation leading to more severe forms of the disorder with each successive generation [18].
This effect modification has been preferentially investigated in genes involved in the physiological regulation of the stress response. A prominent example is the FKBP5 gene, which codes for a co-chaperone regulating the glucocorticoid receptor sensitivity. Previous evidence suggested that subjects carrying the TT genotype of the FKBP5 gene single nucleotide polymorphism (SNP) rs1360780 had an increased susceptibility to adverse effects of experimental stress. We therefore tested the hypothesis of an interaction of childhood abuse with rs1360780 in predicting adult depression in 2076 Caucasian subjects from the general population. We identified significant interaction (p = 0.008) of physical abuse with the TT genotype of rs1360780, increasing the depression scores (BDI-II) to 17.8 (95 % CI 12.3–23.2) compared with 10.6 (8.8–12.4) in exposed CC/CT carriers [T denotes the nucleobase thymine, C denotes cytosine]. Likewise, the adjusted odds ratio (OR) for major depressive disorders (MDD) in exposed TT carriers was 8.9 (95 % CI 2.2–37.2) compared with 1.4 (0.8–2.4) in exposed subjects with CC/CT genotypes [19]. This study revealed interactions between physical abuse and rs1360780 of the FKBP5 gene, confirming its role in an individual’s susceptibility to depression.
Given the large effect sizes, rs1360780 could be included into possibility models for depression in individuals exposed to childhood abuse [20]. Figure 1 depicts a simulation of positive and negative predictive values in carriers of FKBP5 TT-genotype exposed to “physical abuse.” At a given prevalence rate of MDD, e.g., 30 % the risk prediction (positive predictive value) by genotype and abuse status is about 70 %. Those data are encouraging. However, positive and negative predictive values are still far too low to recommend individual risk prediction via this approach. Moreover, those data need independent replication. A further refinement of this model was possible via the inclusion of the status “high or low resilience.” Considering the inclusion criteria “low resilience,” the prevalence rate of MDD increased up to 35 % in the selected subjects. Thus the positive predictive value of the screen-positive subjects (TT-carriers and child abuse) increased to 80 %.
2.3 The Serotonin Transporter (5-HTT) Gene
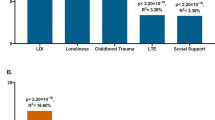
The consequences of childhood trauma and interaction with genetic polymorphisms have been investigated not only in its effects on behavior and health but also on brain structure and function in terms of neuropsychological paradigms elicited by fMRI. A “classical” genetic polymorphism in this respect is the serotonin transporter (5-HTT) gene (SLC6A4, chromosome 17q12). Accordingly, the functional promoter polymorphism of 5-HTT (5-HTTLPR) has often been examined in gene—environment interaction models. In many studies, the short (s) allele of 5-HTTLPR, which is associated with a decreased 5-HTT activity, has been associated with a negative mental outcome after exposure to stress or trauma. A large meta-analysis supported the interaction effect for depressive disorders [21]. Corresponding effects also have been reported for Post-traumatic Stress Disorder (PTSD) [22]. The putative interplay between genes and beneficial environmental conditions like social support on positive outcomes like sense of coherence or resilience has rarely been addressed. “Social support” integrates the perception and actuality that one is cared for, has assistance available from other people, and that one is part of a supportive social network. The questionnaire we have applied assesses social support in dimensions like financial support, care taking in case of illness and emotional support.
We therefore examined the interaction between the serotonin transporter linked polymorphic region (5-HTTLPR) and social support on the sense of coherence (SOC), resilience, and depressive symptoms. Among individuals with the perception of high social support, no significant differences between 5-HTTLPR genotypes regarding all outcome variables were found. However, among those with the perception of low social support, carriers of at least one short allele reported significantly increased levels of SOC and resilience, as well as fewer depressive symptoms than carriers of the l/l genotype. We therefore concluded that in less supportive social environments the impact of distinct genotypes on behavioral outcomes might be more relevant than in supportive environments where social compensation might take place [23]. Morphometrical and functional MRI analyses have demonstrated reduced gray matter volume in short-allele carriers in limbic regions critical for processing negative emotion [24, 25]. Moreover, an amygdala-cingulate feedback circuit critical for emotion regulation was dependent on the polymorphism of the 5-HTTLPR [26]. The magnitude of coupling between amygdala-anterior cingulate cortex inversely predicted almost 30 % of variation in temperamental anxiety [27]. Those data support the view that genetic polymorphisms may alter the brain’s structure and function and thereby behavior, too. Environmental exposure to stress may affect those neuro-circuits that are emotionally responsive and may trigger pathological functions in susceptible subjects.
