Abstract
A central task of the tricarboxylic acid (TCA, Krebs, citric acid) cycle in brain is to provide precursors for biosynthesis of glutamate, GABA, aspartate and glutamine. Three of these amino acids are the partners in the intricate interaction between astrocytes and neurons and form the so-called glutamine–glutamate (GABA) cycle. The ketoacids α-ketoglutarate and oxaloacetate are removed from the cycle for this process. When something is removed from the TCA cycle it must be replaced to permit the continued function of this essential pathway, a process termed anaplerosis. This anaplerotic process in the brain is mainly carried out by pyruvate carboxylation performed by pyruvate carboxylase. The present book chapter gives an introduction and overview into this carboxylation and additionally anaplerosis mediated by propionyl-CoA carboxylase under physiological conditions in the adult and in the developing rodent brain. Furthermore, examples are given about pathological conditions in which anaplerosis is disturbed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
3.1 Introduction
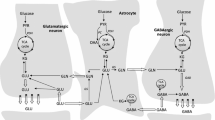
The major fuel for the brain is glucose and relevant metabolic pathways are shown in Fig. 3.1. Glucose metabolism is necessary for glutamate synthesis which is essential for brain function since the blood–brain barrier prevents glutamate from entering the brain. Synthesis of this excitatory amino acid neurotransmitter in neurons is particularly important since 90 % of synapses in the forebrain are glutamatergic (Attwell and Laughlin 2001) and glutamate is the precursor of the other major amino acid neurotransmitter, GABA. The synthesis of the carbon skeleton of these neurotransmitters is dependent on the production of the anaplerotic substrate oxaloacetate from glucose (Fig. 3.2). Sir Hans Kornberg coined the expression: anaplerotic sequences to describe a series of enzymatic reactions or pathways that replenish the pools of metabolic intermediates in the tricarboxylic acid (TCA) cycle. Since both glutamate and GABA are derived from the TCA cycle intermediate α-ketoglutarate the anaplerotic reactions securing the synthesis of α-ketoglutarate in brain are important. However, because of the coupling of this process with cataplerosis, i.e. the exit of intermediates from the cycle, both pathways are equally important for the regulation of amino acid, glucose and fatty acid homeostasis (Sonnewald 2014).
Pathways for glucose metabolism . Gln glutamine, Glu glutamate, KG ketoglutarate, ME malic enzyme, GS glutamine synthetase, NADPH nicotinamide adenine dinucleotide phosphate, PAG phosphate-activated glutaminase, PC pyruvate carboxylase, PCC propionyl-CoA carboxylase, PDH pyruvate dehydrogenase, PPP pentose phosphate pathway, TCA tricarboxylic acid
Schematic presentation of reactions pertinent to pyruvate carboxylation . *In the brain ME appear to operate primarily in the decarboxylation direction, see Section 3.2. KG ketoglutarate, ME malic enzyme, PC pyruvate carboxylase, PDH pyruvate dehydrogenase, PEPCK phosphoenolpyruvate carboxykinase, PK pyruvate kinase
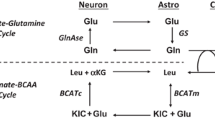
One role of this cycle in all mammalian cells is the oxidation of acetyl-CoA to carbon dioxide and energy production. However, in brain synthesis of precursors for the neurotransmitters glutamate, GABA and aspartate are also an essential part of its function. Pyruvate carboxylase (PC) is the enzyme with major responsibility for oxaloacetate production from glucose and thus anaplerosis in brain (Mohler et al. 1974; Patel 1974b; Kanamatsu and Tsukada 1999). However, pyruvate carboxylation and neurotransmitter synthesis and release do not occur in the same compartment (Fig. 3.3). In the brain only astrocytes express PC (Yu et al. 1983; Shank et al. 1985; Cesar and Hamprecht 1995) but the two amino acid neurotransmitters are produced in the neurons. This dependence of neurons on astrocytes has been investigated extensively in the adult brain of animals and humans (McKenna et al. 2012) and is defined by the details of the glutamate–glutamine cycle (Fig. 3.3) which can also encompass GABA (Berl and Clarke 1983). Using 13C magnetic resonance spectroscopy (MRS) and 13C-labelled glucose it has been shown that pyruvate carboxylation occurs in humans, rats and mice in vivo as well as in vitro in neural tissue and cell preparations (Badar-Goffer et al. 1990; Shank et al. 1993; Hassel and Sonnewald 1995; Griffin et al. 1999; Waagepetersen et al. 2001a; Rae et al. 2005; Mason et al. 2007). An excellent review article gives an overview over the different MRS studies performed to evaluate the relationship between pyruvate carboxylation and dehydrogenation (Hertz 2011). In order to perform de novo synthesis of glutamate, an anaplerotic substrate must be transferred from astrocytes to neurons, since the latter cell type does not contain PC. In contrast to glutamate, glutamine can safely be released to the extracellular milieu without a disturbing interaction with receptors for the two mentioned amino acid neurotransmitters. When glutamine is released from astrocytes into the synapse it is taken up by specific transporters into neurons (Varoqui et al. 2000) where it can be converted to glutamate by phosphate activated glutaminase (PAG) (Hogstad et al. 1988). As can be seen in Fig. 3.3, after release in the neurotransmission process, glutamate is taken up via specialized, high affinity transporters located primarily in astrocytes (Danbolt et al. 1992; Danbolt 2001). In astrocytes , glutamate is either rapidly converted to glutamine via the enzyme glutamine synthetase (GS), localized only in astrocytes (Norenberg and Martinez-Hernandez 1979), or converted to α-ketoglutarate, thereby entering the TCA cycle (McKenna et al. 1996; Schousboe et al. 2014). This sequence of processes is termed the glutamate–glutamine cycle (Fig. 3.3) (references in (McKenna et al. 2012)), which is essential in order to ensure precise neural signalling, preserve carbon atoms for neuronal glutamate synthesis and limit excitotoxicity which may result from excessive glutamate receptor stimulation (Danbolt 2001). Only limited information is available on the above-mentioned processes in the neonatal brain. However, there are indications that the glutamate–glutamine cycle at this stage does not operate as a cycle, but rather as a delivery system of glutamine from astrocytes to neurons, since transport of glutamate from neurons to astrocytes is negligible while that of glutamine from astrocytes to neurons is higher than in the adult brain (Morken et al. 2013; Brekke et al. 2014).
The glutamine–glutamate cycle . AMPA α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, A,B glutamine transport, C,D glutamate transport, E conversion of glutamate to glutamine, GS glutamine synthetase, NADPH nicotinamide adenine dinucleotide phosphate, NMDA N-methyl-d-aspartate receptor, PAG phosphate activated glutaminase, PC pyruvate carboxylase, PCC propionyl-CoA carboxylase, PDH pyruvate dehydrogenase, mGluR metabotropic glutamate receptors
3.2 Carboxylation in the Brain
There are several enzymes that theoretically would have the potential for catalysing carboxylation processes in the brain (Fig. 3.2) such as pyruvate carboxylase (PC), propionyl-CoA carboxylase, phosphoenolpyruvate carboxykinase (PEPCK) and malic enzyme (ME). The latter three enzymes are present both in astrocytes and neurons (Kurz et al. 1993; Alves et al. 1995; McKenna et al. 1995, 2000; Vogel et al. 1998a, b). However, in the brain PEPCK and ME appear to operate primarily if not exclusively in the decarboxylation direction (Kurz et al. 1993; Alves et al. 1995; McKenna et al. 1995, 2000; Vogel et al. 1998a, b).
3.3 Propionyl CoA Carboxylation
The anaplerotic pathway from propionyl-CoA via methylmalonyl-CoA to succinyl-CoA (Fig. 3.2) is known to operate in peripheral tissues. Anaplerotic molecules metabolized to propionyl-CoA and the propionyl-CoA carboxylase pathway include the branched chain amino acids, isoleucine and valine (Bak et al. 2009), propionate and molecules containing fatty acids with an uneven number of carbon atoms, such as triheptanoin (Borges and Sonnewald 2012). This pathway has been shown to be active in brain and supplementation of diet with triheptanoin has been attempted as a treatment against epilepsy (Willis et al. 2010; Borges and Sonnewald 2012; Kim et al. 2013; Marin-Valencia et al. 2013; Smeland et al. 2013).
3.4 Pyruvate Carboxylation
3.4.1 Pyruvate Carboxylation in the Adult
Pyruvate carboxylase is an ATP-dependent mitochondrial enzyme catalysing the carboxylation of pyruvate to form oxaloacetate. It is a nuclear encoded homotetramer found in most tissue from eukaryotes and in many prokaryotes and is a member of the family of biotin-dependent carboxylases (Wallace et al. 1998). In the brain it is involved in various anabolic pathways such as lipogenesis and the synthesis of neurotransmitter substances (Wallace et al. 1998; Schousboe et al. 2015). A defect in the expression or biotinylation of pyruvate is a rare autosomal recessively inherited disorder in humans resulting in developmental delays and failure to thrive starting in the neonatal or early infantile period, early death, or severe debilitating psychomotor retardation (Wallace et al. 1998; Schiff et al. 2006).
While the cellular location of PC in astrocytes but not neurons (Yu et al. 1983) is not disputed, pyruvate carboxylation has been suggested to take place also in neurons (Hassel 2001; Merle et al. 2002). However, a whole range of experiments, using different preparations, in different laboratories have essentially unequivocally confined this carboxylation to glia. Primary mono-cultures of neurons and astrocytes were used to obtain information about the cellular location and magnitude of pyruvate carboxylation using 13C labelled precursors. However, the interpretation of results obtained in these studies may be ambiguous since oxaloacetate can be converted not only to citrate but also via several steps to the symmetrical molecule fumarate (back cycling) and subsequently from oxaloacetate and citrate (cycling). The latter pathway will lead to scrambling of label resulting in misinterpretation of some labelling results. Merle et al. (2002) suggested incomplete back cycling for astrocytes and complete back cycling for cerebellar neurons. If back cycling in neurons is indeed complete, the study mentioned in the previous sentence, utilizing [1-13C]glucose, was not capable of showing carboxylation in neurons. In order to clarify this, Waagepetersen et al. (2001a) have performed a study using [U-13C]glucose or [U-13C]lactate and 3-nitropropionic acid to specifically block the TCA cycle at the succinate dehydrogenase step (Alston et al. 1977). In this way multiple cycling of TCA cycle constituents was avoided and carboxylation could be clearly detected in labelling of the C-3 position, regardless of back cycling. Analysing the 13C labelling patterns in amino acids, pyruvate carboxylation was detected in astrocytes but not in neurons (Waagepetersen et al. 2001a). Furthermore, also in studies of brain tissue slices and cell cultures carboxylation was detected in astrocytes (Griffin et al. 1998, 2003; Waagepetersen et al. 2001a). In a very elegant study using unrestrained awake rats which were continuously infused with a combination of H14CO3 − and [1-13C]glucose in over 50 infusions ranging from 5 to 60 min it was shown that synthesis of glutamine from H14CO3 − was substantial, amounting to 32 % of the glutamate/glutamine cycle (Xu et al. 2004).