3 Individualized Medicine
The conference Anticipation and Medicine prompted several participants (Staiger, Burk, Golubnitschaja, Nadin, among others) to approach this subject. (See respective articles in this volume.) Individualized medicine aims to provide optimal treatment for an individual patient at a given time based on his specific genetic and molecular characteristics [28]. Individualized diagnostic and therapeutic strategies are considered means to improve the efficacy and safety of patient treatment. It might also allow for better individual outcome prediction and risk assessment. Moreover, individualized prevention and early intervention strategies are conceivable. Recently developed high throughput OMICs technologies are thought to enable more targeted diagnostic and treatment approaches [26]. The different conditions and experimental evidence presented so far in this chapter clearly indicate the involvement of psychosocial factors in the prediction of health or disease, even in interaction with biological markers. Therefore, we clearly argue for a broader acknowledgement of traumatization and stress, as well as protective factors such as resiliency, when aiming at anticipating outcomes and modeling the risk structure of diseases. The importance of those factors also advocates early behavioral psychosocial intervention in families with high levels of childhood maltreatment or neglect. Costs to society and the individual’s burden of disease could be reduced by early supportive and protective interventions. The consideration of psychosocial and behavioral factors in disease models and treatment algorithms is important not only to psychiatry, but also to various medical disciplines. Recognizing the earliest symptoms and, even better, the pre-symptomatic indicators of disease is anticipatory medicine in practice, leading to more efficient treatment.
References
Schmitt, A., Malchow, B., Hasan, A., Falkai, P.: The impact of environmental factors in severe psychiatric disorders. Front. Neurosci. 8, 19 (2014)
Carnethon, M.R., Biggs, M.L., Barzilay, J.I., Smith, N.L., Vaccarino, V., Bertoni, A.G., et al.: Longitudinal association between depressive symptoms and incident type 2 diabetes mellitus in older adults: the cardiovascular health study. Arch. Intern. Med. 167(8), 802–807 (2007)
Knol, M., Twisk, J., Beekman, A., Heine, R., Snoek, F., Pouwer, F.: Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia 49, 837–845 (2006)
Vaccarino, V., McClure, C., Johnson, B.D., Sheps, D.S., Bittner, V., Rutledge, T., et al.: Depression, the metabolic syndrome and cardiovascular risk. Psychosom. Med. 70, 40–48 (2008)
Barth, J., Schumacher, M., Herrmann-Lingen, C.: Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom. Med. 66, 802–813 (2004)
Carney, R.M., Freedland, K.E., Miller, G.E., Jaffe, A.S.: Depression as a risk factor for cardiac mortality and morbidity: A review of potential mechanisms. J. Psychosom. Res. 53, 897–902 (2002)
Schulz, A., Becker, M., Van der Auwera, S., Barnow, S., Appel, K., Mahler, J., et al.: The impact of childhood trauma on depression: does resilience matter? Population-based results from the Study of Health in Pomerania. J. Psychosom. Res. 77, 97–103 (2014)
Grabe, H.J., Schwahn, C., Appel, K., Mahler, J., Schulz, A., Spitzer, C., et al.: Childhood maltreatment, the corticotropin-releasing hormone receptor gene and adult depression in the general population. Am. J. Med. Genet Part B. 153, 1483–1493 (2010)
Wingo, A.P., Wrenn, G., Pelletier, T., Gutman, A.R., Bradley, B., Ressler, K.J.: Moderating effects of resilience on depression in individuals with a history of childhood abuse or trauma exposure. J. Affect. Dis. 126, 411–414 (2010)