3.4.2 Pyruvate Carboxylation in the Neonate
Also in the neonatal brain pyruvate carboxylation is predominantly localized in glia since the level of [2,3-13C]glutamine derived from [1,2-13C]glucose is higher than that of [2,3-13C]glutamate (Morken et al. 2013; Brekke et al. 2014). As pointed out earlier, synthesis of the neurotransmitters glutamate and GABA is coupled to pyruvate carboxylation and this is particularly important in development when neurotransmitter levels increase. GABA levels are only slightly lower at postnatal day 7 (P7) compared to later in life, but glutamate has a sharp increase early in brain development (Chowdhury et al. 2007; Morken et al. 2013). In humans, glutamate levels double from gestational week 32 until term and continue to increase during the first year of life (Kreis et al. 2002). In rats, glutamate levels double between P7 and adulthood. This increase in de novo synthesis of glutamate must happen via anaplerosis catalysed by PC.
PC activity in the rat brain is very low at birth, but increases sharply during the first weeks of life (Larsson et al. 1985), coinciding with the major period of gliogenesis (Schousboe 1972; Bandeira et al. 2009). During the first days of life, the activity of the TCA cycle-related enzymes is also low (Wilbur and Patel 1974; Larsson et al. 1985). Thus, at the time of birth, astrocytes may not have the same capacity as in the adult brain for metabolic support of neurons but the relative contribution of PC to pyruvate metabolism might still be significant. Indeed, a larger part of the available glucose is channelled into this pathway in the P7 rat than in the adult (Morken et al. 2013; Brekke et al. 2014). In fact, for every glucose molecule metabolized through glycolysis to become pyruvate, half is metabolized via pyruvate dehydrogenase, and the other half is used for anaplerosis in the P7 rat (Morken et al. 2013; Brekke et al. 2014).
Since pyruvate carboxylation occurs in astrocytes, it is possible to calculate the relative glucose utilization in astrocytes compared to neurons at the level of pyruvate using results from experiments with [1,2-13C]glucose injection. This was calculated to be 34 ± 3 % of glucose metabolism at the level of pyruvate in the P7 rat brain (Morken et al. 2013; Brekke et al. 2014). This is in surprisingly good accordance with results from the adult brain based on a corresponding equation using [U-13C]glucose. Qu et al. (2000) found that astrocytes metabolize maximally 34 % of glucose at the level of pyruvate in the adult brain, and similar results have been reported by Hassel et al. (1995). This could indicate that in rats both astrocytes and neurons have lower glucose metabolism at P7 compared to adult animals. However, the distribution between nonneuronal cells and neurons changes considerably from P7 until adult age, since the number of astrocytes increases dramatically in this period to outnumber neuronal cells at adult age (Baburamani et al. 2013; Bandeira et al. 2009). This may suggest that each astrocyte has a higher metabolic rate relative to each neuron in the neonatal brain compared to later in life.
The exchange of glutamate and glutamine between neurons and astrocytes in rats is radically different in the neonatal brain compared to the adult brain (Morken et al. 2013; Brekke et al. 2014). The necessary transporters for glutamine to enter neurons are present from late gestation (Weiss et al. 2003) (Fig. 3.3B) and the number of glutamine transporters on astrocytes actually reaches higher levels than the adult brain on postnatal day 14 (Boulland et al. 2003) (Fig. 3.3A). However, the expression of astrocytic glutamate transporters (GLT-1 and GLAST) is low early in brain development (Danbolt 2001) (Fig. 3.3E), indicating that the ability of astrocytes to take up glutamate from the synapse may be limited in the neonatal brain. Indeed, the transport of newly synthesized glutamine from astrocytes to glutamatergic neurons is higher in the P7 rat brain than the adult rat brain (Morken et al. 2013, 2014) while the reciprocal transfer of glutamate from neurons to astrocytes was very low at P7 compared to adults. The latter may also be due to low release of glutamate from neurons (Fig. 3.3D) at this stage and/or low rate of conversion into glutamine in the astrocytes (Fig. 3.3F). In the neonatal rat brain the action potentials are smaller in amplitude and the firing rate is lower (McCormick and Prince 1987) and the EEG does not develop an adult pattern until P12 (Snead and Stephens 1983). Moreover, glutamine synthetase activity, just like PC activity, is very low at birth, but increases sharply during the first weeks of life (Wilbur and Patel 1974; Hertz et al. 1978; Juurlink et al. 1981; Larsson et al. 1985). Thus, several factors may lead to this difference regarding astrocyte–neuron interactions in the neonatal and the adult brain.
3.5 What Factors Affect Pyruvate Carboxylation?
3.5.1 Increased Pyruvate Carboxylation
Cerebral hyperammonemia is believed to play a pivotal role in the development of hepatic encephalopathy (HE) , a debilitating condition arising due to acute or chronic liver disease (Butterworth 2003). In the brain, ammonia is thought to be detoxified via the activity of the astrocytic enzyme glutamine synthetase. However, glutamine cannot be synthesized without increased activity of PC as pointed out earlier. Zwingmann (2007) showed that an elevated ammonia concentration increased pyruvate carboxylation both in astrocytes in culture and in animal models. This carboxylation might be coupled to the increased glutamine formation which is a hallmark of ammonia detoxification (Zwingmann 2007). Glutamine formation is the only efficient method of ammonia fixation in brain and also in other cell culture studies increased production of glutamine was detected (Leke et al. 2011). It may be of interest that upon inhibition of the glutamine synthesizing enzyme glutamine synthetase ammonia fixation is brought about by the concerted action of glutamate dehydrogenase and alanine aminotransferase leading to net synthesis of alanine, a process stimulating glycolysis to provide pyruvate for alanine production (Dadsetan et al. 2011, 2013).
The ketogenic diet is a high-fat, adequate-protein, low-carbohydrate diet that is used primarily to treat refractory epilepsy in children (Yudkoff et al. 2004). The diet causes an increase in the metabolism of fatty acids rather than carbohydrates. The effect of ketone bodies on pyruvate carboxylation by rat brain mitochondria has been investigated (Patel 1974a) and it was shown that pyruvate carboxylation increased subsequent to feeding rats a ketogenic diet (Melø et al. 2006). This increase in pyruvate carboxylation was coupled to an elevated pyruvate recycling (Melø et al. 2006), i.e. conversion of oxaloacetate to pyruvate which re-enters the TCA cycle via pyruvate dehydrogenase, as expected since synthesis of a metabolite has to be equalled by its degradation.
3.5.1.1 Neurotransmission
Activation of the glutamatergic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor induces large post-synaptic action potentials (Rao and Finkbeiner 2007) and should therefore stimulate release of neurotransmitter glutamate. In experiments with brain tissue slices metabolizing [1-13C]glucose it was possible to show evidence of pyruvate carboxylation in aspartate which is obtained by transamination of oxaloacetate, the product of pyruvate carboxylation by pyruvate carboxylase (Rae et al. 2009). Increased pyruvate carboxylation was detected when the slices were incubated with AMPA (Rae et al. 2009) suggesting that increased neuronal activity increases carboxylation of pyruvate, a finding compatible with the notion that glutamate biosynthesis requires anaplerosis coupled to pyruvate carboxylation. Furthermore, it was shown that pyruvate carboxylation in the awake rat brain was several fold higher than under deep pentobarbital anaesthesia (Oz et al. 2004).
3.5.2 Decreased Pyruvate Carboxylation
3.5.2.1 Anxiety and Epilepsy
Decreased pyruvate carboxylation has been reported after pentylenetetrazole (PTZ) injection in rats (Eloqayli et al. 2004). PTZ administration is a generally accepted model for epileptic seizures but when given in low doses PTZ can cause anxiety (Eloqayli et al. 2004). Interestingly, the antiepileptic drug carbamazepine (CBZ) decreased the biotin concentration in both humans and rats and decreased pyruvate carboxylase activity in rat brain (Rathman et al. 2003). These results support the use of biotin supplementation as a concurrent strategy during CBZ administration to help maintain pyruvate carboxylation.
3.5.2.2 Ischaemia
Not surprisingly, ischaemia has a profound effect on pyruvate carboxylation, a pathway dependent on adenosine triphosphate availability. In adult rats subjected to 120 min of middle cerebral artery occlusion followed by 120 min of reperfusion it could be shown that the utilization of precursors in astrocytic metabolism originating from the pyruvate carboxylase pathway was markedly reduced compared to that of precursors originating from the pyruvate dehydrogenase pathway. Hence, glutamate synthesis was reduced and that of GABA completely stopped in the ischaemic core area (Håberg et al. 2006). Furthermore, in the re-perfused penumbra, glutamatergic and GABAergic neurons used relatively more astrocytic metabolites derived from the pyruvate carboxylase pathway than in the ischaemic core (Håberg et al. 2006). In the canine cardiac arrest model of global ischaemia, pyruvate carboxylation was decreased in the hippocampus in animals that had undergone hyperoxic resuscitation (Scafidi et al. 2009).
Interestingly, in the Rice–Vannucci model of neonatal hypoxia–ischaemia in postnatal day 7 rats, PC activity is relatively preserved compared to that of the pyruvate dehydrogenase pathway. Labelling via pyruvate dehydrogenase was reduced by 44 % while labelling via pyruvate carboxylase was reduced by 30 % in the hypoxic–ischaemic hemisphere in the Rice–Vannucci model (Brekke et al. 2014) while, as pointed out, in adult models of middle cerebral artery occlusion, PC activity is more profoundly reduced than pyruvate dehydrogenase activity (Haberg, Qu et al. 1998, 2006). This illustrates how the neonatal and adult brain can respond differently to similar insults and may need different treatments.
The consequences of relatively preserved PC activity in the neonatal brain following hypoxia–ischaemia may be ambiguous. As mentioned earlier there is negligible transport of glutamate from neurons to astrocytes during normal conditions in the neonatal brain (Morken et al. 2014). During hypoxia–ischaemia, there is a large release of glutamate that may originate from neurons as well as astrocytes through reversal of glutamate transporters (Puka-Sundvall, Sandberg et al. 1997; Kusaka et al. 2004). However, no increase in transfer of glutamate from neurons to astrocytes was found following neonatal hypoxia–ischaemia at postnatal day 7 in rats. Both glutamate uptake and glutamine synthesis are energy demanding processes, which may explain the lacking augmentation in transfer following hypoxia–ischaemia. Contradicting this, transfer of glutamine from astrocytes to neurons as well as glutamine synthesis and PC activity, which are ATP demanding processes were preserved at this time point following injury suggesting that astrocytes have at least partly maintained or re-established their energy balance (Morken et al. 2014). It is conceivable that preservation of anaplerosis and transfer of newly synthesized glutamine to neurons in the immediate recovery phase following hypoxia–ischaemia may have harmful effects, but this remains to be investigated. On the other hand, following the massive release of glutamate into the extracellular space during hypoxia–ischaemia, neurons might be depleted of TCA cycle intermediates (such as α-ketoglutarate). Under these conditions anaplerosis might be beneficial since de novo synthesized glutamine transferred from astrocytes may be necessary for neurons to re-establish oxidative metabolism.
Surprisingly, at 6 h after hypoxia–ischaemia the transfer of glutamate from neurons to astrocytes was transiently decreased, even though at this point, the energy levels are almost back to normal (Yager et al. 1992). The exact mechanism of this has yet to be explored.
3.5.2.3 Glutamate Exposure
Qu et al. (2001) showed that exogenous glutamate had an inhibitory effect on pyruvate carboxylation in astrocytes, presumably by formation of oxaloacetate from α-ketoglutarate derived from glutamate metabolism. Inhibition of pyruvate carboxylation was also observed when glutamine was added to astrocytes (Hassel and Sonnewald 2002). When metabolism of 13C-labelled glucose was investigated in cultured mouse cerebellar astrocytes, it could be shown that carboxylation of pyruvate was more important for biosynthesis of releasable glutamine and citrate, compared with their intracellular pools (Waagepetersen et al. 2001b). Activation of the cellular energy sensor, AMP-activated protein kinase (AMPK) , has been shown to reduce the metabolism of glutamate in the TCA cycle of astrocytes. Specifically, the synthesis of citrate from glutamate involving malic enzyme and pyruvate carboxylase was reduced. Since AMPK is known to upregulate catabolic processes, this may suggest that glutamate has an anabolic function in astrocytes (Voss et al. 2015).