Ungar, M., Liebenberg, L., Dudding, P., Armstrong, M., van de Vijver, F.J.: Patterns of service use, individual and contextual risk factors, and resilience among adolescents using multiple psychosocial services. Child Abuse Negl. 37, 150–159 (2013)
Collishaw, S., Pickles, A., Messer, J., Rutter, M., Shearer, C., Maughan, B.: Resilience to adult psychopathology following childhood maltreatment: evidence from a community sample. Child Abuse Negl. 31, 211–229 (2007)
Werner, E.E., Smith, R.S., French, F.E.: Kauai’s children come of age: a longitudinal study from the prenatal period to age ten. In: Werner, E.E., Smith, R.S., French, F.E. (eds.). University of Hawaii Press, Honolulu (1971)
Nadin, M.: Anticipation: a spooky computation. In: Dubois, D. (ed.) CASYS, International Journal of Computing Anticipatory Systems, Partial Proceedings of CASYS 99, vol. 6, pp. 3–47. CHAOS, Liege, Belgium (2000)
Wagnild, G.M.: The Resilience Scale Users Guide. The Resilience Center, Worden, MT (2009)
Wagnild, G.M., Young, H.M.: Development and psychometric evaluation of the resilience scale. J. Nurs. Meas. 1, 165–178 (1993)
Nadin, M.: Annotated bibliography: anticipation. In: Klir, G. (ed.) Special Issue of International Journal of General Systems, vol. 39:1, pp. 34–133. Taylor and Blackwell, London (2010)
Nadin, M. (ed.): Anticipation and Medicine. Springer, Cham CH. (2016)
US National Library of Medicine. https://ghr.nlm.nih.gov/primer/inheritance/anticipation
Appel, K., Schwahn, C., Mahler, J., Schulz, A., Spitzer, C., Fenske, K., et al.: Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacol 36, 1982–1991 (2011)
Grabe, H.J., Schwahn, C.: Interaction between psychosocial environments and genes—what is the clinical relevance? Psych. Prax. 38, 55–57 (2011)
Karg, K., Burmeister, M., Shedden, K., Sen, S.: The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch. Gen. Psychiatry 68, 444–454 (2011)
Grabe, H.J., Spitzer, C., Schwahn, C., Marcinek, A., Frahnow, A., Barnow, S., et al.: Serotonin transporter gene (SLC6A4) promoter polymorphisms and the susceptibility to posttraumatic stress disorder in the general population. Am. J. Psych. 166, 926–933 (2009)
Reinelt, E., Barnow, S., Stopsack, M., Aldinger, M., Schmidt, C.O., John, U., et al.: Social support and the serotonin transporter genotype (5-HTTLPR) moderate levels of resilience, sense of coherence, and depression. Am. J. Med. Genet Part B. 168, 383–391 (2015)
Fisher, P.M., Grady, C.L., Madsen, M.K., Strother, S.C., Knudsen, G.M.: 5-HTTLPR differentially predicts brain network responses to emotional faces. Hum. Brain. Map. 36, 2842–2851 (2015)
Dannlowski, U., Kugel, H., Redlich, R., Halik, A., Schneider, I., Opel, N., et al.: Serotonin transporter gene methylation is associated with hippocampal gray matter volume. Hum. Brain. Map. 35, 5356–5367 (2014)
Selvaraj, S., Godlewska, B.R., Norbury, R., Bose, S., Turkheimer, F., Stokes, P., et al.: Decreased regional gray matter volume in S’ allele carriers of the 5-HTTLPR triallelic polymorphism. Mol. Psychiatry. 16(471), 2–3 (2011)
Pezawas, L., Meyer-Lindenberg, A., Drabant, E.M., Verchinski, B.A., Munoz, K.E., Kolachana, B.S., et al.: 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 8, 828–834 (2005)
Grabe, H.J., Assel, H., Bahls, T., Dorr, M., Endlich, K., Endlich, N., et al.: Cohort profile: Greifswald approach to individualized medicine (GANI_MED). J Transl. Med. 12, 144 (2014)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Grabe, H.J. (2017). Environment, Genes, and Mental Health. In: Nadin, M. (eds) Anticipation and Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-45142-8_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-45142-8_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45140-4
Online ISBN: 978-3-319-45142-8
eBook Packages: EngineeringEngineering (R0)