3.5.2.4 Alzheimer’s Disease
Regional hypometabolism of glucose in the brain is a hallmark of Alzheimer’s disease (AD) (Gibson et al. 1998, 1999; Huang et al. 2003; Klivenyi et al. 2004). However, little is known about the specific alterations of neuronal and astrocytic metabolism involved in homeostasis of glutamate and GABA in AD. In a recent study, the effects of amyloid β pathology on energy and neurotransmitter metabolism were investigated in the transgenic McGill-R-Thy1-APP rat model of AD, at age 15 months (Nilsen et al. 2014). Rats were injected with [1-13C]glucose and [1,2-13C]acetate, and brain extracts of the hippocampal formation as well as several cortical regions were analysed using 1H- and 13C nuclear magnetic resonance spectroscopy and high performance liquid chromatography. A decrease of pyruvate carboxylation in the hippocampal formation and retrosplenial/cingulate cortex was observed in the McGill-R-Thy1-APP rat and this decrease might have affected the level of glutamine in the hippocampal formation and levels of glutamate, glutamine, GABA and aspartate in the retrosplenial/cingulate cortex (Nilsen et al. 2014). Future treatment of AD patients might be targeted at increasing anaplerosis in the brain.
Abbreviations
- AD:
-
Alzheimer’s disease
- AMPA:
-
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- AMPK:
-
AMP activated protein kinase
- CBZ:
-
Carbamazepine
- GS:
-
Glutamine synthetase
- ME:
-
Malic enzyme
- MRS:
-
Magnetic resonance spectroscopy
- P:
-
Postnatal day
- PAG:
-
Phosphate activated glutaminase
- PC:
-
Pyruvate carboxylase
- PCC:
-
Propionyl-CoA carboxylase
- PDH:
-
Pyruvate dehydrogenase
- PEPCK:
-
Phosphoenolpyruvate carboxykinase
- PPP:
-
Pentose phosphate pathway
- PTZ:
-
Pentylenetetrazole
- TCA:
-
Tricarboxylic acid
References
Alston TA, Mela L, Bright HJ (1977) 3-Nitropropionate, the toxic substance of Indigofera, is a suicide inactivator of succinate dehydrogenase. Proc Natl Acad Sci U S A 74:3767–3771
Alves PM, McKenna MC, Sonnewald U (1995) Lactate metabolism in mouse brain astrocytes studied by [13C]NMR spectroscopy. Neuroreport 6:2201–2204
Attwell D, Laughlin SB (2001) An energy budget for signaling in the grey matter of the brain. J Cerebr Blood Flow Metab 21:1133–1145
Baburamani AA, Lo C, Castillo-Melendez M, Walker DW (2013) Morphological evaluation of the cerebral blood vessels in the late gestation fetal sheep following hypoxia in utero. Microvasc Res 85:1–9
Badar-Goffer RS, Bachelard HS, Morris PG (1990) Cerebral metabolism of acetate and glucose studied by 13C-n.m.r. spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem J 266:133–139
Bak LK, Iversen P, Sorensen M, Keiding S, Vilstrup H, Ott P, Waagepetersen HS, Schousboe A (2009) Metabolic fate of isoleucine in a rat model of hepatic encephalopathy and in cultured neural cells exposed to ammonia. Metab Brain Dis 24:135–145
Bandeira F, Lent R, Herculano-Houzel S (2009) Changing numbers of neuronal and non-neuronal cells underlie postnatal brain growth in the rat. Proc Natl Acad Sci U S A 106:14108–14113
Berl S, Clarke DD (1983) The metabolic compartmentation concept. In: Hertz L et al (eds) Glutamine, glutamate and GABA in the central nervous system. Alan R. Liss, Inc., New York, pp 205–217
Borges K, Sonnewald U (2012) Triheptanoin—a medium chain triglyceride with odd chain fatty acids: a new anaplerotic anticonvulsant treatment? Epilepsy Res 100:239–244
Boulland J-L, Rafiki A, Levy LM, Storm-Mathisen J, Chaudhry FA (2003) Highly differential expression of SN1, a bidirectional glutamine transporter, in astroglia and endothelium in the developing rat brain. Glia 41:260–275
Brekke EM, Morken TS, Wideroe M, Haberg AK, Brubakk AM, Sonnewald U (2014) The pentose phosphate pathway and pyruvate carboxylation after neonatal hypoxic-ischemic brain injury. J Cereb Blood Flow Metab 34:724–734
Butterworth RF (2003) Hepatic encephalopathy. Alcohol Res Health 27:240–246
Cesar M, Hamprecht B (1995) Immunocytochemical examination of neural rat and mouse primary cultures using monoclonal antibodies raised against pyruvate carboxylase. J Neurochem 64:2312–2318
Chowdhury GM, Patel AB, Mason GF, Rothman DL, Behar KL (2007) Glutamatergic and GABAergic neurotransmitter cycling and energy metabolism in rat cerebral cortex during postnatal development. J Cereb Blood Flow Metab 27:1895–1907
Dadsetan S, Bak LK, Sorensen M, Keiding S, Vilstrup H, Ott P, Leke R, Schousboe A, Waagepetersen HS (2011) Inhibition of glutamine synthesis induces glutamate dehydrogenase-dependent ammonia fixation into alanine in co-cultures of astrocytes and neurons. Neurochem Int 58:482–488
Dadsetan S, Kukolj E, Bak LK, Sorensen M, Ott P, Vilstrup H, Schousboe A, Keiding S, Waagepetersen HS (2013) Brain alanine formation as an ammonia-scavenging pathway during hyperammonemia: effects of glutamine synthetase inhibition in rats and astrocyte-neuron co-cultures. J Cereb Blood Flow Metab 33:1235–1241
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105
Danbolt NC, Storm-Mathisen J, Kanner BI (1992) An [Na+ + K+]coupled L-glutamate transporter purified from rat brain is located in glial cell processes. Neuroscience 51:295–310
Eloqayli H, Dahl CB, Gotestam KG, Unsgard G, Sonnewald U (2004) Changes of glial-neuronal interaction and metabolism after a subconvulsive dose of pentylenetetrazole. Neurochem Int 45:739–745
Gibson GE, Sheu KF, Blass JP (1998) Abnormalities of mitochondrial enzymes in Alzheimer disease. J Neural Transm 105:855–870
Gibson GE, Park LC, Zhang H, Sorbi S, Calingasan NY (1999) Oxidative stress and a key metabolic enzyme in Alzheimer brains, cultured cells, and an animal model of chronic oxidative deficits. Ann N Y Acad Sci 893:79–94
Griffin JL, Rae C, Dixon RM, Radda GK, Matthews PM (1998) Excitatory amino acid synthesis in hypoxic brain slices: does alanine act as a substrate for glutamate production in hypoxia? J Neurochem 71:2477–2486
Griffin JL, Rae C, Radda GK, Matthews PM (1999) Lactate-induced inhibition of glucose catabolism in guinea pig cortical brain slices. Neurochem Int 35:405–409
Griffin JL, Keun H, Richter C, Moskau D, Rae C, Nicholson JK (2003) Compartmentation of metabolism probed by [2-13C]alanine: improved 13C NMR sensitivity using a CryoProbe detects evidence of a glial metabolon. Neurochem Int 42:93–99
Håberg A, Qu H, Haraldseth O, Unsgard G, Sonnewald U (1998) In vivo injection of [1-13C]glucose and [1,2-13C]acetate combined with ex vivo 13C nuclear magnetic resonance spectroscopy: a novel approach to the study of middle cerebral artery occlusion in the rat. J Cereb Blood Flow Metab 18(11):1223–1232
Håberg A, Qu H, Sonnewald U (2006) Glutamate and GABA metabolism in transient and permanent middle cerebral artery occlusion in rat: importance of astrocytes for neuronal survival. Neurochem Int 48:531–540
Hassel B (2001) Pyruvate carboxylation in neurons. J Neurosci Res 66:755–762
Hassel B, Sonnewald U (1995) Glial formation of pyruvate and lactate from TCA cycle intermediates: implications for the inactivation of transmitter amino acids? J Neurochem 65:2227–2234
Hassel B, Sonnewald U (2002) Effects of potassium and glutamine on metabolism of glucose in astrocytes. Neurochem Res 27:167–171
Hassel B, Sonnewald U, Fonnum F (1995) Glial-neuronal interactions as studied by cerebral metabolism of [2-13C]acetate and [1-13C]glucose: an ex vivo 13C NMR spectroscopic study. J Neurochem 64:2773–2782
Hertz L (2011) Astrocytic energy metabolism and glutamate formation—relevance for 13C-NMR spectroscopy and importance of cytosolic/mitochondrial trafficking. Magn Reson Imaging 29:1319–1329
Hertz L, Bock E, Schousboe A (1978) GFA content, glutamate uptake and activity of glutamate metabolizing enzymes in differentiating mouse astrocytes in primary cultures. Dev Neurosci 1:226–238
Hogstad S, Svenneby G, Torgner IA, Kvamme E, Hertz L, Schousboe A (1988) Glutaminase in neurons and astrocytes cultured from mouse brain: kinetic properties and effects of phosphate, glutamate, and ammonia. Neurochem Res 13:383–388
Huang HM, Zhang H, Xu H, Gibson GE (2003) Inhibition of the alpha-ketoglutarate dehydrogenase complex alters mitochondrial function and cellular calcium regulation. Biochim Biophys Acta 1637:119–126
Juurlink BH, Schousboe A, Jorgensen OS, Hertz L (1981) Induction by hydrocortisone of glutamine synthetase in mouse primary astrocyte cultures. J Neurochem 36:136–142
Kanamatsu T, Tsukada Y (1999) Effects of ammonia on the anaplerotic pathway and amino acid metabolism in the brain: an ex vivo 13C NMR spectroscopic study of rats after administering [2-13C]] glucose with or without ammonium acetate. Brain Res 841:11–19
Kim TH, Borges K, Petrou S, Reid CA (2013) Triheptanoin reduces seizure susceptibility in a syndrome-specific mouse model of generalized epilepsy. Epilepsy Res 103:101–105
Klivenyi P, Starkov AA, Calingasan NY, Gardian G, Browne SE, Yang L, Bubber P, Gibson GE, Patel MS, Beal MF (2004) Mice deficient in dihydrolipoamide dehydrogenase show increased vulnerability to MPTP, malonate and 3-nitropropionic acid neurotoxicity. J Neurochem 88:1352–1360
Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Huppi PS (2002) Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med 48:949–958
Kurz GM, Wiesinger H, Hamprecht B (1993) Purification of cytosolic malic enzyme from bovine brain, generation of monoclonal antibodies, and immunocytochemical localization of the enzyme in glial cells of neural primary cultures. J Neurochem 60:1467–1474
Kusaka T, Matsuura S, Fujikawa Y, Okubo K, Kawada K, Namba M, Okada H, Imai T, Isobe K, Itoh S (2004). Relationship between cerebral interstitial levels of amino acids and phosphorylation potential during secondary energy failure in hypoxic-ischemic newborn piglets. Pediatr Res 55(2):273–279
Larsson OM, Drejer J, Kvamme E, Svenneby G, Hertz L, Schousboe A (1985) Ontogenetic development of glutamate and GABA metabolizing enzymes in cultured cerebral cortex interneurons and in cerebral cortex in vivo. Int J Dev Neurosci 3:177–185
Leke R, Bak LK, Anker M, Melo TM, Sorensen M, Keiding S, Vilstrup H, Ott P, Portela LV, Sonnewald U, Schousboe A, Waagepetersen HS (2011) Detoxification of ammonia in mouse cortical GABAergic cell cultures increases neuronal oxidative metabolism and reveals an emerging role for release of glucose-derived alanine. Neurotox Res 19:496–510
Marin-Valencia I, Good LB, Ma Q, Malloy CR, Pascual JM (2013) Heptanoate as a neural fuel: energetic and neurotransmitter precursors in normal and glucose transporter I-deficient (G1D) brain. J Cereb Blood Flow Metab 33:175–182
Mason GF, Petersen KF, de Graaf RA, Shulman GI, Rothman DL (2007) Measurements of the anaplerotic rate in the human cerebral cortex using 13C magnetic resonance spectroscopy and [1-13C] and [2-13C] glucose. J Neurochem 100:73–86
McCormick DA, Prince DA (1987) Post-natal development of electrophysiological properties of rat cerebral cortical pyramidal neurones. J Physiol 393:743–762
McKenna MC, Tildon JT, Stevenson JH, Huang X, Kingwell KG (1995) Regulation of mitochondrial and cytosolic malic enzymes from cultured rat brain astrocytes. Neurochem Res 20:1491–1501
McKenna MC, Sonnewald U, Huang X, Stevenson J, Zielke HR (1996) Exogenous glutamate concentration regulates the metabolic fate of glutamate in astrocytes. J Neurochem 66:386–393
McKenna MC, Stevenson JH, Huang X, Tildon JT, Zielke CL, Hopkins IB (2000) Mitochondrial malic enzyme activity is much higher in mitochondria from cortical synaptic terminals compared with mitochondria from primary cultures of cortical neurons or cerebellar granule cells. Neurochem Int 36:451–459
McKenna M, Gruetter R, Sonnewald U, Waagepetersen HS, Schousboe A (2012) Energy metabolism of the brain. In: Brady ST, Siegel GJ, Albers RW, Price DL (eds) Basic neurochemistry: principles of molecular, cellular, and medical neurobiology, 8th edn. Elsevier Academic, Oxford, pp 200–231
Melø TM, Nehlig A, Sonnewald U (2006) Neuronal-glial interactions in rats fed a ketogenic diet. Neurochem Int 48:498–507
Merle M, Bouzier-Sore AK, Canioni P (2002) Time-dependence of the contribution of pyruvate carboxylase versus pyruvate dehydrogenase to rat brain glutamine labelling from [1-(13)C]glucose metabolism. J Neurochem 82:47–57
Mohler H, Patel AJ, Balazs R (1974) Metabolic compartmentation in the brain: metabolism of a tricarboxylic acid cycle intermediate, (1,4-14C)succinate, after intracerebral administration. J Neurochem 23:1281–1289
Morken TS, Brekke E, Haberg A, Wideroe M, Brubakk AM, Sonnewald U (2013) Neuron-astrocyte interactions, pyruvate carboxylation and the pentose phosphate pathway in the neonatal rat brain. Neurochem Res 39(3):556–569
Morken TS, Brekke E, Haberg A, Wideroe M, Brubakk AM, Sonnewald U (2014) Altered astrocyte-neuronal interactions after hypoxia-ischemia in the neonatal brain in female and male rats. Stroke 45:2777–2785
Nilsen LH, Witter MP, Sonnewald U (2014) Neuronal and astrocytic metabolism in a transgenic rat model of Alzheimer’s disease. J Cereb Blood Flow Metab 34:906–914
Norenberg MD, Martinez-Hernandez A (1979) Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res 161:303–310
Oz G, Berkich DA, Henry PG, Xu Y, LaNoue K, Hutson SM, Gruetter R (2004) Neuroglial metabolism in the awake rat brain: CO2 fixation increases with brain activity. J Neurosci 24:11273–11279
Patel MS (1974a) The effect of ketone bodies on pyruvate carboxylation by rat brain mitochondria. J Neurochem 23:865–867
Patel MS (1974b) The relative significance of CO2-fixing enzymes in the metabolism of rat brain. J Neurochem 22:717–724
Puka-Sundvall M, Sandberg M, Hagberg H (1997) Brain injury after hypoxia-ischemia in newborn rats: relationship to extracellular levels of excitatory amino acids and cysteine. Brain Res 750(1–2):325–328
Qu H, Haberg A, Haraldseth O, Unsgard G, Sonnewald U (2000) (13)C MR spectroscopy study of lactate as substrate for rat brain. Dev Neurosci 22:429–436
Qu H, Eloqayli H, Unsgard G, Sonnewald U (2001) Glutamate decreases pyruvate carboxylase activity and spares glucose as energy substrate in cultured cerebellar astrocytes. J Neurosci Res 66:1127–1132
Rae C, Hansen JT, Bubb WA, Bröer S, Bröer A (2005) Alanine transport, metabolism and cycling in the brain. Proc Intl Soc Magn Reson Med 2481
Rae C, Nasrallah F, Bröer S (2009) Metabolic effects of blocking lactate transport in brain cortical tissue slices using an inhibitor specific to MCT1 and MCT2. Neurochem Res 34:1783–1791
Rao VR, Finkbeiner S (2007) NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci 30:284–291
Rathman SC, Gregory JF III, McMahon RJ (2003) Pharmacological biotin supplementation maintains biotin status and function in rats administered dietary carbamazepine. J Nutr 133:2857–2862
Scafidi S, O’Brien J, Hopkins I, Robertson C, Fiskum G, McKenna M (2009) Delayed cerebral oxidative glucose metabolism after traumatic brain injury in young rats. J Neurochem 109(Suppl 1):189–197
Schiff M, Levrat V, Acquaviva C, Vianey-Saban C, Rolland MO, Guffon N (2006) A case of pyruvate carboxylase deficiency with atypical clinical and neuroradiological presentation. Mol Genet Metab 87:175–177
Schousboe A (1972) Development of potassium effects on ion concentrations and indicator spaces in rat brain-cortex slices during postnatal ontogenesis. Exp Brain Res 15:521–531
Schousboe A, Scafidi S, Bak LK, Waagepetersen HS, McKenna MC (2014) Glutamate metabolism in the brain focusing on astrocytes. Adv Neurobiol 11:13–30
Schousboe A, Walls A, Bak L, Waagepetersen H (2015) Astroglia and brain metabolism: focus on energy and neurotransmitter amino acid homeostasis. In: Verkhratsky A, Parpura V (eds) Colloquium series on neuroglia in biology and medicine from physiology to disease. Morgan & Claypool Life Sciences, San Rafael, pp 1–63
Shank RP, Bennett GS, Freytag SO, Campbell GL (1985) Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res 329:364–367
Shank RP, Leo GC, Zielke HR (1993) Cerebral metabolic compartmentation as revealed by nuclear magnetic resonance analysis of D-[1-13C]glucose metabolism. J Neurochem 61:315–323
Smeland OB, Hadera MG, McDonald TS, Sonnewald U, Borges K (2013) Brain mitochondrial metabolic dysfunction and glutamate level reduction in the pilocarpine model of temporal lobe epilepsy in mice. J Cereb Blood Flow Metab 33(7):1090–1097
Snead OC III, Stephens HI (1983) Ontogeny of cortical and subcortical electroencephalographic events in unrestrained neonatal and infant rats. Exp Neurol 82:249–269
Sonnewald U (2014) Glutamate synthesis has to be matched by its degradation—where do all the carbons go? J Neurochem 131(4):399–406
Varoqui H, Zhu H, Yao D, Ming H, Erickson JD (2000) Cloning and functional identification of a neuronal glutamine transporter. J Biol Chem 275:4049–4054
Vogel R, Hamprecht B, Wiesinger H (1998a) Malic enzyme isoforms in astrocytes: comparative study on activities in rat brain tissue and astroglia-rich primary cultures. Neurosci Lett 247:123–126
Vogel R, Jennemann G, Seitz J, Wiesinger H, Hamprecht B (1998b) Mitochondrial malic enzyme: purification from bovine brain, generation of an antiserum, and immunocytochemical localization in neurons of rat brain. J Neurochem 71:844–852
Voss CM, Pajecka K, Stridh MH, Nissen JD, Schousboe A, Waagepetersen HS (2015) AMPK activation affects glutamate metabolism in astrocytes. Neurochem Res 40(12):2431–2442. doi:10.1007/s11064-015-1558-5
Waagepetersen HS, Qu H, Schousboe A, Sonnewald U (2001a) Elucidation of the quantitative significance of pyruvate carboxylation in cultured cerebellar neurons and astrocytes. J Neurosci Res 66:763–770
Waagepetersen HS, Sonnewald U, Larsson OM, Schousboe A (2001b) Multiple compartments with different metabolic characteristics are involved in biosynthesis of intracellular and released glutamine and citrate in astrocytes. Glia 35:246–252
Wallace JC, Jitrapakdee S, Chapman-Smith A (1998) Pyruvate carboxylase. Int J Biochem Cell Biol 30:1–5
Weiss MD, Derazi S, Rossignol C, Varoqui H, Erickson JD, Kilberg MS, Anderson KJ (2003) Ontogeny of the neutral amino acid transporter SAT1/ATA1 in rat brain. Brain Res Dev Brain Res 143:151–159
Wilbur DO, Patel MS (1974) Development of mitochondrial pyruvate metabolism in rat brain. J Neurochem 22:709–715
Willis S, Stoll J, Sweetman L, Borges K (2010) Anticonvulsant effects of a triheptanoin diet in two mouse chronic seizure models. Neurobiol Dis 40:565–572
Xu Y, Oz G, LaNoue KF, Keiger CJ, Berkich DA, Gruetter R, Hutson SH (2004) Whole-brain glutamate metabolism evaluated by steady-state kinetics using a double-isotope procedure: effects of gabapentin. J Neurochem 90:1104–1116
Yager JY, Brucklacher RM, Vannucci RC (1992) Cerebral energy metabolism during hypoxia-ischemia and early recovery in immature rats. Am J Physiol 262:H672–H677
Yu AC, Drejer J, Hertz L, Schousboe A (1983) Pyruvate carboxylase activity in primary cultures of astrocytes and neurons. J Neurochem 41:1484–1487
Yudkoff M, Daikhin Y, Nissim I, Lazarow A (2004) Ketogenic diet, brain glutamate metabolism and seizure control. Prostaglandins Leukot Essent Fatty Acids 70:277–285
Zwingmann C (2007) The anaplerotic flux and ammonia detoxification in hepatic encephalopathy. Metab Brain Dis 22:235–249
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Brekke, E., Morken, T.S., Walls, A.B., Waagepetersen, H., Schousboe, A., Sonnewald, U. (2016). Anaplerosis for Glutamate Synthesis in the Neonate and in Adulthood. In: Schousboe, A., Sonnewald, U. (eds) The Glutamate/GABA-Glutamine Cycle. Advances in Neurobiology, vol 13. Springer, Cham. https://doi.org/10.1007/978-3-319-45096-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-45096-4_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-45094-0
Online ISBN: 978-3-319-45096-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)